Abstract
Case summary
A 10-month-old domestic shorthair cat was evaluated for severe esophagitis and protracted vomiting and regurgitation secondary to a sliding (type I) hiatal hernia. The hernia and concurrent upper airway obstruction (nasopharyngeal polyp) were diagnosed with a multi-modality approach, including thoracic and abdominal radiographs, abdominal ultrasound, computed tomography and endoscopy. Following unsuccessful attempts at medical management, lower esophageal incompetence was successfully treated by employing a combination of surgical techniques, including herniorrhaphy, esophagopexy and modified (floppy) Nissen fundoplication.
Relevance and novel information
A multi-modality imaging approach was valuable in completely assessing the extent of this cat’s disease. Although an untraditional approach, the authors report herein the first clinical description of the use of combined surgical techniques with the floppy Nissen fundoplication technique (an antireflux procedure) in a cat. This procedure was used as a first-line surgical technique in this cat with severe lower esophageal incompetence, and may be a viable option for cases non-responsive to other therapeutic interventions. Further investigation of this surgical technique is warranted.
Introduction
Hiatal hernia, defined as protrusion of abdominal contents through the esophageal hiatus into the thorax, is a complex entity in humans and animals, with a multifactorial etiology and pathophysiology.1,2 Hiatal widening may be congenital or acquired. The latter leads to decreased movement resistance of the gastric cardia from the abdomen to the thorax.2
Clear association between hiatal herniation and gastroesophageal reflux exists.3 Intrinsic and extrinsic components of the lower esophageal sphincter prevent regurgitation of gastric contents.4 Disruption or displacement of the lower esophagus or gastroesophageal junction contribute to sphincter incompetence and lead to gastroesophageal reflux and esophagitis.3,5,6 Lower esophageal sphincter abnormalities of any nature can exacerbate gastroesophageal reflux.4
Case description
A 10-month-old male intact, 1.4 kg, domestic shorthair cat was evaluated for chronic vomiting and poor body condition despite polyphagia. Clinical signs began at 10 weeks of age and included lethargy, fever, stunted growth and abdominal distention occurring within minutes of eating. No history of trauma existed. Pyrexia and lethargy resolved with empirical treatment with augmented amoxicillin (14 mg/kg orally q12h). Emesis persisted. Famotidine (0.5 mg/kg orally q24h) and metoclopramide (0.3 mg/kg orally q8h) were added to the treatment plan. Results of a serum biochemistry analysis, complete blood count (CBC), serum bile acids, fecal flotation and thyroid testing were normal. Results of referral thoracic radiographs were consistent with aspiration pneumonia. In addition, a well-marginated, 2 cm × 3 cm, soft tissue opacity, caudal esophageal mass was seen. A mural, extramural or luminal caudal esophageal lesion was suspected, with preliminary differentials including esophageal foreign body, hiatal hernia, congenital megaesophagus and esophageal diverticulum.7,8 Neoplasia, although not completely ruled out, was considered unlikely. Upper gastrointestinal endoscopy revealed gastroesophageal mucosal hyperemia, distal esophageal dilation and severe esophageal reflux. Omeprazole (0.9 mg/kg orally q24h) and small, frequent feedings were added to the treatment protocol. Vomiting continued daily. The patient was referred for further diagnostic investigations after 6 months of poor response to medical management.
A body condition score of 3/9, stunted growth and a body weight of 1.4 kg were noted on physical examination.9 Repeat serum biochemistry analysis was normal. Repeat serum bile acid concentrations were marginally increased at 15.7 µmol/l (reference interval [RI] 0–5 µmol/l). A CBC demonstrated a mature neutrophilic leukocytosis (42.2 × 103/µl leukocytes, RI 4.2–15.6 × 103/µl; 34,943 × 103/µl neutrophils, RI 2500–12,500 × 103/µl).
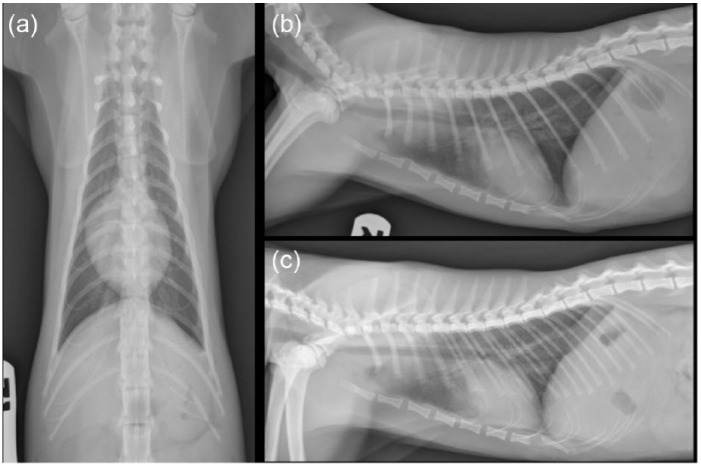
Repeat orthogonal thoracic radiographs showed an intermittently present, large, 5 cm × 2 cm × 3 cm, well marginated, soft tissue opacity within the caudal esophagus with severe gastric distension (of admixed gas/fluid opacity). During imaging the patient demonstrated signs of respiratory distress (increased respiratory effort, rate and stertor), which resolved with discontinuation of applied manual restraint and supplementation of flow-by oxygenation. The increased respiratory effort noted during thoracic imaging acquisition corresponded to a dynamic change in position of the mid- to caudal sternebral segments on the radiographs (Figure 1). This finding was consistent with an upper airway obstruction. Thus, combined radiographic and clinical findings raised the suspicion of sliding hiatal herniation secondary to upper airway obstruction. Given the propensity toward respiratory decline and the dynamic caudal esophageal mass effect, computed tomographic (CT) imaging was deemed the best-suited modality to evaluate the upper airway, esophagus and stomach.
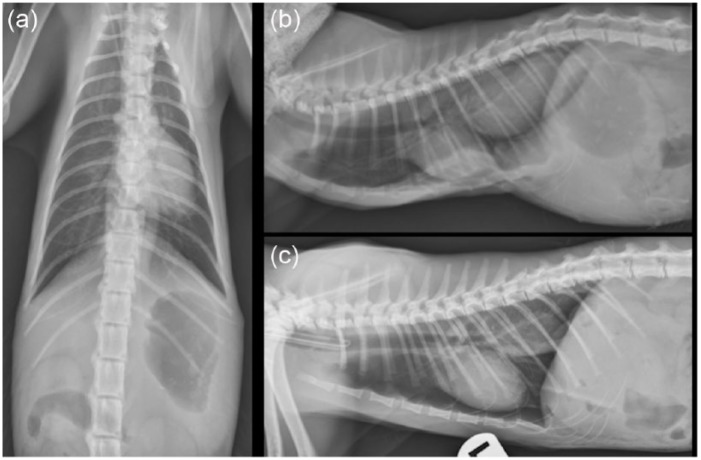
Figure 1.
Preinterventional lateral and dorsoventral thoracic radiographs. (a) Ventrodorsal projection. Note the moderate distention of the caudal esophagus. (b) Right lateral projection. Note the presence of gas within the soft tissue mass at the level of the caudal esophagus and the dorsal displacement of the mid-to-caudal sternebral segments due to increased inspiratory effort and respiratory distress. (c) Left lateral projection with the patient intubated. Note how the patient’s sternebral deformity and the mass at the level of the caudal esophagus resolve with assisted ventilation. Findings were consistent with sliding hiatal herniation. Dynamic changes in the sternebral segments coupled with increased respiratory effort raised suspicion of an upper airway obstruction, which was likely exacerbated by the stress of restraint for thoracic imaging
Abdominal ultrasonography revealed a non-motile, fluid-filled stomach and normal pyloroduodenal junction. The portal vein to aortic ratio was normal (0.89; RI 0.8–1.0), making a portosystemic shunt unlikely. Trypsinogen-like immunoreactivity was normal (36 µg/l; RI 12–82 µg/l).
Although the cat’s episode of dyspnea was brief, it prompted a prioritized diagnostic evaluation of the upper airway with contrast-enhanced CT of the head. Given the intermittent radiographic mass lesion within the caudal thorax, CT of the thorax was also performed followed by a sedated examination of the oropharynx. Premedication, anesthetic induction and maintenance following intubation were maintained with butorphanol (0.1 mg/g IM), midazolam (0.2 mg/kg IM), propofol (2 mg/kg IV) and an isoflurane–oxygen admixture, respectively. Lactated Ringer’s was administered at 10 ml/kg/h. Contrast-enhanced CT demonstrated a non-enhancing, soft tissue density within a thickened and expansile left tympanic bulla (Figure 2). An obstructive, 1 cm diameter, similarly minimally enhancing, ovoid mass was identified within the nasopharynx. Rugal folds were present within the caudal dorsal thorax. The esophagus was fluid and gas distended (Figure 3). CT abnormalities were consistent with a nasopharyngeal polyp extending from the left tympanic bulla/Eustachian tube to the nasopharynx with secondary hiatal herniation.
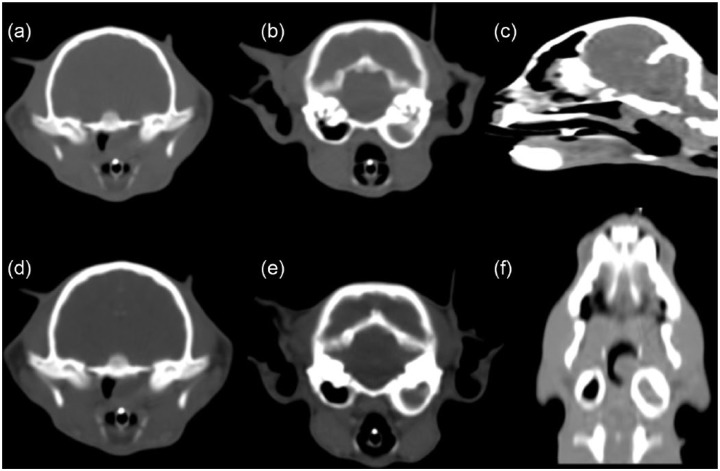
Figure 2.
Computed tomography of the head. (a) Note the non-enhanced images demonstrating the soft tissue dense nasopharyngeal mass lesion. (b) Note the soft tissue density within the expansile left tympanic cavity with thickening of the osseus bulla. (c) Sagittal reconstructed planar image demonstrating the nasopharyngeal mass. (d–f) Postcontrast administration; minimal enhancement was noted. Note the soft tissue densities within the left tympanic cavity and the nasopharynx
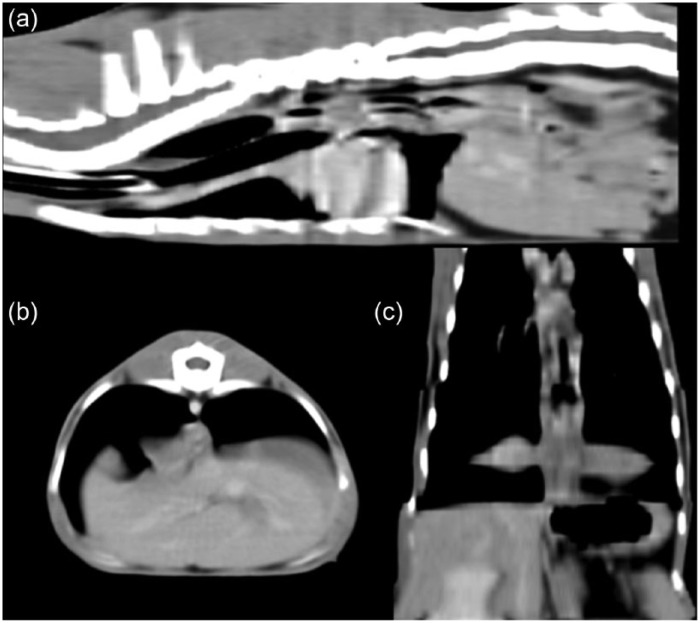
Figure 3.
Computed tomography of the thorax. (a) Sagittal, (b) transverse and (c) dorsal, curved–planar reconstructed images demonstrating rugal folds within the thoracic esophagus just cranial to the hiatus. Note also the diffuse gas distention of the intrathoracic esophagus on all images
Following imaging, the nasopharyngeal mass (presumed to be a polyp) was extracted via gentle traction and submitted for histopathologic confirmation. Ventral bulla osteotomy was considered but not performed owing to anesthesia-induced hypothermia. The patient recovered without complication and was discharged with buprenorphine (0.015 mg/kg orally q4–6h), omeprazole (0.9 mg/kg orally q24h) and cisapride (1.8 mg/kg orally q8h).
Vomiting resolved in the immediate postoperative period and no further respiratory issues were noted. Histopathology confirmed a nasopharyngeal polyp. At the 2 week follow-up, minimal difference was noted in the patient’s overall body condition. However, a 0.2 kg weight gain had occurred. Retching and regurgitation within 3 h of eating had persisted daily during this time. Lower esophageal sphincter incompetence and complications secondary to a sliding hiatal hernia were suspected, with consideration given to esophageal hypotonia and reflux esophagitis. Surgical correction of the hiatal hernia was recommended given continued poor response to medical management.
Thirty-three days following polyp removal, the cat was admitted for endoscopy, esophagoscopy, gastroscopy and hiatal hernia repair using a combined approach and employing an antireflux procedure. Premedication, anesthetic induction and maintenance following intubation were achieved with diazepam (0.5 mg/kg IV), buprenorphine (0.01 mg/kg IM), propofol (3 mg/kg IV) and an isoflorane–oxygen admixture, respectively. Lactated Ringer’s (10 ml/kg/h IV) and a fentanyl continuous rate infusion (0.35 µg/kg/min) were administered. Endoscopy demonstrated severe esophagitis, scarring and erythema of the lower esophageal mucosa, and grossly normal gastric mucosa (Figure 4).
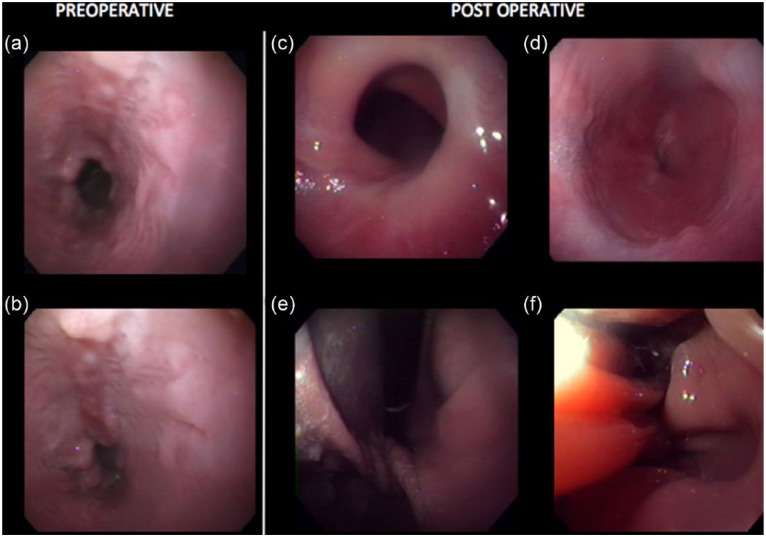
Figure 4.
Pre- and postsurgical endoscopy images. (a,b) Note the severe esophagitis with irregularly marginated, multifocal erosions. A significant amount of bilious reflux was noted. (c) Note the displaced and narrowed esophageal sphincter. (d) Note the mural twist at the cardia just caudal to the diaphragm. (e) Note the normal rugal folds of the fundus. (f) Note the structural abnormalities following the procedure, including the ‘tented’ margins of the lower esophageal wall
Ventral midline celiotomy revealed a large, flaccid stomach and marked dilation of the esophageal hiatus. The stomach was reduced with gentle traction. Blunt dissection of the esophagus, herniorrhaphy (with 3-0 simple interrupted polypropylene simple interrupted diaphragmatic sutures) and diaphragmatic esophagopexy (with 4-0 polydiaxione simple interrupted sutures) were performed followed by a floppy Nissen fundoplication. For this antireflux procedure, a large-bore orogastric tube was placed. The size of the tube was such that it was able to distend the esophagus to some degree but able to be passed easily to the stomach, as per the previously described modified technique.10 The gastric wall was mobilized 360 degrees dorsomedially around the intra-abdominal esophagus and sutured to itself without tension, using 3-0 polypropylene simple interrupted sutures. Peritoneal lavage and closure were routine.
Immediate postoperative complications were not perceived. The cat was kept in the hospital for observation and pain management for 48 h. A fentanyl continuous rate infusion (0.2 µg/kg/min) was administered for postoperative pain management. The cat was discharged from the hospital with no changes in medical management. Two weeks postoperatively the cat was re-evaluated at the referral center for suture removal. The cat’s weight was 1.9 kg (a 0.3 kg weight gain). Normal appetite, bowel movements and an excellent activity level were noted. The owner reported three episodes of non-productive retching, but vomiting had resolved. The referring veterinarian provided a 6 week postoperative follow-up report. At that visit the cat weighed 2.1 kg, having gained an additional 0.4 kg, and the owner noted marked improvement in activity levels at home. Although the owner reported overall improvement of regurgitation, episodes with retching and production of small amounts of brown, frothy fluid persisted and were noted five times weekly, usually within 3 h of consuming a meal.
The cat progressively continued to thrive at home and gain weight, with reports from the owner of once-daily non-productive retching in the 6–16 week interim following surgery. Between this time period, the referring veterinarian provided wellness maintenance with an unchanged regimen of prokinetics and proton pump inhibitors. At the 16 week follow-up at the referral center, the cat’s physical examination was normal. The cat weighed 2.5 kg (an additional 0.4 kg weight gain). This weight gain was attributed to the surgical intervention given that the cat’s body weight had nearly doubled in the 16 week postoperative period. Non-productive retching occurred once daily, but activity levels were excellent. Thoracic radiographs showed a persistent, mild increase in soft tissue opacity in the region of the caudal esophagus (Figure 5). Endoscopy revealed resolved esophagitis with mild reflux within the caudal esophagus and no gross esophageal or gastric mucosal abnormalities. Irregularity of the lower esophagus was considered related to the surgery (Figure 4).
Figure 5.
Postoperative lateral and dorsoventral thoracic radiographs. (a) Ventrodorsal projection. Note the decreased size of the thoracic cavity compared with Figure 1 (a). This finding is likely due to resolved air trapping. Note also the resolution of the previously described moderate esophageal distention. (b) Right lateral projection. Note the lack of gas within the esophagus and the resolution of the previously described soft tissue mass at the level of the caudal esophagus. Mild, normal volume of mixed gas–fluid opacity is present within the fundus. Note also the normal contour of the ventral thoracic body wall with the more normally positioned sternebral segments due to resolved respiratory distress. (c) Left lateral. Note the poorly defined increase in soft tissue opacity just dorsal to the caudal vena cava and cranial to the diaphragm. Note the abnormal small volume of mixed fluid–gas opacity persistent within the fundus and the normal mixed gas–fluid opacity within the pylorus. Findings are consistent with the surgically induced malposition of the stomach
Follow-up by email occurred bi-monthly for 24 months postoperatively, then every 6 months up to 48 months postoperatively. Over that time the owner reported that the cat had non-productive retching of gradually decreasing frequency (episodes stabilized to 2–3 times weekly rather than after every meal). Reduction of prokinetic therapy (cisapride 1.8 mg/kg) from every 8 h to once daily, as per the referral veterinarian, had no effect on retching frequency. Both the proton pump inhibitor and prokinetic agent were discontinued by 21 months postoperatively. The patient’s retching frequency continued to slowly decrease; by 48 months following surgery no retching episodes were noted and the cat was thriving.
Discussion
A multi-modality imaging approach and combined surgical techniques were used in the juvenile cat of this report in order to diagnose and treat a sliding hiatal hernia. Even though respiratory issues were not a primary complaint, restraint for radiographic imaging precipitated a respiratory episode. Dynamic dorsal displacement of the sternebral segments alluded to an upper airway obstruction. Congenital or acquired hiatal herniations were the primary differentials for the soft tissue opaque mass in the caudodorsal thorax seen on the same study. CT was supportive of this clinical suspicion, revealing a large obstructive mass in the nasopharnynx.
Upper airway obstruction has been reported in cases of dogs with hiatal hernia, where it is speculated that increased intraesophageal and intrapleural pressure can pull the esophagus and stomach into the thorax.1 Further evaluation of the upper and lower airway is indicated in patients with suspected respiratory disease. In the patient of this report, CT provided details regarding the inappropriate presence of mixed soft tissue and gas densities with rugal fold architecture at the hiatus. These details would not have otherwise been detected. The diagnosis of hiatal hernia typically relies heavily on signalment, history, clinical signs and diagnostic imaging. Widely available modalities such as radiographs, contrast esophagraphy, fluoroscopy and CT can aid in the diagnosis of the sliding hernia.1,5,11,12 Barium esophagraphy can be especially useful in assessing esophageal motility and caudal esophageal sphincter function.13 As was the case with the cat of this report, endoscopic evaluation was helpful in evaluating the degree of mucosal changes associated with secondary reflux esophagitis, as well as aiding in medical and presurgical treatment planning. Although not a traditional approach, CT was instrumental in a rapid diagnosis for both the cause of the upper airway obstruction and the displaced gastric anatomy through the hiatus.14 Abdominal ultrasonography demonstrated gastric dilation and functionally reduced gastric motility, while ruling out other causes of persistent regurgitation in young cats (such as pyloric stenosis). Endoscopy confirmed the presence of esophagitis, gastritis and gastric flaccidity, and also prescreened the patient for the presence of contraindicated disease for the employment of the modified Nissen fundoplication technique. Repeat endoscopy was valuable in documenting resolved esophagitis. In the private practice setting, this degree of imaging may not be economically or technically feasible. At a minimum, we recommend survey thoracic and abdominal radiographs, and a sedated oropharyngeal exam prior to referral for advanced imaging and surgical intervention of cats with suspected hiatal hernias. In addition, we recommend endoscopy and/or dynamic fluoroscopic evaluation of esophageal motility for cats with evidence of chronic reflux esophagitis and poor response to medical management.
The multi-modality imaging approach resulted in staged treatment. Removal of the nasopharyngeal polyp was prioritized to alleviate the risk of respiratory decompensation. Following a poor response to medical management and protracted episodes of retching and regurgitation, lower esophageal sphincter incompetence due to chronic esophageal reflux was highly suspected and confirmed with presurgical endoscopic evaluation of the lower esophagus. Surgical correction of the sliding hiatal hernia with an antireflux procedure, a modified (floppy) Nissen fundoplication, was then performed. Floppy Nissen fundoplication successfully reduced clinical signs (vomiting and weight loss) in this cat in the short term; in the long term, the cat’s clinical signs completely resolved.
Three main types of hernias are described in humans, cats and dogs.15 A sliding hiatal hernia, also called a type I hernia or axial hernia, is described as cranial displacement of the abdominal esophagus, esophageal junction and, sometimes, a portion of the stomach into the thoracic cavity.16 Hernias are rare in cats, with hiatal hernia being the most common.1,5,15 Congenital sliding hiatal hernias in young patients and acquired sliding hiatal hernias in adults are the most common forms seen.1,5,15 When sliding hiatal hernias occur in young animals between the ages of 2 and 4 months, they are usually described as congenital.13 However, hiatal herniation is a complex entity in humans and animals with an incompletely understood, multifactoral etiology and pathophysiology.2 Given the large obstructive nasopharyngeal mass in the patient of this report, an acquired sliding hiatal hernia was suspected, despite the patient’s young age. Congenital hiatal herniation was also considered, and coincidental occurrence of the polyp could not be ruled out. The patient of this report did not have any history of known trauma.
Patients with sliding (type I) hiatal herniation usually present for emesis and regurgitation.17 Other clinical signs can include ptyalism, nasal discharge, coughing, dysphagia and hematemesis.13 Stunted growth, recurrent gastric dilations, and clinical signs referable to esophagitis and gastroesophageal reflux, such as regurgitation with or without blood, aerophagia, anorexia and weight loss, are often reported. Megaesophagus and aspiration pneumonia may be additional radiographic findings. Some patients with sliding hiatal hernia are asymptomatic.5
Clear association between hiatal hernia and gastroesophageal reflux exists.3 Disruption or displacement of the position of the esophagus, gastroesophageal junction and lower esophageal sphincter contribute to sphincter incompetence, leading to gastroesophageal reflux and esophagitis.3,5 Experimentally, the production of esophageal reflux in cats has been shown to lower gastroesophageal sphincter pressure, resulting in a chronic cycle of reflux and esophagitis.18 Hiatal herniation may be associated with, or exacerbated by, respiratory disease, neuromuscular compromise, megaesophagus, esophageal motility disorders and obesity.2 As with the cat described in this report, respiratory difficulty and obstruction can be a predisposing factor in the occurrence of hiatal hernia both in dogs and cats.1,5,19,20 Intrinsic and extrinsic components of the lower esophageal sphincter prevent regurgitation of gastric contents.2 Entrapment of gastric fluid orad to the hiatus and loosening of the phrenoesophageal attachment may also contribute to movement of the intrinsic component of the lower esophageal sphincter.6
Patients with subclinical sliding hiatal hernia may not require medical or surgical intervention.2 In patients with clinical signs, goals of medical therapy are to eliminate clinical signs and decrease reflux.21 Medical management of gastroesophageal reflux should be attempted for a minimum of 30 days when reasonable, but is often unsuccessful.2 Management consists of a combination of feeding modifications and drug therapy. Small-volume, frequent feedings of a low-fat diet may aid in gastric emptying and decreased acid production.5 Changing the consistency of the food and feeding from an elevated position may also contribute to esophageal clearing.5 Drug therapy aims to decrease acid production, increase gastric emptying and increase the tone of the esophageal gastric junction.16 Frequently employed pharmaceuticals include sucralfate, antacids, H2 blockers, prokinetic agents and proton pump inhibitors. Surgical intervention is recommended if clinical signs (especially regurgitation and high-volume reflux) persist despite medical management.2,17
The occurrence and severity of reflux in patients with hiatal hernia is multifactorial. In immature animals, as with the cat of this report, reflux may be significantly increased because of an immature or poorly developed lower esophageal sphincter. Reduction of the hiatus alone rarely results in complete resolution of the clinical signs.16 Antireflux procedures are considered superior in patients with gastroesophageal reflux and may obviate the inconvenience and expense of lifelong medical management.18 As the majority of clinical signs associated with sliding hiatal hernias are related to gastroesophageal reflux, a procedure that is effective in reducing or eliminating reflux has merit.1 Techniques for repairing hiatal hernias in animals vary and there is much debate in the veterinary community regarding which techniques, or combination thereof, are best depending on the type of hernia.22 Described techniques include herniorrhaphy, gastropexy, esophagopexy, antireflux procedures and feeding tubes, or a combination thereof.23 Hiatal reduction alone may be insufficient.16
Corrective surgical techniques have been described and include different methods of maintenance of hernia reduction and manipulation of the gastroesophageal sphincter.15 The Nissen fundoplication was developed to treat reflux in humans.18,22 In pediatric patients, the modified fundoplication used in conjunction with a gastrostomy tube is commonly used to treat esophageal achalasia or hiatal hernia with severe gastroesophageal reflux.1 An antireflux procedure combined with herniorrhaphy and esophagopexy were chosen in this patient in an attempt to prevent further reflux, which was causing the clinical signs. In cats, modified Nissen fundoplication has experimentally been the most effective procedure in restoring lower esophageal pressure to normal, and the only procedure that helped prevent reflux.4 Two clinical reports of modified fundoplication in cats exist. One cat recovered without complication and had a favorable outcome. The other developed severe necrotizing gastritis in the immediate postoperative period but recovered with medical management. Both cats were radiographically normal and asymptomatic 1 year after surgery.8
Antireflux procedures in small animals have previously been challenged, with opponents arguing that primary lower esophageal sphincter incompetence, the main indication for an antireflux procedure in humans, does not occur in small animals.22 However, lower esophageal sphincter incompetence is thought to be secondary rather than primary in humans.24 Some authors propose that these techniques are surgically demanding, lend themselves to higher complication rates, and that the rationale for pursuing sphincter enhancement techniques is a failure of response to previous repositioning efforts.2,22,23
Given the low number of cases, there is a lack of evidence clearly demonstrating that repositioning techniques lead to irreversible hiatal attenuation and prevention of reflux in the long term. Additionally, performance of techniques such as gastropexy could potentially compromise performance of a subsequent antireflux procedure. Antireflux procedures have not been reported following failure of repositioning techniques in the veterinary literature. In humans, studies have shown that some patients having no reflux preoperatively developed reflux postoperatively when antireflux procedures were not performed.2 In these cases, the development of reflux may have been attributable to dissection of the gastroesophageal junction, reduction of the stomach or insufficient crural repair.2 We recommend that any antireflux procedure be performed by an experienced surgeon.
Multiple limitations of this report exist. Complete evaluation for a functional esophageal motility disorder was not performed prior to or after surgery with dynamic, fluoroscopic, positive contrast esophagography. In patients with esophageal motility disorders, however, an excellent outcome can be achieved with modified Nissen fundoplication, regardless of etiology.2 The procedure should be avoided in cases with aperistalsis, tight fibrous strictures and ‘short esophagus syndrome’.5,21 One previous study suggested that the absence of megaesophagus on survey radiography is consistent with a functional esophagus, while other studies emphasize the importance of evaluating for functional motility disorders with a barium esophagram or dynamic fluoroscopic esophagography.2,13 Additional limitations of this report include lack of esophageal and gastric histopathology and full gastrointestinal panel serum analysis (B12/folate). Although this cat was quite young, the possibility of primary inflammatory bowel disease as a contributing factor was considered.
Conclusions
Advanced imaging was an invaluable tool in the diagnosis, management and employment of combined surgical techniques, which provided an excellent outcome in a young cat with a sliding hiatal hernia, severe lower esophageal incompetence and concurrent reflux esophagitis. Although presumed to be secondary to upper airway obstruction, the sliding hiatal herniation may have been congenital in nature. Failure of response to medical management, degree of reflux noted on endoscopy, lack of a functional lower esophageal sphincter, suspicion of gastric hypomotility and questionable esophageal functional motility prompted the consideration of combined surgical techniques to include an antireflux procedure. Surgical correction consisted of herniorrhaphy, esophagopexy and modified (floppy) Nissen fundoplication. The modified Nissen fundoplication is the gold standard antireflux procedure in humans.25 Regardless of the underlying etiology, advanced imaging and a multi-modality approach can assist in evaluation of candidacy for antireflux procedures. Further studies are needed to investigate short- and long-term outcomes of the floppy Nissen fundoplication antireflux procedure and combined herniorrhaphy/organopexy in feline and canine cases of sliding hiatal hernia.
Acknowledgments
The case presented herein was seen at the Animal Medical Center of New York, NY, and was presented in grand rounds by Dr. DeLucia.
Footnotes
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1. Ellison GW, Lewis DD, Phillips L, et al. Esophageal hiatal hernia in small animals: literature review and modified surgical technique. J Am Anim Hosp Assoc 1987; 23: 391–399. [Google Scholar]
- 2. Sivacolundhu RK, Read RA, Marchevsky AM. Hiatal hernia controversies – a review of pathophysiology and treatment options. Aust Vet J 2002; 80: 48–53. [DOI] [PubMed] [Google Scholar]
- 3. Christensen J, Miftakhov R. Hiatal hernia: a review of evidence for its origin in esophageal longitudinal muscle dysfunction. Am J Med 2000; 108: 35–75. [DOI] [PubMed] [Google Scholar]
- 4. Hananoki M, Haruma K, Tsuga K, et al. Evaluation of lower esophageal sphincter pressure using endoscopic manometric sleeve assembly. J Gastroen Hepatol 2000; 15: 121–126. [DOI] [PubMed] [Google Scholar]
- 5. Bright RM, Sackman JE, Denovo C, et al. Hiatal hernia in the dog and cat: a retrospective study of 16 cases. J Small Anim Pract 1990; 31: 244–250. [Google Scholar]
- 6. Kahrilas PJ, Lin S, Chen J, et al. The effect of hiatus hernia on gastro-esophageal junction pressure. Gut 1999; 44: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasiri AM, Tabrizi SA, Khafi A. Gastroesophageal intussusception in a domestic short-hair cat. Iran J Vet Res 2013; 14: 358–361. [Google Scholar]
- 8. Durocher L, Johnson SE. Esophageal diverticulum associated with a trichobezoar in a cat. J Am Anim Hosp Assoc 2009; 45: 142–146. [DOI] [PubMed] [Google Scholar]
- 9. Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–18. [Google Scholar]
- 10. Donahue PE, Larson GM, Stewardson RH, et al. Floppy Nissen fundoplication. Rev Surg 1977; 34: 223–224. [PubMed] [Google Scholar]
- 11. Clark GN, Spodnick GJ, Rush JE, et al. Belt loop gastropexy in the management of gastroesophageal intussusception in a pup. J Am Vet Med Assoc 1992; 201: 739–742. [PubMed] [Google Scholar]
- 12. Ba-Ssalamah A, Prokop M, Uffmann M, et al. Dedicated multidetector CT of the stomach: spectrum of diseases. Radiographics 2003; 23: 625–644. [DOI] [PubMed] [Google Scholar]
- 13. Callan MB, Washabau RJ, Saunders HM, et al. Congenital esophageal hiatal hernia in the Chinese shar-pei dog. J Vet Intern Med 1993; 7: 210–215. [DOI] [PubMed] [Google Scholar]
- 14. Stadler K, O’Brien R. Computed tomography of nonanesthetized cats with upper airway obstruction. Vet Rad Ultrasound 2013: 54; 231–236. [DOI] [PubMed] [Google Scholar]
- 15. Williams JM. Hiatal hernia in a shar-pei. J Small Anim Pract 1990; 31: 251–254. [Google Scholar]
- 16. Prymak C, Saunders HM, Washabau RJ. Hiatal hernia repair by restoration and stabilization of normal anatomy: an evaluation in four dogs and one cat. Vet Surg 1989; 18: 386–391. [DOI] [PubMed] [Google Scholar]
- 17. Stickle R, Sparschu G, Love N, et al. Radiographic evaluation of esophageal function in Chinese shar pei pups. J Am Vet Med Assoc 1992; 201: 81–84. [PubMed] [Google Scholar]
- 18. Waldron DR, Moon M, Leib MS, et al. Oesophageal hiatal hernia in two cats. J Small Anim Pract 1990; 31: 259–263. [Google Scholar]
- 19. Pratschke KM, Hughes JML, Skelly C, et al. Hiatal herniation as a complication of chronic diaphragmatic herniation. J Small Anim Pract 1998; 39: 33–38. [DOI] [PubMed] [Google Scholar]
- 20. Hardie EM, Ramirez O, Clary EM, et al. Abnormalities of the thoracic bellows: stress fractures of the ribs and hiatal hernia. J Vet Intern Med 1998; 12: 279–287. [DOI] [PubMed] [Google Scholar]
- 21. Ellis FH. Controversies regarding the management of hiatus hernia. Am J Surg 1980; 139: 782–788. [DOI] [PubMed] [Google Scholar]
- 22. Lorinson D, Bright RM. Long-term outcome of medical and surgical treatment of hiatal hernias in dogs and cats: 27 cases (1978–1996). J Am Vet Med Assoc 1998; 213: 381–384. [PubMed] [Google Scholar]
- 23. Guiot LP, Lansdowne JL, Rouppert P, et al. Hiatal hernia in the dog: a clinical report of four Chinese shar peis. J Am Anim Hosp Assoc 2008; 44: 335–341. [DOI] [PubMed] [Google Scholar]
- 24. Sloan S, Rademaker A, Kahrilas P. Determinants of gastroesophageal junction incompetence: hiatal hernia, lower esophageal sphincter, or both? Ann Intern Med 1992; 117: 977–982. [DOI] [PubMed] [Google Scholar]
- 25. Reginald VN, DeMeester SR, Peters JH, et al. Hiatal hernia, lower esophageal sphincter incompetence, and effectiveness of Nissen fundoplication in the spectrum of gastroesophageal reflux disease. J Gastrointest Surg 2009; 13: 602–610. [DOI] [PubMed] [Google Scholar]