Abstract
Inhibition of cyclooxygenase (COX)-derived prostaglandins (PGs) by non-steroidal anti-inflammatory drugs (NSAIDs) mediates leukocyte killing of bacteria. However, the relative contribution of COX 1 versus COX 2 to this process as well as the mechanisms controlling it in mouse and humans are unknown. Indeed, the potential of NSAIDs to facilitate leukocyte killing of drug-resistance bacteria warrants investigation. Therefore, we carried a series of experiments in mouse and humans finding that COX 1 is the predominant isoform active in PG synthesis during infection and that its prophylactic or therapeutic inhibition primes leukocytes to kill bacteria by increasing phagocytic uptake and reactive oxygen intermediate-mediated killing in a cAMP-dependent manner. Moreover, NSAIDs enhance bacterial killing in humans, exerting an additive effect when used in combination with antibiotics. Finally, NSAIDs, through the inhibition of COX prime the innate immune system to mediate bacterial clearance of penicillin-resistant Streptococcus pneumoniae serotype 19A, which is a well recognised vaccine escape serotype of particular concern given its increasing prevalence and multi-antibiotic resistance. Therefore, these data underline the importance of lipid mediators in host responses to infection and the potential of inhibitors of PG signaling pathways as adjunctive therapies, particularly in the context of antibiotic resistance.
Introduction
Antibiotic resistance arising from the selective pressure generated by excessive/inappropriate antibiotic use in human and veterinary practices poses major challenges to the management of infection, particularly with the scarcity of new anti-bacterial drugs1. For this reason, there is considerable interest in developing strategies to counteract multidrug microbial resistance either as an independent pharmaceutical entity or as an adjunct to existing treatment regimes.
COX metabolises phospholipase A2-liberated arachidonic acid to PGH2, which serves as a substrate for down-stream synthases to generate PGs and thromboxane A22. Two isoforms of COX exist with constitutively expressed COX 1 suggested to make PGs to aid physiological processes while COX 2 is inducible at sites of inflammation believed to generate pathophysiological PGs3. During inflammation in response to infection, PGs of the E/D series elevate cAMP by activating EP2/EP4 or DP1 receptors4, respectively. Elevating cAMP inhibits two pivotal steps in NADPH oxidase-mediated bacterial killing, namely the phosphorylation as well as the translocation of the cytosolic p47phox subunit to cell membrane5–8. Moreover, by signaling through EP2, PGE2 inhibits FcγR-mediated phagocytosis9. Therefore, as NSAIDs inhibit PG synthesis10, it is not surprising that targeting COX pathways of arachidonic acid metabolism is attracting current attention as a means of facilitating leukocyte killing of bacteria11. That notwithstanding, the anti-bacterial properties of COX 1 versus COX 2 inhibitors is unknown as is their respective roles in PG synthesis, cAMP expression and therefore cytokine balance during infection in both mouse and in humans. Moreover, it is not known whether NSAIDs interfere with antibiotic-mediated bacterial killing. Finally, it is unknown whether priming the innate response by PG inhibition would enhance leukocyte killing of antibiotic-resistant bacteria by overcoming strategies drug-resistant bacteria have developed to parry antibiotic efficacy.
To investigate this, we carried a series of experiments in mouse and in humans finding that COX 1 is the predominant isoform active in synthesising PGs during infection and that prophylactic as well as therapeutic inhibition of both COX isoforms kills bacteria by increasing phagocytic uptake and reactive oxygen intermediate-mediated killing. Moreover, we report that inhibiting PGs synthesis and/or signaling enhances bacterial killing in humans and that NSAIDs do not interfere with the mode of action of antibiotics but exert an additive effect when used in combination. Finally, we show that priming the innate immune system by PG inhibition enables the effective killing of antibiotic-resistant bacteria. These data highlight the therapeutic potential of inhibitors of PG biosynthetic and/or signaling pathways in combination with existing antibacterial regimes in combating recalcitrant bacterial infections, particularly resistance strains.
Materials and Methods
Preparation of bacteria for in vivo experiments
A clinical isolate of Group B Streptococcus (GBS) from adult humans, NCTC10/84 (serotype V) was a gift from Dr. T Lawrence (Centre d'Immunologie de Marseille, France). Bacteria were grown in 3% Todd-Hewitt Broth (TH Broth) (Difco) without agitation at 37°C in 5% CO2 to an OD600 of between 0.3-0.4. A single loop of culture was transferred to an agar plate made up of 3% TH broth and 2% bactericidal agar (Oxoid) (TH agar) and incubated overnight at 37°C. Agar plates containing GBS colonies were transferred to 4°C and stored for future use. 24h prior to experimentation one colony of GBS was removed from agar and grown in 3ml TH broth at 37°C for 8h without agitation. After this time, 50μl of inoculate was added to 5ml of fresh TH broth and incubated overnight at 37°C. 2ml of this solution was added to 18ml of TH broth and divided into 4 x 50ml falcon tubes (5ml in each), to optimise growth and incubated at 37°C until the OD600 reached 0.4, equivalent to 108 colony forming units (cfu)/ml. GBS was collected by centrifugation at 2000rpm for 5 min, washed with sterile PBS and kept on ice to limit further growth. To elicit peritonitis, GBS (3x107 cfu) was inoculated i.p. in 300μl sterile PBS12.
Preparation of bacteria for human in vitro experiments
Two clinical isolates of S. pneumoniae known to confer different susceptibilities to the beta lactam (β-lactam) antibiotic penicillin, ND6022 (strain 199, serotype 19A, intermediate resistance) and 10839 (strain 193, serotype 19A, susceptible), denoted ST199 and ST193, respectively, were donated by Dr. W. Hanage (Imperial College London). S. pneumoniae was cultured at 37°C in 5% CO2 on 5% blood Columbia agar (Oxoid) plates made using defibrinated horse blood (TCS Biosciences). Working stocks were made by transferring one colony of S. pneumonia or GBS to TH broth (containing 0.5% yeast extract for S. pneumonia) and grown to an OD600 of between 0.3 and 0.4. 10% glycerol was added and bacteria were stored in single use aliquots at -80 °C. The concentration of each stock aliquot was determined by plating tenfold serial dilutions onto Colombia blood agar (S. pneumoniae) or TH agar (GBS), culturing overnight and counting the resulting number of colonies expressed as cfu/ml.
Animal maintenance, peritonitis and drug dosing
Wild type (W/T) and COX 1 deficient mice (COX 1-/-), all C57bl6/J were bred under standard conditions and maintained in a 12h/12h light/dark cycle at 22 ± 1°C and given food and tap water ad libitum in accordance with United Kingdom Home Office regulations. Peritonitis was induced by intraperitoneal (i.p.) injection of either a resolving inoculums of GBS (3x107) in 300μl sterile PBS or 1mg zymosan A (Sigma-Aldrich). Aspirin (200mg/kg, Sigma), indomethacin (3mg/kg, Sigma), NS398 (10mg/kg, Cayman), SC-560 (5mg/kg, Cayman) or vehicle alone (1% w/v gum tragacanth, Sigma) were dosed orally in 100μl either 1h before or 1h after induction of peritonitis. At selected time points after triggering peritonitis the peritoneum was lavaged (sterile PBS containing 3.0% sodium citrate) and cells counted by haemocytometer following separation from edema by centrifugation with both cells and edema stored at -80°C for cytokine, lipid and cAMP quantification. To determine effects of NSAIDs on bacterial counts, either peritoneal washouts or peripheral blood was taken by cardiac puncture 3h after GBS injection and cfu counted after 24h incubation on agar. To rescue effects of NSAIDs on bacterial killing and cytokine synthesis, 100mg/kg of the PGE2 analog, 9-deoxy-9-methylene-16,16-dimethyl PGE2 (meteneprost, Cayman) or 0.1mg/kg of di-butyryl-cAMP (db-cAMP, Sigma-Aldrich) were injected i.p. 55mins after indomethacin and therefore 5min prior to GBS injection. For survival assays, mice were injected i.p. with GBS (6x107) and monitored every 4h. In these experiments, NSAIDs were given orally 1h prior to GBS infection and every 24h thereafter while infliximab (Remicade, from University College London Hospital Pharmacy) was given as a single bolus dose of 20mg/kg i.p. 1h before GBS.
S. pneumoniae and GBS in human whole blood assays
Healthy male volunteers over the age of 18 and not taking NSAIDs for two weeks were recruited according to University College London Research Ethics Committee who approved the protocol (reference number 1309/003) and all subjects gave written informed consent in accordance with the Declaration of Helsinki. 10ml of peripheral blood was collected in EDTA from volunteers with/without oral 500mg naproxen. Blood from volunteers who consumed naproxen was taken 1h after drug ingestion, seeded onto 24 well plates and made up to a final volume of 500μl with RPMI containing 10% FBS and 1% L-Glutamine without antibiotics. Separately, leukocytes isolated from whole human blood without naproxen were pre-treated for 10 min with a pan EP/DP PG receptor antagonist targeted against EP1, EP2, EP3-III, and DP1 receptors (AH6809 at 50, 100 and 300μM) as well as against individual PG receptors including EP4 (L-161,982 at 10μM), DP1 (MK-0524 at 0.5μM) and IP (CAY10441 at 1μM) (all from Cayman) and/or penicillin at a concentration that is ineffective at killing S. pneumoniae ST199 (0.075μg/ml, Sigma-Aldrich). Due to the insolubility of these compounds in aqueous solution, DMSO was used at a final concentration of 0.1% or lower. Cells were stimulated with opsonised-GBS or opsonised-S. pneumoniae (ST193 or ST199) at a ratio of 10:1 (10 bacteria:1 leukocyte) at 37°C in 5% CO2. After 1h incubation surviving bacteria were enumerated by overnight incubation on agar plates.
FACS analysis
0.5x106peritoneal cells isolated at specified time points were pre-incubated at room temperature (10min) with 1μl of mouse SeroBlock FcR (AbD serotec) diluted in FACS buffer (incomplete DMEM, 20mM glucose, 1% w/v BSA). Cells were aliquoted and co-incubated with either F4/80, (eBiosciences), GR1, Ly6G (BD Pharmingen) or CD3/CD19 (Serotec) antibodies for 30 min at room temperature in the dark before FACS analysis using isotype antibodies as controls.
Western blotting, cytokines, cAMP and prostanoids
Western blotting was carried as described previously13. Briefly, total leukocytes, isolated from the peritoneum, were lysed in lysis buffer (50mM Tris HCl, 250mM NaCl, 3mM EDTA pH8, 1% Triton-X-100, 0.5% NP-40, 10% glycerol, 2mM 1,4-dithithreitol [DTT], 0.1mM phenylmethylsufonyl [PMSF], 0.1mM Na3VO4, 1mM NaF, 1μg/ml aprotinin, 1μg/ml pepstatin A, 1μg/ml bestatin and 1μg/ml leupeptin) and protein concentration determined by BCA assay (Pierce). 5μg (COX 1) and 20μg (COX 2) protein were separated by SDS-PAGE using control standards for COX 1 (4μg rat gut mesentery) or COX 2 (5μg RAW.264.7 stimulated with 1μg/ml LPS for 24h). Separated proteins were transferred onto a polyvinylidene fluoride transfer membrane (Immobilon, Millipore) and incubated with COX 1 (1: dilution), COX 2 (1:3000) rabbit polyclonal IgG antibody (Santa Cruz Biotechnology Inc., USA) or β-actin mouse monoclonal IgG1 antibody (1;100,000, Sigma-Aldrich) at 4°C overnight under agitation in Tris-HCl containing 1% Tween-20 (Sigma-Aldrich) supplemented with 5% non-fat milk powder (Marvel) and 1% bovine serum albumin (BSA) (Sigma-Aldrich). After washing, proteins were incubated with HRP-conjugated antibodies for COX 1 and COX 2 (goat anti-rabbit IgG, Santa Cruz Biotechnology Inc., USA) and β-actin (goat anti-mouse IgG, Santa Cruz Biotechnology Inc., USA) for 1h at room temperature under agitation. Membranes were washed and specified proteins visualised by ECL hyperfilm. Levels of mediators in the peritoneal cell-free inflammatory exudate were measured by either ELISA (TNFα and IL-10) (eBiosciences and BD Biosciences, respectively) or EIA (PGE2, 6-keto PGF1α, 2,3-dinor 6-keto PGF1α, PGD2 and cAMP) (Cayman Chemicals) according to manufacturer’s instructions.
Opsonisation
Stock bacteria were removed from -80°C on the day of experimentation and allowed to thaw at room temperature (10 min), incubated in a 25% human serum solution for 30 min at 37°C and centrifuged at 12,000 x g for 10 min, discarding the supernatant. Pooled human serum was obtained from normal human volunteers that had not been vaccinated with the Pneumococcal polysaccharide or conjugate vaccine.
Characterising S. pneumoniae susceptibility to penicillin
To test the susceptibility of S. pneumoniae to penicillin, a minimum inhibitory concentration (MIC) test was performed. Bacteria were cultured at 37 °C on 5% blood Columbia agar in 5% CO2 in the presence of a benzylpenicillin Etest strips (AB BIODISK) with MIC of between 0.016-256μg/ml and 32μg/ml-0.002μg/ml. 24h later, MIC values were determined where the edge of the inhibition ellipse intersected the strip (Supplementary data, Figure S1). It was concluded that the MIC value of ST199 was between 0.094μg/ml and 0.047μg/ml (mean: 0.07μg/ml) and 0.012μg/ml for ST193. Thus, 0.075μg/ml penicillin was used, at which concentration ST193 would be fully susceptible to and ST199 partially resistant to penicillin.
Blood flash lysis
3ml of blood from each volunteer was transferred into 50ml falcon tubes followed by 42ml of deionised water for 19 sec after which time 5ml of 9% saline (to generate final concentration of 0.9%) was added to maintain osmolarity. After the mixture was allowed to equilibrate for 5 min on ice, cells were centrifuged at 400 x g for 10 min at 4°C, the remaining supernatant removed and white blood cell pellet resuspended in PBS
C3 Binding Assay
Complement factor interaction with S. pneumoniae was assessed using flow cytometry assays as described14–15. Briefly, 1x106 cfu S. pneumoniae or GBS was incubated in 10μl of human serum (diluted to 20% or 50% in PBS) for 30 min at 37°C with/without 400μM naproxen, washed twice with 500μl PBS/0/1% Tween-20 and resuspended in 50μl of PBS/0.1% Tween-20 containing 1:300 dilution of fluorescein isothiocyanate (FITC)-conjugated polyclonal goat anti-human C3 antibody (ICN). FITC labeling of bacteria was determined by FACSCalibur (BD Biosciences) with gating based on the analysis of at least 25,000 bacteria as described previously14. The very large differences in results for control and sera with naproxen were compared using the geometric mean fluorescence intensities of bacteria positive for C3b.
Phagocytosis assays
FITC-labelled zymosan (Invitrogen) (0.5mg per mouse) was injected i.p. 1h after oral treatment with gum traganth (control) or 200mg/kg aspirin. Phagocytosis, using naïve peritoneal macrophages, was measured at 10, 15, 30 and 60 min following FITC-labelled zymosan by performing a peritoneal lavage with ice-cold 2mM EDTA (Amresco). Cells was quenched in 0.002% trypan blue for 5 min and then fixed in ice-cold 4% paraformaldehyde (Acros organics). Cells were washed of unbound FITC-labelled zymosan three times before analysing phagocytosis using FACS. Control mice were used at each time-point (mice with 0.5mg zymosan without FITC). For in vitro phagocytosis on human blood leukocytes, S. pneumoniae was fluorescently labeled with 6-carboxyfluorescein succinimidyl ester (FAMSE; Molecular Probes) as described previously16. Human peripheral blood leukocytes were seeded at 2.5x105/24-well plate and pre-treated with/without 400μM naproxen for 30 min at 37°C, 5% CO2. Cells were then stimulated with FAM-SE labeled, opsonised penicillin-susceptible (ST193) S. pneumoniae at a ratio of 10:1. After 1.5, 3 and 5mins 100μl was removed from triplicate wells and fixed using 1% paraformaldehyde (Sigma) and quenched with 500 μl of 0.002% trypan blue and analysed by FACScalibur (Becton Dickinson).
NADPH oxidase activity
1x105 leukocytes were incubated with 50μM Amplex©Red Ultra (Invitrogen), 0.1U/ml horseradish peroxidise (R&D systems) with/without PMA (1ng/ml). Fluorescence was measured using excitation in the range of 530–560nm and emission detection at 590nm every 30s with data analysed using Omega Data Analysis Software (BMG Labtech) determining the rate of O2 consumption between 0-15 min. The absolute concentration of hydrogen peroxide (H2O2) was ascertained by adding an excess of Amplex©Red Ultra and HRP to varying concentrations of H2O2 (Calbiochem), allowing the reaction to proceed for 15 min, which ensured all the H2O2 was converted into O2.
NSAID/live bacterial co-incubation assay
To exclude NSAIDs possessing direct antibiotic properties against bacteria, dual COX inhibitors naproxen and indomethacin as well as the COX 2 specific inhibitor NS398 were incubated with GBS in a 6% plasma/DMEM solution in the absence of mammalian leukocytes. After 1h numbers of surviving bacteria were incubated on Todd Hewitt agar for enumeration 24h later. NS398 and indomethacin were used at concentrations concordant with their IC80 and at levels x10 higher17, while naproxen was used at a concentration equivalent to serum levels of healthy volunteers after ingestion of 500mg twice daily 18 and a dose x10 lower. Penicillin was used as a reference at 0.075μg/ml bringing about greater than 99% killing of bacteria.
Results
COX, PG and cytokine profiles throughout infectious inflammation
Following i.p. injection of GBS to W/T mice, inflammation (total leukocyte infiltrate) peaked between 6/24h and waned slowly thereafter (Figure 1A) with profiles of individual leukocytes illustrated in Figures 1B-C. Unlike COX 2, total leukocyte COX 1 was constitutively expressed in the naive peritoneum and throughout infection (Figure 1D) with cell-free peritoneal exudate levels of PGE2 (Figure 1E) and 6-keto PGF1α (PGI2 metabolite, Figure 1F) peaking at 3h. 2,3-dinor 6-keto PGF1α, the β oxidation metabolite of 6-keto PGF1α and principle urinary metabolite of PGI2 in humans peaked as inflammation resolved, Figure 1F. As with lipids, inflammatory cytokines TNFα (Figure 1G) and IL-10 (Figure 1H) were also maximal early in inflammatory exudates in response to GBS (3h). To discern the relative contribution of COX 1 and COX 2 to PG synthesis during GBS-elicited peritonitis, selective COX inhibitors (SC-560 for COX 1 and NS-398 for COX 2) and non-selective COX inhibitors (aspirin and indomethacin) were used. Results of these experiments revealed that inhibition of COX 1 brought about a greater reduction in PGE2 (Figure 2A) and PGD2 (Figure 2B) at 3h than did inhibition of COX 2; findings supported using COX 1-/- mice (Figure 2C-D) bearing GBS-induced peritonitis. An equally predominant role for COX 1 in PG generation was also found in a non-infectious peritonitis triggered by zymosan (Figure 2E-F). As NS-398 was used at a concentration that did not inhibit plasma TxB2 (Figure 2D), a marker of COX 1 activity, these data reveal a predominant role for COX 1 in the generation of PGs during infectious and non-infectious inflammation, as shown previously19–20.
Figure 1.
Characterisation of infectious peritonitis. Injecting GBS i.p. to W/T mice triggers an immediate (A) leukocytes infiltrate comprising (B) GR1-positive PMNs and F4/80-positive macrophages as well as (C) CD3-positive T and CD19-positive B cells. Total peritoneal leukocytes were prepared for (D) COX 1 and COX 2 protein expression while (E) PGE2, (F) prostacyclin, measured as its stable metabolites 6-keto PGF1α and 2,3-dinor 6-keto PGF1α, (G) TNFα and (H) IL-10 were determined in the cell-free inflammatory exudates. Values are expressed as the mean ± SEM of 5-12 mice/group.
Figure 2.
COX 1 is the predominant isoforms functional during infectious peritonitis. Selective COX inhibitors (SC-560 for COX 1 and NS-398 for COX 2) and non-selective COX inhibitors (aspirin and indomethacin) were dosed orally to W/T animals 1h before GBS injection. Cell-free peritoneal exudate levels of (A) PGE2 and (B) PGD2 were measured 3h after GBS with similar results obtained for (C) PGE2 and (D) PGD2 using COX 1 knockout mice. In addition, COX inhibitors were dosed to mice 1h before i.p. zymosan (non-infectious stimulus) with cell-free peritoneal exudate levels of (E) PGE2 and (F) PGD2 measured 3h later. NS-398 was used at dosing levels that do not inhibit (G) COX 1-derived plasma TxB2 measured 3h after i.p. GBS injection. Data were analysed by one-way ANOVA and Dunnett’s multiple comparison test or by unpaired Student T-test. Values are expressed as the mean ± SEM of between 5-12 mice/group. * P < 0.05; ** P < 0.01 and *** P <0.001.
NSAIDS enhance bacterial killing in rodents
Selective and non-selective COX inhibitors were given either as a single dose 1h before i.p. GBS injection (Figure 3A) or therapeutically 1h after GBS, Figure 3B. 3h after inoculation, peripheral blood (Figures 3A-B) or peritoneal lavage fluid (Figure 3C) were taken and cultured on agar for 24h to determine GBS cfu numbers. Regardless of dosing regimes, there was consistent and significant enhancement of bacterial killing with all categories of NSAIDs by up to 90%. Enhanced bacterial killing was also observed in COX 1 deficient mice (Figure 3D) thereby excluding an off-target effect of COX inhibitors. COX 2-/- mice not used in these studies as they develop spontaneous sterile peritonitis21. Therefore, we have extended the previous findings of others5,9,11 by showing that NSAIDs prime the innate immune response to efficiently kill bacteria when given either prophylactically or therapeutically. We confirmed that these effects were not due to NSAIDs acting directly on bacteria (Figure 3E) finding that naproxen, indomethacin and NS-398 incubated with GBS in the absence of leukocytes did not affect bacteria viability whereas penicillin was 99% efficacious in this in vitro assay.
Figure 3.
COX inhibition enhances bacteria killing in rodents. Mice were given dual (indomethacin/aspirin), COX 1 (SC-560) or COX 2 (NS-398) selective inhibitors orally either (A) 1h before or (B) therapeutically 1h after i.p. inoculation of GBS. To exclude dampened bacterial translocation as a potential explanation for reduced plasma GBS titres following COX inhibition, peritoneal exudate levels of (C) GBS were measured 3h after inoculation. GBS was also injected i.p. to (D) COX 1 knockout mice with plasma taken 3h later for overnight cfu culture number determination as well as (E) incubated in culture media only with NSAIDs in the absence of leukocytes. Data were analysed by one-way ANOVA and Dunnett’s multiple comparison test or by unpaired Student T-test. Values are expressed as the mean ± SEM of between 5-12 mice/group. * P < 0.05 and ** P < 0.01.
cAMP reverses NSAID-induced bacterial killing and cytokine synthesis
In the above experiments where bacterial killing was enhanced after COX inhibition (Figure 3A-C), cAMP levels in the cell-free peritoneal lavage fluid were dampened (Figure 4A) with similar effects obtained with COX 1-/- mice, Figure 4B. This reduction in cAMP was associated with increased peritoneal leukocyte phagocytic capacity (Figure 4C) and NADPH oxidase activity as measured by H2O2 production in both NSAID-treated W/Ts (Figure 4Di-ii) and COX 1 knockouts, Figure 4E. Given that cAMP inhibits pro-inflammatory signalling pathways, inhibition of COX 1 triggered TNFα synthesis in inflammatory exudates (Figure 4F) with a modest reduction in IL-10, Figure 4G. A similar cytokine profile was obtained using COX 1-/- mice, Figure 4H-I. To determine that changes in cAMP consequent to COX inhibition are instrumental in modulating bacterial killing as well as NADPH oxidase activity and cytokine synthesis, mice were given the PGE2 analogue 15(S)-15 methyl PGE2 (Metenprost) or dibutyrl-cAMP 55mins following indomethacin. 1h after indomethacin and therefore 5mins after PGE2/cAMP treatment, mice were injected i.p. with GBS. Overnight culture on agar plates of peripheral blood taken 3h after GBS confirmed that enhanced bacterial killing with indomethacin was reversed by 15(S)-15 methyl PGE2 and dibutyrl-cAMP, Figure 4J. Indomethacin-induced NADPH oxidase activity in peritoneal leukocytes (Figure 4K) as well as changes in exudate levels of TNFα (Figure 4L) and IL-10 (Figure 4M) were also reversed by 15(S)-15 methyl PGE2 or dibutyrl-cAMP. These data implicate cAMP in PG mediated suppression of bacterial killing and pro-inflammatory cytokine synthesis.
Figure 4.
COX inhibition alters phagocytosis, cytokine synthesis and bacterial killing mechanisms in a cAMP-dependent manner. GBS was injected i.p. to (A) W/T mice dosed orally 1h earlier with indomethacin (dual COX inhibitor), SC-560 (COX 1 inhibitor) or NS-398 (COX 2 inhibitor) as well as to (B) COX 1 knockout mice. 3h after GBS injection (A-B) cAMP was measured in cell-free exudates while (C) phagocytosis and (D-E) NADPH oxidase activity was determined in total leukocytes. Cell-free exudate levels of (F-G) TNFα and IL-10 were also determined in NSAID-treated W/Ts and (H-I) COX 1 knockouts. This COX-inhibited differential change in W/T mice (J) bacterial killing, (K) NADPH oxidase activity and (L-M) cytokine synthesis was reversed by 15(S)-15 methyl PGE2 (EP agonist) or db-cAMP given 5mins before GBS and therefore 55mins after NSAIDs. Data is represented and analysed by an unpaired Student’s T-test or ANOVA followed by either Dunnett’s or Bonferroni multiple comparison tests. Values are expressed as the mean ± SEM of 5-12 mice/group. * P < 0.05 and ** P < 0.01. *** P < 0.001.
PG control of cytokine synthesis
TNFα was substantially elevated when COX 1 inhibitors were given prior to infection (Figure 4E). This was associated with enhanced mortality, which was completely reversed by blocking TNFα with infliximab, Figure 5A. However, COX 1 inhibitors did not alter cytokines when given therapeutically, 1h after GBS injection (Figure 5B). NS398 was also without effect on TNFα levels when given before GBS (Figure 4E) and even modestly reduced TNFα (Figure 5B) when administered 1h after infection. These data suggest that PG/cAMP control of TNFα synthesis is COX 1 mediated and occurs within the first 60min of infection. Therefore, prophylactic inhibition of PG synthesis triggers a cytokine storm leading to death, effects not seen when COX is inhibited in a clinically-relevant, therapeutic setting after infection is established.
Figure 5.
Prophylactic but not therapeutic COX inhibition triggers pro-inflammatory cytokine synthesis. W/T mice were injected i.p. with GBS 1h after oral administration of NS-398 (COX 2 inhibitor), SC-560 (COX 1 inhibitor) or SC-560 plus infliximab with (A) complete reversal of COX 1-mediated cytokine storm (TNFα synthesis, Figure 4F) afforded by infliximab. SC-560 and NS-398 were dosed orally 1h after GBS injection causing little effects on (B-C) inflammatory cytokine levels in cell-free inflammatory exudates 3h later. Survival analysis was completed on 8 mice/group and the log-rank test was used to compare each group against one another. ** values represent P < 0.01 for controls versus Sc-560/infliximab. For cytokines, values are expressed as the mean ± SEM of 5-7 mice/group and analysed by one-way ANOVA and Dunnett’s multiple comparison test. *values represent P < 0.05. Survival experiments had 8 mice/group.
COX inhibition enhances bacterial killing in human whole blood assays
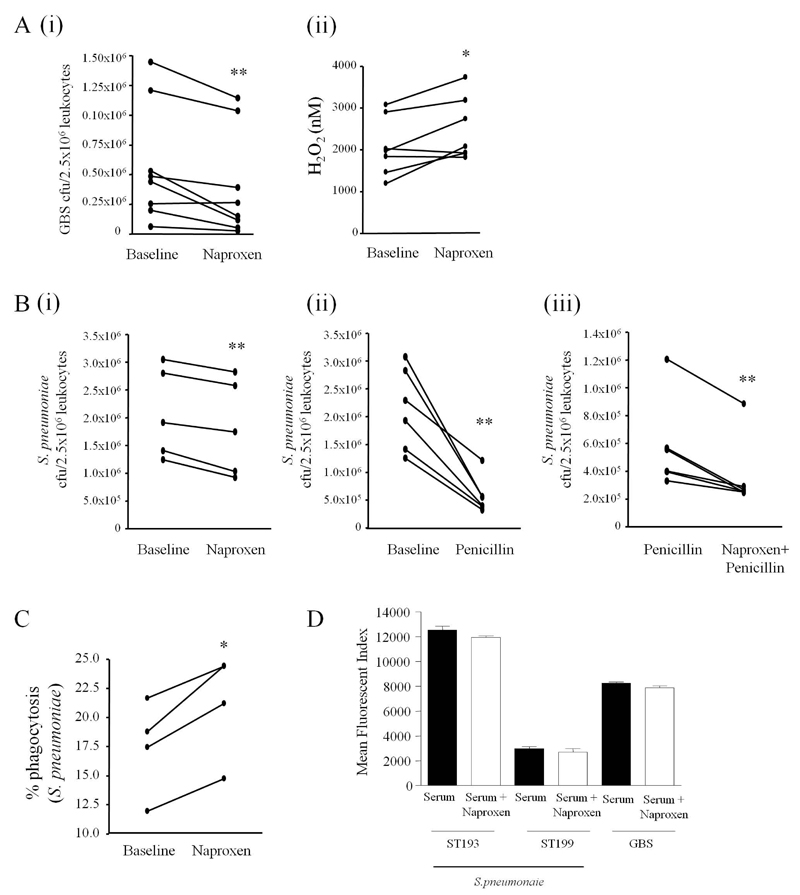
To determine whether COX inhibition is anti-bacterial in human, peripheral blood of 8 healthy males between the ages of 25-50 was taken before and 1h after oral naproxen (500mg) and incubated ex vivo with opsonised GBS. Naproxen significantly enhanced whole blood leukocyte bacterial killing (Figure 6Ai) concomitant with enhanced blood leukocyte NADPH oxidase activity (Figure 6Aii). At these anti-inflammatory doses naproxen did not impair antibiotic activity but worked additively with penicillin as penicillin plus naproxen exerted significantly greater bacterial killing compared to penicillin alone, Figure 6Bi-iii. In these experiments, as with mice (Figure 4C), COX inhibition also enhanced blood leukocyte uptake of FAM-SE labelled S. pneumoniae (ST193, Figure 6C) consistent with PGE2 being and inhibitor of phagocytosis9. Importantly, NSAIDs did not alter opsonisation (C3 deposition) of target bacteria (Figure 6D) providing further evidence of a direct immune-priming effect of NSAIDS on leukocyte anti-microbial activity. Thus, from studies on both humans and rodents we found that inhibiting COX enhances bacterial uptake as well as killing in a cAMP-dependent manner and does not interfere with the mode of action of antibiotics or opsonisation of target bacteria.
Figure 6.
COX inhibition primes human whole blood to kill bacteria. Peripheral blood of 8 healthy male volunteers between the ages of 25-50 was taken before and 1h after ingestion of 500mg naproxen and incubated ex vivo with opsonised GBS. After 60min aliquots were taken to determine (A i) numbers of viable bacteria determined by overnight incubation on agar plates or (A ii) leukocyte NADPH oxidase activity. Blood from volunteers taken naproxen was further incubated with (B i-iii) penicillin (0.075μg/ml) to determine NSAID inhibitory/additive effects on bacterial killing with antibiotics. (C) Blood was also treated 400μM naproxen for 30min and stimulated with FAM-SE labelled S. pneumoniae (ST193) 5min to determine bacterial phagocytosis and to show that (D) NSAIDs do not interfere with opsinisation of bacteria. Data were analysed by paired Student’s T-test. * values represent P < 0.05 and ** values represent P < 0.01.
COX inhibition overcomes antibiotic resistance in human whole blood assays
Finally, we questioned whether COX inhibition would prime innate immune-mediated responses to kill antibiotic resistant bacteria. This hypothesis was tested by incubating human whole blood with a pan EP/DP PG receptor antagonist targeted against EP1, EP2, EP3-III, and DP1 receptors as well as against individual PG receptors including EP4, DP1 and IP with/without penicillin and subsequently spiking human blood with S. pneumoniae serotype 19A which was either penicillin susceptible (ST193) or penicillin resistant (ST199). After 1h, blood samples were taken and incubated overnight on agar. Penicillin and pan-EP/DP1 receptor antagonism significantly reduced ST193 numbers (Figure 7Ai). Although a trend towards a reduction in ST193 was seen when individual EP4, DP1, and IP receptors were antagonised, results were not significant (Figure 7Aii). Importantly, while S. pneumoniae ST199 was refractory to penicillin, inhibiting EP/DP signalling with AH6809 (Figure 7Bi) or specific inhibition of IP (Figure 7Bii) brought about a significant killing of antibiotic resistant S. pneumoniae by whole blood with EP4 and DP1 antagonism causing a trend towards reduced ST199 cfu numbers (Figure 7Bii).
Figure 7.
Inhibiting PG signalling primes human whole blood to kill antibiotic-resistant bacteria. Healthy human peripheral blood was pre-incubated with/without penicillin and a pan EP/DP PG receptor antagonist targeted against EP1, EP2, EP3-III, and DP1 receptors as well as against individual PG receptors including EP4, DP1 and IP. Whole blood was then stimulated with either (A) serum-opsonised penicillin-susceptible (ST193) or (B) intermediate penicillin-resistant (ST199) S. pneumonia for 1h. After which time, numbers of surviving S. pneumoniae (cfu) were quantified 24h later on agar plates. Data were analysed by one-way ANOVA and Bonferroni’s multiple comparison test. * values represent P < 0.05, ** values P < 0.01 and *** values P < 0.001.
Dicsussion
Some PGs elevate cAMP, an intracellular second messenger that differentially regulates cytokines (upregulates IL-10 and inhibits TNFα) but also inhibits phagocytosis as well as reactive oxygen and nitrogen intermediates, phagosomal acidification and lysosomal enzyme release22. Thus, during infection cAMP dampens host responses to bacteria underscored by studies showing that its pharmacological manipulation in vitro7–9,23 and in vivo leads to early lethality and enhanced bacterial load23–24. We found in rodents that prophylactic as well as therapeutic inhibition of both COX isoforms enhanced host ability to kill bacteria by dampening cAMP in a PG-dependent manner. Extending these studies to humans, we showed that NSAIDs in whole blood assays enhanced bacterial phagocytic uptake and killing without interfering with antibiotics, but acting in concert with them to kill bacteria. Finally, we report that by priming the innate immune system through COX inhibition, NSAIDs promote the killing of antibiotic-resistant bacteria by blood leukocytes. Collectively, these data highlight the significance of lipid mediators in host responses to infection and the potential of inhibitors of PG signaling pathways or products of lipoxygenases25–27 as adjunctive therapies in the setting of antibiotic resistance. Of particular relevance to clinical disease, we demonstrate that NSAIDs enhance whole blood killing of penicillin resistant serotype 19A S. pneumoniae. Other investigators have demonstrated that this non-vaccine serotype is currently increasing in prevalence and isolates are frequently resistant to multiple antibiotics. This novel therapeutic use of NSAIDs may potentially enhance antibiotic mediated killing of bacterial pathogens including S. pneumoniae and GBS and reduce the therapeutic level of antibiotic required, thereby reducing the likelihood of antibiotic resistance occurring.
COX 1-/- mice or COX 1 inhibitors given prophylactically (i.e. 1h prior to infection) in W/Ts bearing a GBS-triggered inflammation elevated TNFα. COX 2 inhibitors had little effect on TNFα despite dampening PGs and cAMP. Moreover, neither COX 1 nor COX 2 inhibition modulated TNFα when given therapeutically, 1h after GBS injection. As COX 2 is expressed from 3h and COX 1 is constitutively expressed in the naive cavity, these data suggest that PG control of cAMP-modulated TNFα is COX 1 mediated and occurs within the first 60mins of infection. After this time the regulation of TNFα synthesis becomes COX independent. These results highlight differential roles of COX isoforms in controlling pro-inflammatory cytokine generation during infection/injury and support the historical concern surrounding the use of conventional (i.e. non-selective) NSAIDs in humans for fever and in sepsis due to their propensity to trigger pro-inflammatory cytokines28–29. Indeed, inhibiting COX 1 triggered a cytokine storm (Figures 4E and G) most likely responsible for hastened death in animals given sub-lethal GBS as these effects were completely reversed with infliximab, Figure 5A. Therefore, we suspect that elevated TNFα in humans following non-selective COX inhibition with ibuprofen28–29 stems from the pre-dosing regimen that inhibited constitutive COX 1 as a result of priming the innate immune system to generate a Th1-type cytokine response. Although it is well appreciated that prophylactic treatment with NSAIDs (particularly ibuprofen and indomethacin) cause significant elevations of pro-inflammatory cytokines (i.e. TNFα, IL-6 and IL-8) in the plasma of humans after inducing endotoxemia28–30, it is not known what effect therapeutic dosing with either non-selective or COX 2 selective inhibitors would have on plasma pro-inflammatory cytokine levels. But, in the context of NSAIDs being used therapeutically in combination with antibiotics to treat drug-resistant infections, it is likely that cytokine synthesis will not be triggered. In the event a cytokine storm ensues and despite the reservations of TNFα blockade in sepsis31, concomitant treatment with infliximab may efficiently dampen the associated symptoms.
Reports also suggest that NSAIDs increase the risk of developing Group A Streptococcal (GAS) necrotising fasciitis, impair its diagnosis/management and accelerate its course of infection32–34. However, critical assessment of current data does not support a causal role for NSAIDs in the development of this disease state or a worsening of infection once established35. Indeed, there are suggestions that NSAIDs may alleviate its symptoms resulting in delayed diagnosis and treatment. In support of this a recent case-control study found that NSAIDs do not increase the risk of developing severe sepsis or septic shock as a consequence of necrotizing fasciitis in adults with community acquired bacterial infections36. They contend that NSAID exposure during evolving bacterial infection is associated with delayed prescription of effective antibiotic therapy, which is hypothesised to be due to NSAIDs masking the progression of the disease by suppressing the associated inflammation. Interestingly, a recent genetic study using advanced recombinant inbred mice revealed a potentially major causal role for PGE2 in conferring susceptibility to invasive GAS infections37. They demonstrated that within quantitative trait loci, hypothesized to be involved in modulating severity of GAS infections, genes for both mPGES-1 and mPGES-2, (Ptges and Ptges1, respectively) showed marked upregulation in mice strains susceptible to GAS, but showed no change or slight decrease in resistant strains post-infection. Thus, these results associated high expression of mPGES-1 (and to a lesser extent mPGES-2) with increased susceptibility to death, bacteremia, and bacterial dissemination.
Antibiotics suppress the growth of microorganisms by inhibiting bacteria cell wall synthesis, protein synthesis or nucleic acid/ribonucleic acid metabolism38. Despite this, bacteria have developed mechanisms of drug resistance including modifications leading to loss or decreased affinity of the drug for its target; reducing the number and/or size of external membrane pore diameter thereby limiting antibiotic permeability; producing drug detoxifying enzymes or actively effluxing antibiotics by energy-dependent pumps38. As a result, there is a pressing need to develop alternatives to overcome antibiotic-resistance. Current solutions include efflux pump inhibition 39–40, phage therapy 41 or bacteriocins (bacteria employed in probiotic application)42. However, few such strategies have made it to the clinic. Given its constitutively expressed nature and predominant role in PG synthesis during bacterial infection, potential strategies for targeting drug-resistant bacteria based on COX pathways of arachidonic acid metabolism may include dampening COX 1 and/or inducible mPGES, antagonizing cAMP-elevating PG signaling receptors (EP2, DP1 or IP) or inhibiting COX 2. Selective inhibition of COX 1 may not exert the same cardiovascular side effects as COX 2 inhibitors; in fact it should be as beneficial as low-dose aspirin and cause little gastric toxicity as both COX 1 and COX 2 need to be inhibited to cause gastric ulceration43.
If selective inhibition of constitutively-expressed COX 1 perturbs physiological pathways required to maintain health then inhibiting inducible mPGE2S during inflammation maybe more tractable. From data presented here, inhibiting COX 2 would also be an attractive target as its inhibition effectively kills bacteria without triggering pro-inflammatory cytokine synthesis. Indeed, its cardio-toxic profile is manifested only after protracted exposure, which may not be problematic over the relatively short time frame needed to eradicate drug resistant infections. However, COX 2 inhibition only delayed mortality, Figure 5A, conferring protection up 50h post infection in our studies, after which time clinical signs of systemic inflammation (piloerection, reduced movement, shallow breathing) and mortality was equivalent to that of controls. This was surprising given that NS-398 improved the survival of mice over 7 days when challenged with P. aeruginosa 44, but is consistent with our previous reports of a protective role for COX 2 in the resolution of acute inflammation13,45–47 and those of others regarding a protective role for COX 2-derived lipids as endogenous stop-signals of innate immune-mediated responses48–50. We suspect that while COX 2 inhibition protected animals in the early phase of infection (bacterial killing, conserved IL-10 release) it dampened pro-resolution pathways and exerted a resolution-toxic effect.
In conclusion, as NSAIDs not only possess anti-bacterial properties but also overcome drug resistance in a manner that does not interfere with antibiotic’s mode of action, these data collectively support COX inhibition or strategies that disrupt PG signaling pathways as useful adjunctive therapies in treating persistent/multi-drug resistant infection.
Supplementary Material
Acknowledgements
DWG is a Wellcome Trust Senior Research Fellow while MJS was supported by a studentship from the Medical Research Council, UK. Funding was provided by the Wellcome Trust and Medical Research Council, UK to DWG.
Footnotes
Authorship Contribution
MJS designed and carried our experiments with the help of JN and CH. SSA and JB contributed valuable experimental material and expertise while DWG designed experiments and wrote the paper.
No author has conflicting financial interest.
References
- 1.Livermore DM. Minimising antibiotic resistance. Lancet Infect Dis. 2005;5(7):450–459. doi: 10.1016/S1473-3099(05)70166-3. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978;30(3):293–331. [PubMed] [Google Scholar]
- 3.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56(3):387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka T, Narumiya S. Prostaglandin receptor signaling in disease. ScientificWorldJournal. 2007;7:1329–1347. doi: 10.1100/tsw.2007.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174(2):595–599. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 6.Crawford MA, Aylott CV, Bourdeau RW, Bokoch GM. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J Immunol. 2006;176(12):7557–7565. doi: 10.4049/jimmunol.176.12.7557. [DOI] [PubMed] [Google Scholar]
- 7.Mitsuyama T, Takeshige K, Minakami S. Cyclic AMP inhibits the respiratory burst of electropermeabilized human neutrophils at a downstream site of protein kinase C. Biochim Biophys Acta. 1993;1177(2):167–173. doi: 10.1016/0167-4889(93)90036-o. [DOI] [PubMed] [Google Scholar]
- 8.Hwang TL, Yeh SH, Leu YL, Chern CY, Hsu HC. Inhibition of superoxide anion and elastase release in human neutrophils by 3'-isopropoxychalcone via a cAMP-dependent pathway. Br J Pharmacol. 2006;148(1):78–87. doi: 10.1038/sj.bjp.0706712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173(1):559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 10.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 11.Aronoff DM, Lewis C, Serezani CH, et al. E-Prostanoid 3 Receptor Deletion Improves Pulmonary Host Defense and Protects Mice from Death in Severe Streptococcus pneumoniae Infection. J Immunol. 2009 doi: 10.4049/jimmunol.0900129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong CH, Bebien M, Didierlaurent A, et al. An antiinflammatory role for IKKbeta through the inhibition of "classical" macrophage activation. J Exp Med. 2008;205(6):1269–1276. doi: 10.1084/jem.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 14.Brown JS, Hussell T, Gilliland SM, et al. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc Natl Acad Sci U S A. 2002;99(26):16969–16974. doi: 10.1073/pnas.012669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuste J, Sen A, Truedsson L, et al. Impaired opsonization with C3b and phagocytosis of Streptococcus pneumoniae in sera from subjects with defects in the classical complement pathway. Infect Immun. 2008;76(8):3761–3770. doi: 10.1128/IAI.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jomaa M, Yuste J, Paton JC, Jones C, Dougan G, Brown JS. Antibodies to the iron uptake ABC transporter lipoproteins PiaA and PiuA promote opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 2005;73(10):6852–6859. doi: 10.1128/IAI.73.10.6852-6859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96(13):7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Ouweland FA, Franssen MJ, van de Putte LB, Tan Y, van Ginneken CA, Gribnau FW. Naproxen pharmacokinetics in patients with rheumatoid arthritis during active polyarticular inflammation. Br J Clin Pharmacol. 1987;23(2):189–193. doi: 10.1111/j.1365-2125.1987.tb03028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilroy DW, Tomlinson A, Willoughby DA. Differential effects of inhibitors of cyclooxygenase (cyclooxygenase 1 and cyclooxygenase 2) in acute inflammation. Eur J Pharmacol. 1998;355(2–3):211–217. doi: 10.1016/s0014-2999(98)00508-1. [DOI] [PubMed] [Google Scholar]
- 20.Wallace JL. Selective COX-2 inhibitors: is the water becoming muddy? Trends Pharmacol Sci. 1999;20(1):4–6. doi: 10.1016/s0165-6147(98)01283-8. [DOI] [PubMed] [Google Scholar]
- 21.Morham SG, Langenbach R, Loftin CD, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83(3):473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 22.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39(2):127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bystrom J, Evans I, Newson J, et al. Resolution-phase macrophages possess a unique inflammatory and bactericidal phenotype that is controlled by cAMP. Blood. 2008;112(10) doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares AC, Souza DG, Pinho V, et al. Impaired host defense to Klebsiella pneumoniae infection in mice treated with the PDE4 inhibitor rolipram. Br J Pharmacol. 2003;140(5):855–862. doi: 10.1038/sj.bjp.0705517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancuso P, Nana-Sinkam P, Peters-Golden M. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun. 2001;69(4):2011–2016. doi: 10.1128/IAI.69.4.2011-2016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun. 1998;66(11):5140–5146. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spinas GA, Bloesch D, Keller U, Zimmerli W, Cammisuli S. Pretreatment with ibuprofen augments circulating tumor necrosis factor-alpha, interleukin-6, and elastase during acute endotoxinemia. J Infect Dis. 1991;163(1):89–95. doi: 10.1093/infdis/163.1.89. [DOI] [PubMed] [Google Scholar]
- 29.Martich GD, Danner RL, Ceska M, Suffredini AF. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J Exp Med. 1991;173(4):1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michie HR, Manogue KR, Spriggs DR, et al. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- 31.Fisher CJ, Jr, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334(26):1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 32.Brun-Buisson CJ, Saada M, Trunet P, Rapin M, Roujeau JC, Revuz J. Haemolytic streptococcal gangrene and non-steroidal anti-inflammatory drugs. Br Med J (Clin Res Ed) 1985;290(6484):1786. doi: 10.1136/bmj.290.6484.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veenstra RP, Manson WE, van der Werf TS, et al. Fulminant necrotizing fasciitis and nonsteroidal anti-inflammatory drugs. Intensive Care Med. 2001;27(11):1831. doi: 10.1007/s001340101070. [DOI] [PubMed] [Google Scholar]
- 34.Rimailho A, Riou B, Richard C, Auzepy P. Fulminant necrotizing fasciitis and nonsteroidal anti-inflammatory drugs. J Infect Dis. 1987;155(1):143–146. doi: 10.1093/infdis/155.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aronoff DM, Bloch KC. Assessing the relationship between the use of nonsteroidal antiinflammatory drugs and necrotizing fasciitis caused by group A streptococcus. Medicine (Baltimore) 2003;82(4):225–235. doi: 10.1097/01.md.0000085060.63483.bb. [DOI] [PubMed] [Google Scholar]
- 36.Legras A, Giraudeau B, Jonville-Bera AP, et al. A multicentre case-control study of nonsteroidal anti-inflammatory drugs as a risk factor for severe sepsis and septic shock. Crit Care. 2009;13(2):R43. doi: 10.1186/cc7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdeltawab NF, Aziz RK, Kansal R, et al. An unbiased systems genetics approach to mapping genetic loci modulating susceptibility to severe streptococcal sepsis. PLoS Pathog. 2008;4(4):e1000042. doi: 10.1371/journal.ppat.1000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(12 Suppl):S122–129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 39.Marquez B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie. 2005;87(12):1137–1147. doi: 10.1016/j.biochi.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Bassetti M, Righi E, Viscoli C. Novel beta-lactam antibiotics and inhibitor combinations. Expert Opin Investig Drugs. 2008;17(3):285–296. doi: 10.1517/13543784.17.3.285. [DOI] [PubMed] [Google Scholar]
- 41.Housby JN, Mann NH. Phage therapy. Drug Discov Today. 2009;14(11–12):536–540. doi: 10.1016/j.drudis.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Gillor O, Etzion A, Riley MA. The dual role of bacteriocins as anti- and probiotics. Appl Microbiol Biotechnol. 2008;81(4):591–606. doi: 10.1007/s00253-008-1726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119(3):706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- 44.Sadikot RT, Zeng H, Azim AC, et al. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur J Immunol. 2007;37(4):1001–1009. doi: 10.1002/eji.200636636. [DOI] [PubMed] [Google Scholar]
- 45.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. Faseb J. 2004;18(3):489–498. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 46.Gilroy DW, Colville-Nash PR, McMaster S, Sawatzky DA, Willoughby DA, Lawrence T. Inducible cyclooxygenase-derived 15-deoxy(Delta)12-14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. Faseb J. 2003;17(15):2269–2271. doi: 10.1096/fj.02-1162fje. [DOI] [PubMed] [Google Scholar]
- 47.Rajakariar R, Hilliard M, Lawrence T, et al. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci U S A. 2007;104(52):20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196(8):1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92(21):9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164(4):1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.