Summary
Between September 2012 and January 20, 2017, the World Health Organization (WHO) received reports from 27 countries of 1879 laboratory-confirmed cases in humans of the Middle East respiratory syndrome (MERS) caused by infection with the MERS coronavirus (MERS-CoV) and at least 659 related deaths. Cases of MERS-CoV infection continue to occur, including sporadic zoonotic infections in humans across the Arabian Peninsula, occasional importations and associated clusters in other regions, and outbreaks of nonsustained human-to-human transmission in health care settings. Dromedary camels are considered to be the most likely source of animal-to-human transmission. MERS-CoV enters host cells after binding the dipeptidyl peptidase 4 (DPP-4) receptor and the carcinoembryonic antigen–related cell-adhesion molecule 5 (CEACAM5) cofactor ligand, and it replicates efficiently in the human respiratory epithelium. Illness begins after an incubation period of 2 to 14 days and frequently results in hypoxemic respiratory failure and the need for multiorgan support. However, asymptomatic and mild cases also occur. Real-time reverse-transcription–polymerase-chain-reaction (RT-PCR) testing of respiratory secretions is the mainstay for diagnosis, and samples from the lower respiratory tract have the greatest yield among seriously ill patients. There is no antiviral therapy of proven efficacy, and thus treatment remains largely supportive; potential vaccines are at an early developmental stage. There are multiple gaps in knowledge regarding the evolution and transmission of the virus, disease pathogenesis, treatment, and prospects for a vaccine. The ongoing occurrence of MERS in humans and the associated high mortality call for a continued collaborative approach toward gaining a better understanding of the infection both in humans and in animals.
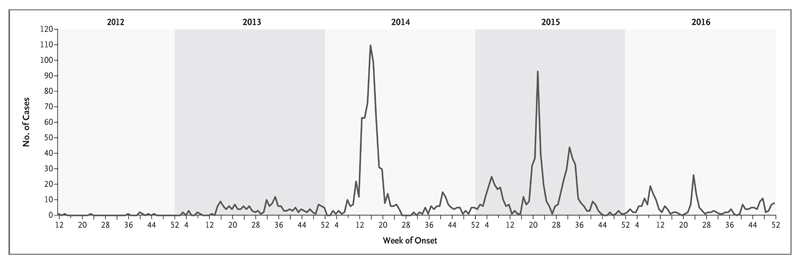
MERS-CoV was first identified in September 20121 in a patient from Saudi Arabia who had hypoxemic respiratory failure and multiorgan illness. Subsequent cases have included infections in humans across the Arabian Peninsula, occasional importations and associated clusters in other regions, and outbreaks of nonsustained human-to-human transmission in health care settings (Fig. 1).
Epidemiology
Overview
As of January 20, 2017, the WHO had received reports from 27 countries of 1879 cases of laboratory-confirmed MERS and at least 659 related deaths (percentage of fatal cases, 35%).2 Approximately 80% of reported cases have been linked to exposure in Saudi Arabia (Fig. 2). Transmission of MERS-CoV between dromedary camels and humans has been documented in several countries. Human-to-human transmission in health care settings accounts for the majority of reported cases to date, although human-to-human transmission in household settings has also been identified (Fig. 3).
Figure 2. Geographic Distribution of the Middle East Respiratory Syndrome.
Data are from the World Health Organization (www.who.int/emergencies/mers-cov/mers-summary-2016.pdf) and were collected through December 2, 2016. At that time, the total number of cases was 1841.
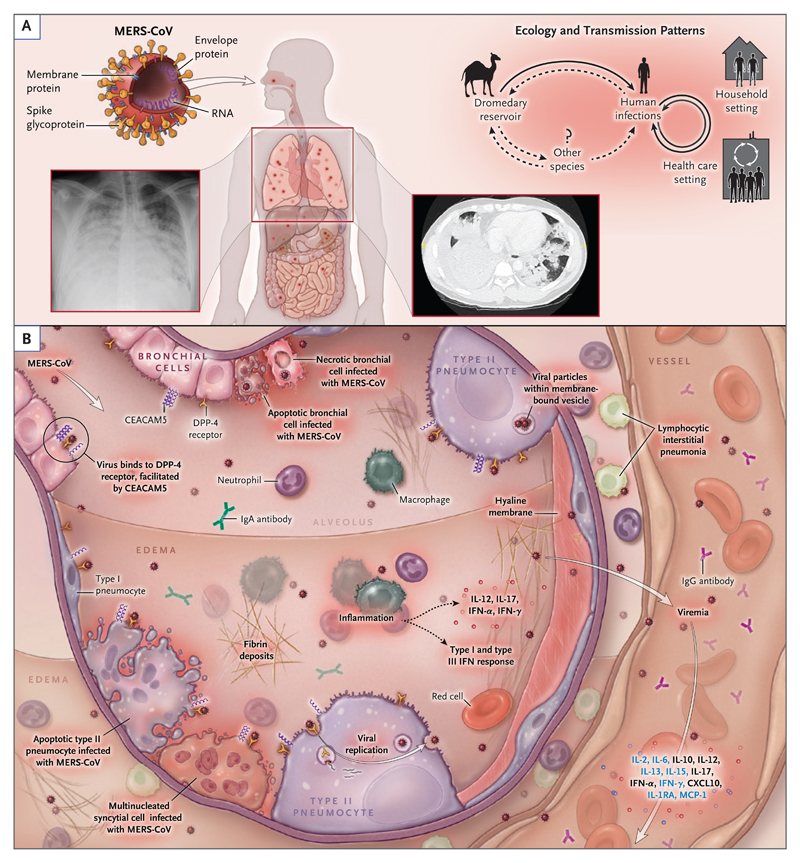
Figure 3. Transmission Patterns and Pathogenesis of the Middle East Respiratory Syndrome Coronavirus.
Panel A shows the structure, ecologic features, and transmission patterns of the Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV). The virion surface is covered with the spike glycoprotein, a 149 kDa glycoprotein that extends outward to create a crownlike appearance. The spike glycoprotein is critical for binding the host-cell receptor, dipeptidyl peptidase 4 (DPP-4), to initiate infection. Dromedary camels are infected with the virus and are believed to be the most likely source of animal-to-human transmission. Human-to-human transmission in household and health care settings has also occurred. Panel B shows the current understanding of key events in MERS pathogenesis, which is based on limited observations in patients and data from animal and cell-culture model systems. After intratracheal inoculation of MERS-CoV in nonhuman primates, the virus infects bronchial epithelial cells through DPP-4 before spreading to lung parenchymal cells, including type I and type II alveolar pneumocytes and endothelial cells. Viral entry is facilitated by another cell-surface protein, carcinoembryonic antigen–related cell-adhesion molecule 5 (CEACAM5), which is also expressed in lung tissue. Inflammatory signaling molecules that are released by infected cells, alveolar macrophages, and neutrophils recruited to infected tissue have been detected in infected patients (black text) and animal models (blue text). A host antiviral type I and type III interferon response occurs, with systemic release of proinflammatory cytokines and chemokines.3,4 The virus may spread into the circulation, possibly from lung parenchyma or through infected endothelial cells. In humans, a high viral copy number has been detected in the lower respiratory tract, including tracheal aspirates and bronchoalveolar lavage specimens, as well as in peripheral blood. In advanced disease, diffuse alveolar damage is seen, with extensive hemorrhagic edema and hyaline membrane deposition. CXCL10 denotes C-X-C motif chemokine 10, IL interleukin, IL-1RA IL-1 receptor antagonist, IFN interferon, and MCP monocyte chemotactic protein.
Animal-to-Human Transmission
Seroepidemiologic studies have shown that antibodies to MERS-CoV are present in dromedary camels (Camelus dromedarius) throughout the Middle East and Africa, and studies indicate that MERS-CoV has circulated among dromedaries for at least three decades. Although MERS-CoV RNA has been detected in respiratory and other bodily secretions, particularly among juvenile dromedaries, infections in dromedaries may cause only mild upper respiratory manifestations. Investigations of animals in close proximity to reported cases in humans support the theory that dromedaries are the most likely source of animal-to-human transmission.5 One case–control study showed that persons presenting with community-acquired MERS were 7 times as likely as controls to have had direct exposure to dromedaries during the preceding 2 weeks.6 In addition, seroepidemiologic studies indicate that infection may be associated with occupational exposures to dromedaries. In one study conducted in Saudi Arabia, the rate of MERS-CoV seropositivity was 15 times as high in shepherds and 23 times as high in slaughterhouse workers as in the general population.7 There is high sequence homology between the viruses from sporadic cases in humans and the implicated dromedaries. Although the routes of transmission remain unclear, they appear to include contact with infectious nasal or other bodily secretions and possibly the consumption of raw dromedary products (e.g., unpasteurized milk). Although dromedary-to-human transmission of MERS-CoV is now well recognized, direct exposure to dromedaries has been documented in only 40% of primary cases.6 (For further details, see Tables S1 and S2 in the Supplementary Appendix, available with the full text of this article at NEJM.org.)
Human-to-Human Transmission
Non–Health Care Settings
Human-to-human transmission appears to be infrequent outside health care settings but has been documented after close household contact with infected persons.8 Among 280 household contacts evaluated in a study conducted in Saudi Arabia, 4.3% had positive results for MERS-CoV on realtime RT-PCR assay or serologic assay.9 In one household cluster in which 24% of 79 extended family members were affected, risk factors for infection included sleeping in an index patient’s room and touching respiratory secretions from an index patient.10 Very little or no household transmission has been detected in other countries in which MERS-CoV infections have been reported,11 and no sustained human-to-human transmission has been documented in any country to date.
One seroepidemiologic study conducted in Saudi Arabia with the use of samples collected in 2012–2013 showed that antibodies to MERS-CoV were present in 0.15% of 10,009 participants; this finding suggests that more than 40,000 unrecognized infections may have occurred.7 During the 2014 outbreak in Jeddah, Saudi Arabia, 25% of MERS cases were reported to be asymptomatic.12 The role of persons with asymptomatic infections in human-to-human transmission remains unclear but may be underappreciated.7,13 Increasingly sensitive surveillance strategies and follow-up for all contacts are contributing to the increased recognition of asymptomatic cases.12,14
Health Care Settings
The earliest cases of MERS were identified retrospectively in a hospital-associated cluster in Jordan in April 2012,15 and transmission within health care settings has remained a prominent epidemiologic feature of MERS. During the 2014 MERS outbreak in Jeddah, 31% of the affected patients were health care personnel, and 88% of the affected patients who were not health care personnel had been admitted to or visited a health care facility within 14 days before symptom onset.12 During 2015 and 2016, nosocomial outbreaks occurred in health care facilities in Jordan,2 South Korea,14 and Saudi Arabia.16 In the Korean outbreak, which has been the largest outbreak outside the Middle East, transmission from a single infected person led to 186 cases and 36 deaths (case fatality rate, 19.4%) across multiple health care facilities.14 Overcrowded emergency departments, delays in diagnosis, and substandard practices for infection control have been identified as contributing factors in outbreaks that occur in health care settings.12,17
Available epidemiologic observations suggest that human-to-human transmission occurs primarily through close contact with a patient who has confirmed MERS, most likely through respiratory droplets. The risk of transmission appears to be greater during the performance of aerosol-generating procedures without adequate personal protection or proper room ventilation.18 In addition, contamination of environmental surfaces in patient rooms with MERS-CoV may result in transmission.19,20 One study isolated the infectious virus from air samples from MERS isolation wards, although this finding requires confirmation.20
Travel to and from the Arabian Peninsula
Persons with MERS who have been identified in regions other than the Arabian Peninsula have either recently traveled to the Arabian Peninsula or have had contact with someone returning from the Arabian Peninsula.21 Despite the large number of persons visiting Saudi Arabia yearly for the Hajj and Umrah pilgrimages, no pilgrimage-related cases of MERS have been reported.22 Although two Dutch pilgrims returning from the 2014 Hajj received a diagnosis of MERS,11 the source of their infection remains unclear, and other members who traveled in the same tour group reported visiting camel markets and consuming raw camel milk during activity unrelated to Hajj. Saudi Arabian authorities have banned the presence of camels near holy sites to minimize exposure to potentially infected camels.23
Host Risk Factors
The majority of severe MERS cases have occurred in adults older than 50 years of age who have coexisting conditions such as diabetes, hypertension, cardiac disease, obesity, chronic respiratory disease, end-stage renal disease, or cancer or in persons receiving immunosuppressive therapy (Table S5 in the Supplementary Appendix). Older age and chronic respiratory illness have been associated with death among infected patients.17 Mild and asymptomatic infections have occurred predominantly among young and healthy persons, including health care workers.12,17 Clinically recognized infections among children are uncommon.24 Host genetic risk factors have not been documented to date.
Virology
MERS-CoV is the sixth coronavirus and the first betacoronavirus of the C phylogenetic lineage known to infect humans.25 (Lineage A includes HCoV-OC43 and HCoV-HKU1, and lineage B includes the severe acute respiratory syndrome [SARS] coronavirus [SARS-CoV].) Similar to other coronaviruses, MERS-CoV is enveloped, contains a very large (30 kb) single-stranded positive-sense RNA genome,26 and replicates in the host-cell cytoplasm. Sixteen nonstructural proteins serve roles in replication, including a novel RNA proofreading exonuclease that regulates replication fidelity27 (Table S3 in the Supplementary Appendix). MERS-CoV tropism is determined primarily by binding of the virus spike glycoprotein to the cell-surface molecule, DPP-4 (Fig. 3). Attachment and entry are further facilitated by a recently discovered cellular cofactor, CEACAM5.28 The spike glycoprotein can be activated to its fusogenic form by the host-cell transmembrane serine protease TMPRSS2, endosomal cathepsins, and furin; this suggests that there are multiple possible pathways of spike maturation, viral entry into the cell, and virion assembly.29,30 Circulating strains of human MERS-CoV to date appear to represent a single serotype.31 Serum from convalescent patients with MERS29 and neutralizing monoclonal antibodies to the receptor-binding site of the spike glycoprotein inhibit infection of cells by the virus.32,33
Human Pathogenesis
Host-Cell Targets
DPP-4 is widely expressed on human cells, including fibroblasts, intestinal epithelial cells, and hepatocytes, and it has weak expression in the lung.34 CEACAM5 has a pattern of expression that is more specific to cell type. In the gene-expression atlas FANTOM5,34 only a small number of tissues have detectable expression of both DPP-4 and CEACAM5, including tissues from the trachea, throat, small intestine, colon, and appendix. In a finding consistent with this observation, MERS-CoV has been shown to replicate well in human cell lines derived from respiratory and intestinal epithelium.35
In an ex vivo model of human lung-tissue infection, DPP-4 expression, MERS-CoV antigen, and viral particles were found in bronchial epithelial cells, type I and type II alveolar pneumocytes, and vascular endothelial cells; the result was cell death and diffuse alveolar damage (Fig. 3).36 Alveolar macrophages appeared to be relatively spared.36 A similar pattern of DPP-4 distribution and viral replication has been seen in nonhuman primate models.37,38
Viral Kinetics
MERS-CoV RNA is detected more frequently and at higher viral loads in samples from the lower respiratory tract than in samples from the upper respiratory tract39 and can persist in some patients, particularly the most severely ill patients, for more than 1 month.40 MERS-CoV RNA has also been detected in blood or serum samples from up to one third of patients presenting with MERS41; positive tests for MERS-CoV RNA in blood are associated with a greater need for mechanical ventilation and extracorporeal respiratory support and with higher mortality.41,42 MERS-CoV RNA has been detected occasionally in stool (in 14.6% of patients with MERS) and urine (in 2.4%).39
Animal Models
Available animal models of MERS-CoV infection do not reliably recapitulate severe human infections. Laboratory rodents, including mice, are not susceptible to MERS-CoV.43 However, MERS-CoV susceptibility in mice has been described after transduction with an adenovirus vector expressing the human version of DPP-4.44 Similar to humans, a transgenic mouse expressing the human DPP-4 receptor has primarily pulmonary disease.45
In rhesus macaques and marmosets, inoculation of the respiratory tract with MERS-CoV induces clinical disease of varying severity. In rhesus macaques, a self-limited pneumonia develops after an incubation period of only 24 hours.37,46 Some studies in marmosets have shown extensive lung disease and virus replication, and detection of MERS-CoV RNA in multiple organs and in blood was associated with death.38,47 However, another study could not reproduce the experimentally induced illness in marmosets.48
Pathology
Insights into the pathobiology of MERS are limited. The principal autopsy finding in a patient with MERS who died approximately 12 days after the onset of illness was diffuse alveolar damage. Double-staining immunoassays showed MERS-CoV antigen in pneumocytes and epithelial syncytial cells. No evidence of extrapulmonary MERS-CoV antigen was detected, even in the kidney, despite the fact that renal insufficiency is a common clinical finding among critically ill patients.49
Immune Responses
Examination of infected mice expressing the human DPP-4 receptor showed that effective interferon signaling and T cell–mediated immunity are required for viral clearance.44 Similar to other coronaviruses and many other pathogens, MERS-CoV subverts host-cell pathways: nonstructural proteins such as nsp1 and nsp3, which are cleaved from the replicase polyprotein, directly target host-cell functions and immune responses.50,51 In addition, MERS-CoV has a unique set of accessory genes, several of which may contribute to immune evasion and virulence (e.g., ORF4a, ORF4b, and ORF5), but it does not have immune-evasion proteins such as SARS-CoV ORF6 protein, which reduces the signaling of signal transducer and activator of transcription 1.52,53
The milieu of cytokines in bronchoalveolar lavage fluid and serum have been studied in two patients with MERS.3 One patient, who did not survive, had persistent viral detection and blunted expression of antiviral sensing and response factors (retinoic acid–inducible gene 1, interferon regulatory factors 3 and 7, and interferon alfa) in bronchoalveolar lavage fluid. The other patient, who survived, had high levels of the antiviral factors in bronchoalveolar lavage fluid and achieved rapid viral clearance. These antiviral factors are part of the innate antiviral response and are induced early in cells exposed to MERS-CoV.54
The kinetics and durability of specific antibody responses to MERS-CoV are not fully characterized. Most patients produce serum IgG and secretory IgA within 2 weeks after the onset of illness.39,55,56 However, the presence of such antibodies has not necessarily been followed by the elimination of MERS-CoV RNA from the lower respiratory tract, although data on titers of infectious virus at this site are lacking.39 A decline in antibody levels over a period of several months has been observed in some convalescent patients.57
Clinical Manifestations
Patients with symptomatic MERS present after an incubation period of 2 to 14 days. Mildly symptomatic cases can be manifested by low-grade fever, runny nose, sore throat, and muscle aches. Severe disease is characterized by progression to the acute respiratory distress syndrome, with a median of 2 days between hospitalization and admission to the intensive care unit.58 In severely ill patients, chest radiographs and computed tomographic scans often show multilobar airspace disease, ground-glass opacities, and occasional pleural effusions. Thoracic imaging is usually normal in patients with mild illness.
Extrapulmonary manifestations are common among severely ill patients. Up to one third of critically ill patients have gastrointestinal symptoms, such as nausea, vomiting, or diarrhea. Acute kidney injury has been reported in up to half of critically ill patients.58 Stillbirth has been reported in a pregnant woman with probable MERS.59 Neurologic manifestations suggestive of encephalitis have been reported in three cases.60 Laboratory findings typically include leukopenia and lymphopenia and occasionally include thrombocytopenia and anemia. Mild-to-moderate elevation of aminotransferase levels is common, especially among critically ill patients. (For further details, see Fig. S1 and Tables S4, S5, and S6 in the Supplementary Appendix.)
Diagnosis
Diagnostic Approach to a Suspected Case
The clinical features of MERS often do not differ from those of other acute febrile respiratory illnesses or pneumonia. Therefore, a diagnostic workup for MERS in a patient with an acute respiratory illness, with or without fever, should be guided by the presence of both clinical suspicion and an epidemiologic link61 (Fig. S1 and Table S7 in the Supplementary Appendix). Once MERS is suspected, immediate precautions for infection control should be taken, and specimens from the upper and lower respiratory tracts should be collected for real-time RT-PCR analysis. Specimens from the lower respiratory tract that are obtained early during the course of the illness (within 7 days after symptom onset) appear to have the best diagnostic sensitivity.62 Repeat testing of samples from the lower respiratory tract over a period of several days increases the diagnostic yield. In hospitalized patients, testing serum specimens with the use of real-time RT-PCR analysis is also recommended. For patients presenting with symptoms that have lasted for 14 days or longer, serologic testing for MERS-CoV–specific antibodies may inform the diagnosis.
In some severely ill patients with laboratory-confirmed MERS, coinfection with other respiratory viral or bacterial pathogens has been detected on presentation.58,63 An appropriate diagnostic workup should be performed and empirical antibiotic or antiviral treatment (or both) should be administered in accordance with the clinical syndrome (e.g., community-acquired or hospital-acquired pneumonia) while the test results for MERS-CoV are pending. Detection of a conventional respiratory viral or bacterial pathogen should not deter clinicians from testing for MERS-CoV in patients for whom a high index of suspicion is present.
Molecular Testing
Laboratory confirmation of MERS by means of real-time RT-PCR assay requires positivity at two different MERS-CoV genomic target sites or detection at a single target site followed by sequence confirmation at a second site (Fig. S1 in the Supplementary Appendix).64,65 Screening and confirmatory molecular assays for MERS-CoV are summarized in Table S8 in the Supplementary Appendix. Currently, an assay targeting the region upstream of the envelope gene (upE) is recommended for screening, and an assay targeting ORF1a is used for confirmation.65
Antibody Detection
MERS can be confirmed by seroconversion in two samples obtained 14 days apart.64 A two-stage approach for MERS-CoV serologic testing has been recommended. First, screening is performed by means of enzyme-linked immunosorbent assay or immunofluorescence assay with the use of the recombinant MERS-CoV spike glycoprotein S1 subunit. Then, confirmatory testing is performed by means of immunofluorescence assay of MERS-CoV–infected cells or virus neutralization assay.7,9
Virus Culture
MERS-CoV has been successfully isolated in cell culture, most commonly from specimens from the lower respiratory tract.66 However, viral culture requires high-level biocontainment facilities.
Treatment
Current Approach
No specific antiviral therapies have been shown to be effective for the treatment of MERS. Prevention of complications and organ support define the current standards of care.
Investigational Studies in Humans
Data from randomized clinical trials investigating whether the use of therapeutics licensed for other indications are effective in patients with MERS are lacking. Retrospective observational reports of treatment with ribavirin and interferons do not indicate consistent clinical benefits (Table S9 in the Supplementary Appendix).42,67 A meta-analysis of observational studies of passive immunotherapy for SARS and severe influenza suggests a decrease in mortality associated with timely use of blood products from convalescent patients, particularly those with neutralizing antibodies.68 Thus, fresh-frozen plasma (or hyperimmune intravenous immune globulin) produced from patients who have recovered from MERS could be an investigational therapy. However, collection of sufficient high-titer plasma for experimental use may prove to be an obstacle to further evaluation and wider therapeutic application.57 Other neutralizing-antibody preparations have been developed that are effective in animal models (Table S9 in the Supplementary Appendix).
Immunomodulatory Agents
Despite the common use of glucocorticoids for severe MERS illness,58 a retrospective analysis involving patients with SARS suggests that glucocorticoid therapy may be associated with increased mortality.69 Because the safety and effectiveness of glucocorticoids in patients with MERS are uncertain, use of glucocorticoids should be avoided unless they are indicated for other clinical reasons or conditions or they are being studied in the context of a clinical trial.
Preclinical Studies with Licensed and Experimental Drugs
Ribavirin has been shown to have inhibitory effects in vitro but at concentrations greatly exceeding those reached with usual clinical doses.70 Nonetheless, among rhesus macaques that had been exposed to MERS-CoV 8 hours previously, treatment with high doses of ribavirin and interferon alfa-2b was associated with modest antiviral effects and with fewer pulmonary changes than was no treatment.4 Marmosets treated with lopinavir–ritonavir or interferon beta-1b had substantially lower morbidity and mortality than marmosets treated with mycophenolate mofetil and than untreated controls.47 Various experimental polyclonal and monoclonal antibodies, peptide constructs, and polymerase inhibitors have been tested in animal models; several are in early clinical development, and one placebo-controlled trial of interferon beta-1b and lopinavir–ritonavir has recently begun (ClinicalTrials.gov number, NCT02845843). Multiple other licensed drugs have shown antiviral effects in vitro or have been proposed as host-directed therapeutics.71 At present, none of these drugs can be recommended for the treatment of MERS outside the context of a clinical trial. (For further details, see Table S9 in the Supplementary Appendix.)
Prevention
MERS-CoV Transmission
Public health authorities, including the WHO, advocate the use of various nonpharmaceutical interventions for the prevention of MERS-CoV transmission (Table S10 in the Supplementary Appendix). Strategies aimed at reducing the risk of animal-to-human transmission, rapid identification of cases, tracing of all contacts in clusters associated with health care settings,61 and appropriate infection control are core components of the control and prevention of MERS-CoV infection.
Vaccines and Chemoprophylaxis
Candidate MERS vaccines are under development for use in humans and potentially for use in dromedaries (Table S11 in the Supplementary Appendix). The primary target for these candidate vaccines is the spike glycoprotein S1 subunit. The range of vaccine approaches includes live attenuated, spike protein subunit, DNA, nanoparticles, and recombinant vector constructs. Major challenges in the development of the vaccines include identifying an appropriate animal model and establishing humoral and cellular correlates of protection in humans, as well as assessing the potential for an up-regulated, harmful immune response upon subsequent exposure to the wildtype virus.72 Data on chemoprophylaxis from controlled studies involving humans are lacking.
Future Directions
Our knowledge about MERS is increasing, but more work is required to characterize certain ecologic and epidemiologic features of MERS-CoV, including the routes of animal-to-human and human-to-human transmission and the sources of infection in sporadic cases. In addition to better characterizing the dynamics of MERS-CoV viral replication and their relationship to clinical manifestations, we need to assess innate and adaptive immune responses and possible host genetic susceptibility factors. Further autopsy studies are essential to inform our understanding of the disease pathology and the extent of extrapulmonary infection. There is a pressing need for evaluation of potential therapies and vaccines through collaborative and coordinated clinical trials conducted across affected regions. Lowering the risk to humans requires more effective control of animal-to-human transmission and, ideally, an effective dromedary vaccine. The lack of adequate studies of clinical characterization and disease pathogenesis, which is common among emerging infectious diseases, can be addressed in part by developing preapproved research protocols for rapid implementation in response to a new or emerging infection.73,74 The continued threat of MERS — including severe illness, periodic nosocomial outbreaks, and international spread — calls for enhanced international collaboration to address these research priorities.
Supplementary Material
Figure 1. Confirmed Cases of the Middle East Respiratory Syndrome.
Data are from the World Health Organization (www.who.int/emergencies/mers-cov/en) and were collected through December 31, 2016.
Acknowledgments
Supported by a Wellcome Trust Intermediate Clinical Fellowship and a Wellcome–Beit Prize (103258/Z/13/Z and 103258/Z/13/A, both to Dr. Baillie), a Biotechnology and Biological Sciences Research Council Institute Strategic Programme Grant (to the Roslin Institute), the Intensive Care Foundation, and an award from the Heart and Stroke Foundation, Ontario Provincial Office (to Dr. Fowler).
Footnotes
Drs. Senga and Shindo are staff members of and Drs. Fowler, Hayden, Nguyen-Van-Tam, and Van Kerkhove are consultants for the World Health Organization. The authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the World Health Organization. Dr. Luke is an employee of the U.S. Federal Government. The views expressed in this article are his and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. This work was prepared as part of his official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Sibylle Bernard Stoecklin for her help in generating the data for Figure 1.
This article is dedicated to all patients and families who have suffered because of Middle East respiratory syndrome, as well as to the memory of Dr. Allison Bermingham, scientist and colleague, who died unexpectedly during the preparation of this work.
References
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) ( http://www.who.int/emergencies/mers-cov/en/)
- 3.Faure E, Poissy J, Goffard A, et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9(2):e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–7. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azhar EI, El-Kafrawy SA, Farraj SA, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499–505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 6.Alraddadi BM, Watson JT, Almarashi A, et al. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller MA, Meyer B, Corman VM, et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15:559–64. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–94. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 9.Drosten C, Meyer B, Müller MA, et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371:828–35. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 10.Arwady MA, Alraddadi B, Basler C, et al. Middle East respiratory syndrome coronavirus transmission in extended family, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:1395–402. doi: 10.3201/eid2208.152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraaij-Dirkzwager M, Timen A, Dirksen K, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) infections in two returning travellers in the Netherlands, May 2014. Euro Surveill. 2014;19(21):20817. doi: 10.2807/1560-7917.es2014.19.21.20817. [DOI] [PubMed] [Google Scholar]
- 12.Oboho IK, Tomczyk SM, Al-Asmari AM, et al. 2014 MERS-CoV outbreak in Jeddah — a link to health care facilities. N Engl J Med. 2015;372:846–54. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Gethamy M, Corman VM, Hussain R, Al-Tawfiq JA, Drosten C, Memish ZA. A case of long-term excretion and subclinical infection with Middle East respiratory syndrome coronavirus in a healthcare worker. Clin Infect Dis. 2015;60:973–4. doi: 10.1093/cid/ciu1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ki M. 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health. 2015;37:e2015033. doi: 10.4178/epih/e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–33. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkhy HH, Alenazi TH, Alshamrani MM, et al. Description of a hospital outbreak of Middle East respiratory syndrome in a large tertiary care hospital in Saudi Arabia. Infect Control Hosp Epidemiol. 2016;37:1147–55. doi: 10.1017/ice.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korea Centers for Disease Control and Prevention. Corrigendum to “Middle East Respiratory Syndrome Coronavirus Outbreak in the Republic of Korea, 2015” [Volume 6, Issue 4, August 2015, 269-278] Osong Public Health Res Perspect. 2016;7:138. doi: 10.1016/j.phrp.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Infection prevention and control during health care for probable or confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection. 2015 Jun 4; ( http://apps.who.int/iris/bitstream/10665/174652/1/WHO_MERS_IPC_15.1_eng.pdf?ua=1)
- 19.Bin SY, Heo JY, Song MS, et al. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin Infect Dis. 2016;62:755–60. doi: 10.1093/cid/civ1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Chang SY, Sung M, et al. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis. 2016;63:363–9. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider E, Chommanard C, Rudd J, Whitaker B, Lowe L, Gerber SI. Evaluation of patients under investigation for MERS-CoV infection, United States, January 2013-October 2014. Emerg Infect Dis. 2015;21:1220–3. doi: 10.3201/eid2107.141888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Tawfiq JA, Omrani AS, Memish ZA. Middle East respiratory syndrome coronavirus: current situation and travel-associated concerns. Front Med. 2016;10:111–9. doi: 10.1007/s11684-016-0446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camel is absent for the second year from the Hajj scene. Alriyadh. 2016 Sep 5; ( http://www.alriyadh.com/1531133) [Google Scholar]
- 24.Memish ZA, Al-Tawfiq JA, Assiri A, et al. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J. 2014;33:904–6. doi: 10.1097/INF.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 25.de Groot RJ, Baker SC, Baric RS, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–2. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Boheemen S, de Graaf M, Lauber C, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3(6):e00473–12. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EC, Blanc H, Surdel MC, Vignuzzi M, Denison MR. Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. PLoS Pathog. 2013;9(8):e1003565. doi: 10.1371/journal.ppat.1003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan CM, Chu H, Wang Y, et al. Carcinoembryonic antigen-related cell adhesion molecule 5 is an important surface attachment factor that facilitates entry of the Middle East respiratory syndrome coronavirus. J Virol. 2016;90:9114–27. doi: 10.1128/JVI.01133-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gierer S, Bertram S, Kaup F, et al. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J Virol. 2013;87:5502–11. doi: 10.1128/JVI.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millet JK, Whittaker GR. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci U S A. 2014;111:15214–9. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muth D, Corman VM, Meyer B, et al. Infectious Middle East respiratory syndrome coronavirus excretion and serotype variability based on live virus isolates from patients in Saudi Arabia. J Clin Microbiol. 2015;53:2951–5. doi: 10.1128/JCM.01368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang L, Wang N, Zuo T, et al. Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci Transl Med. 2014;6:234ra59. doi: 10.1126/scitranslmed.3008140. [DOI] [PubMed] [Google Scholar]
- 33.Tang XC, Agnihothram SS, Jiao Y, et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci U S A. 2014;111:E2018–E2026. doi: 10.1073/pnas.1402074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The FANTOM Consortium and the RIKEN PMI and CLST (DGT) A promoter-level mammalian expression atlas. Nature. 2014;507:462–70. doi: 10.1038/nature13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan JF, Chan KH, Choi GK, et al. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J Infect Dis. 2013;207:1743–52. doi: 10.1093/infdis/jit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hocke AC, Becher A, Knepper J, et al. Emerging human middle East respiratory syndrome coronavirus causes wide-spread infection and alveolar damage in human lungs. Am J Respir Crit Care Med. 2013;188:882–6. doi: 10.1164/rccm.201305-0954LE. [DOI] [PubMed] [Google Scholar]
- 37.de Wit E, Rasmussen AL, Falzarano D, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A. 2013;110:16598–603. doi: 10.1073/pnas.1310744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falzarano D, de Wit E, Feldmann F, et al. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014;10(8):e1004250. doi: 10.1371/journal.ppat.1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–83. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Memish ZA, Assiri AM, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014;29:307–8. doi: 10.1016/j.ijid.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SY, Park SJ, Cho SY, et al. Viral RNA in blood as indicator of severe outcome in Middle East respiratory syndrome coronavirus infection. Emerg Infect Dis. 2016;22:1813–6. doi: 10.3201/eid2210.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129–32. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Doremalen N, Miazgowicz KL, Milne-Price S, et al. Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. J Virol. 2014;88:9220–32. doi: 10.1128/JVI.00676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao J, Li K, Wohlford-Lenane C, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111:4970–5. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agrawal AS, Garron T, Tao X, et al. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol. 2015;89:3659–70. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munster VJ, de Wit E, Feldmann H. Pneumonia from human coronavirus in a macaque model. N Engl J Med. 2013;368:1560–2. doi: 10.1056/NEJMc1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan JF, Yao Y, Yeung ML, et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–13. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson RF, Via LE, Kumar MR, et al. Intratracheal exposure of common marmosets to MERS-CoV Jordan-n3/2012 or MERS-CoV EMC/2012 isolates does not result in lethal disease. Virology. 2015;485:422–30. doi: 10.1016/j.virol.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng DL, Al Hosani F, Keating MK, et al. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186:652–8. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mielech AM, Kilianski A, Baez-Santos YM, Mesecar AD, Baker SC. MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology. 2014;450–451:64–70. doi: 10.1016/j.virol.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lokugamage KG, Narayanan K, Nakagawa K, et al. Middle East respiratory syndrome coronavirus nsp1 inhibits host gene expression by selectively targeting nuclear-transcribed mRNAs but spares mRNAs of cytoplasmic origin. J Virol. 2015;89:10970–81. doi: 10.1128/JVI.01352-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y, Zhang L, Geng H, et al. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein Cell. 2013;4:951–61. doi: 10.1007/s13238-013-3096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu DX, Fung TS, Chong KK, Shukla A, Hilgenfeld R. Accessory proteins of SARS-CoV and other coronaviruses. Antiviral Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Josset L, Menachery VD, Gralinski LE, et al. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. MBio. 2013;4(3):e00165–e13. doi: 10.1128/mBio.00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park WB, Perera RA, Choe PG, et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis. 2015;21:2186–9. doi: 10.3201/eid2112.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22:1554–61. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–97. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 59.Payne DC, Iblan I, Alqasrawi S, et al. Stillbirth during infection with Middle East respiratory syndrome coronavirus. J Infect Dis. 2014;209:1870–2. doi: 10.1093/infdis/jiu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization. Surveillance for human infection with Middle East respiratory syndrome coronavirus (MERS-CoV): interim guidance. 2015 Jun 30; ( http://www.who.int/csr/disease/coronavirus_infections/surveillance-human-infection-mers/en/)
- 62.Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014;210:1590–4. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.WHO Mers-Cov Research Group. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013:5. doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corman VM, Müller MA, Costabel U, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17(49):20334. doi: 10.2807/ese.17.49.20334-en. [DOI] [PubMed] [Google Scholar]
- 65.Corman VM, Eckerle I, Bleicker T, et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17:20285. doi: 10.2807/ese.17.39.20285-en. [DOI] [PubMed] [Google Scholar]
- 66.Drosten C, Muth D, Corman VM, et al. An observational, laboratory-based study of outbreaks of Middle East respiratory syndrome coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60:369–77. doi: 10.1093/cid/ciu812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–5. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Auyeung TW, Lee JS, Lai WK, et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51:98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan JF, Chan KH, Kao RY, et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67:606–16. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zumla A, Azhar EI, Arabi Y, et al. Host-directed therapies for improving poor treatment outcomes associated with the middle east respiratory syndrome coronavirus infections. Int J Infect Dis. 2015;40:71–4. doi: 10.1016/j.ijid.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agrawal AS, Tao X, Algaissi A, et al. Immunization with inactivated Middle East respiratory syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016;12:2351–6. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunning JW, Merson L, Rohde GG, et al. Open source clinical science for emerging infections. Lancet Infect Dis. 2014;14:8–9. doi: 10.1016/S1473-3099(13)70327-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Kerkhove MD, Broberg E, Engelhardt OG, Wood J, Nicoll A. The Consortium for the Standardization of Influenza Seroepidemiology (CONSISE): a global partnership to standardize influenza seroepidemiology and develop influenza investigation protocols to inform public health policy. Influenza Other Respir Viruses. 2013;7:231–4. doi: 10.1111/irv.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.