Abstract
Obesity is primarily due to food intake in excess of the body's energetic requirements, intake that is not only associated with hunger but also the incentive value of food. The 5-hydroxytryptamine 2C receptor (5-HT2CR) is a target for the treatment of human obesity. Mechanistically, 5-HT2CRs are positioned to influence both homeostatic feeding circuits within the hypothalamus and reward circuits within the ventral tegmental area (VTA). Here we investigated the role of 5-HT2CRs in incentive motivation using a mathematical model of progressive ratio (PR) responding in mice. We found that the 5-HT2CR agonist lorcaserin significantly reduced both ad libitum chow intake and PR responding for chocolate pellets and increased c-fos expression in VTA 5-HT2CR expressing γ-aminobutyric acid (GABA) neurons, but not 5-HT2CR expressing dopamine (DA) neurons. We next adopted a chemogenetic approach using a 5-HT2CRCRE line to clarify the function of subset of 5-HT2C receptor expressing VTA neurons in the modulation of appetite and food-motivated behavior. Activation of VTA 5-HT2C receptor expressing neurons significantly reduced ad libitum chow intake, operant responding for chocolate pellets, and the incentive value of food. In contrast, chemogenetic inhibition of VTA 5-HT2C receptor expressing neurons had no effect on the feeding behavior. These results indicate that activation of the subpopulation of 5-HT2CR neurons within the VTA is sufficient to significantly reduce homeostatic feeding and effort-based intake of palatable food, and that this subset has an inhibitory role in motivational processes. These findings are relevant to the treatment of obesity.
Introduction
The activity of the monoamine neurotransmitter 5-hydroxytryptamine (5-HT; serotonin) within the brain has a functional role in the regulation of feeding behavior and body weight (Blundell, 1986; Hill and Blundell, 1990), and as such has been a target for obesity medications (Halford et al, 2011; Vickers and Clifton, 2012). These effects of 5-HT are primarily achieved via action at the G-protein-coupled 5-HT2C receptor (5-HT2CR) subtype, which is mainly, if not exclusively, expressed within the central nervous system. Activation of 5-HT2CRs promotes weight loss by decreasing food intake (Martin et al, 2011; Higgs et al, 2016) through the modulation of the activity of neuronal pathways regulating energy balance, including those within the arcuate nucleus of the hypothalamus (Burke and Heisler, 2015). However, 5-HT2CRs are also positioned to influence motivational circuits processing rewarding stimuli (Kranz et al, 2010). Specifically, 5-HT2CRs within the ventral tegmental area (VTA) (Bubar and Cunningham, 2007; Bubar et al, 2011) exert an inhibitory effect upon the mesoaccumbens dopamine (DA) system (Di Matteo et al, 2000; Hayes et al, 2009) which has been implicated in motivational processes and effort-related choice (Salamone and Correa, 2013). Collectively, these findings suggest that 5-HT2CRs may modulate feeding not solely by influencing energy homeostasis via the hypothalamus and functionally related structures, but also by regulating appetitive motivation (operant responding).
Several behavioral tasks have been used to assess motivation. One such task is the progressive ratio (PR) schedule, in which the number of responses required per reinforcer is progressively increased (Hodos, 1961). The ratio at which the subject stops responding (the ‘breakpoint') is regarded as a measure of motivation (Richardson and Roberts, 1996). The selective 5-HT2CR agonists lorcaserin ((1R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine) (Meltzer and Roth, 2013), Ro-600175 ((αS)-6-chloro-5-fluoro-α-methyl-1H-indole-1-ethanamine) and CP-809101 (2-(3-chlorobenzyloxy)-6-(piperazin-1-yl)pyrazine); and non-selective 5-HTR agonists with high affinity for 5-HT2CR, such as mCPP (m-chlorophenylpiperazine) and MK-212 (6-chloro-2-(l-piperazinyl)pyrazine) reduce the breakpoint for food (Bezzina et al, 2015; Fletcher et al, 2009; Higgins et al, 2012), cocaine (Burbassi and Cervo, 2008, Harvey-Lewis et al, 2015), ethanol (Rezvani et al, 2014), and nicotine (Fletcher et al, 2012), effects that are blocked by 5-HT2CR antagonists (Bezzina et al, 2015; Fletcher et al, 2012). Thus, lorcaserin, recently approved by the US FDA for the treatment of human obesity, may also have therapeutic potential for nicotine (Higgins et al, 2012; Levin et al, 2011) and ethanol dependence (Rezvani et al, 2014). Although 5-HT2CR activation appears to have an inhibitory role in motivational processes (Filip et al, 2012; Fletcher et al, 2012), the subset of 5-HT2C receptor expressing neurons underlying these effects has yet to be identified.
Here we investigated the role of 5-HT2CRs in food motivation in mice. To this end, we used a mathematical model (Bradshaw and Killeen, 2012) derived from Killeen's general theory of schedule-controlled behavior, the Mathematical Principles of Reinforcement (MPR, Killeen, 1994), that enables the experimenter to discriminate between changes in motor capability and the incentive value of food in PR performance. First, we analyzed the effect of systemic administration of 5-HT2CR agonist lorcaserin, on home cage chow intake and operant responding for chocolate pellets. Next, we characterized the neurons expressing 5-HT2CRs within the VTA and their response to lorcaserin as measured by c-fos immunoreactivity (IR). To investigate the role of VTA 5-HT2C receptor expressing neurons in the modulation of appetite and the food-motivated behavior, we used a chemogenetic approach (designer receptors exclusively activated by a designer drug, DREADD) (Urban and Roth, 2015) to acutely activate or inhibit this population of neurons using a recently developed 5-HT2CRCRE mouse line (Burke et al, 2016). These studies identify the therapeutic potential of the activation of a small subset of 5-HT2C receptor expressing neurons in the reduction of homeostatic feeding and also more broadly in incentive motivation.
Materials and methods
Subjects
5-HT2CRCRE mice were intercrossed with ROSA26-stop-enhanced yellow fluorescent protein (YFP) (B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J; Jackson Laboratory) to create a 5-HT2CRCRE:YPF line (Burke et al, 2016). All the mice were group-housed and maintained on a 12 h light/dark cycle with ad libitum access to water and standard laboratory chow diet, unless otherwise stated. All the experiments were in accordance with guidelines and approvals of the U.K. Animals (Scientific Procedures) Act 1986 or the University of Michigan Committee on the Use and Care of Animals.
Drugs
Lorcaserin HCl (3, 7, 10 or 12 mg/kg; LGM Pharma, Nashville, TN) and clozapine-N-oxide (CNO) (2 mg/kg; Sigma-Aldrich, Gillingham, UK) were dissolved in 0.9% NaCl and injected intraperitoneally (i.p.) at a volume of 10 ml/kg body weight.
Stereotaxic Viral Vector Injection
Male and female 5-HT2CRCRE:YFPmice were injected bilaterally with 0.25 μl AAV-hSyn-DIO-hM3D(Gq)-mCherry, AAV-hSyn-DIO-hM4D(Gi)-mCherry or AAV-DIO-mCherry (University of North Carolina Vector Core Facilities, Chapel Hill, NC) into the VTA (stereotaxic coordinates, millimeters from bregma: antero-posterior −3.16 medio-lateral ±0.56; dorso-ventral −4.5) using a stereotaxic frame (see Supplementary Information for further details). We did not observe significant sex differences between treatment responses to food intake (5-HT2CRCRE:YPF::hM3Dq-mCherryVTA: n=14; F1,10=2.78; NS; 5-HT2CRCRE:YPF::hM4Di-mCherryVTA: n=14; F1,10=1.12; NS) or the breakpoint in the ad libitum fed (5-HT2CRCRE:YPF::hM3Dq-mCherryVTA: n=15; F1,11=1.36; NS; 5-HT2CRCRE:YPF::hM4Di-mCherryVTA: n=29; F1,25=0.440; NS) or food-deprived conditions (5-HT2CRCRE:YPF::hM3Dq-mCherryVTA: n=15; F1,11=0.216; NS; 5-HT2CRCRE:YPF::hM4Di-mCherryVTA: n=30; F1,26=0.533; NS) and thereby combined data with male and female mice.
Operant Conditioning
The mice were individually housed and trained on a PR schedule based on an exponential progression derived from the formula (5 × e0.2n)−5, rounded to the nearest integer, where n is the position in the ratio sequence (Richardson and Roberts, 1996; see Supplementary Information for further details). The breakpoint was defined as the last ratio completed before 5 min elapsed without any responding or, when this criterion was not met within the session, the highest completed ratio (Olarte-Sanchez et al, 2012). The mathematical model (Bradshaw and Killeen, 2012) used to analyze PR performance comprises three key equations. The parameters of these equations provide separate numerical indices of the motivational impact of the reinforcer and the motor capability of the organism. The linear waiting equation (Wynne et al, 1996) is used to predict the post-reinforcement pause in each ratio (TP,i) from the time taken to complete the preceding ratio (total time, TTOT,i−1):
where T0 is the initial post-reinforcement pause and k is the slope of the linear waiting function. Two further equations define running response rate, RRUN, and overall response rate, ROVERALL, in successive ratios of the schedule:
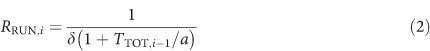 |
The parameters δ and a are the fundamental ‘motor' and ‘motivational' parameters of the model: δ expresses the minimum time needed to execute a response, and a the duration of behavioral activation induced by a single reinforcer (Bradshaw and Killeen, 2012; Killeen, 1994; see Supplementary Information for further details).
Food Intake
The mice were individually housed and habituated for 5 days in TSE Phenomaster chambers (TSE, Bad Homburg, Germany) that record food intake automatically using weight sensors attached to food containers suspended from the ceiling. On each treatment day, 30 min before the onset of the dark cycle, the food hopper was automatically closed and the mice were injected with treatment (saline, lorcaserin or CNO). At the onset of the dark cycle, the food hopper was opened and chow intake was recorded for the next hour.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on coronally sectioned 4% paraformaldehyde fixed brain tissue using methods previously reported (Burke et al, 2016) using the following primary antibodies to detect immunoreactivity (IR) for c-fos (#2672548, 1:5000, Millipore) or immunofluorescence (IF) for 5-HT2CR (#L1813, 1:300; Santa Cruz Biotechnology), c-fos (#L1610; 1:800, Santa Cruz Biotechnology), γ-aminobutyric acid (GABA) synthesizing enzyme glutamic acid decarboxylase (GAD; #J2804; 1:150, Santa Cruz Biotechnology), the dopamine (DA) synthesizing enzyme tyrosine hydroxylase (TH; #2716631; 1:1000, Millipore), green fluorescent protein (GFP; #GR279236-1; 1: 800, Abcam), or mCherry (#31089; 1:800, Rockland). Images were acquired using an Axioskop II microscope (Carl Zeiss, Germany), processed with Adobe Photoshop (Adobe Systems Software, Ireland) and analyzed with Image J software (NIH). For IHC quantification analysis, the VTA was defined using the Mouse Brain Atlas (Paxinos and Franklin, 2001) and sections containing the VTA (from bregma −2.92 to −3.88 mm) were counted bilaterally (see Supplementary Information for further details).
Electrophysiology
Egith-to-twelve–week-old 5-HT2CRCRE:YFP mice were deeply and terminally anesthetized with sodium pentobarbital and then decapitated. The brain was rapidly removed and placed in an ice-cold oxygenated (95%O2/5%CO2) high sucrose ‘slicing' solution. Coronal slices containing the VTA were prepared and immediately transferred to a ‘recording' solution in a continuously oxygenated holding chamber at 35°C for a period of 25 min. Subsequently, the slices were allowed to recover in the ‘recording' solution at room temperature for a minimum of 1 h before recording. All patch clamp recordings were made using multiclamp 700B amplifier, and the data were filtered at 2 kHz and digitized at 10 kHz (see Supplementary Information for further details).
Statistics
For lorcaserin treatment PR and food intake experiments, the weight of the food consumed, the estimates derived from the mathematical model and the breakpoint were analyzed using analysis of variance (ANOVA) with repeated measures for treatment (lorcaserin dose) with Dunnett's post hoc test. For the chemogenetic food intake and PR experiments, the weight of the food consumed, estimates derived from the mathematical model and the breakpoint were analyzed using ANOVA (group [AAV-hM3D(Gq)), AAV-mCherry] × treatment [vehicle, CNO]) or (group [AAV-hM4D(Gi)), AAV-mCherry] × treatment [vehicle, CNO]) with repeated measures for treatment, and post hoc comparisons using Tukey's test. A significance criterion of p<0.05, two-tailed, was adopted in all the statistical analyses.
Results
5-HT2CR Agonist Lorcaserin Reduces Food Intake and Decreases Operant Responding for Food Reward
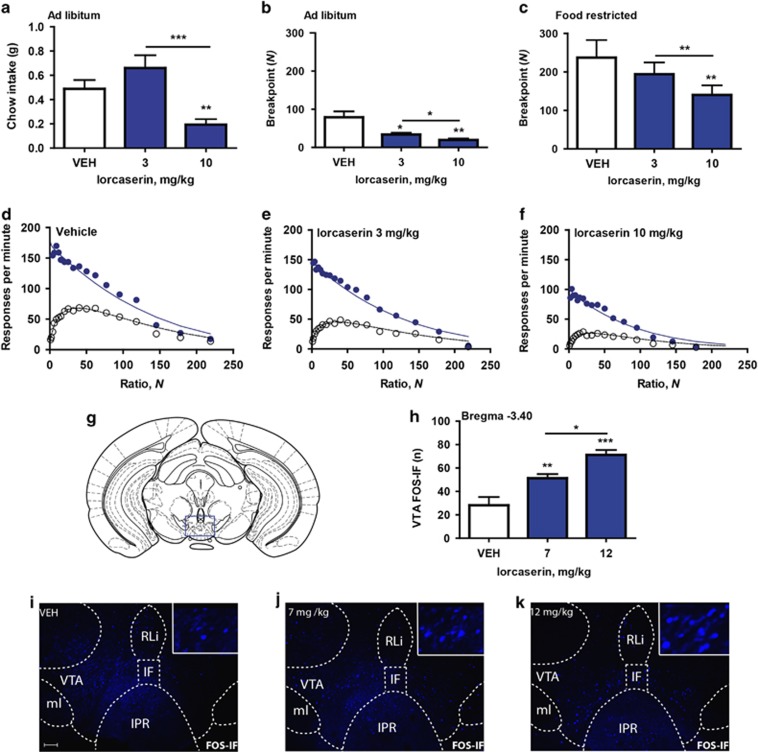
Consistent with previous reports (Fletcher et al, 2009; Thomsen et al, 2008; Burke et al, 2014), lorcaserin significantly reduced home cage ad libitum chow intake compared with saline (n=14; F2,26=11.76; p<0.001; Figure 1a). To assess the effect of lorcaserin on food-motivated behavior, the mice were trained on a PR schedule and tested during two conditions: ad libitum fed and food restricted. In the ad libitum fed condition, lorcaserin 3 and 10 mg/kg significantly reduced the breakpoint compared with vehicle (n=9; F2,16=11.76; p<0.001; Figure 1b). The breakpoint was also reduced by 10 mg/kg lorcaserin during the food restricted condition (n=9; F2,16=10.99; p<0.01; Figure 1c). Further analysis of performance on the PR schedule during the food-restricted condition indicated that RRUN declined monotonically towards zero, whereas ROVERALL rose to a peak before declining towards zero. In accordance with the mathematical model, response rates conformed closely to equations (2) and (3) (R2=0.981 [vehicle], 0.990 [lorcaserin 3 mg/kg], and 0.978 [lorcaserin 10 mg/kg]) and post-reinforcement pause duration in successive ratios was linearly related to the prior inter-reinforcer interval, as indicated by values of R2 above 0.9 (Figures 1d–f). Lorcaserin 3 and 10 mg/kg significantly increased the value of k (n=9; F2,16=14.46; p<0.0001; Supplementary Figure S1D), while having no significant effect on the ‘motivational' parameter, a, (n=9; F2,16=0.31; NS; Supplementary Figure S1A) or T0 (n=9; F2,16=0.93; NS; Supplementary Figure S1C). The ‘motor' parameter, δ, was increased by lorcaserin, the effect of 10 mg/kg being statistically significant (n=9; F2,16=19.6; p<0.0001; Supplementary Figure S1B). These data suggest that lorcaserin significantly reduces operant responding for food reward by impacting motor processes.
Figure 1.
Effect of lorcaserin on food intake and operant responding. (a) Lorcaserin 10 mg/kg reduced 1 h chow intake (n=14). (b) Lorcaserin 3 and 10 mg/kg reduced the breakpoint when mice were in ad libitum condition (n=9). (c) Lorcaserin 10 mg/kg reduced the breakpoint when mice were in food-restricted condition (n=9). (d–f) Effect of lorcaserin on performance on the progressive-ratio schedule. Ordinate, response rate; abscissa, response/reinforcer ratio, N. Points are group mean data: unfilled symbols indicate running response rate, filled symbols indicate overall response rate. The curves are best-fit functions defined by equations (2) and (3). (g) Schematic of the location of the VTA defined by the Mouse Brain Atlas (Paxinos and Franklin, 2001). (h) Lorcaserin 7 and 12 mg/kg increased FOS-IF (blue) within the VTA (n=6–7 per group). (i–k) FOS-IF (blue) within the VTA following administration of vehicle, lorcaserin 7 or 12 mg/kg (scale bar: 100 μm). All the data are presented as mean±SEM. ∗p<0.05; ∗∗p<0.01; ∗∗∗p<0.001.
Lorcaserin Increases the Activity of VTA Neurons
The VTA is a structure involved in motivation, food intake and motor function. To determine whether lorcaserin influences the activity of VTA neurons, the mice were treated with vehicle or lorcaserin (7 or 12 mg/kg) and brains were processed for FOS-IF. First, it was confirmed that 7 or 12 mg/kg of lorcaserin reduced chow intake (n=7–8 per group; F2,19=8.12; p<0.001; Supplementary Figure S2A) and the breakpoint in both the ad libitum (n=25; F2,48=41.65; p<0.0001; Supplementary Figure S2B) and food-restricted conditions (n=25; F2,48=23.58; p<0.0001; Supplementary Figure S2C). Next, we assessed VTA FOS-IF and observed that lorcaserin significantly increased FOS-IF compared with vehicle (n=6–7 per group; F2,17=17.27; p<0.0001; Figures 1g–k). These data indicate that doses of lorcaserin that reduced food intake and operant responding increase the activity of VTA neurons.
Lorcaserin Increases the Activity of VTA 5-HT2CR Expressing GABA-Ergic Neurons
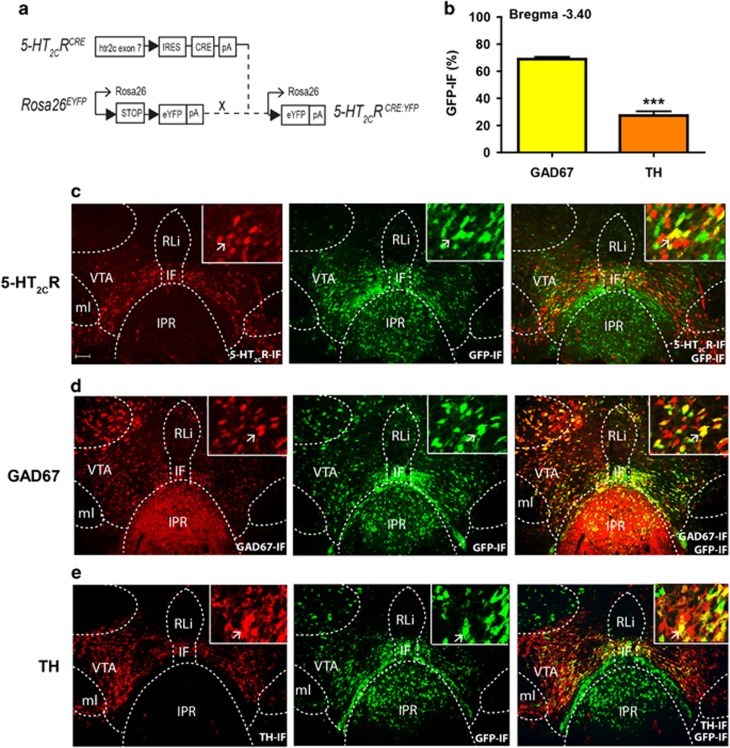
To characterize the neurochemical identity of 5-HT2CR expressing VTA neurons, we intercrossed a 5-HT2CRCRE line with a ROSA26EYFP line (Figure 2a) to generate 5-HT2CRCRE:YPFmice. In agreement with previous reports of endogenous 5-HT2CR expression (Bubar and Cunningham, 2007; Bubar et al, 2011), we observed that 5-HT2CRCRE:YFPpositive cells were widely expressed within the VTA and co-localized with 5-HT2CR-IF (Figure 2c). To explore the neurochemical phenotype of VTA 5-HT2CR expressing neurons, we performed dual-IHC for GPF (5-HT2CR) and GAD67 (GABA) or TH (dopamine). In line with previous reports using a 5-HT2CR antibody (Bubar and Cunningham, 2007; Bubar et al, 2011), we found that 5-HT2CRCRE:YPFis expressed with both GAD67 and TH neurons (Figures 2d and e). Specifically, we observed that the majority of VTA 5-HT2CRCRE:YPFexpressing neurons were GAD67 positive (69%), whereas a significantly smaller percentage co-expressed TH (27%) (n=3 per group; t(4)=11.69; p<0.001; Figure 2b and Supplementary Figure S3). These data suggest that 5-HT2CRs are predominantly positioned to influence the activity of GABAergic VTA neurons.
Figure 2.
5-HT2CRCRE:YFP VTA neurons co-express GABA and dopamine. (a) A 5-HT2CRCRE mouse line in which Cre recombinase is driven by a 5-HT2CR promoter was intercrossed with a ROSA26-stop-EYFP reporter mouse to generate a 5-HT2CRCRE:YFP line to facilitate the visualization of VTA 5-HT2CR expressing cells. (b) VTA 5-HT2CRCRE:YFP containing neurons (GFP-IF) have a greater co-expression with GAD67-IF compared with TH-IF (n=4 per group, p<0.001). (c) VTA 5-HT2CRCRE:YFP (GFP-IF; green) are co-localized (overlay, yellow) with endogenous 5-HT2CR-IF (red). (d) VTA 5-HT2CRCRE:YFP (GFP-IF; green) containing neurons and GAD67 (red) are co-localized (yellow). (e) VTA 5-HT2CRCRE:YFP (GFP-IF; green) containing neurons and TH (red) are co-localized (yellow). All the data are presented as mean±SEM. ∗∗∗p<0.001. Scale bar: 100 μm.
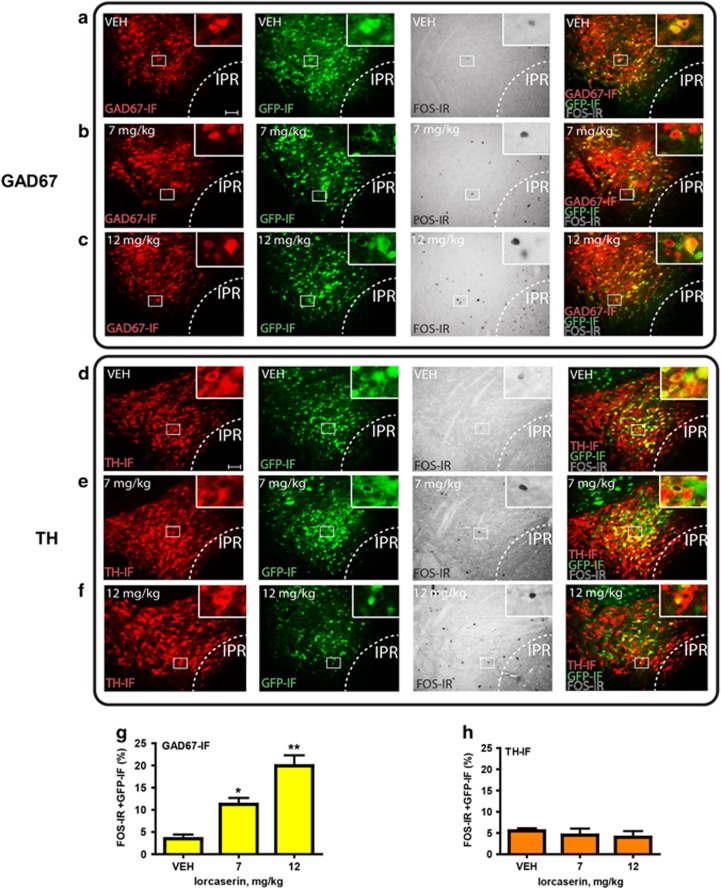
We next evaluated the neurochemical phenotype of 5-HT2CR expressing neurons responsive to lorcaserin treatment. 5-HT2CRCRE:YFP mice were treated with vehicle or lorcaserin (7 or 12 mg/kg) and tissue processed for triple-IHC for GFP, FOS and GAD67 or TH. Lorcaserin significantly and dose-dependently increased the activity (FOS-IR) of GAD67-positive 5-HT2CR expressing VTA neurons (n=3 per group; F2,6=23.3; p<0.001; Figures 3a–c and g), but had no effect on TH-positive 5-HT2CR expressing VTA neurons (n=4 per group; F(2,9)=0.52; NS; Figures 3d–f and h). These findings suggest that lorcaserin at doses that reduce food intake and PR responding for food reward increase the activity of VTA GABAergic, but not dopaminergic neurons.
Figure 3.
Lorcaserin increases c-Fos in VTA 5-HT2CR GABAergic, but not dopamine neurons. (a-c) c-Fos expression (FOS-IR, gray) in VTA 5-HT2CR containing neurons (GFP-IF, green) co-expressing GAD67 (GAD67-IF, red) in 5-HT2CRCRE:YFP mice following (a) vehicle, (b) lorcaserin 7 mg/kg or (c) 12 mg/kg administration. (d–f) c-Fos expression (FOS-IR, gray) in VTA 5-HT2CR containing neurons (GFP-IF, green) co-expressing TH (TH-IF, red) in 5-HT2CRCRE:YFP mice following (d) vehicle, (e) lorcaserin 7 mg/kg or (f) 12 mg/kg administration. (g) Lorcaserin dose-dependently increases FOS-IR in GAD67-positive 5-HT2CR expressing neurons (n=4 per group, p<0.001), but not in (h) TH-positive 5-HT2CR expressing neurons (n=4 per group, NS). All the data are presented as mean±SEM. ∗p<0.05; ∗∗p<0.01. Scale bar: 50 μm.
Chemogenetic Activation of VTA 5-HT2CR Expressing Neurons Reduce Food Intake and Operant Responding
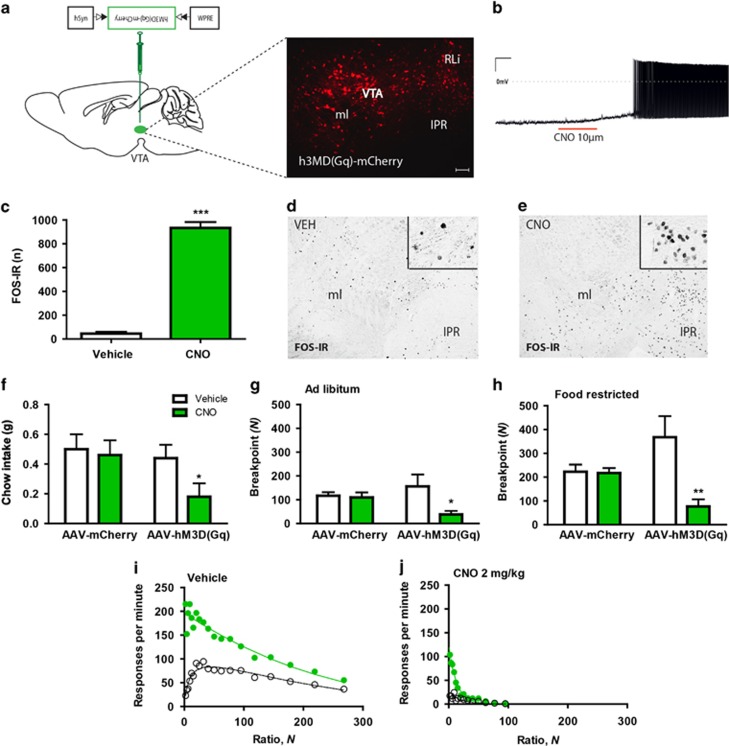
We next considered whether selective activation or inhibition of VTA 5-HT2CR expressing neurons was able to influence ad libitum food intake or operant responding for food reward. To investigate this, 5-HT2CRCRE:YPFmice received bilateral VTA injections with AAVs that mediate the Cre-dependent expression of designer receptors exclusively activated by designer drugs (DREADDs; expressed as DREADD-mCherry fusion proteins, hM3Dq; 5-HT2CRCRE:YPF::hM3Dq-mCherryVTA) (Figure 4a). DREADDs are designer muscarinic receptor variants that can only be activated by an otherwise biologically inert designer drug, CNO (Alexander et al, 2009). We first confirmed that DREADD-fused mCherry reporter protein was expressed in the VTA (Figure 4a). Next, we demonstrated that CNO activated 5-HT2CRCRE:YPF::hM3Dq-mCherryVTA cells with whole-cell electrophysiological recordings in ex vivo VTA slices (n=4/4, 100% responsive; Figure 4b) and in vivo using FOS-IR (n=3 per group; t(4)=17.45; p<0.0001; Figures 4c–e).
Figure 4.
Chemogenetic activation of VTA 5-HT2CR neurons reduces food intake and operant responding for food reward. (a) Representative image of Cre-dependent expression of hM3Dq-mCherry specifically within the VTA of a 5-HT2CRCRE:YFP mouse. (b) Firing rate of 5-HT2CRCRE:YPF::hM3Dq-mCherryVTA neurons increased upon 10 μM CNO application. (c–e) CNO 2 mg/kg increased FOS-IR within 5-HT2CRCRE:YPF::hM3Dq-mCherryVTA compared with vehicle (n=3 per group). (f) CNO 2 mg/kg reduced 1 h chow intake (n=14, p<0.05). (g) CNO 2 mg/kg reduced the breakpoint in the experimental group [AAV-hM3D (Gq)] compared with control [AAV-mCherry] in ad libitum condition (n=15) and (h) food-restricted condition (n=15). (i and j) Effect of chemogenetic activation of VTA 5-HT2CR neurons on performance on a PR schedule. Ordinate, response rate; abscissa, response/reinforcer ratio, N. Points are group mean data: unfilled symbols indicate running response rate, filled symbols indicate overall response rate. The curves are best-fit functions defined by equations (2) and (3). All the data are presented as mean±SEM. ∗p<0.05; ∗∗p<0.01; ∗∗∗p<0.001. Scale bar: 100 μm.
Subsequently, we examined the effect of chemogenetic activation of VTA 5-HT2CR expressing neurons on feeding behavior. The administration of CNO significantly reduced ad libitum chow intake compared with vehicle in the home cage in 5-HT2CRCRE:YPF::hM3Dq-mCherryVTA mice (n=14; F1,12=5.47; p<0.05; Figure 4f). These data suggest that activation of VTA 5-HT2CR expressing neurons is sufficient to significantly reduce food intake. To assess the effect of activation of VTA 5-HT2CR expressing neurons on food-motivated behavior, 5-HT2CRCRE:YPF::hM3Dq-mCherryVTA mice were trained on a PR schedule. The administration of CNO significantly reduced the breakpoint in both the ad libitum fed (n=15; F1,13=5.11; p<0.05; Figure 4g) and food-restricted (n=15; F1,13=21.5, p<0.001; Figure 4h) conditions in 5-HT2CRCRE:YPF::hM3Dq-mCherryVTA mice compared with vehicle treatment. Further analysis revealed that RRUN declined monotonically towards zero, whereas ROVERALL rose to a peak before declining towards zero. In accordance with the mathematical model, response rates conformed closely to equations (2) and (3) (control group [AAV-mCherry]: R2=0.960 [vehicle], 0.947 [CNO 2 mg/kg] experimental group [AAV-hM3D (Gq)]: R2=0.961 [vehicle], 0.979 [CNO 2 mg/kg]) (Figures 4i–j). Quantitative analysis indicated a reduction of a (n=15; F1,13=6.6, p<0.05), but no change in δ (n=15; F1,13= 1.70; NS). These results are consistent with a reduction in the incentive value of the reinforcer without a concomitant impairment of motor competence (Supplementary Figure S2A and B). Activation of VTA 5-HT2CR expressing neurons significantly increased the value of k (n=15; F1,13=12.6; p<0.01; Supplementary Figure S2D), while having no effect on T0 (n=15; F1,13=1.3; NS; Supplementary Figure S2C). Collectively, these data indicate that the selective activation of VTA 5-HT2CR expressing neurons is sufficient to significantly reduce home cage food intake and operant responding for food reward, without altering motor performance.
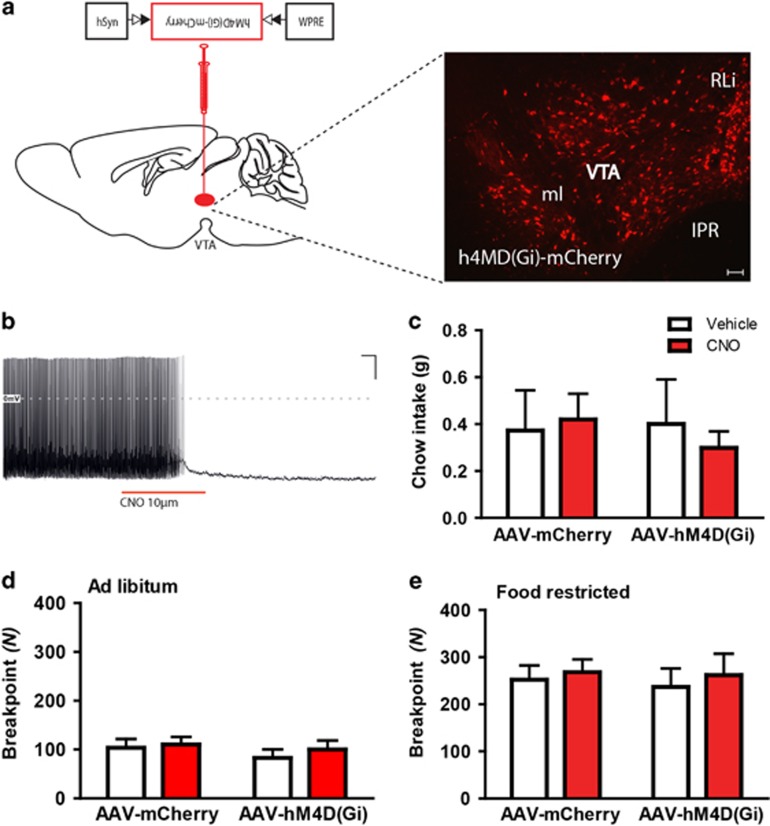
To investigate whether VTA 5-HT2CR expressing neurons are required for normal food intake and reward processing, we chemogenetically silenced VTA 5-HT2CR expressing cells. Specifically, 5-HT2CRCRE:YPFmice received bilateral VTA injections with AAVs that mediate the Cre-dependent expression of inhibitory (Gi) DREADD-mCherry fusion protein, hM4Di; 5-HT2CRCRE:YPF::hM4Di-mCherryVTA (Figure 5a). First, we performed patch-clamp recordings in ex vivo 5-HT2CRCRE:YPF::hM4Di-mCherryVTA slices to confirm the inhibitory effect of CNO on cell activity. Bath application of CNO reduced firing rate in 8/8 5-HT2CRCRE:YPF::hM4Di-mCherryVTA expressing neurons (Figure 5b). The effect of CNO administration on homeostatic food intake and food-motivated behavior in 5-HT2CRCRE:YPF::hM4Di-mCherryVTA mice was then assessed. Treatment with CNO had no effect on home cage chow intake (n=14; F1,12= 0.33; NS; Figure 5c) or PR responding for food reward in ad libitum (n=29; F1,27= 1.93; NS; Figure 5d) or food-restricted conditions (n=30; F1,28=0.05; NS; Figure 5e) compared with vehicle.
Figure 5.
Chemogenetic inhibition of VTA 5-HT2CR neurons does not alter food intake or operant responding for food reward. (a) Representative image of Cre-dependent expression of hM4D(Gi)-mCherry specifically within the VTA of a 5-HT2CRCRE:YFP mouse. (b) Firing rate of 5-HT2CRCRE:YPF::hM4Di-mCherryVTA neurons decreased upon 10 μM CNO application. (c) CNO 2 mg/kg had no effect on 1 h chow intake (n=14). (d) CNO 2 mg/kg had no effect on the breakpoint in the experimental group [AAV-hM4D (Gi)] compared with the control [AAV-mCherry] in ad libitum condition (n=29) or (e) food-restricted condition (n=30).
Though this chemogenetic approach suggests that reducing the activity of VTA 5-HT2CR expressing neurons via 5-HT2CRCRE:YPF::hM4Di-mCherryVTA is not sufficient to alter normal food intake or motivation for food reward, targeted genetic knockdown of VTA 5-HT2CRs is required to establish the specific function of VTA 5-HT2CRs in food intake and motivation for food reward. However, of translational relevance to human obesity treatment, we observed that activation of this discrete subset of VTA neurons is sufficient to produce a significant effect on multiple types of feeding behavior. These include, ad libitum food intake in the home cage and the PR task where the incentive value of food reward is manipulated by food-deprivation levels. Activation of this small subset of 5-HT2CR expressing neurons produced a consistent reduction in the feeding behavior that was not associated with changes in motor performance, suggesting that the effect is directly related to incentive motivation.
Discussion
Due to its prevalence and deleterious health consequences, obesity is one of the primary global healthcare challenge of the 21st century. Discerning a new means to combat the obesity epidemic is therefore of paramount clinical importance. Obesity is primarily due to the consumption of food in excess of the body's energetic requirements, energy that is then stored as fat. 5-HT, via action at 5-HT2CRs, produces a potent inhibitory effect on the feeding behavior. Capitalizing on this effect, the 5-HT2CR agonist lorcaserin, an obesity medication recently approved by the US FDA, reduces body weight by decreasing ad libitum food intake (Martin et al, 2011) and promoting satiety (Higgs et al, 2016).
However, as 5-HT2CRs are expressed throughout the brain, precisely where and how lorcaserin exerts its effects upon feeding behavior has not been fully defined. Here we investigated a specific subset of 5-HT2CR expressing neurons that are sufficient to reduce food intake and moreover, food-motivated behavior. In particular, we were interested in food motivation because the drive or ‘motivation' to consume food in the absence of hunger is a primary behavior underpinning the development of obesity. Targeting and reducing this specific aspect of feeding behavior would be particularly attractive for the treatment of obesity.
Obesity Medication Lorcaserin Reduces Food Intake and Operant Responding for Food Reward
Previous research has focussed on the role of 5-HT2CRs in the modulation of homeostatic feeding by influencing the activity of the hypothalamic melanocortin system (Burke et al, 2016; Doslikova et al, 2013; Heisler et al, 2002; Lam et al, 2008; Xu et al, 2008). Here we observed that lorcaserin produced the expected reduction in home cage ad libitum intake of standard laboratory chow. Given that 5-HT2CRs are also expressed in the VTA (Bubar and Cunningham, 2007; Bubar et al, 2011), a brain region associated with food and drug reward (DiLeone et al, 2012), we investigated lorcaserin's effects on a PR schedule using a mathematical model (Bradshaw and Killeen, 2012) derived from Killeen's Mathematical Principles of Reinforcement (MPR, Killeen, 1994). In agreement with previous reports in rats (Bezzina et al, 2015; Bradshaw and Killeen, 2012; Olarte-Sanchez et al, 2013, 2015; Valencia-Torres et al, 2014), our results indicate that operant behavior maintained by PR schedules is well described by the mathematical model in mice (Bradshaw and Killeen, 2012). Progressive ratio schedules are widely used to assess the effects of neuropharmacological interventions on motivation. This practice is based on the assumption that the traditional index of performance, the breakpoint, provides a quantitative index of motivation, or the incentive value of the reinforcer (Hodos, 1961; see Killeen et al, 2009). However, there is considerable evidence that the breakpoint is sensitive to motor as well as motivational factors (Aberman et al, 1998; Skjoldager et al, 1993). The mathematical model incorporates separate parameters that represent motivational and motor processes (a and δ, respectively); a change in the value of either parameter may give rise to a change in the breakpoint (Bradshaw and Killeen, 2012). Our findings indicated that systemically administered lorcaserin increased the value of δ without affecting a. The present finding is consistent with the results of Bezzina et al (2015) that showed that a reduction of the breakpoint induced by 5-HTR agonists mCPP and Ro-600175 was associated not with a reduction of the value of a, but with an increase of the value of δ, which could be reversed by 5-HT2CR antagonist SB-242084. These findings suggest that systemic treatment with 5-HT2CR agonists suppress operant performance via a detrimental effect on motor processes, thereby confounding interpretations of effects on motivational processes.
Lorcaserin Increases the Activity of VTA 5-HT2CR GABAergic Neurons
Given that 5-HT2CRs are expressed in multiple brain regions where they are positioned to perform different functions (including motor functions), we were interested in discerning a specific subset of 5-HT2CRs capable of modulating food motivation without interfering with motor function. We observed that doses of lorcaserin that reduce food intake and PR responding increase the activity of VTA neurons. As the VTA neuronal population is phenotypically heterogeneous, we characterized the neurochemical phenotype of 5-HT2CR expressing neurons and their response to lorcaserin using of a new mouse line (5-HT2CRCRE:YFP; Burke et al, 2016). We observed that VTA 5-HT2CR expressing neurons were mainly co-expressed with GABA and a much smaller percentage were co-expressed with dopamine. Consistent with this anatomical localization, lorcaserin significantly and dose-dependently increased the activity of GABA-ergic neurons. This is consistent with the notion that activation of 5-HT2C receptor expressing neurons of the VTA predominantly stimulates GABA-ergic neurons thereby suppressing the dopaminergic output of this structure (Filip et al, 2012; Navailles et al, 2008).
Selective Chemogenetic Activation of VTA 5-HT2CR Expressing Neurons Reduces Food Intake and Operant Responding for Food Reward
Next, using a chemogenetic approach (Urban and Roth, 2015), we investigated the specific function of 5-HT2CR expressing cells within the VTA. Here we observed that selective activation of VTA 5-HT2C receptor expressing neurons was sufficient to significantly reduce ad libitum chow intake (59%) to a degree comparable with systemic lorcaserin treatment (60%).
Next, we explored the role of VTA 5-HT2C receptor expressing neurons in food-motivated behavior. We observed that chemogenetic activation of VTA 5-HT2C receptor expressing neurons caused suppression of responding in the PR schedule and a reduction in the breakpoint both when mice were food restricted and fed ad libitum. Further quantitative analysis revealed a reduction of a but no change in δ, consistent with a reduction of the incentive value of the reinforcer with no concomitant impairment of motor competence. This is in line with previous research that demonstrated that 5-HT2CRs within the VTA exert an inhibitory influence upon the mesoaccumbens dopamine (DA) system (Navailles et al, 2008) attenuating the effort-based intake of food (Salamone and Correa, 2013). Systemic administration of lorcaserin may engage additional systems that mask the specific motivational effect seen with acute stimulation of VTA 5-HT2C receptor expressing neurons. For example, 5-HT2CRs are expressed in several structures within the basal ganglia, where they exert influence over extrapyramidal motor functions (Graves et al, 2013; Miguelez et al, 2014).
Although activation of VTA 5-HT2CR expressing neurons produced a potent reduction in the feeding behavior, inhibition of these neurons did not alter food intake or PR responding for food reward. Targeted genetic knockdown studies of VTA 5-HT2CRs are required to establish the necessity of this subpopulation on the regulation of food intake and motivation for food reward.
Summary
Here we identify a new subpopulation of 5-HT2CRs that are capable of reducing homecage food intake and food-motivated behavior without interfering with motor function, findings that reveal a new site of 5-HT2CR activation to pharmacologically exploit in the development of novel obesity medication. Given that one of the primary factors in the development of human obesity is enhanced motivation to consume food, these findings are particularly germane to the development of new selective medications to abate the obesity epidemic.
FUNDING AND DISCLOSURE
The research was funded by Wellcome Trust (WT098012) to LKH; and National Institute of Health (DK056731) and the Marilyn H. Vincent Foundation to MGM. The University of Michigan Transgenic Core facility is partially supported by the NIH-funded University of Michigan Center for Gastrointestinal Research (DK034933). The remaining authors declare no conflict of interest.
Acknowledgments
We thank Dr Celine Cansell, Ms Raffaella Chianese and the staff of the Medical Research Facility for technical assistance. We thank Dr Vladimir Orduña for the scientific advice and technical assistance.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Aberman JE, Ward SJ, Salamone JD (1998). Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav 61: 341–348. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA et al (2009). Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzina G, Body S, Cheung TH, Hampson CL, Bradshaw CM, Glennon JC et al (2015). Evidence for a role of 5-HT2C receptors in the motor aspects of performance, but not the efficacy of food reinforcers, in a progressive ratio schedule. Psychopharmacology 232: 699–711. [DOI] [PubMed] [Google Scholar]
- Blundell JE (1986). Serotonin manipulations and the structure of feeding behaviour. Appetite 7 Suppl: 39–56. [DOI] [PubMed] [Google Scholar]
- Bradshaw CM, Killeen PR (2012). A theory of behaviour on progressive ratio schedules, with applications in behavioural pharmacology. Psychopharmacology 222: 549–564. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA (2007). Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience 146: 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Stutz SJ, Cunningham KA (2011). 5-HT(2C) receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS One 6: e20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbassi S, Cervo L (2008). Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology 196: 15–27. [DOI] [PubMed] [Google Scholar]
- Burke LK, Doslikova B, D'Agostino G, Greenwald-Yarnell M, Georgescu T, Chianese R et al (2016). Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol Metab 5: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LK, Heisler LK (2015). 5-hydroxytryptamine medications for the treatment of obesity. J Neuroendocrinol 27: 389–398. [DOI] [PubMed] [Google Scholar]
- Burke LK, Doslikova B, D'Agostino G, Garfield AS, Farooq G, Burdakov D et al (2014). 5-HT obesity medication efficacy via POMC activation is maintained during aging. Endocrinology 155: 3732–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Taylor JR, Picciotto MR (2012). The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci 15: 1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E (2000). Biochemical and electrophysiological evidence that RO 60-0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors. Brain Res 865: 85–90. [DOI] [PubMed] [Google Scholar]
- Doslikova B, Garfield AS, Shaw J, Evans ML, Burdakov D, Billups B et al (2013). 5-HT2C receptor agonist anorectic efficacy potentiated by 5-HT1B receptor agonist coapplication: an effect mediated via increased proportion of pro-opiomelanocortin neurons activated. J Neurosci 33: 9800–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Spampinato U, McCreary AC, Przegalinski E (2012). Pharmacological and genetic interventions in serotonin (5-HT)(2C) receptors to alter drug abuse and dependence processes. Brain Res 1476: 132–153. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Noble K, Soko AD, Silenieks LB, Le AD et al (2012). Effects of the 5-HT2C receptor agonist Ro60-0175 and the 5-HT2A receptor antagonist M100907 on nicotine self-administration and reinstatement. Neuropharmacology 62: 2288–2298. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA (2009). Characterizing the effects of 5-HT(2C) receptor ligands on motor activity and feeding behaviour in 5-HT(2C) receptor knockout mice. Neuropharmacology 57: 259–267. [DOI] [PubMed] [Google Scholar]
- Graves SM, Viskniskki AA, Cunningham KA, Napier TC (2013). Serotonin(2C) receptors in the ventral pallidum regulate motor function in rats. Neuroreport 24: 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Boyland EJ, Lawton CL, Blundell JE, Harrold JA (2011). Serotonergic anti-obesity agents: past experience and future prospects. Drugs 71: 2247–2255. [DOI] [PubMed] [Google Scholar]
- Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ (2015). The 5-HT receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology 101: 237–245. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Mosher TM, Greenshaw AJ (2009). Differential effects of 5-HT2C receptor activation by WAY 161503 on nicotine-induced place conditioning and locomotor activity in rats. Behav Brain Res 197: 323–330. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL et al (2002). Activation of central melanocortin pathways by fenfluramine. Science 297: 609–661. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD et al (2012). The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 37: 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs S, Cooper AJ, Barnes NM (2016). The 5-HT2C receptor agonist, lorcaserin, and the 5-HT6 receptor antagonist, SB-742457, promote satiety; a microstructural analysis of feeding behaviour. Psychopharmacology 233: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AJ, Blundell JE (1990). Sensitivity of the appetite control system in obese subjects to nutritional and serotoninergic challenges. Int J Obes 14: 219–233. [PubMed] [Google Scholar]
- Hodos W (1961). Progressive ratio as a measure of reward strength. Science 134: 943–944. [DOI] [PubMed] [Google Scholar]
- Killeen PR (1994). Mathematical principles of reinforcement. Behav Brain Sci 17: 105–172. [Google Scholar]
- Killeen PR, Posadas-Sanchez D, Johansen EB, Thrailkill EA (2009). Progressive ratio schedules of reinforcement. J Exp Psychol Anim B 35: 35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R (2010). Reward and the serotonergic system. Neuroscience 166: 1023–1035. [DOI] [PubMed] [Google Scholar]
- Lam DD, Przydzial MJ, Ridley SH, Yeo GSH, Rochford JJ, O'Rahilly S et al (2008). Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology 149: 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A et al (2011). Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther 338: 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Redman LM, Zhang J, Sanchez M, Anderson CM, Smith SR et al (2011). Lorcaserin, A 5-HT2C receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. J Clin Endocr Metab 96: 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Roth BL (2013). Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeteddrugs. J Clin Invest 123: 4986–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguelez C, Morera-Herreras T, Torrecilla M, Ruiz-Ortega JA, Ugedo L (2014). Interaction between the 5-HT system and the basal ganglia: functional implication and therapeutic perspective in Parkinson's disease. Front Neural Circuits 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, Moison D, Cunningham KA, Spampinato U (2008). Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology33: 237–246.. [DOI] [PubMed]
- Olarte-Sanchez CM, Valencia-Torres L, Cassaday HJ, Bradshaw CM, Szabadi E (2013). Effects of SKF-83566 and haloperidol on performance on progressive ratio schedules maintained by sucrose and corn oil reinforcement: quantitative analysis using a new model derived from the Mathematical Principles of Reinforcement (MPR). Psychopharmacology 230: 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olarte-Sanchez CM, Valencia-Torres L, Cassaday HJ, Bradshaw CM, Szabadi E (2015). Quantitative analysis of performance on a progressive-ratio schedule: effects of reinforcer type, food deprivation and acute treatment with Delta(9)-tetrahydrocannabinol (THC). Behav Process 113: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olarte-Sanchez CM, Valencia Torres L, Body S, Cassaday HJ, Bradshaw CM, Szabadi E et al (2012). A clozapine-like effect of cyproheptadine on progressive ratio schedule performance. J Psychopharmacol 26: 857–870. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K (2001) The Mouse Brain in Stereotaxic Coordinates, 2nd edn. Academic Press: San Diego. [Google Scholar]
- Rezvani AH, Cauley MC, Levin ED (2014). Lorcaserin, a selective 5-HT(2C) receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacol Biochem Behav 125: 8–14. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M (2013). Dopamine and food addiction: lexicon badly needed. Biol Psychiatry 73: e15–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjoldager P, Winger G, Woods JH (1993). Effects of GBR 12909 and cocaine on cocaine-maintained behavior in rhesus monkeys. Drug Alcohol Depend 33: 31–39. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D et al (2008). Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325: 577–587. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Roth BL (2015). DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol 55: 399–417. [DOI] [PubMed] [Google Scholar]
- Valencia-Torres L, Bradshaw CM, Bouzas A, Hong E, Orduña V (2014). Effect of streptozotocin-induced diabetes on performance on a progressive ratio schedule. Psychopharmacology 231: 2375–2384. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG (2012). Animal models to explore the effects of CNS drugs on food intake and energy expenditure. Neuropharmacology 63: 124–131. [DOI] [PubMed] [Google Scholar]
- Wynne CD, Staddon JE, Delius JD (1996). Dynamics of waiting in pigeons. J Exp Anal Behav 65: 603–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ et al (2008). 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60: 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.