Abstract
Case series summary
In October 2011, an abnormally large morbidity and mortality event was noted in the intensive care unit (ICU) of a veterinary school hospital in Nantes, France. Cats, and cats only, transferred from the emergency room presented with fever, ulcers on the tongue and cutaneous lesions around venepuncture or surgical incision sites, leading to suspicion of a feline calicivirus-associated virulent systemic disease confirmed with reverse transcriptase-polymerase chain reaction. A total of 14 cats were suspected. The clinical features and the origin of the contamination were described for each cat. The median length of incubation was 4.5 days. Fifty-seven percent of the cats were euthanased (8/14) and 21% died (3/14), with a combined mortality of 79% (11/14) – the highest ever reported. Median survival was 12 days. The recovery rate was 21% (3/14).
Relevance and novel information
Eight outbreaks have been reported, in veterinary clinics or in group-housed cats. The main unusual aspects of the present outbreak were: (1) the extreme flare-up of lesions at sites of skin breach, precluding any puncture/incision; (2) the suggested better survival rate at home than in hospital; and (3) the immediate control of the outbreak after recognition of the disease. Other striking but less unusual features of this outbreak were: (4) the increasing of the virulence of the calicivirus with the passage of time; and (5) the primary role that the caregivers’ hands played in the spread of the outbreak.
Introduction
At the start of October 2011, a month after the beginning of classes, an abnormally large morbidity and mortality event was noted in the intensive care unit (ICU) of a veterinary school hospital. Cats, and cats only, transferred from the emergency room (ER) presented with fever and cutaneous lesions around venepuncture or surgical incision sites. These clinical signs were apparently unrelated to the cause of hospitalisation. At first, no common link was apparent between these cases. Ulcers on the tongue were observed, and attributed to a classical calicivirus. By Friday 7 October a nosocomial infection was suspected and a census of suspected cases was undertaken. On Sunday 9 October lingual ulcerations and very severe generalised signs in a cat that had spent 2 days in the ICU led to the hypothesis of a feline calicivirus-associated virulent systemic disease (FCV-VSD), a hypothesis that was subsequently confirmed.
That evening (9 October 2011) hospital personnel were alerted to the situation by email, and invited to attend an informational meeting the next morning, Monday 10 October. The decision was made to close the hospital to cats for 48 h; dogs were still admitted for appointments. The ER and ICU were closed to all patients, dogs included, for 5 days, to allow for disinfection of the premises and supplies. The hospital was closed to the staff of the laboratory catteries.
Hospitalised cats were transferred to a building located 500 m away from the hospital, outside of the school’s enclosure, separated by a street; it accepted neither animals nor personnel from the hospital. This building was specifically designed to treat infectious diseases. A staffed telephone line in the ER was operated, to inform clients of the closure, and direct sick animals that had recently been in the hospital to the quarantine facility.
Owners of hospitalised cats were notified. All owners of cats that been in the ER or the ICU from 1 September 2011 onwards were contacted by telephone, to question them about their cat’s health. The veterinarians of the region were notified by email.
Biosecurity measures were put in place in the hospital: bleach footbaths, mandatory booties, scrub caps and gloves, disinfection of the hands with an alcohol-based solution (even if gloves had been worn), use of disposable gloves and clothes, and so on. Surfaces and supplies were cleaned with an industrial detergent and disinfectant (Surfanios; Laboratoires ANIOS), then disinfected with bleach, then with fumigation (Nocolyse; Oxypharm). To allow for better cleaning, cages were disassembled. Consumables like anaesthesia tubing, pill guns and tourniquets were discarded. All rooms were sprayed down. These procedures were carried out throughout the entire small animal hospital, even though only three services received infected cats. The ER is located in a separate building located at the entrance to the veterinary school, 200 m away from the rest of the hospital; it receives practically no animals from the other services. The ICU is located at the end of the hospital building; animals from all the services are hospitalised there, but normally an animal hospitalised in the ICU does not return to the original service.
On Friday 7 October 2011, the charts of all the cats presented to the ER or hospitalised in the ICU since the beginning of classes on 1 September 2011 were re-examined. On Monday 10 October, 10 cats were suspected of being infected with a FCV-VSD; we later added 4 cats diagnosed after that date (Table 1).
Table 1.
Summary of 14 cats involved in feline calicivirus-associated virulent systemic disease in Nantes, France
| Reference | Presenting complaint | Clinical signs | Vaccination status | RT-PCR | Origin of the infection | Incubation time | Outcome | Duration (days) |
|---|---|---|---|---|---|---|---|---|
| Cat 0 | Anorexia | Fever (hyperthermia, weakness, anorexia), lingual ulcers | − | Unavailable | Unknown | Unknown | Recovered | 20 |
| Cat 1 | Fracture of the radius-ulna | Fever, lingual ulcers, significant erythema at the surgical site | Unknown | Unavailable | Cat 1 | 6 days | Died | 13 |
| Cat 2 | 10 cm inguinal wound | Fever, lingual ulcers, diarrhoea, pleural effusion | + | Unavailable | Cat 2 | 5 days | Died | 12 |
| Cat 3 | Fracture of the tibia | Fever, lingual ulcers, facial oedema, significant erythema at the surgical site | + | + | Cat 3 | 5 days | Recovered | 40 |
| Cat 4 | Anorexia secondary to dental disease | Fever, lingual ulcers, perineal ulcerations | − | + | Cat 3 | 4 days | Recovered | 12 |
| Cat 5 | Forelimb paralysis | Fever, lingual ulcers, chin ulcers | − | + | Cat 3 | 3 days | Euthanased | 13 |
| Cat 6 | Rupture of the bladder | Fever, lingual ulcers, subcutaneous oedema, significant erythema at surgical site and around catheters | Unknown | Invalid (but necrospy confirmed) | Cat 6 or cat 5 | 3 days | Euthanased | 8 |
| Cat 7 | Fall from the fourth floor | Fever, lingual ulcers, footpad ulcers, oedema of all four limbs | Unknown | + | Cat 6 | 7 days | Euthanased | 8 |
| Cat 8 | Urolithiasis | Fever, lingual ulcers | − | + | Cat 4 | 3 days | Euthanased | 7 |
| Cat 9 | Ataxia | Fever, lingual ulcers, sneezing, nasal discharge, oedema of the face and limbs | − | + | Cat 5 | 9 days | Euthanased | 20 |
| Cat 10 | Dysorexia and exhaustion | Fever, lingual ulcers, icterus | − | + | Cat 5 | 9 days | Euthanased | 22 |
| Cat 11 | Chronic renal failure | Fever, lingual ulcers, icterus | − | + | Cat 5 | 1 day | Euthanased | 5 |
| Cat 12 | Fall of several stories | Fever, lingual ulcers, facial ulcers, limb ulcers, oedema of the face and limbs, sneezing, nasal discharge, epistaxis, vomiting | Unknown | + | Cat 5 | 8 days | Euthanased | 14 |
| Cat 13 | Bacterial cystitis | Fever, lingual ulcers, haematuria, vomiting | + | + | Cat 5 | 2 days | Died | 10 |
| Cat 14 | Anorexia | Fever, lingual ulcers, facial ulcers, limb ulcers | + | Unavailable | Cat 5 or cat 8 | 4 days | Recovered | 10 |
RCT-PCR = reverse transcriptase-PCR
Cat 0 was an apartment cat that presented to the ER on Sunday 3 September with fever (hyperthermia, weakness, anorexia) and lingual ulcers. It was diagnosed with a classical calicivirus. On Monday 4 September an oesophagostomy tube was placed in the cat in the ICU. It left the hospital the morning of Sunday 10 September. Friday 16 September, a check-up established that the cat was progressing well. On September 23 the feeding tube was removed in the ICU, and the cat was healed. This cat did not exhibit signs of FCV-VSD but rather signs of unremarkable FCV infection. As the hospital was closed for the whole of August after a complete disinfection at the end of July, it is likely that this was the first case of calicivirus-induced disease introduced after the beginning of classes. The hospitalisation of this cat in the ICU preceded by 4 days and overlapped by 2 days that of cat 1, which arrived healthy and developed marked generalised signs that led to its death.
Cat 1 was in a road accident and presented with a fracture of the radius-ulna to the ER on Thursday 7 September, and was transferred to the ICU on Friday 8 September. Several wound debridements were carried out by the surgical service, and the fracture was reduced with a plate on Tuesday 13 September. It was hospitalised in the ICU until Wednesday 21 September. Its stay in the ICU overlapped that of cat 0 for 2 days, Friday 8 and Saturday 9 September. On Wednesday 14 September the cat presented with a fever, and on 19 September lingual ulcers and significant erythema at the surgical site were observed. The cat died on 21 September, 14 days after admission.
Cat 2 came in to the ER on Thursday 15 September for a 10 cm inguinal wound, after being impaled on a piece of bamboo. It was transferred to the ICU the next day, Friday 16 September. It left on Monday 19 September, having spent 4 days in the hospital at the same time as cat 1. Two days after leaving, on Wednesday 21 September, the cat presented again to the ER, with hyperthermia (40°C), lingual ulcers, diarrhoea and pleural effusion. It died on Wednesday 28 September, 13 days after admission.
Cat 3 was seen in the ER on Wednesday 21 September for a fractured tibia. It was never hospitalised in the ICU as it was immediately transferred to its own veterinary surgeon for treatment, but on the day it presented cat 2 was present in the ER. On Monday 26 September, in its own veterinary surgery, the cat presented with fever, lingual ulcers and facial oedema. It underwent debridements for 9 days, and was finally amputated on Saturday 1 October, because of significant erythema at the surgical site. It was very ill for the whole of October and received care at home. By the time of a follow-up appointment on Tuesday 8 November it was cured.
Cat 4, aged 16 years, was seen in the ER on Monday 26 September for anorexia secondary to dental disease. It was transferred to the ICU on Wednesday 28 September and underwent dental extractions in the surgery service. The cat spent 24 h in the ICU at the same time as cat 2, Wednesday 28 September. On Sunday 2 October it presented with fever, lingual ulcers and perineal ulcerations. The cat left the hospital on Friday 7 October, 11 days after admission; it did not relapse.
Cat 5 presented to the ER on Tuesday 27 September for forelimb paralysis following a road accident. Abandoned by its owner, this cat was looked after by the ER staff while waiting to put it up for adoption. It remained in the ER from Tuesday 27 September until the date of death. Beginning Friday 30 September, the cat presented with fever, a chin ulcer and tongue ulcers. It was euthanased on Monday 10 October, 13 days after admission.
Cat 5 was the origin of the infection for seven or eight cats (cats 6, 7, 9, 10, 11, 12, 13, 14). By her own admission, the student who cared for cat 5 was under the impression that she had infected these cats in the course of caring for them. The origin of infection in cat 5 was difficult to establish. It never stayed in the ICU, where the only two infected cats were hospitalised (cats 2 and 4). No student from the ICU was on duty in the ER, or vice versa; the ER is located in a building separated from the rest of the hospital. The resistance of calicivirus in the environment in spite of regular cleaning and disinfection procedures might presumably account for infections by fomites. The student who cared for cat 5 also took care of cat 3, 6 days earlier. Cat 3 would reveal the disease it had thus contracted from this student during the 2 h the cat spent in the ER. The student was contaminated via parcipitation in the care of cat 2, which had returned that day to the ER after being infected in the ICU.
Cat 6 presented to and was operated on in the ER on Sunday 2 October for a ruptured bladder. It was transferred to the ICU on recovery. It may have been infected through the intermediary of cat 5 in the ER or cat 4 in the ICU. On 5 October the cat presented with fever, lingual ulcers, subcutaneous oedema and significant erythema at the surgical site and around its catheters. It was euthanased on Monday 10 October, 8 days after admission.
Cat 7 presented to the ER on Sunday 2 October after a fall from the fourth floor. It was transferred to its veterinary surgeon on Tuesday 4 October, after having spent 2 days near cat 5, where it underwent surgery. The cat returned to the ER on Sunday 9 October with fever, ptyalism, lingual ulcers, footpad ulcers and oedema of all four limbs. It was this cat that prompted us to consider the hypothesis of FCV-VSD. The cat was euthanased the next day, Monday 10 October. It was not the source of a secondary outbreak, presumably because its veterinary surgeon was informed, and took appropriate biosecurity measures.
Cat 8 presented to the internal medicine service on Monday 3 October for urolithiasis. This is the only cat in this event that was not seen in the ER. It was hospitalised in the ICU that day in preparation for surgery, which, in fact, it never received. It was infected through the intermediary of cat 4. On Thursday 6 October it presented with a fever and lingual ulcerations. The cat was euthanased on 10 October, 7 days after admission.
With the exception of cat 11, which broke with signs the day after admission, the following cases all showed clinical signs after Monday 10 October, the date of diagnosis; they were received directly into the quarantine area after a telephone interview established possible infection during a recent stay in the hospital.
Cat 9 arrived at the ER on Monday 3 October for ataxia. It stayed in the ER for 2 days, 3 and 4 October, at the same time as cat 5, and then went home. Nine days later, on Wednesday 12 October, in the course of our telephone call, the owner informed us that the cat was in poor condition. Upon its second admission that day, the cat was admitted to the quarantine facility, and presented with fever, lingual ulcers, sneezing, nasal discharge and oedema of the face and limbs. It was euthanased on Sunday 23 October, 20 days after its first admission.
Cat 10 presented to the ER on Wednesday 5 October for dysorexia and exhaustion (no precise diagnosis was ever established). It was never hospitalised, but presented during the time when cat 5 was present. After 9 days, on Friday 14 October, it was admitted to the quarantine area for fever, lingual ulceration and icterus. The cat was euthanased on 27 October, 22 days after its first admission.
Cat 11 presented to the ER on Wednesday 5 October for chronic renal failure. The following day it presented with fever, lingual ulcers and icterus; there is no doubt as to the diagnosis, as reverse transcriptase PCR (RT-PCR) of a blood sample was positive. It was euthanased on 10 October, 5 days after admission. Again, cat 5 was the source of the infection.
Cat 12 presented to the ER on Wednesday 5 October after a fall of several stories. It left the next day, Thursday 6 October, having been infected by cat 5. It returned on Thursday 13 October in very poor health. It presented with fever, lingual ulcers, ulcers on the face and limbs (Figure 1), oedema of the face and limbs, nasal discharge, sneezing, epistaxis and vomiting. It was euthanased on Wednesday 19 October, 14 days after its first admission.
Figure 1.
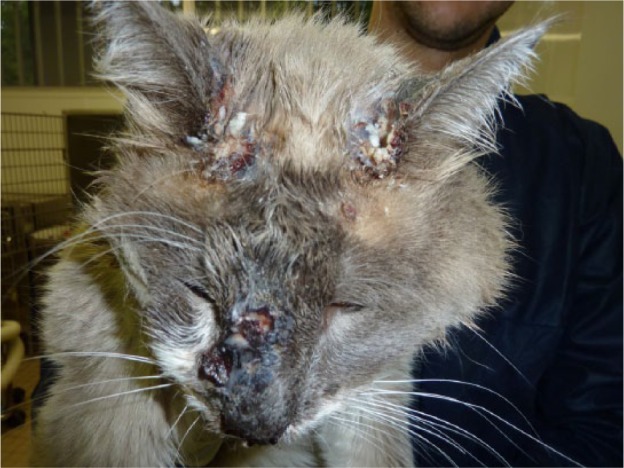
Ulcers on the face of cat 12
Cat 13 presented to the ER Saturday 8 October for bacterial cystitis, and was never hospitalised. It was infected during a blood draw in the ER, which took place in the radiography suite; the student who was caring for cat 5 provided restraint. It presented again on Monday 10 October for fever, lingual ulcers, haematuria and vomiting. It died on Tuesday 18 October, 10 days after its first admission.
Cat 14 belonged to a student who was working in the ICU. On Saturday 8 October the cat’s owner brought the cat in for anorexia of several days’ duration. After a blood draw and radiograph in the ER, where cat 5 was present, cat 14 spent 3 h in the ICU in a cage in which cat 8 had been housed. On Wednesday 12 October cat 14 presented with fever and ulceration of the tongue, face and limbs. Its owner cared for him at home; the cat was healed by Saturday 22 October, 16 days after admission.
Cats 2, 3, 13 and 14 had received one or more vaccines (protocols unknown); cats 0, 4, 5, 8, 9, 10 and 11 had not; vaccine status was unknown for cats 1, 6, 7 and 12.
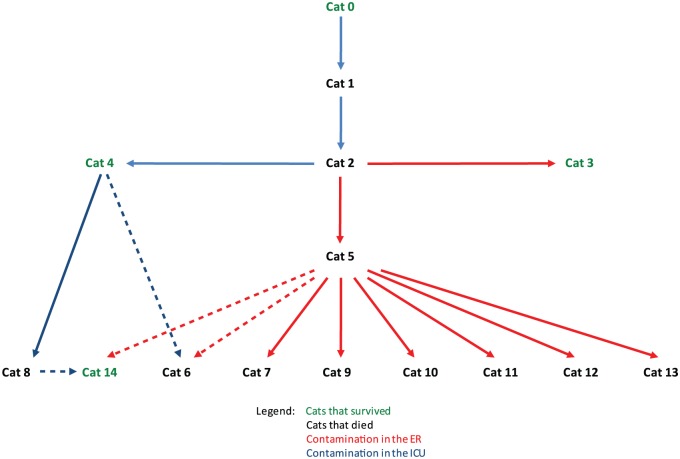
With a single exception (cat 14), none of the infected cats was housed in a cage previously occupied by an infected cat. A meticulous investigation established that: (1) every cat was infected when a previously infected cat was present in the same area (with the exception of cat 5); and (2) every newly infected cat was cared for by a veterinary student who was also caring for an infected cat (without exception). This investigation was not able to resolve arguments in favour of an environmental contamination, even if this is suspected for cat 5. The transmission scheme is presented in Figure 2.
Figure 2.
Origin of the infections . Dashed lines indicate where two sources of contamination are possible. ER = emergency room; ICU = intensive care unit
After the day on which biosecurity measures were established, Monday 10 October, there were no further infections.
RT-PCR confirmation from blood (not oropharyngeal swabs) was obtained for 10/14 cats (cats 3, 4, 5, 7, 8, 9, 10, 11, 12, 13). The blood samples were collected on Monday 10 October for hospitalised cats (cats 5, 6, 7, 8), and the day of their second admission for cats 9, 10 and 11. Analysis was performed on stored refrigerated samples for cats that had left the hospital prior to the assay (cats 3 and 4). The RT-PCR for cat 6 was invalid, but the autopsy and the context were also unequivocal, with subicterus, subcutaneous oedema, pulmonary oedema, hepatitis and a fibrino-necrotic cystitis, while the presenting complaint was traumatic rupture of the bladder. No samples were saved for the first patients (cats 0, 1, 2), while cat 14, which belonged to the student, was not screened; however, for these four cases the context and clinical signs were unequivocal. An autopsy was performed on five cats (cats 6, 8, 9, 11, 12); in each case it revealed multi-organ necrotic and inflammatory lesions and oedema.
At the time of diagnosis, Monday 10 October, the suspected source of the infection was healed (cat 0), two cats had died (cats 1, 2) and two cats were recovering at home (cats 3, 4). That day, 12 cats were hospitalised in the ICU. The five cats that were strongly suspected of FCV-VSD were euthanased that day (cats 5, 6, 7, 8, 11); four infections were confirmed by RT-PCR performed on blood, and infection in cat 6 was confirmed by necropsy. Six more hospitalised cats were transferred to the quarantine building; all had negative RT-PCRs and were returned to their owners. Cat 14 was treated at home by its owner, a veterinary student, and survived.
Four cats (cats 9, 10, 12, 13) that had stayed in the hospital presented directly to the quarantine area after 10 October; all were confirmed by RT-PCR performed on blood. Each cat was assigned two pairs of students. These students did not own cats, were relieved of all other duties and were forbidden access to the rest of the veterinary school. Strict biosecurity procedures were established in the quarantine building. With no specific treatment, care primarily consisted of hygiene of the mouth and skin and nutritional support. The pain associated with the lesions observed responds poorly to opioids, and as the latter caused nausea and loss of appetite, they were not used. It was quickly noted that any invasive procedure such as a blood draw or catheter placement caused a flare-up of lesions at the site involved. Blood draws were discontinued, and catheters withdrawn. Naso-oesophageal tubes were placed, as all the cats were anorexic, and oral intake of water, food or medication was impossible given the oral lesions. Each tube was associated with a purulent rhinitis. Liquid antibiotics were administered through the feeding tubes in the four cases. No sign of improvement was ever seen. All of the cats died despite attentive care (washing and cleaning ulcerations, feeding by hand and by naso-oesophageal tube, topical analgesia through application of oral anaesthetic dressings, grooming, and so on).
The last death occurred on Thursday 27 October, 43 days (around 8 weeks) after the first signs of systemic disease in the first cat (cat 2).The cost of care and diagnosis for all the animals was born by the hospital. Including closing the hospital, labour costs, disinfection products and medical materials used, the cost of this outbreak is estimated at 50,000 euros. Damage to the hospital’s reputation was limited by great transparency with owners and referring veterinarians, by the scope of our response to the outbreak, and by the fact that all of our response was free of charge to our clients and referring veterinarians.
Discussion
FCV is an RNA virus common in the cat, which causes a relatively mild range of upper respiratory signs in which ulcers of the end of the tongue figure prominently.1,2 The virus is highly mutable and can give rise to a hypervirulent systemic form of the disease – so-called FCV-VSD.1,2
The first description of a FCV-VSD is attributed to Pedersen et al, who in 2000 published an account of an epidemic that occurred in 1998 in California, affecting 11 cats.3,4 Previously, isolated cases of systemic calicivirus infections had been reported.5–7 Seven outbreaks have since been reported, in veterinary clinics (two in the USA, one in France and one in Germany),8,10–12 or in group-housed cats (one in the USA, one in England and one in Germany)9,12,13 (Table 2). FCV-VSD has been observed in isolated cats, in a cat infected experimentally with the normal strain and in captive exotic felids.14–19 The disease has appeared in isolated, independent epizootics. The strains involved were genetically distinct from each other.11,17,18,20–25 In our outbreak, we propose the hypothesis that cat 0, infected with a classical calicivirus, infected cat 1, which developed a lethal hypervirulent form. The calicivirus would have mutated in our ICU or in cat 1.
Table 2.
Outbreaks of feline calicivirus-associated virulent systemic disease reported in the world
| Year of outbreak | Year of publication | First author | Location | Place | Cases (n) | Mortality (%) |
|---|---|---|---|---|---|---|
| 1998 | 2000 | Pedersen | Sacramento/UC Davis, CA, USA | A private small animal hospital/an experimental animal facility | 7/4 | 43/50 |
| 2001 | 2003 | Schorr-Evans | Bellingham, MA, USA | A private small animal hospital | 24 | 38 |
| 2002 | 2004 | Hurley | Los Angeles, CA, USA | Three veterinary practices and a rescue organisation | 54 | 65 |
| 2003 | 2006 | Coyne | Staffordshire, England | Two neighbouring private households | 5 | 60 |
| 2005 | 2009 | Reynolds | Toulouse, France | A veterinary teaching hospital | 8 | 63 |
| 2005 | 2011 | Schulz | State of Hessen/State Bavaria, Germany | An animal shelter/a veterinary teaching hospital | 55/4 | 22/75 |
| 2011 | 2015 | Deschamps | Nantes, France | A veterinary teaching hospital | 14 | 79 |
As has been observed in previous outbreaks, vaccinated animals may be affected by FCV-VSD. As feline calicivirus has a high mutation rate, vaccine protection depends upon the adequacy between the infecting strain (regardless of whether or not it is hypervirulent) and the vaccine strain(s) administered. Work is underway to improve vaccine protection.4,22,25–27
The median length of incubation was 4.5 days (range 1–9) (Table 1), similar to that found in other outbreaks. Clinical signs most frequently observed were fever (100%) (hyperthermia, anorexia, weakness), lingual ulcers (100%), ulcers and/or oedema of the face (4/14; 29%), ulcers and/or oedema of the limbs (4/14; 29%), significant erythema at surgical or venepuncture sites (3/14; 21%), icterus (2/14; 14%) and death (11/14; 79%). Fever and lingual ulceration were present in all cases. In a cat presenting with lingual ulcers, a hypervirulent strain of calicivirus should be suspected in the presence of cutaneous effects (oedema or ulcers) on the face or limbs or the site of incisions or venepuncture, or in the presence of icterus, or in cases of mortality.
Twenty-five days passed between the first observation of systemic symptoms in cat 1 on Wednesday 14 September and the first suspicion of FCV-VSD put forward on Sunday 9 October (cat 7).
FCV is such a ubiquitous virus that full demonstration of the epizootic would have required virus gene sequencing establishing that the same strain of FCV infected several of the cats.9 Despite the lack of this data, positive RT-PCRs performed on blood samples (not on oropharyngeal swabs) demonstrated the presence of calicivirus in the blood. Given the fact that viraemia occurs during FCV infection, even when it causes classical signs, the RT-PCR must be interpreted in the light of epidemiological and clinical data. In the present context, the systemic nature of the infection is doubtless from cat 1.
In this outbreak, the virulence of the calicivirus seems to have increased with the passage of time. The more the outbreak progressed, the more numerous and spectacular the signs became, and the more grave the outcome (Table 1). Icterus was only observed beginning with cats 10 and 11. Of the three survivors (cats 3, 4, 14), two were among the first four patients. Of the three survivors, two were cats which were cared for in their owners’ homes (cats 3 and 14). Only cat 4 survived in the ICU. This is an illness that is care-dependent: lesions flared at surgical incisions and catheter or venepuncture sites, so much so that any break in the skin was precluded and catheters were removed. No oesophagostomy tubes were placed, in order to avoid cutaneous eruptions. Fifty-seven percent of the cats were euthanased (8/14) and 21% died (3/14), giving a combined mortality rate of 79% (11/14), the highest ever reported (Table 2). Median survival was 12 days. The recovery rate was 21% (3/14); recovered cats did not relapse. Global mortality rate is, to some extent, dependent on the criteria that led to a decision perform euthanasia. Except for cat 8, whose lesions were only moderate, the serious lesions observed clinically and on autopsy, and the failure of treatment of cats 9, 10, 12 and 13, strengthened the decisions for euthanasia and made us think that the mortality is probably not an overestimate. No euthanasia was implemented as a sanitary measure to control the outbreak, as we have isolated premises and adequate staffing. A detailed review of all the records reassured us that the morbidity was not significantly underestimated.
It is probable that among group-housed cats direct transmission from cat to cat by the nasal route predominates, but in a hospital setting, in which there is no direct contact between cats, indirect infection is most plausible. In this outbreak, the investigation led to the conclusion that all infections took place at the hands of the veterinary students who cared for the cats: every newly infected cat was cared for by a student who was caring for a sick cat at the same time. Viral contamination of caretakers’ hands and clothes may occur not only during care with contact with the oral mucosa of an infected cat, but also during simple handling of an affected cat, especially when cutaneous lesions are present.
Cats may be infected with calicivirus by the nasal, oral or conjunctival route;1 the students examine the mucous membranes of the animals at least twice daily, by raising the lips and eyelids of the cats. When a veterinarian examines the oral or conjunctival mucosa, or gives an oral medication to an infected cat, the next cat will be infected if the veterinarian does not disinfect his or her hands. The procedures that are taught and posted require washing of the hands between patients, drying the hands and disinfecting the hands with a hydro-alcohol solution,28 but these protocols are not always followed. Moreover, FCV is quite resistant in the environment and not sensitive to the antiviral effects of all disinfectants, including hand sanitisers.29 As antimicrobial soaps or alcohol-based hand rubs may not be sufficient,29 the use of disposable gloves is necessary to prevent transmission. A student contaminated her hands while handling cat 2 and probably infected cats 3, 5, 6, 7, 9, 10, 11, 12 and 13, and perhaps 14. She alone may have been the source of 9–10 (64–70%) of the 14 infections. However, cat 14 may have been infected at home by its owner, a student who worked in the ICU; this has been observed previously.11 For cat 13, infected during a blood draw in the radiography suite, and for cat 14, there does not seem to have been any contact with the oral mucosa. The delay of 6 days between the presence of cat 2 in the ICU and the infection of cat 5 is surprising. The possibility that the student concerned never used an effective disinfectant on her hands during these 6 days must be considered. It is not excluded that a fomite like a thermometer, a pill gun, a cage or an examination table could have served as the source, despite the fact that they are disinfected after every use and that no other animal has been infected. For the environment, commercial sodium hypochlorite is the optimal disinfectant.30
Conclusions
This outbreak of FCV-VSD had all the characteristics of a nosocomial infection: it was propagated in a hospital setting, in highly active services, in weakened animals, during treatment, by caretaking staff, because of a break in hygiene that could easily have been prevented. The main unusual aspects of the present outbreak were: (1) the extreme flare-up of lesions at sites of skin breach, precluding any puncture/incision; (2) the suggested better survival rate at home than in hospital; and (3) the immediate control of the outbreak after recognition of the disease. Other striking but less unusual features of this outbreak were: (4) the increasing of the virulence of the calicivirus with the passage of time; and (5) the primary role that the caregivers’ hands played in the spread of the outbreak. The suspicion of a FCV-VSD was quite delayed. The outbreak was easily halted as soon as it was identified. Faster recognition of the disease is key to preventing or improving management of such potentially devastating outbreaks.
Acknowledgments
We thank all those who made a significant contribution to the response to this crisis: the ICU junior staff members – Dr Corinne Panier, Dr Pauline Vanbelle and Dr Maria Martinez – the hospital staff, especially M Jean-Marc Pouilly, and the director of the hospital, Pr Francis Fiéni, as well as the hundred or so students and interns being trained in the small animal hospital at the time.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Radford AD, Coyne KP, Dawson S, et al. Feline calicivirus. Vet Res 2007; 38: 319–335. [DOI] [PubMed] [Google Scholar]
- 2. Radford AD, Addie D, Belak S, et al. Feline calicivirus infection. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pedersen NC, Elliott JB, Glasgow A, et al. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet Microbiol 2000; 73: 281–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Hess J, Gill M, et al. A dual-strain feline calicivirus vaccine stimulates broader cross-neutralization antibodies than a single-strain vaccine and lessens clinical signs in vaccinated cats when challenged with a homologous feline calicivirus strain associated with virulent systemic disease. J Feline Med Surg 2010; 12: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper LM, Sabine M. Paw and mouth disease in a cat. Aust Vet J 1972; 48: 644. [DOI] [PubMed] [Google Scholar]
- 6. Ellis TM. Jaundice in a Siamese cat with in utero feline calicivirus infection. Aust Vet J 1981; 57: 383–385. [DOI] [PubMed] [Google Scholar]
- 7. Love DN, Zuber RM. Feline calicivirus associated with pyrexia, profound anorexia and oral and perianal ulceration in a cat. Aust Vet Pract 1987; 17: 136–137. [Google Scholar]
- 8. Schorr-Evans EM, Poland A, Johnson WE, et al. An epizootic of highly virulent feline calicivirus disease in a hospital setting in New England. J Feline Med Surg 2003; 5: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hurley KE, Pesavento PA, Pedersen NC, et al. An outbreak of virulent systemic feline calicivirus disease. J Am Vet Med Assoc 2004; 224: 241–249. [DOI] [PubMed] [Google Scholar]
- 10. Pesavento PA, MacLachlan NJ, Dillard-Telm L, et al. Pathologic, immunohistochemical, and electron microscopic findings in naturally occurring virulent systemic feline calicivirus infection in cats. Vet Pathol 2004; 41: 257–263. [DOI] [PubMed] [Google Scholar]
- 11. Reynolds BS, Poulet H, Pingret JL, et al. A nosocomial outbreak of feline calicivirus associated virulent systemic disease in France. J Feline Med Surg 2009; 11: 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulz BS, Hartmann K, Unterer S, et al. Two outbreaks of virulent systemic feline calicivirus infection in cats in Germany. Berl Munch Tieratztl Wochenshr 2011; 124: 186–193. [PubMed] [Google Scholar]
- 13. Coyne KP, Jones BR, Kipar A, et al. Lethal outbreak of disease associated with feline calicivirus infection in cats. Vet Rec 2006; 158: 544–550. [DOI] [PubMed] [Google Scholar]
- 14. Bordes F, Le Net JL. Insuffisance rénale aiguë lors de calicivirose systémique chez un chat. Point Vet 2011; 316: 60–63. [Google Scholar]
- 15. Meyer A, Kershaw O, Klopfleisch R. Feline calicivirus-associated virulent systemic disease: not necessarily a local epizootic problem. Vet Rec 2011; 168: 589. [DOI] [PubMed] [Google Scholar]
- 16. Gouvernayre F, Faudou H, Bergamo P. Un cas isolé de forme systémique sévère de calicivirose féline. Point Vet 2011; 326: 52–57. [Google Scholar]
- 17. Battilani M, Vaccari F, Carelle MS, et al. Virulent feline calicivirus disease in a shelter in Italy: a case description. Res Vet Sci 2013; 95: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohe K, Takahashi T, Hara D, et al. FCV-VBS isolated from cats with typical symptoms caused VSD in experimental cats. Vet Res Commun 2008; 32: 145–158. [DOI] [PubMed] [Google Scholar]
- 19. Harrison TM, Sikarskie J, Kruger J, et al. Systemic calicivirus epidemic in captive exotic felids. J Zoo Wildl Med 2007; 38: 292–299. [DOI] [PubMed] [Google Scholar]
- 20. Abd-Eldaim M, Potgieter L, Kennedy M. Genetic analysis of feline caliciviruses associated with a hemorrhagic-like disease. J Vet Diagn Invest 2005; 17: 420–429. [DOI] [PubMed] [Google Scholar]
- 21. Foley J, Hurley K, Pesavento PA, et al. Virulent systemic feline calicivirus infection: local cytokine modulation and contribution of viral mutants. J Feline Med Surg 2006; 8: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rong S, Slade D, Floyd-Hawkins K, et al. Characterization of a highly virulent feline calicivirus and attenuation of this virus. Virus Res 2006; 122: 95–108. [DOI] [PubMed] [Google Scholar]
- 23. Ossiboff RJ, Sheh A, Shotton J, et al. Feline caliciviruses (FCVs) isolated from cats with virulent systemic disease possess in vitro phenotypes distinct from those of other FCV isolates. J Gen Virol 2007; 88: 506–517. [DOI] [PubMed] [Google Scholar]
- 24. Schultz RD, Thiel B, Mukhtar E, et al. Age and long-term protective immunity in dogs and cats. J Comp Pathol 2010; 142 Suppl 1: S102–S108. [DOI] [PubMed] [Google Scholar]
- 25. Rong S, Lowery D, Floyd-Hawkins K, et al. Characterization of an avirulent FCV strain with a broad serum cross-neutralization profile and protection against challenge of a highly virulent vs feline calicivirus. Virus Res 2014; 188: 60–67. [DOI] [PubMed] [Google Scholar]
- 26. Poulet H, Jas D, Lemeter C, et al. Efficacy of a bivalent inactivated non-adjuvanted feline calicivirus vaccine: relation between in vitro cross-neutralization and heterologous protection in vivo. Vaccine 2008; 26: 3647–3654. [DOI] [PubMed] [Google Scholar]
- 27. Harrison TM, Harrison SH, Sikarskie JG, et al. Humoral response to calicivirus in captive tigers given a dual-strain vaccine. J Zoo Wildl Med 2014; 45: 23–28. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. WHO guidelines on hand hygiene in health care. http://www.ncbi.nlm.nih.gov/books/NBK144013/ (2009, accessed ??). [DOI] [PubMed]
- 29. Lages SL, Ramakrishnan MA, Goyal SM. In-vivo efficacy of hand sanitisers against feline calicivirus: a surrogate for norovirus. J Hosp Infect 2008; 68: 159–163. [DOI] [PubMed] [Google Scholar]
- 30. Chiu S, Skura B, Petric M, et al. Efficacy of common disinfectant/cleaning agents in inactivating murine norovirus and feline calicivirus as surrogate viruses for human norovirus. Am J Infect Control 2015; 43: 1208–1212. [DOI] [PubMed] [Google Scholar]