Abstract
Epithelial barriers play an important role in regulating mucosal homeostasis. Upon injury, the epithelium and immune cells orchestrate repair mechanisms that re-establish homeostasis. This process is highly regulated by protein and lipid mediators such as Annexin A1. In this review, we focus on the pro-repair properties of Annexin A1.
Keywords: annexin A1, mucosa, resolution of inflammation, wound repair
Introduction
Epithelial barriers interface external environment from tissue compartments. Thus injury to this barrier can have detrimental effects on tissue homeostasis. Efficient resealing of such injuries or wounds is critical for not only re-establishing the epithelial barrier, but also in the resolution of inflammation and restoration of mucosal homeostasis. Following injury, epithelial cells migrate and proliferate to repair denuded mucosal surfaces. Inflammatory cells are recruited to sites of injury where they not only contribute to host defense but also actively participate in repair of epithelial wounds. A number of pro-resolving mediators are released from the leukocytes as well as the epithelium into the wound bed where they orchestrate tissue repair. In this review we focus on the pro-resolving mediator, Annexin A1 that has been shown to have therapeutic effects in promoting mucosal wound repair.
ANNEXIN A1: a pro-resolving mediator
The inflammatory phase is an essential component of mucosal tissue repair. Mucosal injury is associated with rapid onset of inflammation, which increases over the subsequent few days and then subsides. The inflammatory response and wound repair are delicately balanced to ensure restoration of mucosal homeostasis. In chronic inflammatory diseases, such resolution of inflammation is compromised resulting in sustained inflammation and impaired wound healing (Fullerton and Gilroy, 2016).
Following injury pro-resolving mediators that include lipids (lipoxins, resolvins, protectins, and maresins) and proteins such as Annexin A1 (ANXA1) are released into the epithelial milieu to orchestrate clearance of inflammation, wound repair and restoration of mucosal homeostasis (Leoni et al., 2015c; Serhan, 2014). Pro-resolving mediators achieve these functions by a number of mechanisms that include decreased endothelial activation, reduced leukocyte infiltration, and activation of neutrophil apoptosis by scavenger macrophages through efferocytosis (Basil and Levy, 2016; Headland and Norling, 2015; Ortega-Gomez et al., 2013; Serhan, 2014).
ANXA1 is a 37 kDa calcium- and phospholipid-binding protein expressed in monocytes, macrophages, neutrophils and epithelial cells (Babbin et al., 2007; Flower and Rothwell, 1994). It is regulated by glucocorticoids (CGs) and has been reported to mediate their anti-inflammatory activity (Croxtall and Flower, 1994; Perretti et al., 1996; Taylor et al., 1994). GCs not only induce the ANXA1 gene but also increase secretion of the protein from existing intracellular pools by stimulating PKC activity (Solito et al., 2003).
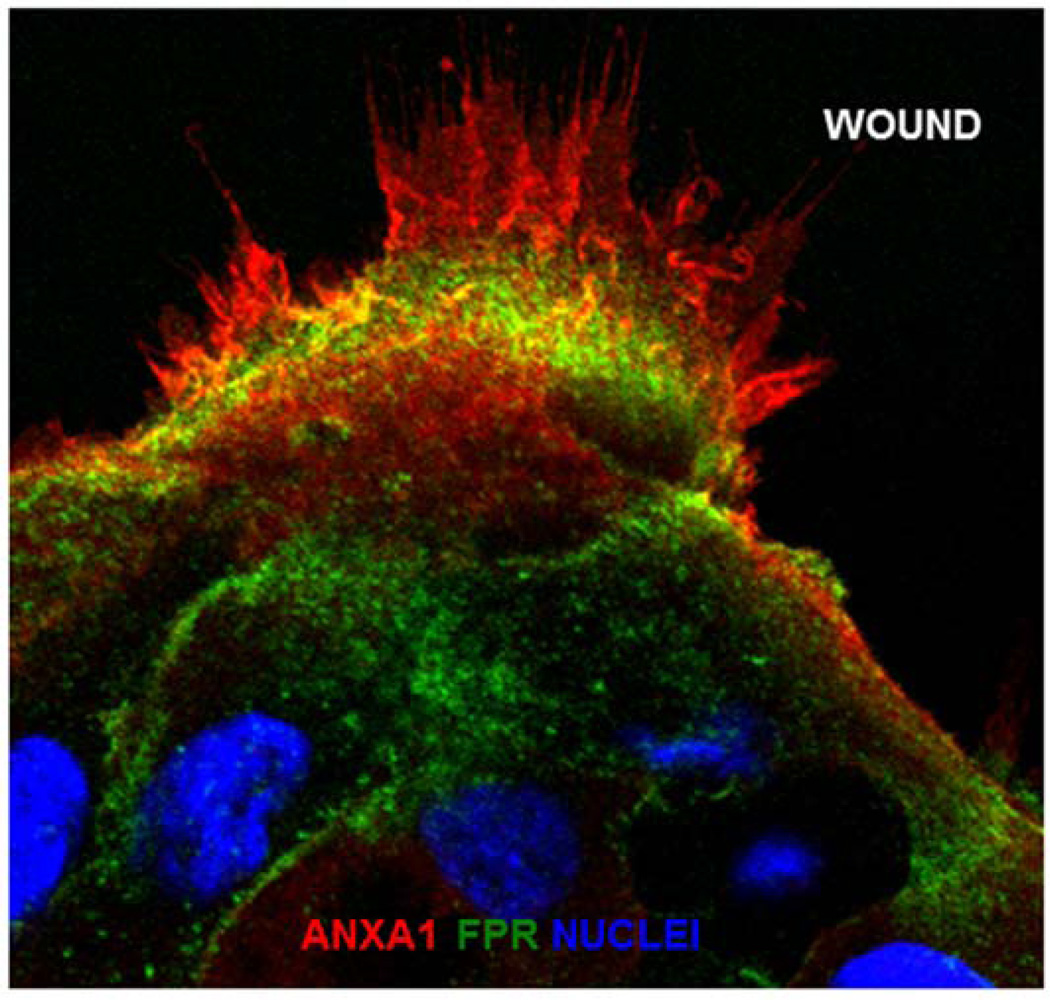
ANXA1 exerts its biological responses by activation Formyl peptide receptors (FPRs) (Perretti, 2003; Perretti and D'Acquisto, 2009). The three human FPRs (FPR1, FPR2/ALX, and FPR3) are G protein-coupled receptors that share significant sequence homology (Ye et al., 2009). While function of these receptors has been extensively explored in leukocytes, recent studies have addressed contribution of FPR signaling in mediating repair of epithelial surfaces (Alam et al., 2014; Babbin et al., 2007; Leoni et al., 2013, 2015a; Wentworth et al., 2010, 2011). Several pro- and anti- inflammatory ligands bind FPR1 and FPR2/ALX (Le et al., 2002). The biological response of the ANXA1 protein and its cleavage product Ac2-26 peptide are mediated by FPR1 and FPR2/ALX (Leoni et al., 2015a; Perretti, 2003; Perretti et al., 2002). Intestinal epithelial cells express ANXA1 and its receptors (FPR1 and FPR2/ALX). Shown in Figure 1 is the expression of these proteins at the leading edge of epithelial cells migrating to reseal a wound (Riesselman et al., 2007). Cooray and coworkers reported FPR2/ALX homo-dimerization and activation of p38 mitogen-activated protein kinase signaling by the full-length ANXA1 protein. In contrast Ac2-26 promotes hetero-dimerization of FPR1 and FPR2/ALX, and activation of c-Jun N-terminal kinase (JNK)-signaling (Cooray et al., 2013). We recently demonstrated that endogenous ANXA1 is released as a component of extracellular vesicles (EVs) derived from intestinal epithelial cells, and ANXA1 EVs activate mucosal wound repair circuits (Leoni et al., 2015b). EVs are emerging as important mechanisms of intercellular communication by transferring proteins and other cellular components to target cells. Thus, ANXA1 derived from wound associated cells, including leukocytes and epithelial cells, exerts paracrine and autocrine effects on the epithelium to facilitate wound closure and enhance barrier recovery (Babbin et al., 2006, 2008; Leoni et al., 2013). In addition to naturally occurring ANXA1 in EVs, hydrogels containing ANXA1 peptide mimetic Ac2-26 have been generated and shown to promote wound repair (Del Gaudio et al., 2015).
Figure 1.
ANXA1 expression in migrating human epithelial cells.
Representative images of human epithelial cells (SK-CO15) after scratch wound-induced injury. Frozen sections were stained with antibodies against ANXA1 (red), NFPR1 (green), and nuclei (TO-PRO-3, blue).
ANXA1 facilitates resolution of inflammation and repair by a number of mechanisms that include inhibition of leukocytes recruitment by decreasing their adhesion/recruitment and transmigration (Chatterjee et al., 2005; Getting et al., 1997; Mancuso et al., 1995). Interestingly, anti-allergic drugs referred to as cromones induce the release of ANXA1 which decreases neutrophil recruitment (Yazid et al., 2010). Hydrogen sulfide (H2S), a gaseous mediator increased during sepsis and under inflammatory conditions also promotes ANXA1 mobilization, which in turn controls leukocyte trafficking (Brancaleone et al., 2014). The existence of this positive loop between ANXA1 and H2S represents an important mechanism which harnesses the biological properties of this gaseous mediator.
McArthur and colleagues identified a novel mechanism by which ANXA1 released from apoptotic neutrophils recruits monocytes that clear apoptotic cells thereby protecting the surrounding healthy tissue (McArthur et al., 2015). ANXA1 induces a favorable macrophage M2a phenotype that release interleukin-10 and TGFβ (Li et al., 2011). In line with these results, ANXA1 administration suppressed M1 activation of liver macrophages. Interestingly, ANXA1 skewed M1 macrophages to anti-inflammatory M2-like cells, attenuating the expression of IL-6, IL-1β, and TNF-α (Li et al., 2011). Additionally, ANXA1 and its receptor FPR2/ALX have been reported to promote transition of pro-inflammatory M1 macrophages in the acute phase of renal injury to anti-inflammatory M2 macrophages in the chronic phase of disease (Locatelli et al., 2014; Zhang et al., 2012). ANXA1 and its mimetic peptide increase the clearance rate of apoptotic neutrophils by human macrophages also referred to as efferocytosis (Blume et al., 2012; Maderna et al., 2005). ANXA1-null mice provided further evidence for a functional role of ANXA1 in efferocytosis, as bone marrow derived macrophages from these mice were defective in clearance of apoptotic cells (Maderna et al., 2005). Another study confirmed the importance of ANXA1 expression on bone marrow- derived macrophages involved in the recognition and phagocytosis of apoptotic neutrophils (Dalli et al., 2012). Recent studies have identified apoptosis as an important host defense mechanism against microbial infection. ANXA1 absence is in fact correlated with reduced phagocytosis in the presence of bacterial and fungal particles (Yona et al., 2006). These properties confirm the importance of ANXA1 and Ac2-26 in mediating host defense and resolution of inflammation (Buckley et al., 2014; Serhan, 2014; Vago et al., 2016). Additionally, ANXA1 contributes to the transfer of antigens from apoptotic vesicles to dendritic cells for activation of CD8+ T cells (Tzelepis et al., 2015). ANXA1 in fact, controls the immune response to Mycobacterium tuberculosis infection (Tzelepis et al., 2015). A recent study also identified release of ANXA1 by murine macrophages expressing purigenic P2X7 receptor that contribute to its pro-resolving response (de Torre-Minguela et al., 2016). In addition to the cell types mentioned above, ANXA1 is expressed in brain microvascular endothelial cells and regulates blood-brain barrier (BBB) integrity. ANXA1 knockout mice show significantly increased BBB permeability that is associated with compromised function of endothelial tight and adherens junctions (Cristante et al., 2013). Furthermore, ANXA1 influences mast cell response in inflammation by limiting their degranulation and activation (Sinniah et al., 2016). In summary, ANXA1 and its receptors on epithelial cells, macrophages, neutrophils, endothelial cells and mast cells represent important targets for pro-resolution pathways in diseases involving inflammation and tissue injury.
ANNEXIN A1 as a potential therapeutic tool to reduce mucosal inflammation
Chronic inflammatory disorders such as inflammatory bowel disease (IBD) encompassing ulcerative colitis (UC) and Crohn’s disease (CD) are characterized by compromised epithelial barrier function, aberrant inflammatory response and mucosal wounds (Cosnes et al., 2011). Analysis of mucosal tissue from Ulcerative Colitis (UC) patients implicates a relationship between ANXA1 secretion and severity of the inflammatory response (Vergnolle et al., 2004; Vong et al., 2012). Previous studies have demonstrated that ANXA1 is localized in neutrophils in biopsies of human patients with active disease and in macrophages during the disease remission (resolution phase). Reduction of pro-inflammatory cytokine TNF-alpha signaling has been observed to amplify ANXA1 levels in the intestinal mucosa. Furthermore, biopsies from UC patients with anti-TNF-α therapy during disease remission revealed increased mucosal ANXA1 protein. Additionally, TNF- α inhibition increased ANXA1 expression in the intestinal epithelium and promoted resolution of inflammation in a murine colitis model (Sena et al., 2015). Interestingly, ANXA1 knockout mice have increased susceptibility to Dextran Sulfate Sodium (DSS) – induced colitis and delayed recovery from colitis (Leoni et al., 2013). ANXA1 suppresses indomethacin-induced leukocyte adherence to the vascular endothelium (Zanardo et al., 2005) further supporting its role in resolution of inflammation. Another ANXA1 peptide, MC-12 has been reported to have beneficial effects in suppressing NF-κB-dependent inflammatory signaling (Ouyang et al., 2012). Treatment with MC-12, inhibited the inflammatory response and promoted repair in the intestinal mucosa (Ouyang et al., 2012).
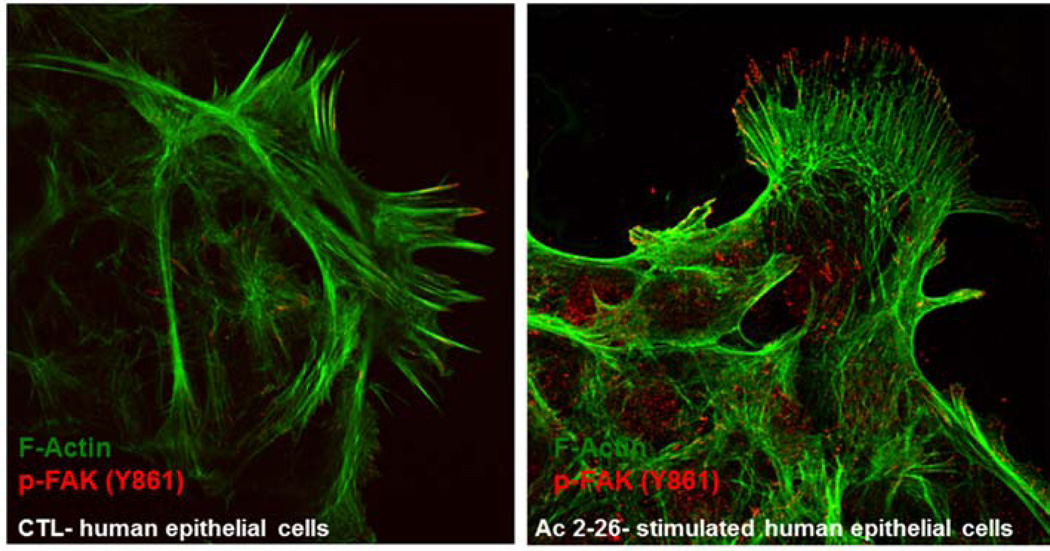
The therapeutic effects of ANXA1 were observed during the resolution and repair phase of colitis as reported above. This pro-repair response of ANXA1 in the intestinal epithelium was mediated by activation of a small GTPase Rac1 and epithelial oxidase NOX1 resulting in reactive oxygen species generation and oxidative modification of phosphatases involved in controlling activation/phosphorylation of focal cell matrix adhesion proteins such as Focal Adhesion Kinase (FAK). As shown in Figure 2 increased phosphorylated FAK is visualized in the migrating epithelial sheet incubated with Ac2-26 (Leoni et al., 2013). Recently, we also identified wound-mucosa-associated microbiota that activate the ANXA1 receptor, FPR1 to promote intestinal mucosal wound repair (Alam et al., 2016).
Figure 2.
ANXA1 mimetic peptide Ac2-26 stimulates phosphorylation of focal adhesion kinase.
Laser confocal micrographs of FAK p-Y861 (red) and F-actin (green) in migrating SK-CO15 cells with or without treatment with Ac2-26 (3 µM) for 15 minutes.
Externalization of ANNEXIN A1 - and what NEXT?
ANXA1 protein resides on the inner leaflet of the plasma membrane and can be externalized through a number of mechanisms that involve membrane transporters and vesicular trafficking. Perretti and colleagues identified ANXA1 in the gelatin granules of resting neutrophils (Murav'ev et al., 2003; Perretti et al., 2000). Following neutrophil adhesion to endothelial cells, gelatinase granules were observed to translocate and fuse with the plasma membrane, leading to the release of ANXA1 in the extracellular compartment and its association with the cell surface (Euzger et al., 1999). This process was reported to be controlled by ANXA1-binding protein expressed at the cell surface (Goulding et al., 1996). During an inflammatory response, secreted free-ANXA1 is cleaved by serine proteases with generation of an inactive state that may result in autoantibody production (Pederzoli-Ribeil et al., 2010). These antibodies may be responsible for neutralizing the protein directly, as well as reducing its levels in the plasma (Yazid et al., 2015). ANXA1 in the extracellular milieu is localized in extracellular vesicles (EVs) that include microparticles (MPs) and membrane-coated vesicles that originate from the plasma membrane (Raposo and Stoorvogel, 2013). Dalli and coworkers identified ANXA1 containing MPs that are released from activated neutrophils which mediate its anti-inflammatory activity (Tsai et al., 2012). These results suggest that generation and delivery of ANXA1-rich microparticles in the inflamed microcirculation could potentially be used to reduce neutrophil recruitment and promote resolution of inflammation. In addition to MPs, ANXA1 has been identified in smaller EVs referred to as exosomes (40 nm to 100 nm) that are derived from the endocytic compartment (Raposo and Stoorvogel, 2013). ANXA1 is released in exosomes derived from cancer cells as well as from leukocytes and epithelial cell (Aalberts et al., 2012; Boudhraa et al., 2016). ANXA1 containing EVs were identified during resolution of colitis and harvested ANXA1 EVs had functional effects in promoting wound repair by activation of FPR1 and FPR2/ALX signaling (Leoni et al., 2015b). Furthermore, Headland et al. also identified ANXA1 containing EVs in the synovial fluid of patients with rheumatoid arthritis (Headland et al., 2015). In addition to mediating a resolution response, ANXA1 containing EVs administered by intra-articular injection had beneficial effects by reducing cartilage degradation and transforming growth factor-beta (TGF-β) signaling in chondrocytes (Headland et al., 2015). These studies further highlight the therapeutic potential of using ANXA1 EVs to promote resolution of inflammation and repair. Of additional importance, increased ANXA1 EVs were detected in the circulation during the active stage of mucosal inflammatory disease suggesting that they could also serve as a biomarker of active disease (Headland et al., 2015; Leoni et al., 2015b).
Repair and tissue regeneration in injured skeletal myofibers involves fusion of intracellular vesicles with sarcolemma and also fusion of muscle progenitor cells. In vitro studies have identified a role of ANXA1 in both these fusion events. Lack of ANXA1 delays muscle regeneration after injury and lowers the number of differentiating myoblasts (Leikina et al., 2015). Another study identified ANXA1 peptide cleaved by calpain that is secreted from skeletal muscle cells during contraction and promotes repair (Goto-Inoue et al., 2016). Systemic levels of pro-resolving mediators are increased in a number of diverse chronic inflammatory diseases ranging from intestinal disorders, as described above to central nervous system diseases such as Alzheimer’s disease. However, in the latter scenario, ANXA1 signaling may defective (Leoni et al., 2015b; Wang et al., 2015). ANXA1 receptor, FPR2/ALX expression is decreased in patients with asthma, which might account for the inability of increased ANXA1 to have beneficial effects (Planaguma et al., 2008).
In the context of leukocyte migration, ANXA1 has been shown to induce L-Selectin shedding on neutrophils and the detachment of adhering leukocytes from the endothelium, by reducing α4β1 integrin clustering and activation (Gavins and Hickey, 2012). Administration of ANXA1 inhibits neutrophil rolling and capture and the Ac2-26 peptide antagonizes neutrophil adhesion and chemotaxis (Hayhoe et al., 2006). Recruitment of inflammatory cells from the circulation and their transendothelial migration represents an early phase of atherosclerosis. Administration of ANXA1 mimetic peptide Ac2-26 attenuates early atherogenesis which resulted in reduction of plaques. Drechsler and colleagues demonstrated that Ac2-26 inhibits CCL5-induced switch of β2 integrin conformation into its activated state in neutrophils and monocytes (Drechsler et al., 2015). Interestingly, Fredman et al. showed that collagen IV (Col IV)– nanoparticles (NPs) containing Ac2-26 targeted to lesions led to a marked improvement in plaque properties such as reduction of plaque necrosis and oxidative stress and improved stability of advanced atherosclerotic lesions (Fredman et al., 2015). Kusters and coworkers also demonstrated that ANXA1 treatment suppressed atherogenesis in a murine model of atherosclerosis (Kusters et al., 2015). These studies further highlight the therapeutic potential of ANXA1 in atherosclerosis.
Recent advances in the understanding of mechanisms underlying both physiological and pathological repair of tissue injury have identify potentially new therapeutic targets to reduce inflammation and associated fibrosis. In addition to promoting resolution of inflammation and repair, ANXA1 has also been shown to influence the fibrotic response that contributes to tissue repair. While ANXA1 biosynthesis and release can be induced by the pro-fibrotic cytokine TGF-β, ANXA1 inhibits the cytokine effects on α -SMA and collagen A1 gene expression. In synovial fibroblasts from patients with rheumatoid arthritis, ANXA1 enhanced secretion of matrix metalloproteinase 1, an enzyme involved in the degradation of extracellular matrix components (Damazo et al., 2011; Morand et al., 2006; Tagoe et al., 2008). Furthermore, ANXA1 absence in mice is associated with aggravated bleomycin-induced pulmonary fibrosis (Damazo et al., 2011). Thus, this ligand–receptor interaction may represent a novel therapeutic target to inhibit fibrosis in chronic kidney disease (Neymeyer et al., 2015). The ANXA1 mimetic peptide Ac2-26 also has beneficial effects on lung function and pathology in mice with silicosis suggesting that it could be used as a therapeutic agent in lung disease (Trentin et al., 2015).
ANNEXIN A1 as modulator of tumor- targeting immune strategies
Although advances in new diagnostic tools and treatments have reduced mortality rates, cancer remains a leading cause of death. Recently, Lin and colleagues analyzed 115 patients with oral carcinoma and observed high expression of ANXA1 in the nucleus of epithelial tumor cells (Lin et al., 2008). ANXA1 expression has been reported to be associated with a highly invasive basal-like breast cancer, a particularly aggressive molecular subtype defined by a robust cluster of genes expressed by epithelial cells in the basal or outer layer of the adult mammary gland. ANXA1 promotes metastasis by enhancing TGFβ/Smad signaling in breast cancer cells (de Graauw et al., 2010). Up-regulation of ANXA1/FPR2 expression in cancer cells was correlated with down-regulation of inflammatory cytokines (IL-6, IL-8 and MCP-1) and MMP2 (Gastardelo et al., 2014). Rossi et al. described dysregulated ANXA1 protein expression in pre-cancerous gastric lesions, suggesting its involvement in the early stages of gastric carcinogenesis (Rossi et al., 2014). Furthermore, it has been shown that ANXA1 is post-transcriptionally regulated by miR-196a in response to VEGF, which contributes to endothelial cell migration (Pin et al., 2012). Accordingly, the absence of ANXA1 in mice is associated with defects in tumor growth, metastasis and angiogenesis implicating a role of ANXA1 in tumor progression (Murav'ev et al., 2003).
Technologies are needed to map and image inflammation and also, cancer in vivo. Proteomic and imaging analyses demonstrated that a post-translationally modified form of ANXA1 is selectively concentrated in human and rodent tumor caveolae. To follow trafficking of cancer cells, the authors have designed a specific ANXA1 antibody that targets caveolae in the tumor endothelium and the proteomic imaging strategy represents an important tool for future detections of cancer in human patients (Oh et al., 2014). It is important to keep in mind that the success of anticancer chemotherapy is linked to a durable tumor-targeting immune response (Zitvogel and Kroemer, 2015). Recent studies confirmed that FPR1 and its ligand, ANXA1, promoted stable interactions between dying cancer cells and leukocytes. Thus, FPR1 and its ligand ANXA1 (and Ac2-26) might contribute to chemotherapy-induced anticancer immune responses (Vacchelli et al., 2015; Zitvogel et al., 2015).
Concluding remarks
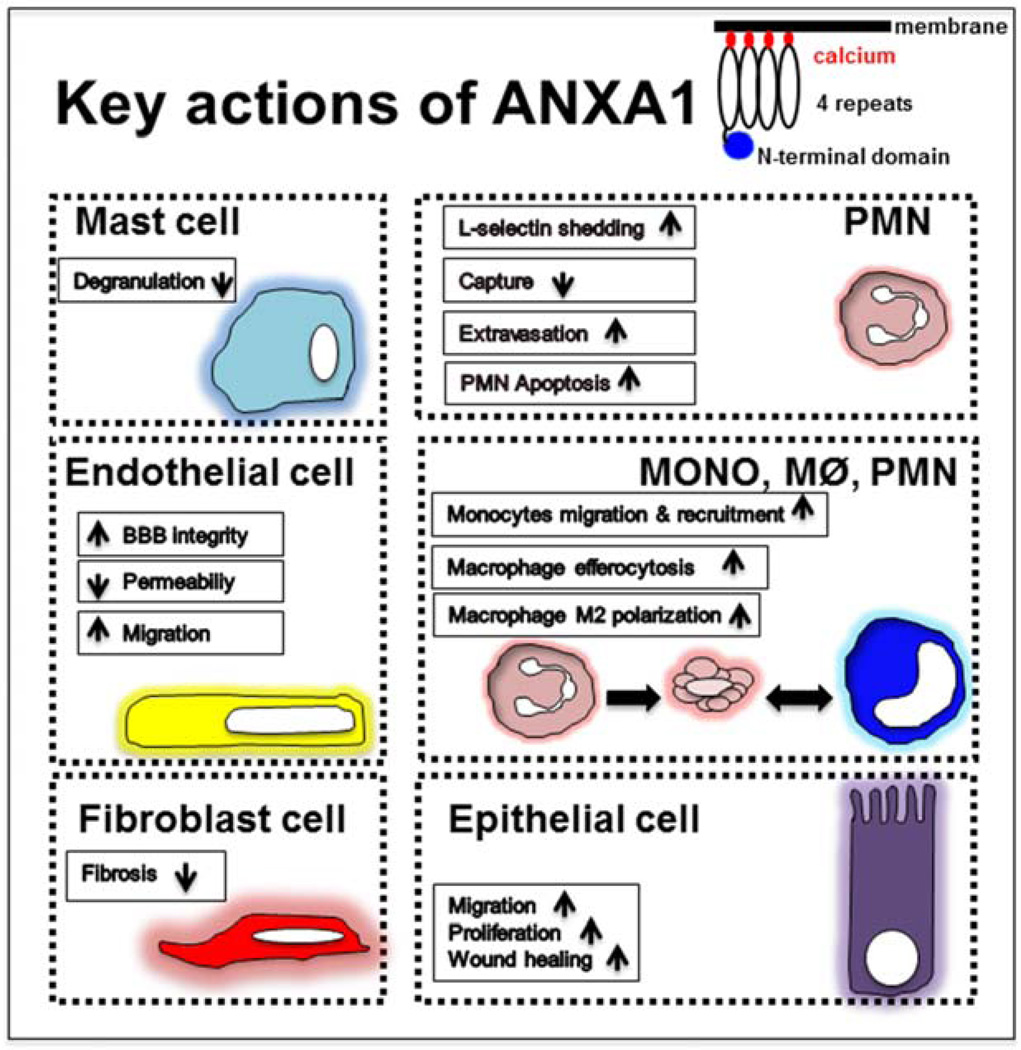
In summary, ANXA1 participates in a number of important biological processes that encompass cell migration, recruitment, permeability, apoptosis, phagocytosis and proliferation (Figure 3). Accumulating evidence supports an important role of ANXA1 in facilitating resolution of inflammation and mucosal wound repair. In addition to serving as a biomarker for active inflammation, ANXA1 administration has therapeutic potential to promote resolution and repair after injury.
Figure 3.
Summary of key functions of ANXA1 in different cell populations.
Footnotes
Biol Chem ‘Just Accepted’ Papers have undergone the complete peer-review process. However, none of the additional editorial preparation, which includes copy editing, typesetting and proofreading, has been performed. Therefore, there may be errors in articles published as Biol Chem ‘Just Accepted’ Papers that will be corrected in the final print and online version of the Journal. Any use of these articles is subject to the explicit understanding that the papers have not yet gone through the full quality control process prior to advanced publication.
References
- Aalberts M, van Dissel-Emiliani FM, van Adrichem NP, van Wijnen M, Wauben MH, Stout TA, Stoorvogel W. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol Reprod. 2012;86:82. doi: 10.1095/biolreprod.111.095760. [DOI] [PubMed] [Google Scholar]
- Alam A, Leoni G, Quiros M, Wu H, Desai C, Nishio H, Jones RM, Nusrat A, Neish AS. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol. 2016;1:15021. doi: 10.1038/nmicrobiol.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A, Leoni G, Wentworth CC, Kwal JM, Wu H, Ardita CS, Swanson PA, Lambeth JD, Jones RM, Nusrat A, et al. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 2014;7:645–655. doi: 10.1038/mi.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbin BA, Jesaitis AJ, Ivanov AI, Kelly D, Laukoetter M, Nava P, Parkos CA, Nusrat A. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J Immunol. 2007;179:8112–8121. doi: 10.4049/jimmunol.179.12.8112. [DOI] [PubMed] [Google Scholar]
- Babbin BA, Laukoetter MG, Nava P, Koch S, Lee WY, Capaldo CT, Peatman E, Severson EA, Flower RJ, Perretti M, et al. Annexin A1 regulates intestinal mucosal injury, inflammation, and repair. J Immunol. 2008;181:5035–5044. doi: 10.4049/jimmunol.181.7.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbin BA, Lee WY, Parkos CA, Winfree LM, Akyildiz A, Perretti M, Nusrat A. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem. 2006;281:19588–19599. doi: 10.1074/jbc.M513025200. [DOI] [PubMed] [Google Scholar]
- Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume KE, Soeroes S, Keppeler H, Stevanovic S, Kretschmer D, Rautenberg M, Wesselborg S, Lauber K. Cleavage of annexin A1 by ADAM10 during secondary necrosis generates a monocytic "find-me" signal. J immunol. 2012;188:135–145. doi: 10.4049/jimmunol.1004073. [DOI] [PubMed] [Google Scholar]
- Boudhraa Z, Bouchon B, Viallard C, D'Incan M, Degoul F. Annexin A1 localization and its relevance to cancer. Clin Sci. 2016;130:205–220. doi: 10.1042/CS20150415. [DOI] [PubMed] [Google Scholar]
- Brancaleone V, Mitidieri E, Flower RJ, Cirino G, Perretti M. Annexin A1 mediates hydrogen sulfide properties in the control of inflammation. J Pharmacol Exp Ther. 2014;351:96–104. doi: 10.1124/jpet.114.217034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40:315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee BE, Yona S, Rosignoli G, Young RE, Nourshargh S, Flower RJ, Perretti M. Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro . J Leukoc Biol. 2005;78:639–646. doi: 10.1189/jlb.0405206. [DOI] [PubMed] [Google Scholar]
- Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the Gprotein- coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci USA. 2013;110:18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Cristante E, McArthur S, Mauro C, Maggioli E, Romero IA, Wylezinska-Arridge M, Couraud PO, Lopez-Tremoleda J, Christian HC, Weksler BB, et al. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc Natl Acad Sci USA. 2013;110:832–841. doi: 10.1073/pnas.1209362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxtall JD, Flower RJ. Antisense oligonucleotides to human lipocortin-1 inhibit glucocorticoid-induced inhibition of A549 cell growth and eicosanoid release. Biochem Pharmacol. 1994;48:1729–1734. doi: 10.1016/0006-2952(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Dalli J, Jones CP, Cavalcanti DM, Farsky SH, Perretti M, Rankin SM. Annexin A1 regulates neutrophil clearance by macrophages in the mouse bone marrow. FASEB J. 2012;26:387–396. doi: 10.1096/fj.11-182089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damazo AS, Sampaio AL, Nakata CM, Flower RJ, Perretti M, Oliani SM. Endogenous annexin A1 counter-regulates bleomycin-induced lung fibrosis. BMC Immunol. 2011;12:59. doi: 10.1186/1471-2172-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graauw M, van Miltenburg MH, Schmidt MK, Pont C, Lalai R, Kartopawiro J, Pardali E, Le Devedec SE, Smit VT, van der Wal A, et al. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc Natl Acad Sei USA. 2010;107:6340–6345. doi: 10.1073/pnas.0913360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torre-Minguela C, Barbera-Cremades M, Gomez AI, Martin-Sanchez F, Pelegrin P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci Rep. 2016;6:22586. doi: 10.1038/srep22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gaudio P, De Cicco F, Aquino RP, Picerno P, Russo P, Dal Piaz F, Bizzarro V, Belvedere R, Parente L, Petrella A. Evaluation of in situ injectable hydrogels as controlled release device for ANXA1 derived peptide in wound healing. Carbohydr Polym. 2015;115:629–635. doi: 10.1016/j.carbpol.2014.09.040. [DOI] [PubMed] [Google Scholar]
- Drechsler M, de Jong R, Rossaint J, Viola JR, Leoni G, Wang JM, Grommes J, Hinkel R, Kupatt C, Weber C, et al. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ Res. 2015;116:827–835. doi: 10.1161/CIRCRESAHA.116.305825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euzger HS, Flower RJ, Goulding NJ, Perretti M. Differential modulation of annexin I binding sites on monocytes and neutrophils. Mediators Inflamm. 1999;8:53–62. doi: 10.1080/09629359990720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower RJ, Rothwell NJ. Lipocortin-1: cellular mechanisms and clinical relevance. Trends Pharmacol Sci. 1994;15:71–76. doi: 10.1016/0165-6147(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Science Translat Med. 2015;7:275ra220. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016 doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- Gastardelo TS, Cunha BR, Raposo LS, Maniglia JV, Cury PM, Lisoni FC, Tajara EH, Oliani SM. Inflammation and cancer: role of annexin A1 and FPR2/ALX in proliferation and metastasis in human laryngeal squamous cell carcinoma. PLoS One. 2014;9:e111317. doi: 10.1371/journal.pone.0111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavins FN, Hickey MJ. Annexin A1 and the regulation of innate and adaptive immunity. Front Immunol. 2012;3:354. doi: 10.3389/fimmu.2012.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol. 1997;120:1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto-Inoue N, Tamura K, Motai F, Ito M, Miyata K, Manabe Y, Fujii NL. A fragmented form of annexin A1 is secreted from C2C12 myotubes by electric pulse-induced contraction. Mol Cell Biochem. 2016;411:173–180. doi: 10.1007/s11010-015-2579-8. [DOI] [PubMed] [Google Scholar]
- Goulding NJ, Pan L, Wardwell K, Guyre VC, Guyre PM. Evidence for specific annexin I-binding proteins on human monocytes. Biochem J. 1996;316:593–597. doi: 10.1042/bj3160593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- Headland SE, Jones HR, Norling LV, Kim A, Souza PR, Corsiero E, Gil CD, Nerviani A, Dell'Accio F, Pitzalis C, et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Science Translat Med. 2015;7:315ra190. doi: 10.1126/scitranslmed.aac5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headland SE, Norling LV. The resolution of inflammation: Principles and challenges. Semin Immunol. 2015;27:149–160. doi: 10.1016/j.smim.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Kusters DH, Chatrou ML, Willems BA, De Saint-Hubert M, Bauwens M, van der Vorst E, Bena S, Biessen EA, Perretti M, Schurgers LJ, et al. Pharmacological treatment with annexin A1 reduces atherosclerotic plaque burden in LDLR−/− mice on Western type diet. PLoS One. 2015;10:e0130484. doi: 10.1371/journal.pone.0130484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- Leikina E, Defour A, Melikov K, Van der Meulen JH, Nagaraju K, Bhuvanendran S, Gebert C, Pfeifer K, Chernomordik LV, Jaiswal JK. Annexin A1 Deficiency does not Affect Myofiber Repair but Delays Regeneration of Injured Muscles. Sci Rep. 2015;5:18246. doi: 10.1038/srep18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni G, Gripentrog J, Lord C, Riesselman M, Sumagin R, Parkos CA, Nusrat A, Jesaitis AJ. Human neutrophil formyl peptide receptor phosphorylation and the mucosal inflammatory response. J Leukoc Biol. 2015a;97:87–101. doi: 10.1189/jlb.4A0314-153R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G, et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015b;125:1215–1227. doi: 10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni G, Neumann PA, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune-epithelial interactions. Mucosal Immunol. 2015c;8:959–968. doi: 10.1038/mi.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y, Hao H, Tang K, Yi P, Liu M, et al. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene. 2011;30:3887–3899. doi: 10.1038/onc.2011.112. [DOI] [PubMed] [Google Scholar]
- Lin CY, Jeng YM, Chou HY, Hsu HC, Yuan RH, Chiang CP, Kuo MY. Nuclear localization of annexin A1 is a prognostic factor in oral squamous cell carcinoma. J Surg Oncol. 2008;97:544–550. doi: 10.1002/jso.20992. [DOI] [PubMed] [Google Scholar]
- Locatelli I, Sutti S, Jindal A, Vacchiano M, Bozzola C, Reutelingsperger C, Kusters D, Bena S, Parola M, Paternostro C, et al. Endogenous annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology. 2014;60:531–544. doi: 10.1002/hep.27141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderna P, Yona S, Perretti M, Godson C. Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac(2–26) J Immunol. 2005;174:3727–3733. doi: 10.4049/jimmunol.174.6.3727. [DOI] [PubMed] [Google Scholar]
- Mancuso F, Flower RJ, Perretti M. Leukocyte transmigration, but not rolling or adhesion, is selectively inhibited by dexamethasone in the hamster post-capillary venule. Involvement of endogenous lipocortin 1. J immunol. 1995;155:377–386. [PubMed] [Google Scholar]
- McArthur S, Gobbetti T, Kusters DH, Reutelingsperger CP, Flower RJ, Perretti M. Definition of a novel pathway centered on lysophosphatidic acid to recruit monocytes during the resolution phase of tissue inflammation. J Immunol. 2015;195:1139–1151. doi: 10.4049/jimmunol.1500733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand EF, Hall P, Hutchinson P, Yang YH. Regulation of annexin I in rheumatoid synovial cells by glucocorticoids and interleukin-1. Mediators Inflamm. 2006;2006:73835. doi: 10.1155/MI/2006/73835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murav'ev RA, Fomina VA, Rogovin VV. Gelatinase granules of the neutrophil granulocytes. Izvestiia Akademii nauk Seriia biologicheskaia / Rossiiskaia akademiia nauk. 2003:389–394. [PubMed] [Google Scholar]
- Neymeyer H, Labes R, Reverte V, Saez F, Stroh T, Dathe C, Hohberger S, Zeisberg M, Muller GA, Salazar J, et al. Activation of annexin A1 signalling in renal fibroblasts exerts antifibrotic effects. Acta Physiol. 2015;215:144–158. doi: 10.1111/apha.12586. [DOI] [PubMed] [Google Scholar]
- Oh P, Testa JE, Borgstrom P, Witkiewicz H, Li Y, Schnitzer JE. In vivo proteomic imaging analysis of caveolae reveals pumping system to penetrate solid tumors. Nat Med. 2014;20:1062–1068. doi: 10.1038/nm.3623. [DOI] [PubMed] [Google Scholar]
- Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang N, Zhu C, Zhou D, Nie T, Go MF, Richards RJ, Rigas B. MC-12, an annexin A1-based peptide, is effective in the treatment of experimental colitis. PLoS One. 2012;7:e41585. doi: 10.1371/journal.pone.0041585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederzoli-Ribeil M, Maione F, Cooper D, Al-Kashi A, Dalli J, Perretti M, D'Acquisto F. Design and characterization of a cleavage-resistant Annexin A1 mutant to control inflammation in the microvasculature. Blood. 2010;116:4288–4296. doi: 10.1182/blood-2010-02-270520. [DOI] [PubMed] [Google Scholar]
- Perretti M. The annexin 1 receptor(s): is the plot unravelling? Trends Pharmacol Sci. 2003;24:574–579. doi: 10.1016/j.tips.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Perretti M, Ahluwalia A, Harris JG, Harris HJ, Wheller SK, Flower RJ. Acute inflammatory response in the mouse: exacerbation by immunoneutralization of lipocortin 1. Br J Pharmacol. 1996;117:1145–1154. doi: 10.1111/j.1476-5381.1996.tb16709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Christian H, Wheller SK, Aiello I, Mugridge KG, Morris JF, Flower RJ, Goulding NJ. Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol Int. 2000;24:163–174. doi: 10.1006/cbir.1999.0468. [DOI] [PubMed] [Google Scholar]
- Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- Pin AL, Houle F, Fournier P, Guillonneau M, Paquet ER, Simard MJ, Royal I, Huot J. Annexin-1-mediated endothelial cell migration and angiogenesis are regulated by vascular endothelial growth factor (VEGF)-induced inhibition of miR-196a expression. J Biol Chem. 2012;287:30541–30551. doi: 10.1074/jbc.M112.393561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesselman M, Miettinen HM, Gripentrog JM, Lord CI, Mumey B, Dratz EA, Stie J, Taylor RM, Jesaitis AJ. C-terminal tail phosphorylation of N-formyl peptide receptor: differential recognition of two neutrophil chemoattractant receptors by monoclonal antibodies NFPR1 and NFPR2. J Immunol. 2007;179:2520–2531. doi: 10.4049/jimmunol.179.4.2520. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Duarte MC, Poltronieri AB, Valsechi MC, Jorge YC, de-Santi Neto D, Rahal P, Oliani SM, Silva AE. Deregulation of annexin-A1 and galectin-1 expression in precancerous gastric lesions: intestinal metaplasia and gastric ulcer. Mediators of Inflamm. 2014;2014:478138. doi: 10.1155/2014/478138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena AA, Pedrotti LP, Barrios BE, Cejas H, Balderramo D, Diller A, Correa SG. Lack of TNFRI signaling enhances annexin A1 biological activity in intestinal inflammation. Biochem Pharmacol. 2015;98:422–431. doi: 10.1016/j.bcp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinniah A, Yazid S, Perretti M, Solito E, Flower RJ. The role of the Annexin-A1/FPR2 system in the regulation of mast cell degranulation provoked by compound 48/80 and in the inhibitory action of nedocromil. Int Immunopharmacol. 2016;32:87–95. doi: 10.1016/j.intimp.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solito E, Mulla A, Morris JF, Christian HC, Flower RJ, Buckingham JC. Dexamethasone induces rapid serine-phosphorylation and membrane translocation of annexin 1 in a human folliculostellate cell line via a novel nongenomic mechanism involving the glucocorticoid receptor, protein kinase C, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase. Endocrinology. 2003;144:1164–1174. doi: 10.1210/en.2002-220592. [DOI] [PubMed] [Google Scholar]
- Tagoe CE, Marjanovic N, Park JY, Chan ES, Abeles AM, Attur M, Abramson SB, Pillinger MH. Annexin-1 mediates TNF-α-stimulated matrix metalloproteinase secretion from rheumatoid arthritis synovial fibroblasts. J Immunol. 2008;181:2813–2820. doi: 10.4049/jimmunol.181.4.2813. [DOI] [PubMed] [Google Scholar]
- Taylor AD, Loxley HD, Flower RJ, Buckingham JC. The role of lipocortin 1 (LC1) in the steroid feedback control of hypothalamo-pituitary-adrenocortical function. In vivo studies. Ann NY Acad Sci. 1994;746:446–448. doi: 10.1111/j.1749-6632.1994.tb39281.x. [DOI] [PubMed] [Google Scholar]
- Trentin PG, Ferreira TP, Arantes AC, Ciambarella BT, Cordeiro RS, Flower RJ, Perretti M, Martins MA, Silva PM. Annexin A1 mimetic peptide controls the inflammatory and fibrotic effects of silica particles in mice. Br J Pharmacol. 2015;172:3058–3071. doi: 10.1111/bph.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WH, Chien HY, Shih CH, Lai SL, Li IT, Hsu SC, Kou YR, Hsu HC. Annexin A1 mediates the anti-inflammatory effects during the granulocytic differentiation process in all-trans retinoic acid-treated acute promyelocytic leukemic cells. J Cell Physiol. 2012;227:3661–3669. doi: 10.1002/jcp.24073. [DOI] [PubMed] [Google Scholar]
- Tzelepis F, Verway M, Daoud J, Gillard J, Hassani-Ardakani K, Dunn J, Downey J, Gentile ME, Jaworska J, Sanchez AM, et al. Annexin1 regulates DC efferocytosis and cross-presentation during Mycobacterium tuberculosis infection. J Clin Invest. 2015;125:752–768. doi: 10.1172/JCI77014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350:972–978. doi: 10.1126/science.aad0779. [DOI] [PubMed] [Google Scholar]
- Vago JP, Tavares LP, Sugimoto MA, Lima GL, Galvao I, de Caux TR, Lima KM, Ribeiro AL, Carneiro FS, Nunes FF, et al. Proresolving Actions of Synthetic and Natural Protease Inhibitors Are Mediated by Annexin A1. J Immunol. 2016;196:1922–1932. doi: 10.4049/jimmunol.1500886. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Pages P, Guimbaud R, Chaussade S, Bueno L, Escourrou J, Comera C. Annexin 1 is secreted in situ during ulcerative colitis in humans. Inflamm Bowel Dis. 2004;10:584–592. doi: 10.1097/00054725-200409000-00013. [DOI] [PubMed] [Google Scholar]
- Vong L, Ferraz JG, Dufton N, Panaccione R, Beck PL, Sherman PM, Perretti M, Wallace JL. Up-regulation of Annexin-A1 and lipoxin A(4) in individuals with ulcerative colitis may promote mucosal homeostasis. PLoS One. 2012;7:e39244. doi: 10.1371/journal.pone.0039244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu M, Hjorth E, Cortes-Toro V, Eyjolfsdottir H, Graff C, Nennesmo I, Palmblad J, Eriksdotter M, Sambamurti K, et al. Resolution of inflammation is altered in Alzheimer's disease. Alzheimer's Dement. 2015;11:40–50. e41–e42. doi: 10.1016/j.jalz.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth CC, Alam A, Jones RM, Nusrat A, Neish AS. Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3. J Biol Chem. 2011;286:38448–38455. doi: 10.1074/jbc.M111.268938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth CC, Jones RM, Kwon YM, Nusrat A, Neish AS. Commensal-epithelial signaling mediated via formyl peptide receptors. Am J Pathol. 2010;177:2782–2790. doi: 10.2353/ajpath.2010.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazid S, Gardner PJ, Carvalho L, Chu CJ, Flower RJ, Solito E, Lee RW, Ali RR, Dick AD. Annexin-A1 restricts Th17 cells and attenuates the severity of autoimmune disease. J Autoimmun. 2015;58:1–11. doi: 10.1016/j.jaut.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Yazid S, Leoni G, Getting SJ, Cooper D, Solito E, Perretti M, Flower RJ. Antiallergic cromones inhibit neutrophil recruitment onto vascular endothelium via annexin-A1 mobilization. Arteriosclerosis Thromb Vasc Biol. 2010;30:1718–1724. doi: 10.1161/ATVBAHA.110.209536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Heinsbroek SE, Peiser L, Gordon S, Perretti M, Flower RJ. Impaired phagocytic mechanism in annexin 1 null macrophages. Br J Pharmacol. 2006;148:469–477. doi: 10.1038/sj.bjp.0706730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardo RC, Perretti M, Wallace JL. Annexin-1 is an endogenous gastroprotective factor against indomethacin-induced damage. Am J Physiol Gastrointest Liver Physiol. 2005;288:G481–G486. doi: 10.1152/ajpgi.00299.2004. [DOI] [PubMed] [Google Scholar]
- Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519–4532. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Galluzzi L, Viaud S, Vetizou M, Daillere R, Merad M, Kroemer G. Cancer and the gut microbiota: an unexpected link. Science Transl Med. 2015;7:271ps271. doi: 10.1126/scitranslmed.3010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Kroemer G. Cancer: antibodies regulate antitumour immunity. Nature. 2015;521:35–37. doi: 10.1038/nature14388. [DOI] [PubMed] [Google Scholar]