ABSTRACT
Oropharyngeal candidiasis (OPC), caused predominantly by Candida albicans, is a prevalent infection in patients with advanced AIDS, defects in Th17 immunity, and head and neck cancer. A characteristic feature of OPC is fungal invasion of the oral epithelial cells. One mechanism by which C. albicans hyphae can invade oral epithelial cells is by expressing the Als3 and Ssa1 invasins that interact with the epidermal growth factor receptor (EGFR) on epithelial cells and stimulate endocytosis of the organism. However, the signaling pathways that function downstream of EGFR and mediate C. albicans endocytosis are poorly defined. Here, we report that C. albicans infection activates the aryl hydrocarbon receptor (AhR), leading to activation of Src family kinases (SFKs), which in turn phosphorylate EGFR and induce endocytosis of the fungus. Furthermore, treatment of oral epithelial cells with interferon gamma inhibits fungal endocytosis by inducing the synthesis of kynurenines, which cause prolonged activation of AhR and SFKs, thereby interfering with C. albicans-induced EGFR signaling. Treatment of both immunosuppressed and immunocompetent mice with an AhR inhibitor decreases phosphorylation of SFKs and EGFR in the oral mucosa, reduces fungal invasion, and lessens the severity of OPC. Thus, our data indicate that AhR plays a central role in governing the pathogenic interactions of C. albicans with oral epithelial cells during OPC and suggest that this receptor is a potential therapeutic target.
KEYWORDS: Candida albicans, aryl hydrocarbon receptor, epithelial cells, host cell invasion, interferon-gamma
IMPORTANCE
OPC is caused predominantly by the fungus C. albicans, which can invade the oral epithelium by several mechanisms. One of these mechanisms is induced endocytosis, which is stimulated when fungal invasins bind to epithelial cell receptors such as EGFR. Receptor binding causes rearrangement of epithelial cell microfilaments, leading to the formation of pseudopods that engulf the fungus and pull it into the epithelial cell. We discovered AhR acts via SFKs to phosphorylate EGFR and induce the endocytosis of C. albicans. Our finding that a small molecule inhibitor of AhR ameliorates OPC in mice suggests that a strategy of targeting host cell signaling pathways that govern epithelial cell endocytosis of C. albicans holds promise as a new approach to preventing or treating OPC.
INTRODUCTION
Oropharyngeal candidiasis (OPC) is one of the most common opportunistic infections in HIV-infected individuals, occurring in up to 90% of those with advanced immune suppression (1, 2). The prevalence of OPC and esophageal candidiasis remains high in patients newly diagnosed with HIV, especially in Asia, Africa, and Latin America (3–6). Candida albicans causes at least 80% of cases of OPC in patients with HIV/AIDS (7, 8) and is also the most common cause of OPC in patients with Sjogren’s syndrome, diabetes mellitus, and cancer of the head and neck (9–11). The predominance of C. albicans as the cause of OPC suggests that this organism possesses unique characteristics that enable it to colonize the oropharynx and, when host defenses are impaired, cause OPC.
A characteristic finding during OPC is invasion of the superficial epithelium (12). Indeed, transmission electron microscopy studies of biopsy specimens from patients with OPC demonstrate organisms within the oral epithelial cells (13, 14). Candidal invasion of epithelial cells is a continuous process during OPC, occurring both when a focus of infection is initiated and as the lesion progressively expands. C. albicans can invade epithelial cells by two different mechanisms: active penetration and induced endocytosis (15–19). The latter process occurs when the C. albicans Als3 and Ssa1 invasin proteins bind to epithelial cell E-cadherin and a heterodimer consisting of the epidermal growth factor receptor (EGFR) and HER2. Binding to these receptors triggers rearrangement of epithelial cell microfilaments, leading to the formation of pseudopods that surround the organism and pull it into the epithelial cell (20–22).
As a prototypic Th1 cytokine, interferon gamma (IFN-γ) has been used as adjunctive therapy for patients with both hematogenously disseminated candidiasis and multidrug-resistant OPC (23, 24). When administered prophylactically to patients with advanced HIV infection, IFN-γ appears to reduce the frequency of OPC (25). The salutary effects of IFN-γ on the host’s defense against C. albicans infection have been thought to be due to enhanced antigen presentation and phagocyte activity (26). However, IFN-γ also has effects on nonmyeloid cells. Previously, we found that treatment with IFN-γ protects endothelial cells from C. albicans infection in vitro by inhibiting endothelial cell endocytosis of the organism (27). In the present study, we investigated the capacity of IFN-γ to protect oral epithelial cells from invasion by C. albicans. We found that 24 h of exposure of oral epithelial cells to IFN-γ activates indoleamine 2,3-deoxygenase (IDO), leading to the synthesis of kynurenines, which activate the aryl hydrocarbon receptor (AhR) and Src family kinases (SFKs). Prolonged activation of SFKs inhibits the phosphorylation of EGFR and reduces endocytosis of C. albicans. Pharmacological inhibition of the AhR inhibits SFK activation and endocytosis in vitro and reduces the severity of OPC in mice, indicating that this cytoplasmic receptor plays a vital role in the endocytosis of C. albicans, both in vitro and in vivo.
RESULTS
IFN-γ treatment inhibits endocytosis of C. albicans by oral epithelial cells.
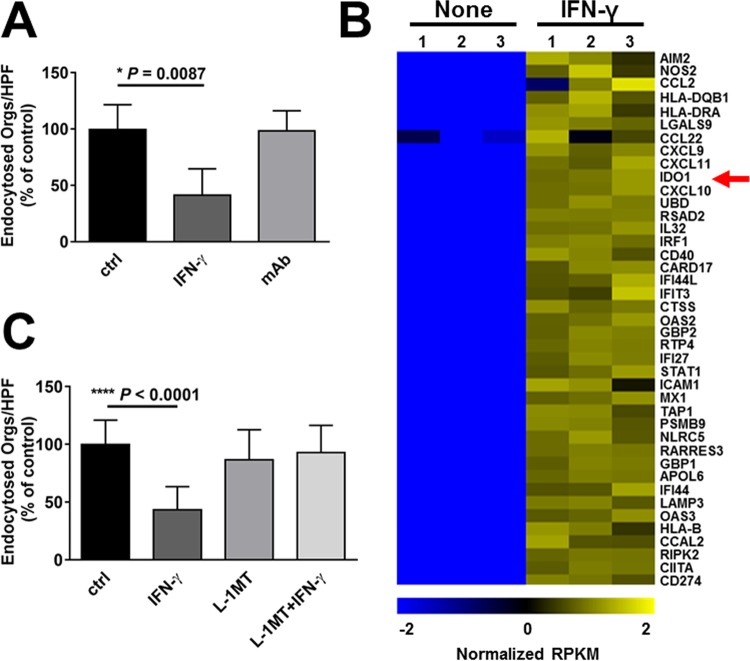
To investigate the effects of IFN-γ on the endocytosis of C. albicans, the OKF6/TERT-2 oral epithelial cell line (28) was incubated with either IFN-γ or medium alone for 24 h and then infected with C. albicans strain SC5314. The number of organisms endocytosed by the oral epithelial cells was measured by our standard differential fluorescence assay, in which endocytosed/internalized organisms fluoresced red, whereas nonendocytosed organisms fluoresced both red and green (20, 21, 29). We found that incubation of epithelial cells with IFN-γ reduced the endocytosis of C. albicans by approximately 60% (Fig. 1A). The inhibitory effect of IFN-γ was reversed by a monoclonal antibody that blocked the epithelial cell IFN-γ receptor.
FIG 1 .
Interferon gamma (IFN-γ) inhibits endocytosis of C. albicans by oral epithelial cells. The OKF6/TERT-2 oral epithelial cell line was incubated with IFN-γ for 24 h in the absence and presence of an anti-IFN-γ monoclonal antibody (mAb). The cells were infected with C. albicans SC5314 for 2.5 h, after which the number of endocytosed organisms was determined using a differential fluorescence assay. Results are means ± standard deviations (SD) from three experiments, each performed in triplicate. Orgs/HPF, organisms per high-power field; ctrl, control. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). (B) RNA-seq analysis of the effects of IFN-γ on the transcriptional response of oral epithelial cells to C. albicans. OKF6/TERT-2 epithelial cells were incubated in the presence or absence of IFN-γ for 24 h and then infected with C. albicans for 5 h. RNA was extracted and analyzed by RNA-seq. The heat map shows normalized, log-transformed RPKM values of the top 40 IFN-γ-responsive genes. The red arrow indicates the IDO1 gene. (C) Effects of IDO inhibition with l-1-methyl-tryptophan (l-1MT) on OKF6/TERT-2 oral epithelial cell endocytosis of C. albicans. Results are means ± SD from three experiments, each performed in triplicate. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05).
To determine if IFN-γ influenced epithelial cell invasion via active penetration, we treated the epithelial cells with this cytokine for 24 and then fixed them with paraformaldehyde. After rinsing the cells extensively, we infected them with live C. albicans cells in the presence of IFN-γ. Although we detected active penetration of C. albicans into the fixed cells, this process was not affected by IFN-γ (see Fig. S1A in the supplemental material). Furthermore, IFN-γ had no detectable effect on C. albicans hyphal formation (Fig. S1B and C) or adherence to the epithelial cells (9.9 ± 3.7 cell-associated organisms per high-power field for control epithelial cells versus 10.3 ± 3.6 for IFN-γ-treated cells; n = 18, P = 0.73). Collectively, these data indicate that IFN-γ inhibits invasion of C. albicans by reducing its endocytosis by oral epithelial cells.
IFN-γ has no effect on active penetration into epithelial cells or hyphal growth. (A) OKF6/TERT-2 oral epithelial cells were incubated with IFN-γ for 24 h, fixed, and infected for 2.5 h with C. albicans cells in the presence of IFN-γ, after which the number of internalized organisms was determined by a differential fluorescence assay. Results are means ± SD from 3 experiments, each performed in triplicate. (B) Microscopic images of C. albicans cells after a 2.5-h incubation with oral epithelial cells that had been exposed to the indicated conditions for 24 h. The fungal cells were stained with an anti-Candida antiserum conjugated with Alexa Fluor 488. (C) Hyphal length after incubation for 2.5 h on oral epithelial cells that had been exposed to the indicated conditions for 24 h. Results are means ± SD from 50 organisms. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). ctrl, control; NS, not significant. Download FIG S1, PDF file, 0.2 MB (160.4KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
IFN-γ upregulates its canonical targets in oral epithelial cells in vitro.
To gain more comprehensive insight into how IFN-γ decreases the endocytosis of C. albicans, we used transcriptome sequencing (RNA-seq) to analyze the transcriptional response of oral epithelial cells that were treated with IFN-γ and then infected with C. albicans. As expected, exposure to this cytokine resulted in upregulation of multiple IFN-γ target genes (Fig. 1B; see Table S1 in the supplemental material). Gene Ontology (GO) term analysis indicated that many of the upregulated genes were involved in the response to interferons (see Table S2 in the supplemental material). In contrast, treatment with IFN-γ did not significantly affect the mRNA levels of the EGFR, ERBB2 (HER2), or CDH1 (E-cadherin) genes that encode the epithelial cell receptors for C. albicans (Table S1).
List of epithelial cell genes whose gene expression was altered by IFN-γ treatment. Download TABLE S1, XLS file, 0.4 MB (412.5KB, xls) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
GO term analysis of genes whose expression was up- or downregulated by IFN-γ treatment. Download TABLE S2, XLSX file, 0.1 MB (32.1KB, xlsx) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Among the known IFN-γ-responsive genes, the IDO1 gene was one of the genes most highly upregulated by IFN-γ treatment (Fig. 1B, red arrow). By real-time PCR, we verified that IFN-γ induced almost a 100-fold increase in IDO1 gene transcript levels in the oral epithelial cells (see Fig. S2 in the supplemental material), similar to what has been reported by others (30). To determine if IDO played a role in IFN-γ-mediated inhibition of endocytosis, epithelial cells were treated with IFN-γ in the presence of the IDO inhibitor l-1-methyl-tryptophan (l-1MT). We found that although l-1MT had no effect on the endocytosis of C. albicans by control epithelial cells, it completely reversed the inhibitory effects of IFN-γ (Fig. 1C), indicating that IFN-γ inhibits endocytosis by stimulating IDO activity.
Effect of IFN-γ on epithelial cell IDO1 mRNA expression, as measured by real-time PCR. Results are means ± SD from 2 experiments, each performed in triplicate. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). ctrl, control. Download FIG S2, PDF file, 0.3 MB (268.3KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
IFN-γ inhibition of endocytosis is mediated by kynurenine.
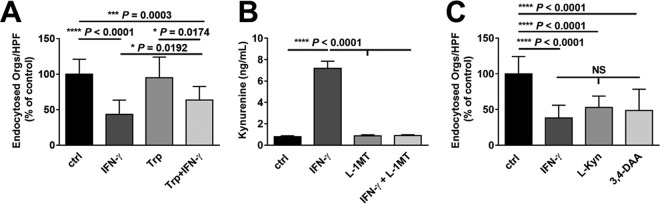
As the rate-limiting enzyme of tryptophan catabolism by the kynurenine pathway, IDO both degrades tryptophan and initiates the production of kynurenines (31). To determine if inhibition of endocytosis by IFN-γ was mediated by tryptophan depletion, we incubated epithelial cells with IFN-γ in the presence of exogenous l-tryptophan. Addition of l-tryptophan caused a small, but statistically significant reduction in IFN-γ-mediated inhibition of endocytosis (Fig. 2A). Next, we investigated whether the effect of IFN-γ on endocytosis was due to the enhanced production of kynurenine. First, we verified that treatment of oral epithelial cells with IFN-γ stimulated the release of l-kynurenine and that this process was blocked by the IDO inhibitor L-1MT (Fig. 2B). Next, we incubated oral epithelial cells for 24 h with either exogenous l-kynurenine or N-(3,4-dimethoxycinnamoyl)-anthranilic acid (3,4-DAA), the stable analog of a kynurenine metabolite (32). Both l-kynurenine and 3,4-DAA inhibited endocytosis of C. albicans similarly to IFN-γ (Fig. 2C). Collectively, these results support the model that IFN-γ stimulates IDO activity, leading to depletion of tryptophan and enhanced production of kynurenines and their metabolites, which inhibit the endocytosis of C. albicans.
FIG 2 .
The tryptophan metabolite kynurenine inhibits the endocytosis of C. albicans. (A) Effects of IFN-γ and the indicated compounds on epithelial cell endocytosis of C. albicans. OKF6/TERT-2 oral epithelial cells were incubated for 24 h with tryptophan (Trp), either alone or in combination with IFN-γ, and then infected with C. albicans for 2.5 h. Results are means ± SD from three experiments, each performed in triplicate. (B) Kynurenine production by epithelial cells after incubation with the indicated compounds for 24 h. Results are means ± SD from three experiments. (C) Effects of 24 h of exposure to l-kynurenine (L-Kyn) or the kynurenine analog N-(3,4-dimethoxycinnamoyl)-anthranilic acid (3,4-DAA) on epithelial cell endocytosis of C. albicans. Results are means ± SD from three experiments, each performed in triplicate. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). NS, not significant; ctrl, control.
AhR activation of SFKs is required for maximal endocytosis of C. albicans.
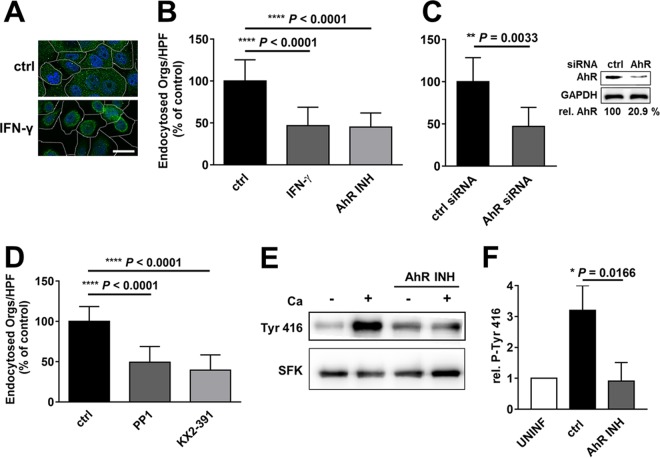
Kynurenines are endogenous ligands for AhR, which is located in the cytoplasm and forms a complex with SFKs (33, 34). When a ligand binds to AhR, the receptor translocates to the nucleus, while SFKs dissociate from the complex and become active, phosphorylating numerous substrates, including EGFR (35, 36). Using indirect immunofluorescence and confocal microscopy, we determined that treatment with IFN-γ caused AhR to translocate from the cytoplasm to the nucleus (Fig. 3A). Treatment with l-kynurenine also induced translocation of AhR (see Fig. S3 in the supplemental material), indicating that both IFN-γ and l-kynurenine activate AhR in oral epithelial cells.
FIG 3 .
IFN-γ activates the aryl hydrocarbon receptor (AhR) and Src family kinases (SFKs), which govern the endocytosis of C. albicans. (A) Confocal micrographs of OKF6/TERT-2 oral epithelial cells incubated in the presence and absence of IFN-γ for 24 h. The cells were stained for AhR (green), and the nuclei were stained with DAPI (blue). The perimeters of the cells were determined by differential interference contrast and are indicated by the dashed lines. Scale bar, 20 µm. (B) Endocytosis of C. albicans by oral epithelial cells treated with IFN-γ for 24 h or the AhR inhibitor for 1 h. (C) Endocytosis of C. albicans by oral epithelial cells transfected with either control siRNA or AhR siRNA. The inset is a representative immunoblot showing knockdown of AhR. (D) Effects of 1 h of exposure to the indicated SFK inhibitors on the endocytosis of C. albicans. All endocytosis data are means ± SD from three experiments, each performed in triplicate. (E and F) Effects of C. albicans and the AhR inhibitor on SFK phosphorylation. OKF6/TERT-2 cells were pretreated for 1 h with the indicated inhibitor and then infected with C. albicans for 1 h, after which the extent of SFK phosphorylation was determined by immunoblotting. (E) Representative immunoblot. (F) Densitometric analysis of 3 immunoblots such as the one shown in panel E. Results are means ± SD from 3 experiments. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). ctrl, control; INH, inhibitor; Ca, C. albicans; UNINF, uninfected.
l-Kynurenine activates the aryl hydrocarbon receptor (AhR) in oral epithelial cells. Shown are confocal micrographs of OKF6/TERT-2 oral epithelial cells incubated in the presence and absence of l-kynurenine for 24 h. The cells were stained for AhR (green), and the nuclei were stained with DAPI (blue). The perimeters of the cells were determined by differential interference contrast and are indicated by the dashed lines. Scale bar, 20 µm. ctrl, control. Download FIG S3, PDF file, 0.1 MB (153.5KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To investigate whether AhR influences the endocytosis of C. albicans by oral epithelial cells, we incubated the cells for 1 h with the AhR inhibitor CH-223191 (37) prior to infection. This inhibitor reduced the endocytosis of C. albicans by the same extent as IFN-γ (Fig. 3B). Knockdown of AhR with small interfering RNA (siRNA) also significantly decreased C. albicans endocytosis (Fig. 3C). Therefore, AhR function is necessary for maximal endocytosis of the fungus.
Activation of AhR leads to derepression of SFKs, which undergo autophosphorylation and in turn phosphorylate and activate EGFR (35, 36). To determine whether SFKs govern epithelial cell endocytosis of C. albicans, we tested two structurally distinct SFK inhibitors, PP1 and KX2-391. Both inhibitors significantly reduced the endocytosis of the fungus (Fig. 3D). By immunoblotting with a phosphospecific antibody, we also determined that C. albicans infection of oral epithelial cells induced the tyrosine phosphorylation of SFKs (Fig. 3E and F). This phosphorylation was blocked when epithelial cells were incubated with the AhR inhibitor, indicating that AhR activation is required for SFK activity, which in turn is necessary for maximal epithelial cell endocytosis of C. albicans.
IFN-γ, AhR, and SFKs govern endocytosis via phosphorylation of EGFR.
Our next objective was to investigate the relationship between IFN-γ and the epithelial cell receptors for C. albicans. One potential explanation for the inhibitory effects of IFN-γ on the endocytosis of C. albicans is that the cytokine downregulates the expression of one or more epithelial cell receptors for C. albicans. However, by real-time PCR, we verified our RNA-seq findings that IFN-γ did not change the mRNA levels of the genes encoding E-cadherin, EGFR, or HER2 (see Fig. S4A in the supplemental material). Furthermore, flow cytometric analysis indicated that IFN-γ treatment did not reduce the surface expression of these receptors (Fig. S4B). Therefore, IFN-γ must inhibit endocytosis by acting on another step in the endocytosis signaling pathway.
IFN-γ treatment has no effect on the expression of host cell receptors for C. albicans. (A) mRNA levels of the indicated epithelial cell receptors after 24 h of IFN-γ treatment. Results are means ± SD from two independent experiments performed in triplicate. (B) Effects of IFN-γ on the expression of E-cadherin, EGFR, and HER2 on the surface of epithelial cells as determined by flow cytometry. Control cells (stained with a control monoclonal antibody [MAb]) are shown in light gray, untreated cells (stained with the specific MAb) are shown in dark gray, and the IFN-γ-treated cells (stained with the specific MAb) are shown in red. Download FIG S4, PDF file, 0.3 MB (304.7KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
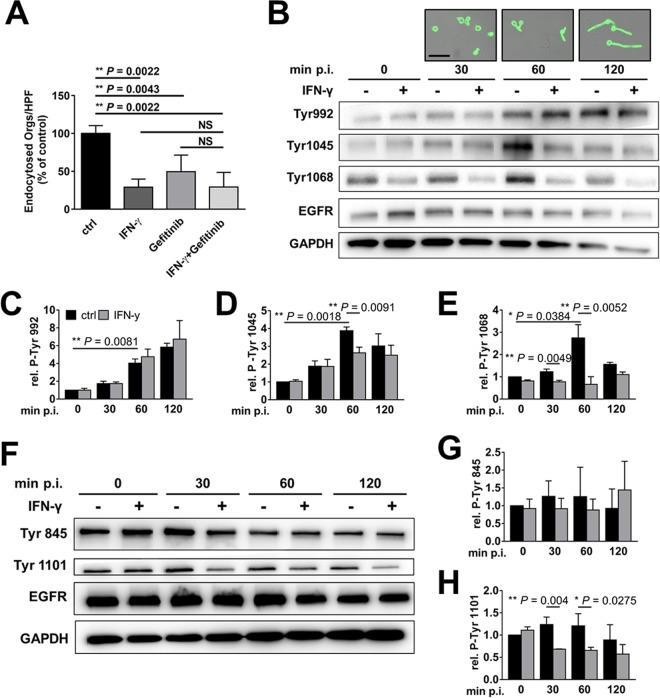
To investigate whether IFN-γ influences signaling through EGFR, we analyzed the effects of IFN-γ and the EGFR inhibitor gefitinib on endocytosis. Treatment of epithelial cells with either IFN-γ or gefitinib alone significantly reduced the endocytosis of C. albicans (Fig. 4A). Moreover, the inhibitory effect of combined treatment with both IFN-γ and gefitinib was similar to that of IFN-γ alone, suggesting that IFN-γ and gefitinib reduce endocytosis by inhibiting the same pathway.
FIG 4 .
IFN-γ inhibits EGFR phosphorylation. (A) Effects of IFN-γ (24 h) and/or the EGFR inhibitor gefitinib (1 h) on the endocytosis of C. albicans by OKF6/TERT-2 oral epithelial cells. Results are the means ± SD from three experiments, each performed in triplicate. (B to E) Effects of IFN-γ on C. albicans-induced autophosphorylation of the indicated tyrosine residues of EGFR. The oral epithelial cells were incubated in the presence and absence of IFN-γ for 24 and then infected with C. albicans for the indicated time points. The phosphorylation of the specific EGFR tyrosine residues was determined by immunoblotting with specific monoclonal antibodies. (B) Representative immunoblots. The images above the blot show the C. albicans morphology at the indicated time points. Scale bar, 20 µm. (C to E) Densitometric analysis of the immunoblots. Results are means ± SD from 3 experiments. (F to H) Effects of IFN-γ on SFK-dependent phosphorylation of the indicated tyrosine residues of EGFR. (F) Representative immunoblots. (G and H) Densitometric analysis of the immunoblots. Results are means ± SD from 3 experiments. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). NS, not significant; ctrl, control; p.i., postinfection.
EGFR is a receptor tyrosine kinase that, when activated, is autophosphorylated on multiple tyrosine residues, including Y992, Y1045, and Y1068 (38). To determine if IFN-γ alters C. albicans-induced autophosphorylation of EGFR, we treated oral epithelial cells with the cytokine and infected them with yeast-phase C. albicans. We observed that at 60 min postinfection, the organisms began to germinate, forming nascent germ tubes (Fig. 4B). By 120 min, these hyphae had grown considerably in length. At each time point, we lysed the cells and analyzed the extent of EGFR phosphorylation on specific tyrosine residues by immunoblotting with phosphospecific monoclonal antibodies. IFN-γ treatment and C. albicans infection altered the autophosphorylation of specific EGFR tyrosine residues in two distinct patterns. The phosphorylation of Y992 and Y1045 increased progressively during C. albicans infection, but this increase was essentially unaffected by IFN-γ (Fig. 4B to D). In contrast, the phosphorylation of Y1068 increased, especially at 60 min postinfection, and this increase was blocked by IFN-γ (Fig. 4B and E).
SFKs phosphorylate EGFR on Y845 and Y1101, enhancing EGFR signaling (36). While C. albicans infection did not induce phosphorylation of either tyrosine residue, IFN-γ treatment significantly inhibited the phosphorylation of Y1101 (Fig. 4F to H). Collectively, these data suggest that the inhibitory effects of IFN-γ on the endocytosis of C. albicans are due to reduced phosphorylation of EGFR on Y1068 and/or Y1101. The finding that C. albicans and IFN-γ had the greatest effect on phosphorylation at the 60-min time point suggests that phosphorylation of these tyrosine residues may be required to prime the endocytosis signaling pathway.
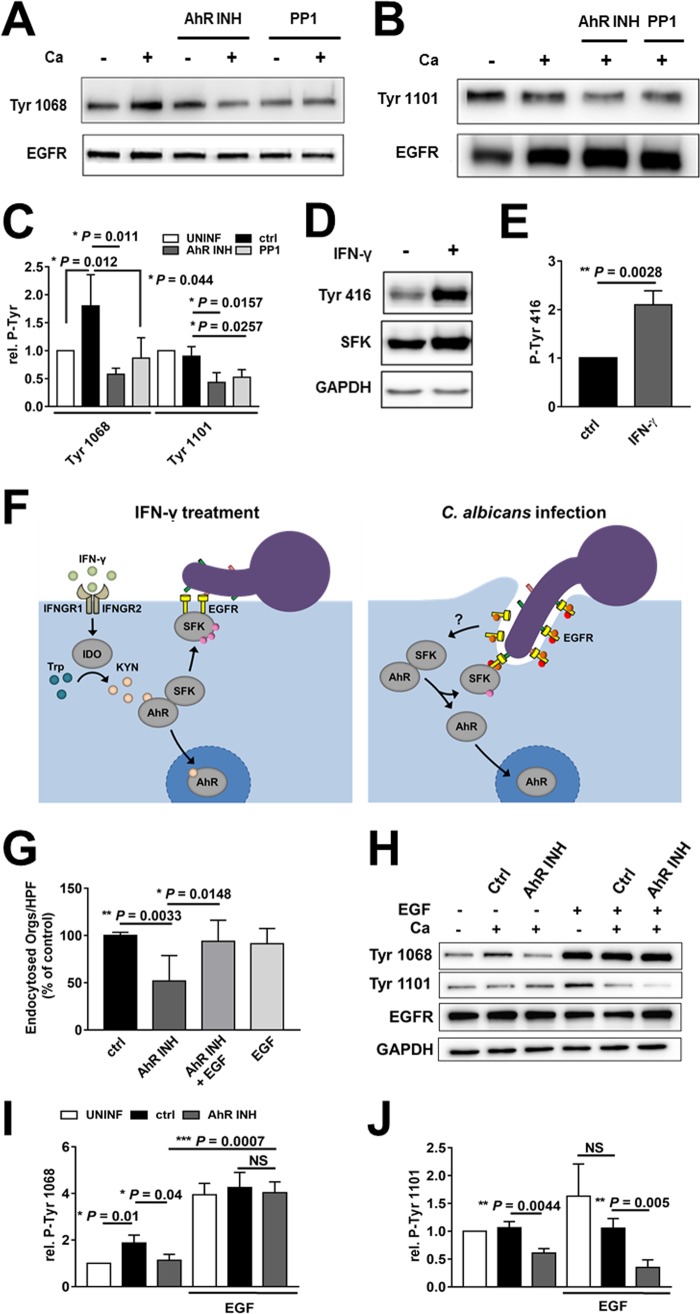
Next, we analyzed the effects of blocking AhR and SFKs on the phosphorylation of these tyrosine residues. Both the AhR and SFK inhibitors decreased C. albicans-induced phosphorylation of EGFR at Y1068 and Y1101 (Fig. 5A to C), similarly to what we observed with IFN-γ (Fig. 4). Incubation of epithelial cells with l-kynurenine for 24 h also inhibited phosphorylation of Y1068 and Y1101 (Fig. S5). Furthermore, incubating the epithelial cells with IFN-γ for 24 h stimulated the phosphorylation of SFKs (Fig. 5D and E), even though it inhibited phosphorylation of EGFR. These results suggest that prolonged stimulation of SFKs leads to compensatory downregulation of EGFR phosphorylation.
FIG 5 .
C. albicans-induced phosphorylation of EGFR depends on AhR and SFK activity. (A to C). Effects of inhibition of AhR and SFKs on C. albicans-induced phosphorylation of EGFR. OKF6/TERT-2 epithelial cells were pretreated for 1 h with the indicated inhibitor and then infected with C. albicans for 1 h. (A and B) Representative immunoblots showing EGFR phosphorylation at Y1068 (A) and Y1101 (B). (C) Densitometric analysis of the immunoblots in panels A and B. Results are means ± SD from 3 experiments. (D and E) Effects of IFN-γ on SFK phosphorylation. (D) Representative immunoblot. (E) Densitometric analysis of the immunoblots in panel D. Results are means ± SD from 3 experiments. (F, left panel) Proposed model of how IFN-γ inhibits the endocytosis of C. albicans by activating IDO, leading to the production of kynurenines that induce prolonged activation of AhR and SFKs, thereby preventing C. albicans-induced activation of EGFR and inhibiting endocytosis of the organism. (Right panel) Proposed model in which C. albicans activates AhR, stimulating SFKs that phosphorylate EGFR, leading to the endocytosis of the fungus. (G to J) Effects of the epidermal growth factor (EGF) and the AhR inhibitor on endocytosis (G) and EGFR phosphorylation (H to J). Results are means ± SD from three experiments, each performed in triplicate. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). Ca, C. albicans; INH, inhibitor; UNINF, uninfected; ctrl, control; Kyn, kynurenines; NS, not significant.
Effects of l-kynurenine on C. albicans-induced phosphorylation of EGFR. OKF6/TERT-2 epithelial cells were incubated with l-kynurenine for 24 h and then infected with C. albicans for 1 h (A) Representative immunoblots showing EGFR phosphorylation at Y1068 and Y1101. (B) Densitometric analysis of the immunoblots in panel A. Results are means ± SD from 3 experiments. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). Ca, C. albicans; UNINF, uninfected; L-Kyn, l-kynurinine. Download FIG S5, PDF file, 0.1 MB (154.2KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Collectively, these data support a model in which prolonged exposure to IFN-γ upregulates epithelial cell IDO, stimulating the production of kynurenines and activating AhR and SFKs. Prolonged SFK activation inhibits phosphorylation of EGFR on Y1068 and Y1101 and blocks the endocytosis of C. albicans (Fig. 5F). Furthermore, by activating SFKs and inducing the phosphorylation of EGFR, AhR plays a key role in initiating the endocytosis of C. albicans by oral epithelial cells.
This model predicts that the effects of the AhR inhibitor could be reversed if EGFR remains phosphorylated. To test this prediction, we added epidermal growth factor (EGF), the natural ligand of EGFR, to oral epithelial cells that had been infected with C. albicans in the presence or absence of the AhR inhibitor. As predicted, EGF restored C. albicans endocytosis by epithelial cells treated with the AhR inhibitor but had no effect on endocytosis by untreated cells (Fig. 5G). We also analyzed the effects of EGF on the phosphorylation of EGFR at Y1068 and Y1101. EGF strongly stimulated the phosphorylation of Y1068, both in the presence and in the absence of C. albicans. This phosphorylation of was not reduced by the AhR inhibitor (Fig. 5H and I). In contrast, EGF did not enhance the phosphorylation of Y1101 in the presence of C. albicans, and the phosphorylation of this tyrosine residue was inhibited by the AhR inhibitor (Fig. 5H and J). These results suggest that AhR-induced phosphorylation of EGFR on Y1068, but not Y1101, is necessary for oral epithelial cells to endocytose C. albicans.
IFN-γ, AhR, and SFKs have different effects on C. albicans-induced epithelial cell damage and cytokine release.
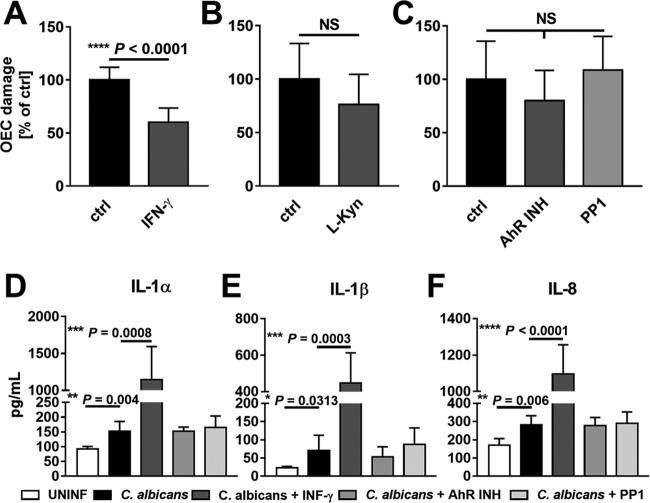
In addition to inducing its own endocytosis by epithelial cells, C. albicans damages these cells and stimulates them to produce proinflammatory cytokines (39). We investigated the effects of IFN-γ and the inhibition of AhR and SFKs on these responses. While treatment with IFN-γ significantly inhibited the extent of C. albicans-induced epithelial cell damage, treatment with l-kynurenine or the AhR or SFK inhibitor did not (Fig. 6A to C). Also, IFN-γ markedly enhanced the release of interleukin-1α (IL-1α), IL-1β, and IL-8 by epithelial cells in response to C. albicans infection (Fig. 6D to F). In contrast, neither the AhR nor the SFK inhibitor significantly altered the release of these cytokines by the infected epithelial cells. Collectively, these results indicate that while IFN-γ inhibits epithelial cell endocytosis of C. albicans by acting via IDO, AhR, and SFKs, it decreases epithelial cell damage and stimulates the release of proinflammatory cytokines via a different pathway or pathways.
FIG 6 .
Effects of IFN-γ, AhR, and SFKs on epithelial cell damage and cytokine release induced by C. albicans (A to C). Oral epithelial cells were incubated with IFN-γ (A) or l-kynurenine (B) for 24 h or with the AhR or SFK inhibitor (C) for 1 h and then infected with C. albicans for 7 h. The extent of epithelial cell damage was measured using a 51Cr release assay. (D and E) Oral epithelial cells were incubated with the indicated compounds as in panels A to C and infected with C. albicans for 8 h, after which the supernatant was collected and analyzed for the concentration of interleukin-1α (IL-1α [D]), IL-1β (E), and IL-8 (F). Results are means ± SD from three experiments, each performed in triplicate. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). OEC, oral epithelial cells; ctrl, control; NS, not significant; INH, inhibitor; UNINF, uninfected; L-Kyn, l-kynurenine.
Inhibition of AhR ameliorates disease in the mouse model of OPC.
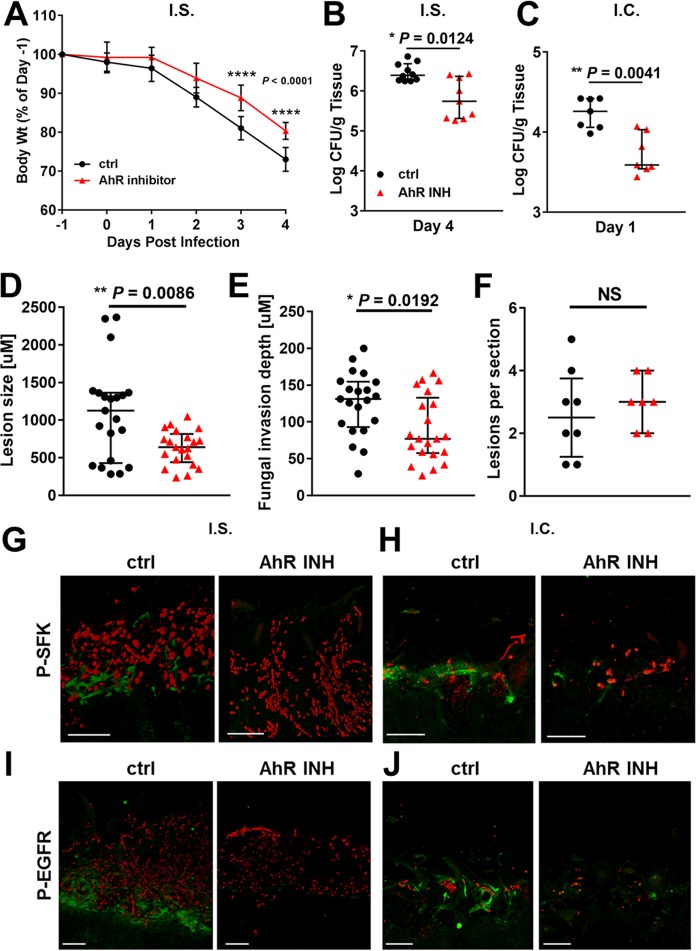
To determine if AhR is required for the pathogenic interactions of C. albicans with epithelial cells in vivo, we analyzed the effects of the AhR inhibitor on disease severity in the mouse model of OPC. In mice that were immunosuppressed with cortisone acetate prior to induction of OPC, treatment with the AhR inhibitor limited the extent of weight loss and reduced the oral fungal burden by 8-fold relative to control mice that received the vehicle alone (Fig. 7A and B). In immunocompetent mice, treatment with the AhR inhibitor also significantly decreased the oral fungal burden (Fig. 7C). Quantitative analysis of thin sections of the tongues of the immunosuppressed mice demonstrated that the fungal lesions of mice treated with the AhR inhibitor were smaller and that the maximal depth of fungal invasion was shallower relative to the control mice (Fig. 7D and E). However, the numbers of fungal lesions were similar in both groups (Fig. 7F). Of note, the AhR inhibitor did not affect the length of the fungal hyphae, either in vitro or in vivo (see Fig. S6 in the supplemental material). In addition, the AhR inhibitor had no effect on the capacity of neutrophils to kill C. albicans (see Fig. S7 in the supplemental material). Consistent with our in vitro data, oral infection with C. albicans induced phosphorylation of SFKs and EGFR in both immunosuppressed and immunocompetent mice (Fig. 7G to J). Moreover, treatment with the AhR inhibitor significantly inhibited this phosphorylation. These results indicate that signaling through AhR is necessary for C. albicans to activate SFKs and EGFR and to invade oral epithelial cells during the pathogenesis of OPC.
FIG 7 .
Inhibition of AhR reduces severity of disease during experimental OPC. Immunosuppressed (I.S.) and immunocompetent (I.C.) mice were treated with either diluent (control [ctrl]) or the AhR inhibitor (INH) and then orally inoculated with C. albicans. (A) Daily body weights of the immunosuppressed mice. (B and C) Oral fungal burden of the immunosuppressed mice after 4 days of infection (B) and of the immunocompetent mice after 1 day of infection (C). Results in panels A and B are medians ± interquartile ranges from the combined results of two separate experiments for a total of 9 to 10 mice per experimental group. Results in panel C are medians ± interquartile ranges from a single experiment with 7 mice per experimental group. (D to F) Analysis of the fungal lesions in tongues of immunosuppressed mice after 4 days of infection. (D) Length of the fungal lesions. (E) Depth of maximal fungal invasion. (F) Number of fungal lesions per tongue section. Results in panels D to F are medians ± interquartile ranges from the analysis of 7 to 8 thin sections from two separate experiments using a total of 6 mice per experimental group. (G to J) Inhibition of AhR reduces phosphorylation of SFKs and EGFR in the oral mucosa. Shown are confocal images of thin sections of the tongues of immunosuppressed (G and I) and immunocompetent (H and J) mice that were administered either diluent alone (ctrl [left panels]) or the AhR inhibitor (right panels) and then infected with C. albicans as in panels A to C. The thin sections in panels G and H were stained for phospho-SFK Y416 (green), and the thin sections in panels I and J were stained for phospho-EGFR Y1068 (green). All sections were also stained with an anti-Candida antiserum (red). Scale bar, 50 µm. Statistical significance was determined using the Mann-Whitney test (P ≤ 0.05).
The AhR inhibitor has no effect on hyphal length. (A) Oral epithelial cells were incubated in the presence or absence of the AhR inhibitor for 1 h and infected with C. albicans for 2.5 h, after which the length of the fungal hyphae was determined. Results are means ± SD of 50 organisms. (B) Hyphal length of C. albicans in the tongues of mice with OPC after 4 days of infection. To detect C. albicans hyphae, thin sections of the infected tongues were rehydrated in PBS and then blocked. They were stained with an anti-Candida antibody conjugated with Alexa Fluor 568 and then imaged by confocal microscopy. The length of the elongated cells (>10 µm) was measured using LAS AF lite software. Results are means ± SD from 50 organisms in the tongues of 3 mice per experimental group. NS, not significant; ctrl, control. Download FIG S6, PDF file, 0.2 MB (183.7KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effects of the AhR inhibitor on the capacity of neutrophils to kill C. albicans. Human neutrophils were incubated with C. albicans cells at a ratio of 1:1 for 3 h in the presence of 10% pooled human serum, with or without the AhR inhibitor. The percentage of organisms killed was determined by colony counting. Results are means ± SD from 3 experiments. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). ctrl, control; INH, inhibitor. Download FIG S7, PDF file, 0.1 MB (119.2KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
Invasion of oral epithelial cells is a vital step in the pathogenesis of OPC. By investigating the mechanism by which IFN-γ treatment protects oral epithelial cells from candidal invasion, we discovered that AhR plays a central role in governing EGFR-mediated endocytosis of C. albicans by oral epithelial cells, both in vitro and in vivo. This conclusion is supported by our findings that both prolonged activation of AhR by IFN-γ or l-kynurenine and inhibition of AhR by either siRNA or a small molecule inhibitor reduced the phosphorylation of EGFR and the endocytosis of C. albicans in vitro. Furthermore, treatment of both immunocompetent and immunocompromised mice with the AhR inhibitor ameliorated experimental OPC.
AhR is known to modulate the host inflammatory response to infectious agents via its effects on leukocytes. This receptor is required for maximal interleukin-10 (IL-10) production by NK cells (40). Also, by inhibiting NLRP3 expression in macrophages, AhR reduces the inflammatory response and inhibits apoptosis during infection (41, 42) In dendritic cells, AhR activates SFKs, which phosphorylate IDO, leading to increased enzyme activity and production of tolerogenic kynurenines (43). In the gastrointestinal mucosa, AhR induces IL-22 production by innate lymphoid cells, thereby augmenting the antifungal resistance of gastrointestinal epithelial cells (44). IL-22 is also necessary for the host’s defense against OPC (45); in contrast, the data presented here demonstrate that AhR has a proinfective function—it induces the endocytosis of C. albicans by acting through SFKs to stimulate the phosphorylation of EGFR, a key epithelial cell receptor for this organism.
Previously, we reported that when pregerminated C. albicans hyphae were added to oral epithelial cells, they stimulated EGFR phosphorylation within 10 min (21). The capacity of C. albicans hyphae to induce phosphorylation of EGFR so rapidly suggests that the fungus quickly stimulates AhR, leading to activation of one or more SFKs that phosphorylate EGFR. Because AhR is located in the cytoplasm, C. albicans must activate this receptor indirectly. Although kynurenines are one of the numerous endogenous AhR ligands, it seems unlikely that C. albicans stimulates IDO activity and induces sufficient synthesis of kynurenines to activate AhR within just 10 min. Thus, it is more probable that C. albicans stimulates AhR by inducing the release of a preformed endogenous ligand, the identity of which remains to be determined.
A notable finding was that although C. albicans infection stimulated the phosphorylation of multiple tyrosine residues in EGFR, the phosphorylation of only two of these residues, Y1068 and Y1101, was governed by IFN-γ. It has been reported that 15 min of exposure of A431, HeLa, and HEK-293 epithelial cells to IFN-γ induces phosphorylation of multiple tyrosine residues in EGFR, including Y1068, and that this phosphorylation can be blocked by inhibition of SFKs (46). In contrast, another group found that a 48-h treatment of human T84 colonic epithelial cells with IFN-γ downregulates the phosphorylation of Y1068 in response to EGF (47). Although the effects of IFN-γ on the phosphorylation of Y1101 were not tested by either of these groups, these data are consistent with our findings that IFN-γ activates SFKs and that prolonged exposure to IFN-γ induces a compensatory downregulation of EGFR phosphorylation.
Although C. albicans infection stimulated the autophosphorylation of multiple tyrosine residues of EGFR in oral epithelial cells, Y1068 appears to be the most important in governing the endocytosis of the fungus. Not only was Y1068 phosphorylated in response to C. albicans hyphae, but this phosphorylation was blocked by prolonged exposure to IFN-γ, l-kynurenine, and inhibition of either AhR or SFKs. Furthermore, treatment of epithelial cells with EGF reversed the inhibitory effect of the AhR inhibitor on Y1068 phosphorylation and restored endocytosis of C. albicans. Y1068 is known to bind to growth factor receptor binding protein 2 (Grb2) (48), an adapter protein that is required for EGFR to be internalized via clathrin-coated pits (49). Previously, we found that endocytosis of C. albicans is mediated by a clathrin-dependent mechanism (50). The present data suggest that C. albicans stimulates the phosphorylation of Y1068 of EGFR, which in turn activates the clathrin endocytosis pathway, leading to internalization of the fungus.
Although C. albicans did not induce the phosphorylation of EGFR Y1101, the basal level of phosphorylation of this tyrosine residue was reduced by IFN-γ, l-kynurenine, and the AhR and SFK inhibitors. However, when epithelial cells were treated with both EGF and the AhR inhibitor, they were able to endocytose C. albicans, even though phosphorylation of Y1101 was reduced. Thus, it is highly probable that Y1101 phosphorylation is dispensable for the induction of endocytosis.
Although treatment with IFN-γ and the AhR and SFK inhibitors had very similar effects on epithelial cell endocytosis of C. albicans, only IFN-γ inhibited fungus-induced epithelial cell damage and enhanced the release of proinflammatory cytokines. Previously, we had found that IFN-γ likewise protects endothelial cells from damage by C. albicans (27). The present data indicate that IFN-γ must protect oral epithelial cells from damage and augment cytokine release via signaling pathways that are independent of AhR and SFKs.
Cancer cell lines are a powerful tool dissecting the interactions of fungi with host cells. However, SFKs and EGFR are overexpressed in many epithelial cell lines (51, 52). The OKF6/TERT-2 oral epithelial cell line was developed by the forced expression of the human telomerase gene in oral keratinocytes from a healthy individual (28). Recently, we determined that the transcriptional response of OKF6/TERT-2 cells to C. albicans infection was highly similar to that of oral mucosa in mice with OPC. Specifically, C. albicans infection in OKF6/TERT-2 cells and OPC in mice activated the same signaling pathways, including the EGFR, IL-17, tumor necrosis factor (TNF), Toll-like receptor (TLR), and NF-κB pathways (53). In the present study, we found that AhR and SFKs are crucial for regulating EGFR signaling during the pathogenic interactions of C. albicans with OKF6/TERT-2 cells in vitro and during OPC in both immunosuppressed and immunocompetent mice. Thus, OKF6/TERT-2 cells constitute a powerful tool for elucidating the receptors and signaling pathways that govern the epithelial cell response to C. albicans during OPC.
Previously, we found that treatment of corticosteroid-treated mice with GW2974, an inhibitor of EGFR and HER2, blocked C. albicans-induced phosphorylation of these receptors and reduced the severity of OPC, demonstrating the importance of receptor-mediated fungal invasion of epithelial cells in the pathogenesis of this disease (21). In the present work, we determined that in corticosteroid-treated mice, a small molecule inhibitor of AhR markedly decreased the phosphorylation of SFKs and EGFR, and it ameliorated OPC similarly to GW2974. The AhR inhibitor was also efficacious in immunocompetent mice, although these animals clear C. albicans from the oral cavity very rapidly and do not exhibit overt OPC symptoms (45, 54). These results suggest that because AhR is essential for C. albicans to subvert EGFR signaling and invade epithelial cells in vivo, it is a potential therapeutic target.
MATERIALS AND METHODS
Ethics statement.
All animal work was approved by the Institutional Animal Care and Use Committee (IACUC) of the Los Angeles Biomedical Research Institute. The collection of blood from human volunteers for neutrophil isolation was also approved by the Institutional Review Board of the Los Angeles Biomedical Research Institute.
Cells and cell lines.
C. albicans SC5314 (55) was used in all experiments. It was maintained on yeast extract-peptone dextrose agar (YPD). For use in the experiments, the organisms were grown for 18 h in YPD broth in a shaking incubator at 30°C. The next day, the fungal cells were harvested by centrifugation, washed twice with phosphate-buffered saline (PBS), and counted using a hemacytometer.
The human oral epithelial cell line OKF6/TERT-2 was kindly provided by J. Rheinwald (Harvard University, Cambridge, MA) (28) and was cultured as previously described (20). Recombinant IFN-γ (PeproTech) was reconstituted in Dulbecco’s PBS containing 0.1% bovine serum albumin (BSA) (Sigma) and stored in aliquots at −80°C. In all experiments, OKF6/TERT-2 cells were incubated with IFN-γ at a final concentration of 25 ng/ml for 24 h prior to infection with C. albicans, and the IFN-γ was present in the medium for the duration of the infection.
Measurement of epithelial cell endocytosis.
The endocytosis of C. albicans by oral epithelial cells was quantified by a differential fluorescence assay as described previously (13). Briefly, OKF6/TERT-2 cells were grown to confluence on fibronectin-coated circular glass coverslips in 24-well tissue culture plates. They were infected with 2 × 105 yeast-phase C. albicans cells per well and incubated for 2.5 h, after which they were fixed, stained, and mounted inverted on microscope slides. The coverslips were viewed with an epifluorescence microscope, and the number of endocytosed organisms per high-power field was determined, counting at least 100 organisms per coverslip. Each experiment was performed at least three times in triplicate.
To determine the effects of the antibodies, exogenous ligands, and inhibitors on endocytosis, the host cells were incubated with an anti-IFN-γ receptor monoclonal antibody (25 µg/ml; R&D Systems), 1-methyl-d-tryptophan (0.2 mM; Sigma-Aldrich), l-kynurenine (100 µM; Sigma-Aldrich), levo-1-methyl tryptophan (l-1MT) (0.2 mM; Sigma-Aldrich), 3,4-DAA (200 µM; Cayman Chemical), CH-223191 (10 µM; Sigma-Aldrich), gefitinib (1 µM; Selleckchem), PP1 (100 nM; Cell Signaling), KX2-391 (100 nM; Selleckchem), or EGF (50 ng/ml; Life Technologies, Inc.). The inhibitors were added to the host cells 60 min before infection with C. albicans, and they remained in the medium for the entire incubation period. Control cells were incubated with a similar concentration of the diluent (dimethyl sulfoxide [DMSO]) at final concentrations ranging from 0.1 to 0.2%.
As described previously (21), siRNA was used to deplete AhR from the epithelial cells. OKF6/TERT-2 cells were transfected with random control siRNA (Qiagen) or AhR siRNA (80 pmol; Santa Cruz Biotechnology) using Lipofectamine 2000 (Thermo Fisher Scientific) following the manufacturer’s instructions.
RNA-seq and real-time PCR.
For RNA-seq, OKF6/TERT-2 cells in six-well tissue culture plates were treated with either recombinant IFN-γ or medium alone for 24 h and then infected with 1 × 107 C. albicans yeast cells for 5 h in biological triplicates. Total epithelial cell RNA was isolated using the RiboPure yeast kit (Ambion), according to the manufacturer’s instructions. The RNA was subjected to poly(A) enrichment by the TruSeq protocol, after which RNA-seq libraries (non-strand-specific, paired end) were prepared with the TruSeq RNA kit (Illumina). Using the HiSeq platform, 100 nucleotides of sequence was determined from each end of the cDNA fragments. Sequencing reads were aligned to the human reference genome Ensemble GRCh38 (56) using TopHat2 (57). The alignment files were then used to generate read counts for each gene, and a statistical analysis of differential gene expression was assessed using the DESeq package from Bioconductor (58). Reads per kilobase million (RPKM) values for each gene in each sample were generated using in-house scripts. For real-time PCR, host RNA was extracted using the RiboPure yeast kit, according to the manufacturer’s instructions. After preparing cDNA, the transcript levels of the genes of interest were measured by real-time PCR using the primers listed in Table S3 in the supplemental material. The relative transcript level of each gene was normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) by the threshold cycle (2−ΔΔCT) method.
Oligonucleotides used in the experiments. Download TABLE S3, DOCX file, 0.1 MB (35KB, docx) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Kynurenine measurement.
OKF6/TERT-2 cells in 24-well tissue culture plates were incubated with medium alone, IFN-γ, l-1MT, or IFN-γ plus l-1MT. After 24 h, the medium above the cells was collected, clarified by centrifugation, and stored at −80°C. The amount of l-kynurenine in the conditioned medium was determined by enzyme-linked immunosorbent assay (ELISA) (MyBioSource) according to the manufacturer’s instructions.
Detection of protein phosphorylation.
OKF6/TERT-2 cells in six-well tissue culture plates were incubated in tissue culture medium with or without IFN-γ for 24 h and then infected with 4.5 × 106 C. albicans cells. At various time points, the cells were rinsed with cold PBS containing protease and phosphatase inhibitor cocktails and removed from the plate with a cell scraper. The cells were collected by centrifugation and boiled in sample buffer. The lysates were separated by SDS-PAGE, and the phosphorylation of specific tyrosine residues of EGFR was detected by immunoblotting with specific antibodies (phospho-EGF receptor antibody sampler kit 9922 from Cell Signaling and EGFR–p-Tyr1101 from ECM Biosciences). Next, the blot was stripped, and total EGFR was detected by immunoblotting with an anti-EGFR antibody (catalog no. sc-101; Santa Cruz Biotechnology). Following a similar approach, SFK phosphorylation on Y416 was determined using the antibodies in the Src antibody sampler kit 9935 (Cell Signaling). Each experiment was performed at least 3 times.
Indirect immunofluorescence.
To determine the intracellular location of AhR, OKF6/TERT-2 cells were incubated in tissue culture medium with or without IFN-γ or l-kynurenine for 24 h. Next, the cells were fixed with 3% paraformaldehyde, blocked with 10% BSA, and incubated with an anti-AhR antibody (catalog no. sc-133088; Santa Cruz Biotechnology), followed by an Alexa 488-conjugated mouse anti-rabbit antibody. To visualize the nuclei, the cells were also stained with DAPI (4′,6-diamidino-2-phenylindole). The cells were then imaged by confocal microscopy. To visualize the perimeters of the epithelial cells, they were also imaged by differential interference contrast.
Flow cytometry.
The expression of EGFR, HER2, and E-cadherin on the surface of the oral epithelial cells was quantified by flow cytometry. Briefly, OKF6/TERT-2 cells in 6-well tissue culture plates were incubated with tissue culture medium with or without IFN-γ for 24 h and then infected with 5 × 105 C. albicans cells. After 75 min, the cells were scraped from the wells with a cell scraper, fixed with 3% paraformaldehyde, blocked with 1% goat serum, and then stained with specific antibodies (for EGFR, sc-101, and for HER2, sc-33684, from Santa Cruz Biotechnology; for E-cadherin, ab1416 from Abcam, Inc.), followed by an Alexa 488-conjugated goat or mouse anti-rabbit antibody (Life Technologies, Inc.). Control epithelial cells were incubated in a similar concentration or mouse or rabbit IgG (Abcam, Inc.). The fluorescence of the cells was determined by flow cytometry, analyzing at least 10,000 cells per condition.
Host cell damage assay.
The extent of oral epithelial cell damage caused by the different treatments was measured using our previously described 51Cr release assay (22). Briefly, OKF6/TERT-2 cells were grown to 95% confluence in 96-well tissue culture plates with detachable wells (Corning) and loaded with 5 µCi/ml Na251CrO4 (PerkinElmer) in the presence or absence of IFN-γ or l-kynurenine for 24 h. After removing the unincorporated 51Cr by rinsing, the epithelial cells were infected with 6 × 105 C. albicans cells. When the AhR and SFK inhibitors were used, they were added to the cells 60 min before infection with C. albicans, and they remained in the medium for the entire incubation period. After 7 h, the amount of 51Cr released into the medium and retained by the cells was determined by gamma counting. Each experiment was performed three times in triplicate.
Cytokine production.
To measure the release of cytokines, OKF6/TERT-2 cells in a 96-well tissue culture plate were incubated with IFN-γ for 24 h or the AhR and SFK inhibitors for 60 min prior to infection. Next, 3 × 105 yeast-phase C. albicans cells were added to the cells. After 8 h, the supernatant was collected, clarified by centrifugation, and stored at −80°C. The concentrations of IL-8/CXCL8, IL-1α, and IL-1β in the medium were determined using the Luminex multiplex assay (R&D Systems). Each experiment was performed three times in triplicate.
Mouse model of oropharyngeal candidiasis.
The effect of AhR inhibitor on the severity of OPC was determined in both immunocompromised and immunocompetent mice following our standard protocol (59). Male BALB/c mice were fed an oral solution of the AhR inhibitor (10 mg/kg/day), administered in divided doses twice daily in 0.05 ml of a 1:1 mixture of propylene glycol and water starting on day −1 relative to infection. Control mice received an equal volume of the vehicle alone. When immunocompromised mice were used, cortisone acetate (2.25 mg/kg) was administered subcutaneously on days −1, 1, and 3 (59). For inoculation, the animals were sedated with ketamine and xylazine, and a swab saturated with 106 C. albicans cells was placed sublingually for 75 min. Immunocompetent mice were inoculated similarly, except that the swab was saturated with 2 × 107 organisms. The immunocompromised and immunocompetent mice were sacrificed after 4 days and 1 day of infection, respectively. Next the tongue and attached tissues were harvested and divided longitudinally. One hemisection was weighed, homogenized, and quantitatively cultured, and the other was processed for histology.
To detect phosphorylation of EGFR, and SFKs, 2-μm-thick sections of OCT-embedded tongues were fixed with cold acetone. Next, the cryosections were rehydrated in PBS and then blocked. They were stained with EGFR–p-Tyr1068 (Cell Signaling) and P-Src-Tyr416 (Cell Signaling) primary antibodies and then rinsed and stained with an Alexa Fluor 488 secondary antibody. To detect C. albicans, the sections were also stained with an anti-Candida antiserum (Biodesign International) conjugated with Alexa Fluor 568 (Thermo Fisher Scientific). The sections were imaged by confocal microscopy. To enable comparison of fluorescence intensities among slides, the same image acquisition settings were used for each experiment.
For histopathologic analysis, thin sections of paraffin-embedded tongues were stained with periodic acid-Schiff stain (PAS). The sections were imaged by light microscopy, and the length of the individual fungal lesions and the depth of fungal invasion relative to surface of the tongue were determined using Infinity Analysis software (Lumenera).
Neutrophil killing.
The effects of the AhR inhibitor on neutrophil killing of C. albicans were determined as described elsewhere (60). Briefly, neutrophils were isolated from the blood of healthy volunteers and incubated with the AhR inhibitor in RPMI 1640 medium plus 10% fetal bovine serum for 1 h at 37°C. Next, the neutrophils were mixed with an equal number of C. albicans cells. After a 3-h incubation, the neutrophils were lysed by sonication, and the number of viable C. albicans cells was determined by quantitative culture.
Statistics.
Data were compared by Mann-Whitney or unpaired Student’s t test using GraphPad Prism (v. 6) software. P values of <0.05 were considered statistical significant.
Accession number(s).
All of the raw sequencing reads have been submitted to the NCBI Sequence Read Archive (SRA; https://www.ncbi.nlm.nih.gov/sra) under ID code SRP077728, BioSample numbers SAMN05150838, SAMN05150839, SAMN05150840, SAMN06392618, SAMN06392619, and SAMN06392620.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants 1R01DE026600 (S.G.F. and V.M.B.), U19AI110820 (S.G.F. and V.M.B.), R01DE022550 (S.L.G.), and UL1TR001881 (S.G.F.).
We thank Samuel W. French and Edward Vitocruz for histopathology and members of the Division of Infectious Diseases at Harbor-UCLA Medical Center for critical suggestions.
N.V.S., M.S., S.L.G., V.M.B., and S.G.F. designed the experiments. N.V.S., M.S., and V.M.B. performed the experiments. N.V.S., M.S., S.L.G., V.M.B., and S.G.F. analyzed the data. N.V.S., M.S., and S.G.F. wrote the paper.
S.G.F. is a cofounder of and shareholder in NovaDigm Therapeutics, Inc.
Footnotes
Citation Solis NV, Swidergall M, Bruno VM, Gaffen SL, Filler SG. 2017. The aryl hydrocarbon receptor governs epithelial cell invasion during oropharyngeal candidiasis. mBio 8:e00025-17. https://doi.org/10.1128/mBio.00025-17.
REFERENCES
- 1.Phelan JA, Saltzman BR, Friedland GH, Klein RS. 1987. Oral findings in patients with acquired immunodeficiency syndrome. Oral Surg Oral Med Oral Pathol 64:50–56. doi: 10.1016/0030-4220(87)90116-2. [DOI] [PubMed] [Google Scholar]
- 2.Feigal DW, Katz MH, Greenspan D, Westenhouse J, Winkelstein W Jr, Lang W, Samuel M, Buchbinder SP, Hessol NA, Lifson AR. 1991. The prevalence of oral lesions in HIV-infected homosexual and bisexual men: three San Francisco epidemiological cohorts. AIDS 5:519–525. doi: 10.1097/00002030-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong-James D, Meintjes G, Brown GD. 2014. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol 22:120–127. doi: 10.1016/j.tim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Kerdpon D, Pongsiriwet S, Pangsomboon K, Iamaroon A, Kampoo K, Sretrirutchai S, Geater A, Robison V. 2004. Oral manifestations of HIV infection in relation to clinical and CD4 immunological status in northern and southern Thai patients. Oral Dis 10:138–144. doi: 10.1046/j.1601-0825.2003.00990.x. [DOI] [PubMed] [Google Scholar]
- 5.Chidzonga MM. 2003. HIV/AIDS orofacial lesions in 156 Zimbabwean patients at referral oral and maxillofacial surgical clinics. Oral Dis 9:317–322. doi: 10.1034/j.1601-0825.2003.00962.x. [DOI] [PubMed] [Google Scholar]
- 6.Castro LÁ, Álvarez MI, Martínez E. 2013. Pseudomembranous candidiasis in HIV/AIDS patients in Cali, Colombia. Mycopathologia 175:91–98. doi: 10.1007/s11046-012-9593-0. [DOI] [PubMed] [Google Scholar]
- 7.Sangeorzan JA, Bradley SF, He X, Zarins LT, Ridenour GL, Tiballi RN, Kauffman CA. 1994. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med 97:339–346. doi: 10.1016/0002-9343(94)90300-X. [DOI] [PubMed] [Google Scholar]
- 8.Revankar SG, Dib OP, Kirkpatrick WR, McAtee RK, Fothergill AW, Rinaldi MG, Redding SW, Patterson TF. 1998. Clinical evaluation and microbiology of oropharyngeal infection due to fluconazole-resistant Candida in human immunodeficiency virus-infected patients. Clin Infect Dis 26:960–963. doi: 10.1086/513950. [DOI] [PubMed] [Google Scholar]
- 9.Redding SW, Zellars RC, Kirkpatrick WR, McAtee RK, Caceres MA, Fothergill AW, Lopez-Ribot JL, Bailey CW, Rinaldi MG, Patterson TF. 1999. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J Clin Microbiol 37:3896–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodus NL, Bloomquist C, Liljemark W, Bereuter J. 1997. Prevalence, density, and manifestations of oral Candida albicans in patients with Sjogren’s syndrome. J Otolaryngol 26:300–305. [PubMed] [Google Scholar]
- 11.Willis AM, Coulter WA, Fulton CR, Hayes JR, Bell PM, Lamey PJ. 1999. Oral candidal carriage and infection in insulin-treated diabetic patients. Diabet Med 16:675–679. [DOI] [PubMed] [Google Scholar]
- 12.Swidergall M, Filler SG. 2017. Oropharyngeal candidiasis: fungal invasion and epithelial cell responses. PLoS Pathog 13:e1006056. doi: 10.1371/journal.ppat.1006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montes LF, Wilborn WH. 1968. Ultrastructural features of host-parasite relationship in oral candidiasis. J Bacteriol 96:1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cawson RA, Rajasingham KC. 1972. Ultrastructural features of the invasive phase of Candida albicans. Br J Dermatol 87:435–443. doi: 10.1111/j.1365-2133.1972.tb01591.x. [DOI] [PubMed] [Google Scholar]
- 15.Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, E Edwards JE Jr, Filler SG. 2005. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol 7:499–510. doi: 10.1111/j.1462-5822.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 16.Wächtler B, Citiulo F, Jablonowski N, Förster S, Dalle F, Schaller M, Wilson D, Hube B. 2012. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One 7:e36952. doi: 10.1371/journal.pone.0036952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalle F, Wächtler B, L’Ollivier C, Holland G, Bannert N, Wilson D, Labruère C, Bonnin A, Hube B. 2010. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol 12:248–271. doi: 10.1111/j.1462-5822.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu W, Filler SG. 2010. Interactions of Candida albicans with epithelial cells. Cell Microbiol 12:273–282. doi: 10.1111/j.1462-5822.2009.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakikhany K, Naglik JR, Schmidt-Westhausen A, Holland G, Schaller M, Hube B. 2007. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol 9:2938–2954. doi: 10.1111/j.1462-5822.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- 20.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE, Filler SG. 2007. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu W, Phan QT, Boontheung P, Solis NV, Loo JA, Filler SG. 2012. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A 109:14194–14199. doi: 10.1073/pnas.1117676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, Liu Y, Dongari-Bagtzoglou A, Edgerton M, Filler SG. 2010. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog 6:e1001181. doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delsing CE, Gresnigt MS, Leentjens J, Preijers F, Frager FA, Kox M, Monneret G, Venet F, Bleeker-Rovers CP, van de Veerdonk FL, Pickkers P, Pachot A, Kullberg BJ, Netea MG. 2014. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infect Dis 14:166. doi: 10.1186/1471-2334-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodasing N, Seaton RA, Shankland GS, Pithie A. 2002. Gamma-interferon treatment for resistant oropharyngeal candidiasis in an HIV-positive patient. J Antimicrob Chemother 50:765–766. doi: 10.1093/jac/dkf206. [DOI] [PubMed] [Google Scholar]
- 25.Riddell LA, Pinching AJ, Hill S, Ng TT, Arbe E, Lapham GP, Ash S, Hillman R, Tchamouroff S, Denning DW, Parkin JM. 2001. A phase III study of recombinant human interferon gamma to prevent opportunistic infections in advanced HIV disease. AIDS Res Hum Retroviruses 17:789–797. doi: 10.1089/088922201750251981. [DOI] [PubMed] [Google Scholar]
- 26.Gozalbo D, Maneu V, Gil ML. 2014. Role of IFN-gamma in immune responses to Candida albicans infections. Front Biosci 19:1279–1290. doi: 10.2741/4281. [DOI] [PubMed] [Google Scholar]
- 27.Fratti RA, Ghannoum MA, Edwards JE Jr., Filler SG. 1996. Gamma interferon protects endothelial cells from damage by Candida albicans by inhibiting endothelial cell phagocytosis. Infect Immun 64:4714–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. 2000. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol 20:1436–1447. doi: 10.1128/MCB.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phan QT, Eng DK, Mostowy S, Park H, Cossart P, Filler SG. 2013. Role of endothelial cell septin 7 in the endocytosis of Candida albicans. mBio 4:e00542-13. doi: 10.1128/mBio.00542-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Han Y, Song J, Luo R, Jin X, Mu D, Su S, Ji X, Ren YF, Liu H. 2015. Interferon-γ regulates the function of mesenchymal stem cells from oral lichen planus via indoleamine 2,3-dioxygenase activity. J Oral Pathol Med 44:15–27. doi: 10.1111/jop.12224. [DOI] [PubMed] [Google Scholar]
- 31.Mellor AL, Munn DH. 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Guillemin GJ. 2009. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res 2:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. 2010. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. 2011. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 35.Enan E, Matsumura F. 1996. Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem Pharmacol 52:1599–1612. doi: 10.1016/S0006-2952(96)00566-7. [DOI] [PubMed] [Google Scholar]
- 36.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. 1999. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez JM, Brembilla NC, Sorg O, Chicheportiche R, Matthes T, Dayer JM, Saurat JH, Roosnek E, Chizzolini C. 2010. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol 40:2450–2459. doi: 10.1002/eji.201040461. [DOI] [PubMed] [Google Scholar]
- 38.Yamaoka T, Frey MR, Dise RS, Bernard JK, Polk DB. 2011. Specific epidermal growth factor receptor autophosphorylation sites promote mouse colon epithelial cell chemotaxis and restitution. Am J Physiol Gastrointest Liver Physiol 301:G368–G376. doi: 10.1152/ajpgi.00327.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villar CC, Kashleva H, Dongari-Bagtzoglou A. 2004. Role of Candida albicans polymorphism in interactions with oral epithelial cells. Oral Microbiol Immunol 19:262–269. doi: 10.1111/j.1399-302X.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 40.Wagage S, John B, Krock BL, Hall AO, Randall LM, Karp CL, Simon MC, Hunter CA. 2014. The aryl hydrocarbon receptor promotes IL-10 production by NK cells. J Immunol 192:1661–1670. doi: 10.4049/jimmunol.1300497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimura A, Abe H, Tsuruta S, Chiba S, Fujii-Kuriyama Y, Sekiya T, Morita R, Yoshimura A. 2014. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int Immunol 26:209–220. doi: 10.1093/intimm/dxt067. [DOI] [PubMed] [Google Scholar]
- 42.Huai W, Zhao R, Song H, Zhao J, Zhang L, Zhang L, Gao C, Han L, Zhao W. 2014. Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription. Nat Commun 5:4738. doi: 10.1038/ncomms5738. [DOI] [PubMed] [Google Scholar]
- 43.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, Iannitti R, Tissi L, Volpi C, Belladonna ML, Orabona C, Bianchi R, Lanz TV, Platten M, Della Fazia MA, Piobbico D, Zelante T, Funakoshi H, Nakamura T, Gilot D, Denison MS, Guillemin GJ, DuHadaway JB, Prendergast GC, Metz R, Geffard M, Boon L, Pirro M, Iorio A, Veyret B, Romani L, Grohmann U, Fallarino F, Puccetti P. 2014. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. 2013. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burova E, Vassilenko K, Dorosh V, Gonchar I, Nikolsky N. 2007. Interferon gamma-dependent transactivation of epidermal growth factor receptor. FEBS Lett 581:1475–1480. doi: 10.1016/j.febslet.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Paul G, Marchelletta RR, McCole DF, Barrett KE. 2012. Interferon-γ alters downstream signaling originating from epidermal growth factor receptor in intestinal epithelial cells: functional consequences for ion transport. J Biol Chem 287:2144–2155. doi: 10.1074/jbc.M111.318139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang F, Sorkin A. 2005. Growth factor receptor binding protein 2-mediated recruitment of the RING domain of Cbl to the epidermal growth factor receptor is essential and sufficient to support receptor endocytosis. Mol Biol Cell 16:1268–1281. doi: 10.1091/mbc.E04-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang X, Huang F, Marusyk A, Sorkin A. 2003. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol Biol Cell 14:858–870. doi: 10.1091/mbc.E02-08-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreno-Ruiz E, Galán-Díez M, Zhu W, Fernández-Ruiz E, d’Enfert C, Filler SG, Cossart P, Veiga E. 2009. Candida albicans internalization by host cells is mediated by a clathrin-dependent mechanism. Cell Microbiol 11:1179–1189. doi: 10.1111/j.1462-5822.2009.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. 2006. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Irby RB, Yeatman TJ. 2000. Role of Src expression and activation in human cancer. Oncogene 19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 53.Conti HR, Bruno VM, Childs EE, Daugherty S, Hunter JP, Mengesha BG, Saevig DL, Hendricks MR, Coleman BM, Brane L, Solis N, Cruz JA, Verma AH, Garg AV, Hise AG, Richardson JP, Naglik JR, Filler SG, Kolls JK, Sinha S, Gaffen SL. 2016. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe 20:606–617. doi: 10.1016/j.chom.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamai Y, Kubota M, Kamai Y, Hosokawa T, Fukuoka T, Filler SG. 2001. New model of oropharyngeal candidiasis in mice. Antimicrob Agents Chemother 45:3195–3197. doi: 10.1128/AAC.45.11.3195-3197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fonzi WA, Irwin MY. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cunningham F, Amode MR, Barrell D, Beal K, Billis K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fitzgerald S, Gil L, Girón CG, Gordon L, Hourlier T, Hunt SE, Janacek SH, Johnson N, Juettemann T, Kähäri AK, Keenan S, Martin FJ, Maurel T, McLaren W, Murphy DN, Nag R, Overduin B, Parker A, Patricio M, Perry E, Pignatelli M, Riat HS, Sheppard D, Taylor K, Thormann A, Vullo A, Wilder SP, Zadissa A, Aken BL, Birney E, Harrow J, Kinsella R, Muffato M, Ruffier M, Searle SM, Spudich G, Trevanion SJ, Yates A, Zerbino DR, Flicek P. 2015. Ensembl 2015. Nucleic Acids Res 43:D662–D669. doi: 10.1093/nar/gku1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol 11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solis NV, Filler SG. 2012. Mouse model of oropharyngeal candidiasis. Nat Protoc 7:637–642. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. 2010. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J Infect Dis 201:1718–1728. doi: 10.1086/652407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IFN-γ has no effect on active penetration into epithelial cells or hyphal growth. (A) OKF6/TERT-2 oral epithelial cells were incubated with IFN-γ for 24 h, fixed, and infected for 2.5 h with C. albicans cells in the presence of IFN-γ, after which the number of internalized organisms was determined by a differential fluorescence assay. Results are means ± SD from 3 experiments, each performed in triplicate. (B) Microscopic images of C. albicans cells after a 2.5-h incubation with oral epithelial cells that had been exposed to the indicated conditions for 24 h. The fungal cells were stained with an anti-Candida antiserum conjugated with Alexa Fluor 488. (C) Hyphal length after incubation for 2.5 h on oral epithelial cells that had been exposed to the indicated conditions for 24 h. Results are means ± SD from 50 organisms. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). ctrl, control; NS, not significant. Download FIG S1, PDF file, 0.2 MB (160.4KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List of epithelial cell genes whose gene expression was altered by IFN-γ treatment. Download TABLE S1, XLS file, 0.4 MB (412.5KB, xls) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
GO term analysis of genes whose expression was up- or downregulated by IFN-γ treatment. Download TABLE S2, XLSX file, 0.1 MB (32.1KB, xlsx) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effect of IFN-γ on epithelial cell IDO1 mRNA expression, as measured by real-time PCR. Results are means ± SD from 2 experiments, each performed in triplicate. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). ctrl, control. Download FIG S2, PDF file, 0.3 MB (268.3KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
l-Kynurenine activates the aryl hydrocarbon receptor (AhR) in oral epithelial cells. Shown are confocal micrographs of OKF6/TERT-2 oral epithelial cells incubated in the presence and absence of l-kynurenine for 24 h. The cells were stained for AhR (green), and the nuclei were stained with DAPI (blue). The perimeters of the cells were determined by differential interference contrast and are indicated by the dashed lines. Scale bar, 20 µm. ctrl, control. Download FIG S3, PDF file, 0.1 MB (153.5KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
IFN-γ treatment has no effect on the expression of host cell receptors for C. albicans. (A) mRNA levels of the indicated epithelial cell receptors after 24 h of IFN-γ treatment. Results are means ± SD from two independent experiments performed in triplicate. (B) Effects of IFN-γ on the expression of E-cadherin, EGFR, and HER2 on the surface of epithelial cells as determined by flow cytometry. Control cells (stained with a control monoclonal antibody [MAb]) are shown in light gray, untreated cells (stained with the specific MAb) are shown in dark gray, and the IFN-γ-treated cells (stained with the specific MAb) are shown in red. Download FIG S4, PDF file, 0.3 MB (304.7KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effects of l-kynurenine on C. albicans-induced phosphorylation of EGFR. OKF6/TERT-2 epithelial cells were incubated with l-kynurenine for 24 h and then infected with C. albicans for 1 h (A) Representative immunoblots showing EGFR phosphorylation at Y1068 and Y1101. (B) Densitometric analysis of the immunoblots in panel A. Results are means ± SD from 3 experiments. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). Ca, C. albicans; UNINF, uninfected; L-Kyn, l-kynurinine. Download FIG S5, PDF file, 0.1 MB (154.2KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The AhR inhibitor has no effect on hyphal length. (A) Oral epithelial cells were incubated in the presence or absence of the AhR inhibitor for 1 h and infected with C. albicans for 2.5 h, after which the length of the fungal hyphae was determined. Results are means ± SD of 50 organisms. (B) Hyphal length of C. albicans in the tongues of mice with OPC after 4 days of infection. To detect C. albicans hyphae, thin sections of the infected tongues were rehydrated in PBS and then blocked. They were stained with an anti-Candida antibody conjugated with Alexa Fluor 568 and then imaged by confocal microscopy. The length of the elongated cells (>10 µm) was measured using LAS AF lite software. Results are means ± SD from 50 organisms in the tongues of 3 mice per experimental group. NS, not significant; ctrl, control. Download FIG S6, PDF file, 0.2 MB (183.7KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effects of the AhR inhibitor on the capacity of neutrophils to kill C. albicans. Human neutrophils were incubated with C. albicans cells at a ratio of 1:1 for 3 h in the presence of 10% pooled human serum, with or without the AhR inhibitor. The percentage of organisms killed was determined by colony counting. Results are means ± SD from 3 experiments. Statistical significance was determined using the unpaired Student’s t test (P ≤ 0.05). ctrl, control; INH, inhibitor. Download FIG S7, PDF file, 0.1 MB (119.2KB, pdf) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oligonucleotides used in the experiments. Download TABLE S3, DOCX file, 0.1 MB (35KB, docx) .
Copyright © 2017 Solis et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.