Abstract
Background
Given the rapid increase in e-cigarette (EC) popularity and paucity of longitudinal health-related data associated with this, there is an urgent need to assess the potential risks of long-term EC use.
Objective
To compare exposure to nicotine, tobacco-related carcinogens and toxicants among cigarette-only smokers, and smokers and ex-smokers with long-term EC use or with use of nicotine replacement therapy (NRT; a product with known safety profile).
Design
Cross-sectional study.
Setting
United Kingdom.
Participants
Five groups were purposively recruited: (1) cigarette-only users, (2) ex-smokers with long-term (≥6 months) EC-only or (3) NRT-only use, and (4) long-term dual cigarette-EC or (5) dual cigarette-NRT users (N=36-37 per group, total N=181).
Measurements
Socio-demographic and smoking characteristics were assessed; participants provided urine and saliva samples, analysed for biomarkers of nicotine, tobacco-specific nitrosamines (TSNAs) and volatile organic compounds (VOCs).
Results
After controlling for confounders, there were no clear group differences in salivary or urinary biomarkers of nicotine intake. EC-only and NRT-only users had significantly lower metabolite levels for TSNAs (including the carcinogenic metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, NNAL) and for VOCs (including metabolites of the toxicants acrolein, acrylamide, acrylonitrile, 1,3-butadiene, ethylene oxide) compared with cigarette-only, dual cigarette-EC or cigarette-NRT users. EC-only users had significantly lower NNAL levels than all other groups. Cigarette-only, dual cigarette-NRT and cigarette-EC users had largely similar levels of TSNA and VOC metabolites.
Limitations
Cross-sectional design with self-selected sample.
Conclusions
Ex-smokers with long-term EC-only or NRT-only use may achieve approximately similar nicotine intake to cigarette-only smokers but results were variable. Long-term NRT-only and EC-only use, but not dual use with cigarettes, is associated with substantially reduced levels of measured carcinogens and toxicants relative to cigarette-only smoking.
Primary source of funding
Cancer Research UK (C27061/A16929).
Keywords: electronic cigarettes, nicotine replacement therapy, biomarkers, nicotine, TSNA, VOC, carcinogens, toxicants, harm reduction, long-term use, tobacco control
Introduction
E-cigarettes (EC) are increasingly popular devices (1) that produce an aerosol by heating a solvent (e-liquid) usually containing nicotine through a battery-powered heating element. Unlike smoked tobacco, nicotine can therefore be delivered to the respiratory tract without combustion (2). Despite this possible advantage, health concerns about EC remain regarding potential cytotoxicity, delivery of carcinogens (3), including carbonyls (4, 5), tobacco-specific N-nitrosamines (TSNAs, (6)) and heavy metals (4), effects on cardiovascular and respiratory function and inflammation (7) and nicotine delivery (8). Data on the effects of long-term EC use are needed to assess their risks and potential effectiveness accurately and to inform health professionals encountering EC users (9).
To date, most studies have looked at toxicant concentrations in EC liquids or aerosol (e.g. (4, 6)) using cell-line or animal models (e.g. (7)). However, this may not provide accurate information as user characteristics, together with device characteristics and their interaction, determine actual body-level exposure, and thus potential health consequences (10). Three studies that have assessed body-level exposure found lower levels for carcinogens, including TSNAs, in recent ex-smokers using EC compared with a historic sample of smokers (11), and reductions in toxicants over a two- or four-week period in smokers switching to EC with or without concurrent smoking (12, 13). However, none of the studies involved long-term users, which is important given observed learning effects in EC use (14) (15), or included real-world control groups to reduce the risk of confounding when interpreting results of observational studies.
Nicotine replacement therapy (NRT) users would be an appropriate control. Dual use with cigarettes of either EC or NRT is common, and there is some long-term use of both (16, 17). Both have been advocated as harm reduction products used to reduce risks associated with combustible tobacco use (18). However, unlike EC, the NRT safety profile is well established (e.g. (19)) and NRT effectiveness for smoking cessation through intial partial (20) or complete substitution (21) has been demonstrated. NRT is therefore recommended as a harm reduction strategy in several countries (22).
While longitudinal cohort studies and randomised controlled trials will provide the best data to answer questions concerning the safety and efficacy for smoking cessation of EC use, these designs are time- and resource-intensive. In the absence of long-term data, a more pragmatic approach is to compare smokers and ex-smokers as a function of EC use in real life settings. This study aimed to address the gap in the existing literature by measuring biomarker levels in long-term users of EC compared with an appropriate control, NRT users. Specifically, this study assessed whether long-term EC-only, NRT-only, dual cigarette-EC or dual cigarette-NRT use is associated with differences in metabolites of a) nicotine; b) TSNAs and c) volatile organic compounds (VOC) compared with cigarette-only smokers.
Methods
Study design and procedure
This cross-sectional study, carried out in January–June 2014 in London, UK sought to evaluate the range of toxicant levels measured in smokers and ex-smokers with or without concurrent long-term use of EC or NRT. The study methodology has been described elsewhere (23). Briefly, participants visited the laboratory for a single session, lasting 30 minutes, after abstaining from eating, drinking or using cigarettes or nicotine products an hour before their visit to standardise assessment. At the laboratory, after providing written consent, participants completed a short questionnaire assessing socio-demographic, smoking and product use characteristics and provided breath, saliva and urine samples. Expired air was assessed for carbon monoxide (CO) with a breathalyser (Micro IV Smokerlyzer, Bedfont Scientific, Kent, England). In addition, two saliva samples were collected with sterile dental rolls (Salivette®, Sarstedt Ltd, Leicester, England) which participants were asked to gently chew for about two minutes or until saturated. Urine was collected in a sealable, sterilised cup by participants on site and transferred by staff into cryovials. Urine and saliva samples were then kept frozen at -20°C until shipment in dry ice for analysis to laboratories at Roswell Park Cancer Institute (RPCI) and the U.S. Centers for Disease Control and Prevention (CDC). All participants were reimbursed for time and travel (£25). The study was approved by the University College London Ethics Committee (Project ID 0483/002).
Participants
Participants were purposively recruited in the greater London area using a variety of methods to increase sample diversity, including newspapers and online adverts, posters in pharmacies, and the use of marketing companies. Participants had to be ever smokers and were eligible to take part if they fulfilled the following inclusion criteria: current smokers had to smoke an average of five or more cigarettes per day for at least six months, and ex-smokers had to have stopped using tobacco products (including cigarettes, waterpipe, cigars, smokeless products such as snus or chewing tobacco) for at least six months. Because this study sought to evaluate the impact of long-term use of non-combustible nicotine delivery devices (NRT and EC), smokers (i.e. dual cigarette-EC or cigarette-NRT users) and ex-smokers (i.e. EC-only or NRT-only users) had to have been using these products at least weekly for six or more months (users of nicotine-free products, e.g. using e-liquid without nicotine, were excluded). In practice, however, participants used products daily as indicated by latency to last product use across groups (Cigarettes-only: 1.4h; Dual cigarette-NRT: 4.3h; Dual cigarette-EC: 1.3h; NRT-only: 24h; EC-only: 5.4h). Product use was verified by asking participants to bring in the NRT or EC that they were currently using, and smoking status was verified with CO readings (10 ppm cut-off (24)). Participants who used both NRT and EC were excluded as were those below 18 years of age, pregnant or with a history of heart or lung disease or bleeding gums, illness, or an active infection within 24 hours of their scheduled appointment.
Measures
Biomarkers of exposure
Level of nicotine exposure was determined to assess effectiveness of nicotine-delivery products using two different methodologies. Saliva samples were analysed for nicotine, and its major metabolite cotinine, using established gas chromatography methodology (25, 26). Urine samples were analysed for main nicotine metabolites to derive total nicotine equivalents and for minor tobacco alkaloids using validated tandem mass spectrometry methodology (27, 28).
Levels of urinary TSNA and VOC metabolites were determined using liquid chromatography atmospheric pressure ionization tandem mass spectrometry (29) and ultra-high performance liquid chromatography coupled with electrospray ionisation and tandem mass spectrometry (30), respectively, to assess the potential risk of nicotine-delivery products. While a comprehensive battery of metabolites was assessed (see Table S1), we focus here on well-established metabolites of compounds that are known to contribute significantly to smoking-related toxicological and carcinogenic risks (31–39) (see Table 1). All analyses of urinary and salivary biomarkers were carried out by the CDC and RPCI, respectively.
Table 1.
Major toxicants and carcinogens related to tobacco use
| Parent compound | Biomarker/Metabolite | Rationale for inclusion |
|---|---|---|
| Tobacco-specific N-nitrosamines | ||
| 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) | 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) | A potent lung carcinogen (40) and major contributor to cancer risk (34); Group 1 carcinogen (39); WHO Tobreg list: one out of nine toxicant recommended for mandated lowering in tobacco smoke (36) |
| Volatile organic compounds | ||
| Acrolein | N-Acetyl-S-(3-hydroxypropyl)-L-cysteine (3HPMA) | A major contributor to respiratory effects (34, 35); Group 3 carcinogen (41);WHO Tobreg list: one out of nine toxicant recommended for mandated lowering in tobacco smoke (36) |
| Acrylamide | N-Acetyl-S-(2-carbamoylethyl)-L-cysteine (AAMA) | Group 2A carcinogen (37); a neurotoxin (71) |
| Acrylonitrile | N-Acetyl-S-(2-cyanoethyl)-L-cysteine (CYMA) | A major contributor to cancer risk (34), highly specific VOC biomarker for tobacco use (33); Group 2B carcinogen (37); WHO Tobreg list: one out of nine toxicant considered high priority for disclosure and monitoring (36) |
| 1,3-Butadiene | N-Acetyl-S-(4-hydroxy-2-buten-1-yl)-L-cysteine (MHBMA3)* | A major contributor to cancer risk (34, 35) ; Group 1 carcinogen (42); WHO Tobreg list: one out of nine toxicant recommended for mandated lowering in tobacco smoke (36) |
| Ethylene Oxide | N-Acetyl-S-(2-hydroxyethyl)-L-cysteine (HEMA)† | Group 1 carcinogen (37) |
More selective metabolite of parent compound than N-Acetyl-S-(3,4-dihydroxybutyl)-L-cysteine (DHBMA) (33)
Major urinary metabolite of ethylene oxide exposure, a minor metabolite of acrylonitrile and vinyl chloride exposure (toxic tobacco smoke constituents); WHO Tobreg - World Health Organisation study group on tobacco product regulation; VOC – volatile organic compound; International Agency for Research on Cancer (IARC) classification of carcinogen groups: Group 1 - Carcinogenic to humans; Group 2A - Probably carcinogenic to humans; Group 2B - Possibly carcinogenic to humans; Group 3 - Not classifiable as to its carcinogenicity to humans; Group 4 - Probably not carcinogenic to humans
Covariates
Socio-demographic characteristics (age, sex, ethnicity, education, marital status) were assessed in addition to self-reported recently resolved physical illness (chest infection, cold/flu, sore throat, fever), subjective well-being (happiness and satisfaction, both assessed with established single item measures (40)) and C-reactive protein (CRP) as a marker of inflammation (and thus potential health problems), measured in saliva and analysed with the ELISA method by Salimetrics Europe Ltd, UK (41). Smoking characteristics assessed included current and past daily cigarette consumption as a measure of dependence for smokers and ex-smokers, respectively, age at which participants had started smoking and the proportion of family or friends who smoke to gauge environmental tobacco smoke exposure.
Analysis
As this was a cross-sectional study, exposure biomarkers including metabolites of known tobacco-related carcinogens and toxicants were used as proxies for future disease risk. Previous research on the association of the carcinogenic metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) with lung cancer suggests that medium-large reductions in NNAL levels (Cohen’s f=0.25-0.40) would result in an appreciable reduction in risk (42) and could thus be considered clinically meaningful in magnitude and warrant further investigation (43). A priori power calculation showed that 180 participants (36 per group) would provide 90% power to detect between-group differences of a medium effect size (Cohen’s f=0.3) in NNAL levels when comparing five groups, using analysis of variance (44). However, this calculation did not account for multiple outcomes being tested, and based on 35 biomarker outcomes reported here, power to detect such an effect size across all biomarkers would have been reduced to 54%. The sample size therefore only provided sufficient power (≥80%) to detect effects at the upper range of the estimate (Cohen’s f≥ 0.36) when accounting for multiple comparisons.
Analyses were conducted with SPSS Version 22.0. In initial analysis of group differences on covariates, one-way ANOVAs were used for continuous and chi-square analysis for categorical covariates, respectively. Prior to the main analysis, urinary metabolites were standardised algebraically to account for individual differences in urine concentration by dividing metabolite data by the ratio of observed to age-, sex-, and ethnicity-adjusted creatinine values and creatinine (measured by standard colorimetric method at RCPI) was also included as covariate in analysis (see Method 7 in 45). Due to non-normal distribution of data, generalised linear models with a log link and gamma distribution were used to assess group differences in outcome measures, adjusted for all covariates and latency to product use. B coefficients were exponentiated to obtain percent change in biomarker levels in all groups compared with cigarette-only smokers. For pre-specified tests of main effects of group, Type I errors were controlled using the false discovery rate (46) separately for socio-demographic comparisons (N=13) and biomarker comparisons (N=35). Where overall omnibus effects were considered significant, the Sidak correction was used in post-hoc analysis to determine between which (if any) groups differences persisted. Biomarker values below the limit of detection (LOD) were imputed using standard methodology (LOD divided by square root of 2 (47)), and biomarkers with 50% or more of values below the LOD were not analysed.
Role of funding source
This work was supported by Cancer Research UK (C27061/A16929) with additional funding from Cancer Research UK (C1417/A14135; C36048/A11654). JB’s post is funded by a fellowship from the Society for the Study of Addiction and CRUK also provide support (C1417/A7972; C44576/A19501). AM and RW are part of the UK Centre for Tobacco and Alcohol Studies, a UK Clinical Research Collaboration Public Health Research: Centre of Excellence. Funding from the Medical Research Council, British Heart Foundation, Cancer Research UK, Economic and Social Research Council and the National Institute for Health Research under the auspices of the UK Clinical Research Collaboration is gratefully acknowledged (MR/K023195/1). MLG was supported by the National Institute on Drug Abuse (NIDA) and the National Cancer Institute (NCI) of the National Institutes of Health under Award Number R01DA037446 and P30 CA016056, respectively, and by an award from Roswell Park Alliance Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and the US Food and Drug Administration. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Overall, participants were relatively young, mainly male, white, had at least a high school education, and about half were single (see Table 2). On average, participants had started smoking in their late teens, smoking nearly one pack a day, with a substantial proportion (16%-51%) of their family or friends smoking. Salivary CRP levels were within the range observed for healthy adults (0.05-64.3 μg/L) (48) and reported well-being levels comparable to that of representative population samples (40). There were some group differences: the proportion of females varied from 19.4% in EC-only users to 61.1% in dual cigarette-NRT users, fewer EC-only users were female; NRT-only users had started smoking the latest and EC-only users had the lowest proportion of family or friends who smoke. There was also considerable variation between groups in terms of ethnicity, marital status, cigarette consumption, recent illness and reported happiness levels (Table 2).
Table 2.
Socio-demographic, smoking, physical health and subjective well-being characteristics of study participants
| Smokers |
Ex-smokers |
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total (N=181) | Cigarette-only (N=37) | Dual cigarette-NRT users (n=36) | Dual cigarette-EC users (n=36) | NRT-only users (n=36) | EC-only users (n=36) | P-value |
| Mean age (SD) | 37.8 (11.8) | 34.4 (14.0) | 36.4 (8.5) | 39.3 (13.1) | 40.3 (11.1) | 38.5 (11.1) | 0.27 |
| % Female (N) | 39.2 (71) | 43.2 (16) | 61.1 (22) | 30.6 (11) | 41.7 (15) | 19.4 (7) | 0.024 |
| % White (N) | 72.4 (131) | 81.1 (30) | 58.3 (21) | 75.0 (27) | 63.9 (23) | 83.3 (30) | 0.11 |
| % High school (N) | 77.3 (140) | 67.6 (25) | 83.3 (30) | 80.6 (29) | 77.8 (28) | 77.8 (28) | 0.61 |
| % Single (N) | 53.6 (97) | 70.3 (26) | 58.3 (21) | 50.0 (18) | 36.1 (13) | 52.8 (19) | 0.10 |
| Mean age started smoking (SD) | 17.8 (4.3) | 16.6 (3.2) | 18.2 (3.4) | 17.3 (3.1) | 20.3 (6.4) | 16.6 (3.2) | 0.012 |
| Mean cigarettes per day (SD)* | 13.3 (8.7) | 13.9 (9.0) | 10.8 (4.6) | 11.9 (9.6) | 14.7 (10.3) | 15.9 (8.3) | 0.10 |
| Mean proportion of friends/family who smoke (SD) | 35.6 (27.5) | 50.9 (23.6) | 39.8 (24.1) | 38.0 (32.4) | 33.2 (27.7) | 15.6 (15.2) | <0.001 |
| % Recent Illness (N) | 23.2 (42) | 37.8 (14) | 8.3 (3) | 19.4 (7) | 27.8 (10) | 22.2 (8) | 0.10 |
| Geometric mean salivary CRP levels, in μg/L (IQR)† | 1.8 (2.5) | 2.0 (3.4) | 1.3 (1.1) | 1.6 (3.5) | 1.9 (2.1)‡ | 2.2 (3.7) | 0.86 |
| Mean global life satisfaction (SD)§ | 3.9 (1.0) | 4.1 (0.9) | 3.8 (1.1) | 3.7 (1.1) | 3.9 (0.9) | 3.9 (1.1) | 0.60 |
| Mean happiness levels (SD)‖ | 5.0 (1.5) | 4.6 (1.7) | 5.6 (1.1) | 4.7 (1.7) | 5.3 (1.3) | 5.0 (1.6) | 0.10 |
Statistical comparison conducted on log-transformed values (not shown)
N=1 missing
Assessed by asking: “All things considered, how satisfied are you with your life as a whole?” with response options (1) “very dissatisfied” to (5) “very satisfied”
Assessed by asking: “Some people are very generally very happy. They enjoy life regardless of what is going on, getting the most out of everything. To what extent does this characterisation describe you?” with response option from (1) “not at all” to (7) “a great deal”; NRT – nicotine replacement therapy; EC – electronic cigarette; CRP – C-reactive protein; IQR – interquartile range; P-value – omnibus test result, adjusted for the reported comparisons in this table using the false discovery rate (46)
As previously reported, length of product use was broadly similar across group at around 17 months, and mean daily NRT and EC use, measured by self-reported nicotine dose, was higher for NRT-only and EC-only users than for dual cigarette-NRT and cigarette-EC users (see (23) for details). In terms of the product type used, first generation ‘cig-a-likes’ with replaceable or disposable cartridges were most popular among dual cigarette-EC users (60.0%) and third/fourth generation advanced personal vaporisers most popular among EC-only users (47.2 %), with refillable pen-style, second generation EC equally popular among dual cigarette-EC (31.4%) and EC-only (36.1%) users. For both dual cigarette-NRT and NRT-only users, gum (44.4% and 33.3%, respectively) and patches (both 33.3%) were the most popular products, and a similar proportion (27.8%) used more than one NRT product.
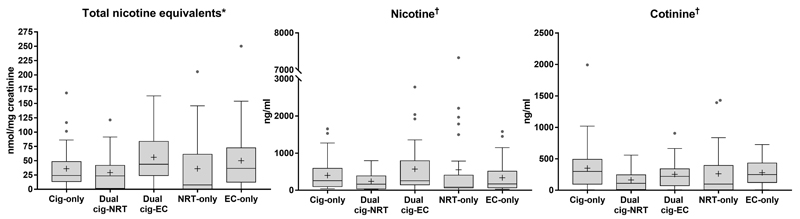
a). Nicotine levels
Nicotine intake from different products was roughly comparable (Figure 1), although there was some variation across groups (Table S1). In terms of urinary biomarkers, users of all products had levels of total nicotine equivalents at least as high as cigarette-only smokers in adjusted analysis (see Table 3). Findings in relation to salivary biomarkers varied. Dual cigarette-NRT users had relatively low nicotine and cotinine levels and EC-only users had relatively low nicotine levels, at around half that of cigarette-only users, with other groups obtaining levels slightly below or above those from cigarette-only users (Table 3). The minor tobacco alkaloids anabasine and anatabine which are specific to tobacco as opposed to nicotine exposure, clearly distinguished between smokers and ex-smokers, with significantly lower levels compared with cigarette-only, dual cigarette-NRT or cigarette-EC users (Table S1).
Figure 1. Urinary and salivary nicotine metabolite levels by group^.
^Boxplots show median with interquartile range, IQR (25%-75%); error bars show Tukey’s whiskers and cross indicates arithmetic mean (geometric means are provided in Table S1); Solid grey circles show outliers; *Measured in urine: data are raw values divided by ratio of observed to covariate-adjusted creatinine levels; values below the limit of detection (LOD) were imputed by LOD divided by square root of 2; †Measured in saliva; There were no significant differences between groups; NRT – Nicotine replacement therapy; EC – Electronic cigarette; Cig-Cigarette
Table 3.
Adjusted biomarker levels by group as proportion of cigarette-only smoker levels*
| Smokers |
Ex-smokers |
||||
|---|---|---|---|---|---|
| Parent Compound | Biomarker/Metabolite | Dual cigarette-NRT users (n=36) | Dual cigarette-EC users (n=36) | NRT-only users (n=36) | EC-only users (n=36) |
| Alkaloids | Percent (95% confidence interval)† | ||||
| Nicotine | Total nicotine equivalents† | 104.2 (64.3-168.9) | 156.8 (105.1-233.8) | 121.6 (62.5-236.8) | 126.9 (82.1-196.2) |
| Nicotine‡ | 64.2 (39.2-104.9) | 152.2 (90.7-255.1) | 135.1 (68.1-268.0) | 60.4 (35.8-101.8) | |
| Cotinine‡ | 46.8 (26.3-83.3) | 69.7 (42.1-115.3) | 82.1 (42.9-157.3) | 75.1 (45.3-124.4) | |
|
Tobacco Specific
N-Nitrosamines | |||||
| 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) | 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) | 57.1 (33.1-98.4) | 81.2 (49.7-132.8) | 11.6 (6.3-21.3) | 2.5 (1.5-4.2) |
|
Volatile Organic Compounds | |||||
| Acrolein | N-Acetyl-S-(3-hydroxypropyl)-L-cysteine (3HPMA) | 107.1 (71.8-159.7) | 91.2 (60.2-138.2) | 35.3 (23.5-53.0) | 33.3 (20.9-53.1) |
| Acrylamide | N-Acetyl-S-(2-carbamoylethyl)-L-cysteine (AAMA) | 80.2 (57.9-111.1) | 115.9 (80.8-166.1) | 45.4 (32.4-63.5) | 42.9 (31.1-59.2) |
| Acrylonitrile | N-Acetyl-S-(2-cyanoethyl)-L-cysteine (CYMA) | 85.6 (48.7-150.4) | 102.7 (63.7-165.6) | 10.5 (5.4-20.6) | 2.9 (1.7-4.7) |
| 1,3-Butadiene | N-Acetyl-S-(4-hydroxy-2-buten-1-yl)-L-cysteine (MHBMA3) | 101.9 (64.6-160.7) | 115.0 (73.2-180.6) | 19.9 (12.8-30.7) | 11.0 (7.5-16.1) |
| Ethylene oxide, acrylonitrile, vinyl chloride | N-Acetyl-S-(2-hydroxyethyl)-L-cysteine (HEMA) | 86.6 (58.7-127.8) | 104.0 (73.9-146.4) | 54.2 (38.4-76.5) | 43.5 (30.8-61.3) |
Levels as a proportion of cigarette-only smoker levels are estimated from a model that adjusted for all variables in Table 2, latency to product use and creatinine. For urinary metabolites, inputs to the model were divided by the ratio of observed to covariate-adjusted creatinine;
Sum of cotinine, nicotine, trans-3’-hydroxycotinine, cotinine N-oxide, nicotine 1’-oxide, norcotinine, nornicotine measured in urine;
Measured in saliva (all other metabolites measured in urine); NRT – nicotine replacement therapy; EC – electronic cigarette
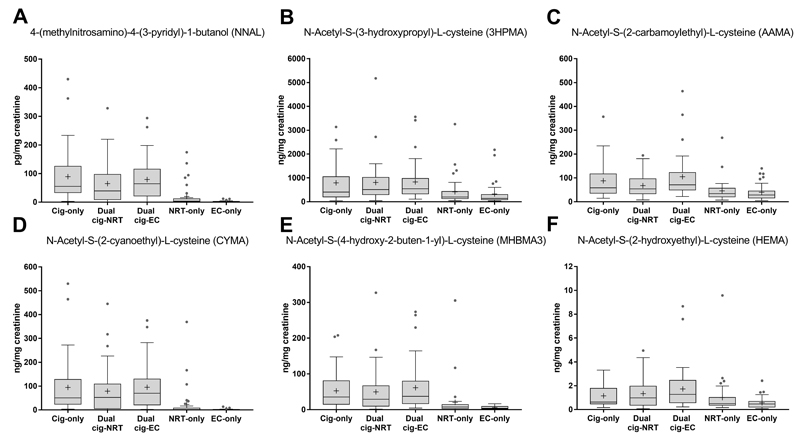
b). TSNA levels
There were clear differences in levels of the carcinogen metabolite NNAL (Figure 2). NRT-only and EC-only users had markedly lower levels than cigarette-only, dual cigarette-NRT and cigarette-EC users (p<0.001), with EC-only users having significantly lower levels than all other groups, at less than 3% of cigarette-only smoker levels (Table 3). Compared with cigarette-only smokers, there were no large differences in NNAL levels for dual cigarette-EC users but dual cigarette-NRT users had somewhat lower levels. Results followed a similar, albeit less pronounced, pattern for the other TSNAs measured (Table S1).
Figure 2. Urinary metabolite levels^ for selected toxicants by group*: (A) Tobacco- specific N-nitrosamine (NNK), (B) Acrolein, (C) Acrylamide, (D) Acrylonitrile, (E) 1,3-butadiene, (F) Ethylene oxide.
^Data are raw values divided by ratio of observed to covariate-adjusted creatinine levels; values below the limit of detection (LOD) were imputed by LOD divided by square root of 2; *Boxplots show median with interquartile range (25%-75%); error bars show Tukey’s whiskers and cross indicates arithmetic mean (geometric means are provided in Table S1); Solid grey circles show outliers; Significant pairwise comparisons are presented in Table S1; NRT – Nicotine replacement therapy; EC – Electronic cigarette; Cig-Cigarette
c). VOC levels
EC-only users had the lowest overall levels of the major urinary VOC metabolites, with acrylonitrile levels as low as 2.9% of cigarette-only smokers, and NRT-only users had the second lowest overall, with acrylonitrile levels as low as 10.5% of cigarette-only smokers (Table 3). By contrast, dual cigarette-NRT, cigarette-EC and cigarette-only users all had very similar VOC metabolite levels (Figure 2). Compared with all other groups, NRT-only and EC-only users at least halved the reference values of cigarette-only smokers (see Table 3), and had significantly lower levels of all major metabolites of selected toxicant and carcinogen VOCs (all p<0.001, Table S1).
Results were largely confirmed when looking at other VOC metabolites that were assessed, with EC-only users generally displaying the lowest levels, followed by NRT-only users and no detectable differences between dual cigarette-NRT, cigarette-EC and cigarette-only users (see Table S1). The only exceptions were metabolites of benzene (N-Acetyl-S-(phenyl)-L-cysteine (PMA) and muconic acid (MU)), carbon disulphide (2-thioxothiazolidine-4-carboxylic acid (TTCA)) and styrene (N-Acetyl-S-(1 and 2-phenyl-2-hydroxyethyl)-L-cysteine (PHEMA) and phenylglyoxylic acid (PGA)). Dual cigarette-EC users had somewhat higher PMA, MU and PHEMA levels and dual cigarette-NRT and dual cigarette-EC users had somewhat higher PGA levels than other groups (Table S1). There were no appreciable group differences in TTCA levels. However, these metabolites were either non-specific to the parent VOC measured (MU, TTCA have dietary contributions, PGA is a metabolite of ethylbenzene and styrene exposure) or had low detection rates (PMA and PHEMA; see Table S2).
Discussion
This is, to our knowledge, the first direct comparison of nicotine, important carcinogen and toxicant metabolite levels in long-term users of EC or NRT. We find that ex- smokers who had switched to exclusive EC or NRT use obtained roughly similar levels of nicotine compared with cigarette-only smokers, but results were variable. Exclusive use of NRT, and in particular of EC, but not dual use with cigarettes, was associated with lower levels of known tobacco-related carcinogens and toxicants measured in this study compared with cigarette-only use.
The finding that NRT-only or EC-only use is associated with roughly similar nicotine intake to cigarette-only use supports the view that users seek a particular level of nicotine intake, irrespective of the delivery system (49), and adjust product use accordingly (50). The finding is consistent with more recent (51) but not older (8) studies on nicotine delivery from EC and may reflect the improved design of newer generations of EC products (52), highlighting the importance of focusing on experienced, long-term rather than naïve, short-term users. Similarly, efficient nicotine intake from exclusive NRT use has been observed in long-term (53) but not short and intermediate-term NRT users (54). The fact that intake was largely similar for both groups also suggests that better craving reductions observed in users of EC compared with NRT (23, 55) may be due to factors other than nicotine delivery, such as the greater behavioural similarity of EC use (as opposed to NRT use) with smoking. This is consistent with research on non-nicotine sensory factors that have been shown to influence tobacco withdrawal (56). However, it should be noted that this study was not powered to detect anything other than relatively large effects, so results are indeterminate regarding smaller differences in nicotine intake between these groups.
The lower carcinogen and toxicant levels associated with NRT-only and EC-only use in this study confirm the known low risk for long-term NRT product use (57). They also underscore the translation of greatly reduced concentrations of some carcinogens and toxicants from e-liquids and aerosol (4, 6, 58) to body-level exposure, contrary to worries that long-term EC use would result in substantial harmful exposure (59). Given the involvement of these TSNAs and VOCs with cancer, cardiovascular and pulmonary diseases (e.g. 42, 60), these results suggest that complete substitution may result in reducing disease risk and supports the assertion that EC use may to be less harmful than smoking (2, 61–63). In this study, there was no evidence that long-term EC-only use is associated with greater carcinogen or toxicant levels than NRT-only use; if anything, on some measures it was associated with lower levels. While this could be due to occasional cigarette smoking lapses by long-term NRT-only users, this is unlikely to have made a substantial contribution given very low levels of tobacco-specific (as opposed to nicotine-specific) biomarkers for acrylonitrile, anabasine and anatabine (64, 65) in this group. Alternatively, these differences may reflect typical low-level contamination in these products (e.g. with nitrosamines from tobacco-derived nicotine (66)), non-specificity of the metabolite for the toxicant (e.g. muconic acid for benzene (67)), or non-smoking related environmental sources of toxicant exposure (e.g. for styrene (68)). Contrary to findings from a recent short-term switching study (12), dual cigarette-NRT or cigarette-EC use was not associated with appreciable reductions in carcinogen and toxicant levels. This may be because participants in the current study may have been even heavier smokers prior to starting concurrent EC or NRT use, thus masking the benefit of potential partial substitution in our cross-sectional study, or because dual users used non-combustible products to bridge times of non-smoking and thus did not actually reduce their cigarettes consumption. Alternatively, it may reflect either differences in study design, e.g. different usage pattern in long-term as opposed to short-term users, or the relatively low power to detect smaller, yet meaningful, effects in this study. Further longitudinal research is needed to differentiate between these explanations.
The findings have several implications. While complete long-term switching to EC may produce a net benefit for the health outcomes of the smoking population, given the association with very low levels of dangerous constituents measured in this study similar in magnitude to NRT, it is only likely to be beneficial if complete cessation of cigarettes is achieved. Thus, dual users should be encouraged to cease using combustible products to reduce long-term health risks. Our results also indicate that machine-derived and actual body-level exposure to toxicants can be very different as shown, for instance, by greatly reduced aldehyde levels in EC users here compared with reportedly high levels in EC aerosol under certain laboratory conditions ((5) but see (69)). Lastly, it should be noted that while exclusive EC and NRT use was associated with marked reductions in carcinogen and toxicant levels compared with cigarette-only smokers, it did not eliminate exposure (and thus possible health risks) completely. Full cessation of all nicotine products remains the best option to avoid harm.
The study had several limitations. Even though participants were recruited using diverse methods, resulting in a sample broadly similar to the population of NRT/EC users (16, 70), and we controlled for important confounders, group differences may not generalise and reflect self-selection. The sample was too small to allow more sophisticated analyses to evaluate the association of different types of EC or NRT (and other characteristics such as EC flavourings) with intake, and we may not have picked up small but important differences in exposure levels. In particular, the lack of group differences in nicotine intake has to be interpreted cautiously given the low power to detect smaller effects and the variability across different urinary and salivary measures. Lastly, we did not assess indirect exposure and the analysis was limited by the number of biomarkers available and spot sampling, which can only provide a snapshot of exposure. However, given the lack of long-term data, we chose this pragmatic design to evaluate quickly potentially important associations of EC use with carcinogen and toxicant intake to inform further longitudinal work. Moreover, the relatively slow pharmacokinetics of the assessed metabolites (71) provides stable estimates of recent exposure and should militate against variations associated with different usage patterns of different products. Future work should attempt to sample a larger range of biomarkers over a longer period of time, including biomarkers of actual harm such as lung function measures, and evaluate the impact of potential interactions of user with device characteristics on delivery of toxicants to users and bystanders.
In conclusion, exclusive long-term NRT or EC use among ex-smokers is associated with substantially reduced levels of select carcinogens and toxicants compared with cigarette smoking; however, concurrent use with cigarettes appears not to be. We found no evidence that EC-only compared with NRT-only use is associated with greater carcinogen and toxicant levels measured in this study. Nicotine delivery, though variable, is roughly comparably to cigarettes, but smaller meaningful differences may exist.
Supplementary Materials
Acknowledgement
We would like to thank Kate Sheals and Victoria Nelson for their help with data collection and CDC reviewers for providing a thorough review of the manuscript.
Footnotes
Contributors
LS conceived the original idea for this study, obtained funding and managed the day-to-day running of the study. LS undertook the data analyses and wrote the initial draft with further input from MLG, JB and AM. MLG, BCB, KUA, JF & LW undertook analysis of biological samples. LS is guarantor for this article. All authors read, reviewed and approved the final version. All researchers listed as authors are independent from the funders and all final decisions about the research were taken without constraint by the investigators. LS & MLG had full access to all the data in the study and LS had final responsibility for the decision to submit for publication.
Conflict of Interest
LS has received an honorarium for a talk, an unrestricted research grant and travel expenses to attend meetings and workshops from Pfizer, a pharmaceutical company that makes smoking cessation products, and has acted as paid reviewer for grant awarding bodies and as a paid consultant for health care companies. MLG reports research grants from and served as an advisory board member to pharmaceutical companies that manufacture smoking cessation medications. JB has received unrestricted research funding from Pfizer to study smoking cessation. RW has received travel funds and hospitality from, and undertaken research and consultancy for, pharmaceutical companies that manufacture or research products aimed at helping smokers to stop. The other authors have no conflicts of interest to declare.
References
- 1.Vardavas CI, Filippidis FT, Agaku IT. Determinants and prevalence of e-cigarette use throughout the European Union: a secondary analysis of 26 566 youth and adults from 27 Countries. Tob Control. 2015;24(5):442–8. doi: 10.1136/tobaccocontrol-2013-051394. [DOI] [PubMed] [Google Scholar]
- 2.Hajek P, Etter JF, Benowitz N, Eissenberg T, McRobbie H. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction. 2014;109(11):1801–10. doi: 10.1111/add.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–86. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–9. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–4. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Shin HS. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2013;1291:48–55. doi: 10.1016/j.chroma.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19(2):98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 9.Etter JF, Bullen C, Flouris AD, Laugesen M, Eissenberg T. Electronic nicotine delivery systems: a research agenda. Tob Control. 2011;20(3):243–8. doi: 10.1136/tc.2010.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15(1):267–70. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BW, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17(6):704–9. doi: 10.1093/ntr/ntu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McRobbie H, Phillips A, Goniewicz ML, Smith KM, Knight-West O, Przulj D, et al. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev Res (Phila) 2015;8(9):873–8. doi: 10.1158/1940-6207.CAPR-15-0058. [DOI] [PubMed] [Google Scholar]
- 13.Goniewicz M, Gawron M, Smith DM, Peng M, Jacob P, III, Benowitz N. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: A longitudinal within-subjects oservational study. Nicotine Tob Res. 2016 doi: 10.1093/ntr/ntw160. First published online: August 17, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YH, Gawron M, Goniewicz ML. Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes. Addict Behav. 2015;48:1–4. doi: 10.1016/j.addbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McQueen A, Tower S, Sumner W. Interviews with “vapers”: implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13(9):860–7. doi: 10.1093/ntr/ntr088. [DOI] [PubMed] [Google Scholar]
- 16.Silla K, Beard E, Shahab L. Characterization of long-term users of nicotine replacement therapy: evidence from a national survey. Nicotine Tob Res. 2014;16(8):1050–5. doi: 10.1093/ntr/ntu019. [DOI] [PubMed] [Google Scholar]
- 17.McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- 18.Le Houezec J, McNeill A, Britton J. Tobacco, nicotine and harm reduction. Drug Alcohol Rev. 2011;30(2):119–23. doi: 10.1111/j.1465-3362.2010.00264.x. [DOI] [PubMed] [Google Scholar]
- 19.Murray RP, Connett JE, Zapawa LM. Does nicotine replacement therapy cause cancer? Evidence from the Lung Health Study. Nicotine Tob Res. 2009;11(9):1076–82. doi: 10.1093/ntr/ntp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ. 2009;338:b1024. doi: 10.1136/bmj.b1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence. NICE guidelines [PH45] London: NICE; 2013. [accessed 12/08/2016]. Smoking: Harm reduction. https://www.nice.org.uk/guidance/ph45. [Google Scholar]
- 23.Nelson VA, Goniewicz ML, Beard E, Brown J, Sheals K, West R, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: A cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300–5. doi: 10.1016/j.drugalcdep.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brose LS, Tombor I, Shahab L, West R. The effect of reducing the threshold for carbon monoxide validation of smoking abstinence--evidence from the English Stop Smoking Services. Addict Behav. 2013;38(10):2529–31. doi: 10.1016/j.addbeh.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Jacob P, 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222(1):61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- 26.Jacob P, 3rd, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3',3'-d2 in humans. Biol Mass Spectrom. 1991;20(5):247–52. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 27.McGuffey JE, Wei B, Bernert JT, Morrow JC, Xia B, Wang L, et al. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers' urine. PLoS One. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei B, Feng J, Rehmani IJ, Miller S, McGuffey JE, Blount BC, et al. A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin Chim Acta. 2014;436:290–7. doi: 10.1016/j.cca.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia B, Xia Y, Wong J, Nicodemus KJ, Xu M, Lee J, et al. Quantitative analysis of five tobacco-specific N-nitrosamines in urine by liquid chromatography-atmospheric pressure ionization tandem mass spectrometry. Biomed Chromatogr. 2014;28(3):375–84. doi: 10.1002/bmc.3031. [DOI] [PubMed] [Google Scholar]
- 30.Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS) Anal Chim Acta. 2012;750:152–60. doi: 10.1016/j.aca.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12(4):424–30. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haussmann HJ. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem Res Toxicol. 2012;25(4):794–810. doi: 10.1021/tx200536w. [DOI] [PubMed] [Google Scholar]
- 33.Burns DM, Dybing E, Gray N, Hecht S, Anderson C, Sanner T, et al. Mandated lowering of toxicants in cigarette smoke: a description of the World Health Organization TobReg proposal. Tob Control. 2008;17(2):132–41. doi: 10.1136/tc.2007.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon: IARC; 2004. Tobacco smoke and involuntary smoking. [PMC free article] [PubMed] [Google Scholar]
- 35.Smith CJ, Livingston SD, Doolittle DJ. An international literature survey of “IARC Group I carcinogens” reported in mainstream cigarette smoke. Food Chem Toxicol. 1997;35(10–11):1107–30. doi: 10.1016/s0278-6915(97)00063-x. [DOI] [PubMed] [Google Scholar]
- 36.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. Lyon: IARC; 2007. Smokeless tobacco and some tobacco-specific N-nitrosamines. [PMC free article] [PubMed] [Google Scholar]
- 37.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91(14):1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 38.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 63. Lyon: IARC; 1995. Dry cleaning, some chlorinated solvents and otehr industrial chemicals. [PMC free article] [PubMed] [Google Scholar]
- 39.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 100F. Lyon: IARC; 2012. Chemical Agents and Related Occupations. [PMC free article] [PubMed] [Google Scholar]
- 40.Shahab L, West R. Differences in happiness between smokers, ex-smokers and never smokers: cross-sectional findings from a national household survey. Drug Alcohol Depend. 2012;121(1–2):38–44. doi: 10.1016/j.drugalcdep.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Ouellet-Morin I, Danese A, Williams B, Arseneault L. Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain Behav Immun. 2011;25(4):640–6. doi: 10.1016/j.bbi.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Stepanov I, Sebero E, Wang R, Gao YT, Hecht SS, Yuan JM. Tobacco-specific N-nitrosamine exposures and cancer risk in the Shanghai Cohort Study: remarkable coherence with rat tumor sites. Int J Cancer. 2014;134(10):2278–83. doi: 10.1002/ijc.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990–6. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien KM, Upson K, Cook NR, Weinberg CR. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ Health Perspect. 2016;124(2):220–7. doi: 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - A Practical and Powerful Approach to Multiple Testing. J R Statist Soc, B. 1995;57(1):289–300. [Google Scholar]
- 47.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 48.Dillon MC, Opris DC, Kopanczyk R, Lickliter J, Cornwell HN, Bridges EG, et al. Detection of homocysteine and C-reactive protein in the saliva of healthy adults: comparison with blood levels. Biomark Insights. 2010;5:57–61. doi: 10.4137/bmi.s5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clin Pharmacol Ther. 2005;77(3):145–58. doi: 10.1016/j.clpt.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Fagerstrom KO, Tejding R, Westin A, Lunell E. Aiding reduction of smoking with nicotine replacement medications: hope for the recalcitrant smoker? Tob Control. 1997;6(4):311–6. doi: 10.1136/tc.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl) 2014;231(2):401–7. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- 52.Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shahab L, Dobbie F, Hiscock R, McNeill A, Bauld L. Prevalence and Impact of Long-term Use of Nicotine Replacement Therapy in UK Stop-Smoking Services: Findings From the ELONS Study. Nicotine Tob Res. 2016 doi: 10.1093/ntr/ntw258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shahab L, Beard E, Brown J, West R. Prevalence of NRT Use and Associated Nicotine Intake in Smokers, Recent Ex-Smokers and Longer-Term Ex-Smokers. PLoS One. 2014;9(11):e113045. doi: 10.1371/journal.pone.0113045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–37. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 56.Rose JE, Salley A, Behm FM, Bates JE, Westman EC. Reinforcing effects of nicotine and non-nicotine components of cigarette smoke. Psychopharmacology (Berl) 2010;210(1):1–12. doi: 10.1007/s00213-010-1810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shields PG. Long-term nicotine replacement therapy: cancer risk in context. Cancer Prev Res (Phila) 2011;4(11):1719–23. doi: 10.1158/1940-6207.CAPR-11-0453. [DOI] [PubMed] [Google Scholar]
- 58.Farsalinos KE, Gillman IG, Melvin MS, Paolantonio AR, Gardow WJ, Humphries KE, et al. Nicotine Levels and Presence of Selected Tobacco-Derived Toxins in Tobacco Flavoured Electronic Cigarette Refill Liquids. Int J Environ Res Public Health. 2015;12(4):3439–52. doi: 10.3390/ijerph120403439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schraufnagel DE, Blasi F, Drummond MB, Lam DC, Latif E, Rosen MJ, et al. Electronic cigarettes. A position statement of the forum of international respiratory societies. Am J Respir Crit Care Med. 2014;190(6):611–8. doi: 10.1164/rccm.201407-1198PP. [DOI] [PubMed] [Google Scholar]
- 60.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151(2):362–7. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67–86. doi: 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McNeill A, Brose LS, Calder R, Hitchman S, Hajek P, McRobbie H. E-cigarettes: an evidence update. London, UK: Public Health England; 2015. [DOI] [PubMed] [Google Scholar]
- 63.Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14:18. doi: 10.1186/1471-2458-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacob P, III, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1668–73. [PubMed] [Google Scholar]
- 65.Jain RB. Distributions of selected urinary metabolites of volatile organic compounds by age, gender, race/ethnicity, and smoking status in a representative sample of U.S. adults. Environ Toxicol Pharmacol. 2015;40(2):471–9. doi: 10.1016/j.etap.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 66.Stepanov I, Carmella SG, Briggs A, Hertsgaard L, Lindgren B, Hatsukami D, et al. Presence of the carcinogen N'-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res. 2009;69(21):8236–40. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver VM, Buckley T, Groopman JD. Lack of specificity of trans,trans-muconic acid as a benzene biomarker after ingestion of sorbic acid-preserved foods. Cancer Epidemiol Biomarkers Prev. 2000;9(7):749–55. [PubMed] [Google Scholar]
- 68.Cohen JT, Carlson G, Charnley G, Coggon D, Delzell E, Graham JD, et al. A comprehensive evaluation of the potential health risks associated with occupational and environmental exposure to styrene. J Toxicol Environ Health B Crit Rev. 2002;5(1–2):1–265. doi: 10.1080/10937400252972162. [DOI] [PubMed] [Google Scholar]
- 69.Farsalinos KE, Voudris V, Poulas K. E-cigarettes generate high levels of aldehydes only in 'dry puff' conditions. Addiction. 2015;110(8):1352–6. doi: 10.1111/add.12942. [DOI] [PubMed] [Google Scholar]
- 70.Brown J, West R, Beard E, Michie S, Shahab L, McNeill A. Prevalence and characteristics of e-cigarette users in Great Britain: Findings from a general population survey of smokers. Addict Behav. 2014;39(6):1120–5. doi: 10.1016/j.addbeh.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gregg EO, Minet E, McEwan M. Urinary biomarkers of smokers' exposure to tobacco smoke constituents in tobacco products assessment: a fit for purpose approach. Biomarkers. 2013;18(6):467–86. doi: 10.3109/1354750X.2013.821523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.