Abstract
Objectives
The aim of this retrospective analysis was to determine the seroprevalence of feline leukaemia virus (FeLV) antigenaemia among owned cats in Vienna and the surrounding area.
Methods
Samples were tested between 1996 and 2011 by the Department of Clinical Virology at the University of Veterinary Medicine, Vienna, Austria. All samples were sent to the university as part of routine diagnostic procedures, either to determine infection in clinically symptomatic individuals or to rule out infection prior to vaccination. To allow for statistical comparison, samples analysed between 2008 and 2011 were pooled into one population (n = 444) and evaluated against samples tested in 1996 (n = 840). Furthermore, analyses of subgroups were undertaken to determine the effect of sex and age on the prevalence of FeLV antigenaemia.
Results
With respect to the samples tested at the University of Veterinary Medicine in Vienna, it was determined that the level of FeLV antigenaemia in eastern Austria between 1996 and 2011 was 5.6%. The proportion of FeLV antigenaemic cats was highly variable and has not fallen significantly over this period, despite advances in vaccination, and the education of pet owners and animal welfare charities.
Conclusions and relevance
This study confirms the importance of continued and regular vaccination against FeLV among Austrian cats, particularly those allowed access to the outdoors. Within the remit of this retrospective study, it was not possible to follow-up results of repeat testing or of other assays (PCR) of individual cats. As a result of this, no conclusions can be drawn as to the possibility of transient antigenaemic cats or false-positive enzyme-linked immunosorbent assay results.
Introduction
Feline leukaemia virus (FeLV) is a gammaretrovirus, first described in 1964 at the University of Glasgow.1 Since the 1980s, significant decreases have been observed in countries with testing and vaccination programmes,2–5 while prevalence remains high in some regions.6
In Europe, a Belgian study in 2002 calculated a prevalence of 3.8% in 346 stray cats,7 and a study in 2009 reported a prevalence of FeLV of 3.6% among >17,000 owned felines in Germany.5 Samples taken from healthy cats in the UK in 1989 demonstrated a prevalence of 5%,2 while a later study of pet cats determined a prevalence of just 1.4% in clinically healthy animals in 2002.3 Similarly, a 2011 study screening healthy cats in Ireland reported a prevalence rate of FeLV of 1.1%.8 These lower rates appear to demonstrate the effectiveness of control programmes throughout the UK.
Mediterranean countries generally have higher levels than the rest of Europe, with a 2006 Italian study reporting a prevalence of 8.4% in clinically healthy cats,9 and a study in Madrid stating a prevalence of 15.6% among healthy animals in 2000.10 More recently, however, an Italian study of stray cats in 2012 reported a lower rate of 3.8%, indicating that the prevalence of FeLV may also be falling in these regions.11
Data on the prevalence of FeLV in Austria are limited. For this reason, we decided to carry out a retrospective analysis of routinely collected data from eastern Austria, including the city of Vienna; the results of which are presented here.
Materials and methods
This was a retrospective study including samples from all owned cats serologically tested for FeLV antigen at the University of Veterinary Medicine, Vienna, Austria, between 1996 and 2011. Cats tested at other laboratories in the region or in-house at veterinary practices were not included in this survey.
Data collated included date of testing, material tested, sex and age of cat tested, and enzyme-linked immunosorbent assay (ELISA) result. Details regarding whether cats were healthy or clinically symptomatic, free roaming, living in a multi-cat household, or were pedigree breeds were not routinely provided at the time of testing. Owing to the geographical location of the laboratory in Vienna, samples included in this study relate to cats in eastern regions of Austria only.
As the annual number of samples tested decreased substantially over the 16 year period, samples from 2008 to 2011 were pooled to compare with the baseline year of 1996.
Between 1996 and 2000, the FASTest FeLV ELISA (Megacor Diagnostik GmbH; sensitivity 94.7% [95% confidence interval {CI} 82.7–98.5] and specificity 98.8% [95% CI 97.3–99.4]12) was used to test samples. From 2001 to 2004, the Megascreen FeLV ELISA (Megacor Diagnostik GmbH; sensitivity and specificity not retrospectively available) was used. From 2005 onwards, samples were tested with the ViraCHEK FeLV ELISA (Synbiotics Corporation, Megacor Diagnostik GmbH; sensitivity 94.9% [95% CI 83.1–98.6] and specificity 98.4% [95% CI 96.8–99.2]12). These ELISAs are based on monoclonal antibodies to p27 antigen and are direct techniques. They detect FeLV antigenaemic rather than viraemic cats. A positive ELISA result indicated antigenaemia (ie, an infected cat, either transiently or persistently viraemic), or a false-positive result, and a negative result showed no antigenaemia present at that time (ie, non-infected cat or a latently infected cat with no antigen detectable in blood) or a false-negative result.
Descriptive statistics were calculated for all demographic parameters and all groups analysed. Prevalence data, odds ratios (ORs) and χ2 values were calculated for a pooled analysis of data collected from 2008 to 2011 compared with data from the baseline year of 1996. P values were calculated using the CHITEST function of Microsoft Excel 2007. Furthermore, χ2 values were calculated with respect to the age and sex of cats found to be FeLV antigenaemic.
Results
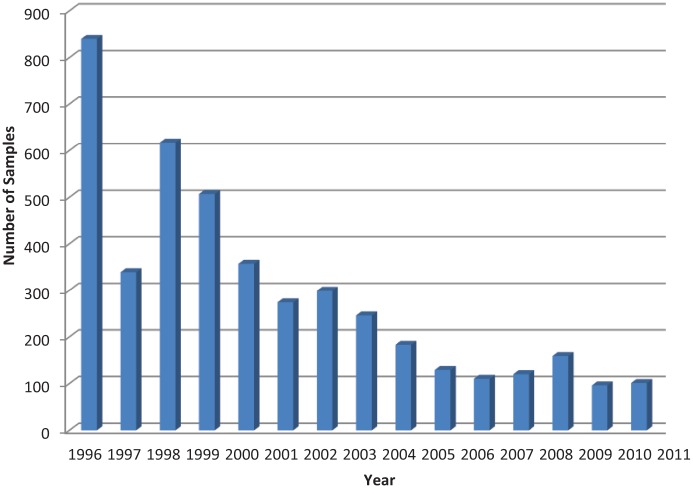
The total number of samples tested for the presence of FeLV antigen between 1996 and 2011 decreased gradually (Figure 1). Over the 16 year period, 4465 samples were analysed.
Figure 1.
Total number of samples tested for the presence of feline leukaemia virus antigen at the Department of Clinical Virology, University of Veterinary Medicine, Vienna, Austria
The age distribution of cats sampled in 1996 and of the pooled analysis from 2008 to 2011 was comparable, with the largest proportion (>36%) of all cats aged <1 year (Table 1). No age was given for >50% of cats included in the 2008 group.
Table 1.
Descriptive statistics – age distribution of 1996 population vs pooled population (2008–2011)
| Year | n | Mean age (years) | 95% confidence interval of the mean age | Median age (years) | SD |
|---|---|---|---|---|---|
| 1996 | 840 | 2.70 | 2.47–2.92 | 1.00 | 3.35 |
| 2008–2011 | 444 | 3.02 | 2.66–3.37 | 1.00 | 3.80 |
The sex distribution of cats analysed in 1996 and in 2008–2011 was evenly distributed. Owing to a lack of data for the 2008 population, >25% of animals in the pooled analysis were classed as sex unknown.
The prevalence of FeLV antigenaemic cats between 1996 and 2011 is shown in Table 2. The OR was calculated for each year (1997–2011) compared with the baseline year of 1996.
Table 2.
Prevalence of feline leukaemia virus (FeLV) antigenaemic cats (excluding borderline positive values)
| Year | n | Prevalence (%) | 95% CI | OR* |
|---|---|---|---|---|
| 1996 | 840 | 7.38 | 7.32–7.44 | – |
| 1997 | 339 | 7.67 | 7.52–7.82 | 1.04 |
| 1998 | 617 | 2.76 | 2.69–2.83 | 0.36 |
| 1999 | 507 | 4.34 | 4.26–4.42 | 0.57 |
| 2000 | 357 | 4.76 | 4.64–4.88 | 0.63 |
| 2001 | 274 | 5.47 | 5.31–5.63 | 0.73 |
| 2002 | 299 | 6.35 | 6.19–6.51 | 0.62 |
| 2003 | 246 | 6.10 | 5.91–6.29 | 0.81 |
| 2004 | 183 | 5.46 | 5.22–5.70 | 0.73 |
| 2005 | 129 | 5.43 | 5.09–5.77 | 0.72 |
| 2006 | 110 | 3.63 | 3.30–3.96 | 0.47 |
| 2007 | 120 | 7.50 | 7.07–7.93 | 1.02 |
| 2008 | 159 | 6.29 | 5.99–6.59 | 0.84 |
| 2009 | 96 | 5.21 | 4.76–5.66 | 0.69 |
| 2010 | 101 | 5.94 | 5.48–6.40 | 0.79 |
| 2011 | 88 | 5.68 | 5.16–6.20 | 0.76 |
| Overall | 4465 | 5.58 | 5.57–5.59 |
Odds ratio (OR): <1 indicates lower probability of FeLV antigenaemia than in baseline year of 1996; OR 1 indicates the same probability of FeLV antigenaemia as in 1996; OR >1 indicates higher probability of FeLV antigenaemia than in 1996
CI = confidence interval; n = number
The OR for the pooled analysis group (2008–2011) compared with 1996 was 0.78, meaning that cats tested between 2008 and 2011 were less likely to test positive for the presence of FeLV antigen than those tested in 1996. Data from 1996 and from 2008–2011 were entered into a 2 × 2 contingency table and analysed using the χ2 test (Table 3). The χ2 value (with Yates correction) equals 1.135 with 1 degree of freedom. The prevalence of FeLV antigenaemic animals in 1996 was 7.4% compared with 5.9% in 2008–2011. The difference between the year groups is therefore 1.5% (95% CI −1.3 to 4.3) and is not statistically significant at the 5% level (P = 0.3036).
Table 3.
χ2 test – prevalence of feline leukaemia virus antigenaemia in 1996 and 2008–2011
| 1996 | 2008–2011 | Total | |
|---|---|---|---|
| Positive | 62 | 26 | 88 |
| Negative | 778 | 418 | 1196 |
| Total | 840 | 444 | 1284 |
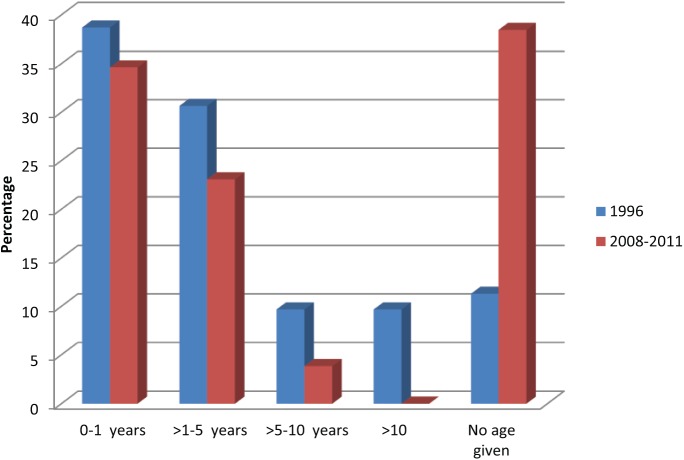
The age distribution of FeLV antigenaemic animals is shown in Figure 2. In 2011, 80% of FeLV-positive cats were aged >1–5 years, whereas in 2008–2010, the majority (>72%) of FeLV-positive cats were 0–1 years of age.
Figure 2.
Distribution of age among feline leukaemia virus antigenaemic cats (pooled analysis)
Using the χ2 test, age data from FeLV antigenaemic cats were analysed as shown in Table 4. The difference between year groups with respect to age is not statistically significant at the 5% level. However, as the χ2 value is relatively high, there is a low probability that the differences between FeLV-positive cats aged ⩽5 years or >5 years occurred by chance alone.
Table 4.
χ2 test – age of feline leukaemia virus antigenaemic cats
| Age (years) | 1996 | 2008–2011 | Total | χ2 value (with Yates correction) | P value |
|---|---|---|---|---|---|
| 0–1 | 24 | 9 | 33 | ||
| >1 | 31 | 7 | 38 | ||
| Total | 55 | 16 | 71 | 0.854 | 0.3733 |
|
| |||||
| ⩽5 | 43 | 15 | 58 | ||
| >5 | 12 | 1 | 13 | ||
| Total | 55 | 16 | 71 | 2.506 | 0.1564 |
No correlation could be made between sex and FeLV antigenaemia. The χ2 value (with Yates correction) equals 0.451 with 1 degree of freedom (P = 0.5416).
Discussion
This was a retrospective analysis to assess the seroprevalence of FeLV antigen among owned cats tested between 1996 and 2011 at the University of Veterinary Medicine in Vienna, Austria. The cat population included in this analysis was made up of felines living in Vienna and eastern Austria. FeLV antigenaemia was determined to be 7.4% in 1996 compared with 5.9% in 2008–2011. The difference between the two periods was not statistically significant at the 5% level (P = 0.3036). The majority (82%) of FeLV antigenaemic cats were aged ⩽5 years.
Data on the prevalence of FeLV in Austria are extremely limited. To date, only one other study has been carried out: Kölbl reported an overall FeLV prevalence of 9.7% among cats from eastern regions of Austria tested at the Austrian Agency for Health and Food Safety between 1985 and 1995.13 Studies on neighbouring countries in the 1990s reported similar FeLV prevalences to the Austrian example: Knotek et al determined a prevalence of 13.2% among owned cats in the Czech Republic,14 and Fuchs et al reported a prevalence of 13.4% in Germany.4 Prior to the availability of a FeLV vaccine and of a rapid in-clinic test, cats tested in the mid-1980s were likely to be clinically symptomatic, leading to much higher prevalence levels than those reported today. Regular testing of seemingly healthy individuals was not common until the introduction of the FeLV vaccine. FeLV prevalence in the preselected sick cat population in the 1980s was extremely high (33.3–56.3% in 1985–198613), and the introduction of the FeLV vaccination onto the Austrian market in 1988 was expected to have a substantial impact on reports of disease prevalence. It is, however, important to bear in mind that the 1985–1995 study did not necessarily represent the general domestic cat population and, in this respect, the current study provides a more realistic picture of FeLV antigenaemia in the region of eastern Austria.
Unlike the situation in Germany, where prevalence fell from 13.4% in 1999 to between 2% and 5% in 2007–2009, and a study in Munich reported a drop from 6% in 1993 to 1% in 2002,4,5,15–18 the prevalence of FeLV antigenaemic cats in eastern Austria appears to have stagnated rather than decreased over the past 16 years. The reasons for this can only be speculated upon, but may include initial efficacy issues with the vaccine, safety concerns regarding fibrosarcomas,19 low owner compliance, confusion as to the necessity of FeLV vaccination, the geographical location of Austria allowing the unhindered import of cats from Eastern Europe (particularly since the removal of border controls in 1997), and insufficient quarantine and testing in animal shelters.20 However, none of these factors can fully explain why the FeLV prevalence in eastern Austria has not fallen to the much lower levels reported in neighbouring Germany.
The number of samples routinely tested for FeLV decreased dramatically from 1985 to 2011: from 22,053 samples between 1985 and 1995 to 4465 samples between 1996 and 2011 in the current study.13 However, this study still includes a much larger cat population than similar studies in neighbouring countries such as Germany (n = 390),18 the Czech Republic (n = 727)14 and Italy (n = 203),9 and covers a longer period than many other European studies. Reasons for the reduction in FeLV testing shown here are likely to be the availability of in-house ELISAs to general practitioners and an overall reduction in the number of combined feline coronavirus/FeLV tests undertaken at the university laboratory in Vienna.
The age distribution of cats tested between 1996 and 2011 was strongly skewed towards young animals, with >40% of all cats tested being under 1 year old. This distribution was expected, as it was assumed that the vast majority of the samples sent to the university were part of routine prevaccination screening. However, in 1996, and 2008–2010, cats <1 year of age also made up the largest proportion of FeLV antigenaemic individuals. It could be hypothesised that this is due to the fact that younger cats at an early stage in the disease process may be more likely to be antigen-positive than older cats. Hofmann-Lehmann et al determined that 10% of the cats tested in their Swiss population (n = 597) were FeLV provirus positive, in spite of a negative ELISA result.21 The proportion of FeLV-antigenaemic animals older than 5 years of age remained below 10% for all periods in the current study.
The sex distribution of cats tested was relatively equally divided between male and female animals. Among FeLV antigenaemic individuals, no statistically significant difference was noted with respect to sex in 1996 and 2008–2011 (P = 0.5416). This corresponds with the results of studies in Switzerland, in Italy and in Germany,9,16,18,22 but contrasts with the study of Gleich et al in Germany where male cats were found to be significantly more likely to be infected with FeLV.5
A number of factors influenced the results of this analysis. Most important of all was the fact that the tested population was preselected by external veterinary surgeons and that the health status of the cats tested was not routinely provided. While the majority of these samples may have been sent for prevaccination screening, some may have been from clinically symptomatic animals, which could account for the relatively high prevalence determined here.
In this retrospective analysis, ELISAs, which detect FeLV antigenaemic rather than viraemic cats, were used. ELISA-positive but virus isolation-negative cats have previously been described and may occur at the beginning or end of an infection, or may be due to localised/atypical infections.23,24 It is impossible to rule out such a result in the present study as only a single antigen test was included in the analysis and, for this reason, we refer to the presence of FeLV antigenaemia rather than infection.
ELISAs are the most commonly used FeLV diagnostic tests in general practice, particularly since the introduction of simple and quick ‘snap’ test kits, and they have substantially improved compared with the first available tests. The sensitivity of a variety of market in-house ELISAs when compared with the gold standard of virus isolation has been reported to be between 92.1% and 96.8%, with specificity ranging between 95.4% and 99.8%.12 Data available on the ELISAs used in the present study showed that they had comparable sensitivity (>94.7%) and specificity (>98.4%) rates. However, it is important to note that false-positive test results are possible and occur more frequently in populations with low disease prevalence (ie, a majority of healthy cats). In healthy cats, in particular, transient viraemia/antigenaemia should always be considered.
In a study by Hartmann et al,12 the positive predictive values of samples in two different in-house ELISAs ranged from 81.4% to a maximum of 96.7%, depending on which assays were used. Therefore, repeat testing with ELISA or another test method is essential in every healthy cat with a positive FeLV antigen test result. The European Advisory Board on Cat Diseases suggests the use of immunofluorescence assay or provirus PCR as confirmatory tests for FeLV infection.25 For this purpose, PCR was used in the Department of Clinical Virology at the University of Veterinary Medicine in Vienna. However, it was not feasible to assign follow-up and confirmatory tests to individual cats in this retrospective analysis, so that the percentage of false-positive test results and transiently antigenaemic cats cannot be estimated here. Additionally, such tests may have been performed in other external diagnostic laboratories. For this reason, it was impossible to tell whether cats testing negative were regressively infected, as such animals do not have free antigen circulating in their blood stream but retain provirus in their bone marrow.16 In order to estimate accurately the true prevalence of FeLV infection, random samples from the entire Austrian cat population and additional virus isolation or PCR would have been required, which were not available for this retrospective analysis of existing data. A Swiss study has reported that up to 10% of cats tested and found to be negative for FeLV antigen are provirus positive when analysed by PCR.21 Similarly, the assessment of antigenaemic animals did not include the possibility of abortive infections, where the FeLV is eliminated by the cat’s immune system prior to antigenaemia occurring.
Demographic data sent with the sample were highly variable, with the year 2008 being particularly poor with respect to data quality of this kind. For this reason, analyses of the effect of age or sex on FeLV-positive results were less statistically powerful than the analysis of overall prevalence. It would also have been relevant to have analysed factors relating to the cats’ household situations, such as whether the animal was clinically symptomatic, indoor dwelling, free roaming, vaccinated, and so on, as well as whether the animals were undergoing an initial FeLV test or were being retested. Unfortunately, given the retrospective nature of this study, such data were not available.
It is very important to note that a single positive result in an antigen test for FeLV should never be assumed to be accurate in a clinically healthy cat. To ensure that no cats with false-positive results are ever erroneously euthanased, a second diagnostic test should always be carried out, ideally with a provirus PCR approximately 6 weeks later.25
Conclusions
This retrospective analysis determined that, with respect to the samples tested at the University of Veterinary Medicine in Vienna, the overall prevalence of FeLV antigenaemia in eastern Austria between 1996 and 2011 was 5.6% and has not fallen significantly over this period. This study therefore confirms the importance of continued and regular vaccination against FeLV among Austrian cats, particularly those allowed access to the outdoors.
Acknowledgments
We would like to thank S Lehr PhD for his advice on statistical testing.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this short communication.
Conflict of interest: The authors do not have any potential conflicts of interest to declare.
References
- 1. Jarrett WF, Crawford EM, Martin WB, et al. Leukaemia in the cat: a virus-like particle associated with leukaemia (lymphosarcoma). Nature 1964; 202: 567–569. [DOI] [PubMed] [Google Scholar]
- 2. Hosie MJ, Robertson C, Jarrett O. Prevalence of feline leukaemia virus and antibodies to feline immunodeficiency virus in cats in the United Kingdom. Vet Rec 1989; 125: 293–297. [DOI] [PubMed] [Google Scholar]
- 3. Muirden A. Prevalence of feline leukaemia virus and antibodies to feline immunodeficiency virus and feline coronavirus in stray cats sent to an RSPCA hospital. Vet Rec 2002; 150: 621–625. [DOI] [PubMed] [Google Scholar]
- 4. Fuchs A, Binzel L, Lonsdorfer M. Epidemiologie der FeLV- und FIV-Infektion in der Bundesrepublik Deutschland [article in German]. Tierarztl Prax 1994; 22: 273–277. [PubMed] [Google Scholar]
- 5. Gleich SE, Krieger S, Hartmann K. Prevalence of feline immunodeficiency virus and feline leukaemia virus among client-owned cats and risk factors for infection in Germany. J Feline Med Surg 2009; 11: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sukhumavasi W, Bellosa ML, Lucio-Forster A, et al. Serological survey of Toxoplasma gondii, dirofilaria immitis, feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) infections in pet cats in Bangkok and vicinities, Thailand. Vet Parasitol 2012; 188: 25–30. [DOI] [PubMed] [Google Scholar]
- 7. Dorny P, Speybroeck N, Berkvens D, et al. Serological survey of Toxoplasma gondii, feline immunodeficiency virus and feline leukaemia virus in urban stray cats in Belgium. Vet Rec 2002; 151: 626–629. [DOI] [PubMed] [Google Scholar]
- 8. Juvet F, Brennan S, Mooney CT. Assessment of feline blood for transfusion purposes in the Dublin area of Ireland. Vet Rec 2011; 168: 352. [DOI] [PubMed] [Google Scholar]
- 9. Bandecchi P, Dell’Omodarme M, Magi M, et al. Feline leukaemia virus (FeLV) and feline immunodeficiency virus infections in cats in the Pisa district of Tuscany, and attempts to control FeLV infection in a colony of domestic cats by vaccination. Vet Rec 2006; 158: 555–557. [DOI] [PubMed] [Google Scholar]
- 10. Arjona A, Escolar E, Soto I, et al. Seroepidemiological survey of infection by feline leukemia virus and immunodeficiency virus in Madrid and correlation with some clinical aspects. J Clin Microbiol 2000; 38: 3448–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spada E, Proverbio D, della Pepa A, et al. Seroprevalence of feline immunodeficiency virus, feline leukaemia virus and Toxoplasma gondii in stray cat colonies in northern Italy and correlation with clinical and laboratory data. J Feline Med Surg 2012; 14: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hartmann K, Griessmayr P, Schulz B, et al. Quality of different in-clinic test systems for feline immunodeficiency virus and feline leukaemia virus infection. J Feline Med Surg 2007; 9: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kölbl S. Untersuchungen über die Verbreitung von Retrovirusinfektionen bei Katzen in Österreich unter besonderer Berücksichtigung der Infektion mit dem Felinen Leukämievirus (FeLV) [thesis in German]. Higher Doctorate thesis, Veterinary University Vienna, 1997. [Google Scholar]
- 14. Knotek Z, Hájková P, Svoboda M, et al. Epidemiology of feline leukaemia and feline immunodeficiency virus infections in the Czech Republic. J Vet Med Ser B Zentralblatt für Veterinärmedizin R B 1999; 46: 665–671. [DOI] [PubMed] [Google Scholar]
- 15. Gleich S, Hartmann K. Hematology and serum biochemistry of feline immunodeficiency virus-infected and feline leukemia virus-infected cats. J Vet Intern Med 2009; 23: 552–558. [DOI] [PubMed] [Google Scholar]
- 16. Englert T, Lutz H, Sauter-Louis C, et al. Survey of the feline leukemia virus infection status of cats in Southern Germany. J Feline Med Surg 2012; 14: 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sand C, Englert T, Egberink H, et al. Evaluation of a new in-clinic test system to detect feline immunodeficiency virus and feline leukemia virus infection. Vet Clin Pathol 2010; 39: 210–214. [DOI] [PubMed] [Google Scholar]
- 18. Meichner K, Kruse BD, Hirschberger J, et al. Changes in prevalence of progressive feline leukaemia virus infection in cats with lymphoma in Germany. Vet Rec 2012; 171: 348. [DOI] [PubMed] [Google Scholar]
- 19. Hartmann K, Day MJ, Thiry E, et al. Feline injection-site sarcoma: ABCD guidelines on prevention and management. J Feline Med Surg 2015; 17: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lutz H, Addie D, Belák S, et al. Feline leukaemia ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hofmann-Lehmann R, Huder JB, Gruber S, et al. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats. J Gen Virol 2001; 82: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 22. Lutz H, Lehmann R, Winkler G, et al. Das Feline Immunschwächevirus in der Schweiz: Klinik und Epidemiologie im Vergleich mit dem Leukämie- und dem Coronavirus [article in German]. Schweiz Arch Tierheilkd 1990; 132: 217–225. [PubMed] [Google Scholar]
- 23. Hartmann K, Werner RM, Egberink H, et al. Comparison of six in-house tests for the rapid diagnosis of feline immunodeficiency and feline leukaemia virus infections. Vet Rec 2001; 149: 317–320. [DOI] [PubMed] [Google Scholar]
- 24. Jarrett O, Pacitti AM, Hosie MJ, et al. Comparison of diagnostic methods for feline leukemia virus and feline immunodeficiency virus. J Am Vet Med Assoc 1991; 199: 1362–1364. [PubMed] [Google Scholar]
- 25. Möstl K, Addie DD, Boucraut-Baralon C, et al. Something old, something new: update of the 2009 and 2013 ABCD guidelines on prevention and management of feline infectious diseases. J Feline Med Surg 2015; 17: 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]