Abstract
The efficacy of checkpoint inhibitor therapy illustrates that cancer immunotherapy, which aims to foster the host immune response against cancer to achieve durable anticancer responses, can be successfully implemented in a routine clinical practice. However, a substantial proportion of patients does not benefit from this treatment, underscoring the need to identify alternative strategies to defeat cancer. Despite the demonstration in the 1990’s that the detection of danger signals, including the nucleic acids DNA and RNA, by dendritic cells (DCs) in a cancer setting is essential for eliciting host defense, the molecular sensors responsible for recognizing these danger signals and eliciting anticancer immune responses remain incompletely characterized, possibly explaining the disappointing results obtained so far upon the clinical implementation of DC-based cancer vaccines. In 2008, STING (Stimulator of Interferon Genes), was identified as a protein that is indispensable for the recognition of cytosolic DNA. The central role of STING in controlling anticancer immune responses was exemplified by observations that spontaneous and radiation-induced adaptive anticancer immunity was reduced in the absence of STING, illustrating the potential of STING-targeting for cancer immunotherapy. Here, we will discuss the relevance of manipulating the STING signaling pathway for cancer treatment and integrating STING-targeting based strategies into combinatorial therapies to obtain long-lasting anticancer immune responses.
Keywords: Cancer immunotherapy, danger signal, DNA, anticancer therapies, adaptive immunity, innate immunity, STING
1. Introduction
Immunologists have long considered that the primary function of the immune system is to distinguish between self and non-self. However, the idea that the immune system only reacts to foreign organisms and is tolerant to self was difficult to reconcile with observations that individuals could feature antibodies to self-antigens, including DNA. In 1994, Polly Matzinger challenged the so-called Self-Nonself theory and proposed instead that the driving force that makes the immune system effective lies on its ability to recognize danger(1). Among the immune cell types able to detect danger, dendritic cells (DCs) are of central importance because of their ability to capture, process, and present antigens to T cells(2). The detection of danger by DCs relies on their expression of pattern recognition receptors (PRRs), which permit sensing, integration and transmission of danger signals to induce adaptive immunity. PRRs include membrane C-type lectins, Toll-like receptors (TLRs), cytoplasmic NOD-like receptors (NLRs) and DNA/RNA sensors(3, 4). These receptors allow DCs to sense pathogens as well as endogenous danger signals released from dying cells such as DNA(5, 6). These recognition mechanisms in DCs can be harnessed to generate more efficient cancer vaccines. For instance, immunogenicity of peptide-protein vaccines can be enhanced by the addition of adjuvants. These include agonists of various TLRs such as TLR3 (poly I:C), TLR4 (monophosphoryl lipid A; MPL), and TLR9 (CpG) (7–12).
The functional properties of DCs prompted their use as a tool in cancer immunotherapy with the aim of inducing anticancer immune responses. Initially, the use of non-targeted short peptides captured by DCs in vivo demonstrated that MHC class I-restricted antigen-specific CD8+ T cell immunity could be mounted in patients with metastatic disease(13–15). The clinical successes were yet limited, possibly because of the lack of CD4+ T cell help necessary for the generation of potent cytotoxic T lymphocytes (CTLs) and long-lived memory CD8+ T cells (16–18). While the clinical ineffectiveness of dendritic cell-based vaccines is attributable to the immunosuppressive cancer microenvironment that curtails the induction of anticancer immune responses(19, 20), the impressive successes of checkpoint inhibitor therapies, which result in 20-40% complete responses in some metastatic cancers, illustrate that cancer-induced immunosuppression can be pharmacologically overcome and anticancer immunity restored(21, 22). This altogether suggests that a better knowledge of DC biology is required to design DC vaccines able to reverse tumor-induced immunosuppression and elicit long-term anticancer responses.
DNA is a potent immune stimulatory molecule widely used as vaccine adjuvant to drive immunity(4, 23). Initially, TLR9 was identified as the sensor for DNA. TLR9 recognizes pathogen derived CpG DNA to trigger innate immune signaling predominantly in plasmacytoid dendritic cells (pDCs)(24). TLR9 was also shown to be responsible for the detection of self-DNA, leading to autoimmunity(25, 26). While TLR9 was promoting immune signaling following its interaction with DNA in endosomes, the mechanisms responsible for the detection of cytosolic DNA were unclear until the characterization of STING (Stimulator of Interferon Genes).
In 2008, STING was described as a transmembrane component of the endoplasmic reticulum (ER) essential for the production of type I IFN in fibroblasts, macrophages and dendritic cells (DCs) in response to cytoplasmic double-stranded DNA (dsDNA) as well as select DNA viruses and intracellular bacteria(27, 28). Interestingly, STING does not share homology with any known immunosensor and seems to represent a novel category of proteins involved in immune signaling in the context of cytosolic DNA presence, with an ability to link the majority of DNA sensors to immune signaling(29, 30). The detection of DNA indeed relies on a variety of cytoplasmic DNA sensors, including the cyclic GMP-AMP synthase (cGAS)(31). The discovery of cGAS in 2013 actually represented a significant advance in our understanding of the signaling mechanisms underpinning innate DNA sensing. After binding to cytosolic DNA species from viruses, bacteria, or self -DNA from the nucleus or mitochondria, cGAS catalyzes the production of a type of cyclic dinucleotide (CDN) named cGAMP (cyclic GMP–AMP)(32, 33). Following binding to CDNs, STING activation leads to the phosphorylation of interferon regulatory factor 3 (IRF3) and nuclear factor-κB (NFκB) and the subsequent induction of cytokines and proteins, such as the type I interferons (IFN) that exert anti-pathogen activities(28, 34). STING was proposed to be activated by other cytoplasmic DNA sensors, including DAI, DHX9, DHX36, IFI204 (IFI16), DDX41, DXX60, Pol III, LRRFIP1, DNA-PK, cGAS and the DNA repair protein Mre11(35), that bind DNA directly and act upstream of STING to induce type I IFNs(30). This together defines STING as an adaptor protein that is essential for immune signaling following pathogen DNA detection by cytoplasmic DNA sensors (reviewed in (36)). Recent reports have also indicated that potent activators of the STING pathway may also include self-DNA that has leaked from the nucleus of the host cell, perhaps following cell division or as a consequence of DNA damage(37). STING is thus central to the induction of immune responses following DNA detection.
In this review, we discuss recent findings illustrating the links between STING signaling in immune and cancer cells and cancer progression. We also describe emerging strategies that exploit the STING signaling pathway to enhance anticancer immune responses. We eventually highlight the relevance of modulating the STING pathway for cancer immunotherapy.
2. STING signaling in tumor promotion
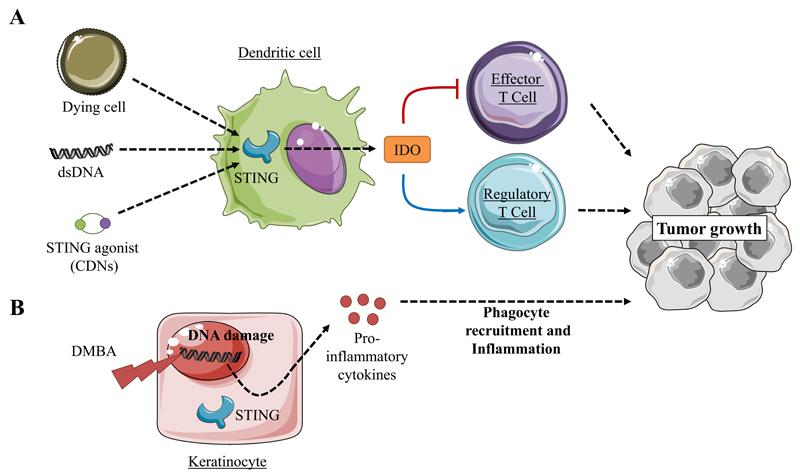
Studies have shown that STING activation could lead to inflammatory responses that promote tumorigenesis. Lemos and colleagues reported that DNA, apoptotic cells and STING agonists could induce STING-dependent tolerogenic responses. Mechanistically, they found that DNA sensing via the STING/IFN-β pathway induces indoleamine 2,3 dioxygenase (IDO), which catabolizes tryptophan to suppress effector and helper T-cell responses and activate regulatory T cells(38, 39) (Figure 1A). Moreover, IDO activity induced by STING in the tumor microenvironment promoted the growth of Lewis lung adenocarcinoma (LLC). In the LLC model, STING deficiency enhanced CD8+ T-cell infiltration and tumor cell killing while decreasing myeloid-derived suppressor cell infiltration and IL-10 production in the tumor microenvironment(40). In a model of cutaneous skin tumors induced by 7,12-dimethylbenz[α]anthracene (DMBA), a potent carcinogen causing DNA damage, STING-driven inflammation also promoted tumor growth. In this context, DNA damage resulted in the leakage of DNA into the cytosol and the intrinsic chronic activation of the STING pathway was associated to the recruitment of phagocytes, inflammation and tumor development (41) (Figure 1B). Accordingly, STING deficiency protected against DMBA-induced tumorigenesis(41).
Figure 1.
Involvement of STING in the promotion of tumor growth
A) The stimulation of the STING signaling pathway by DNA elicits IDO dependent inhibition of effector T cells while promoting regulatory T cell activity resulting in enhanced tumor growth(38–40).
B) The leakage of DNA induced by 7,12-dimethylbenz[α]anthracene(DMBA), a potent carcinogen, can result in intrinsic chronic activation of STING signaling which drives phagocyte recruitment inflammation and tumor growth(41).
3. STING in spontaneous anti-cancer immunity
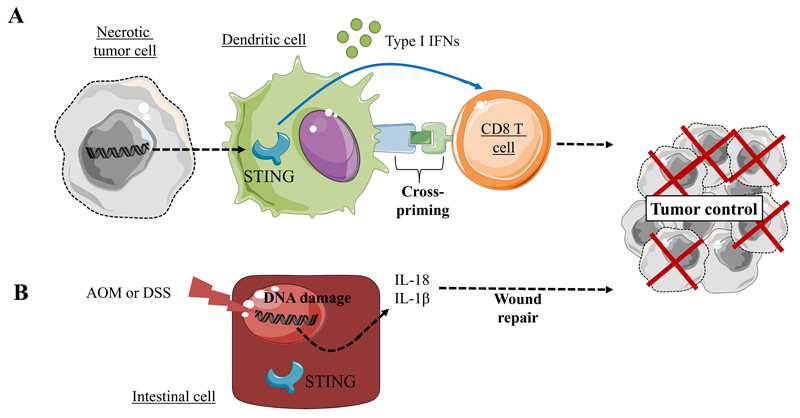
While the findings discussed above suggest that STING-driven chronic inflammation leads to cancer, STING-dependent DNA detection was also found to trigger anticancer immunity(29, 31). Importantly, activation of the STING pathway was correlated to the induction of a spontaneous antitumor T cell response involving the expression of Type I interferon (IFN) genes(29, 31). These observations are in line with several studies identifying type I IFN as critical mediator in the spontaneous priming of antitumor CD8+ T cell responses(42, 43). Accordingly, Woo and colleagues reported that the spontaneous CD8+ T cell priming against tumors was defective in mice lacking STING. Moreover, STING-deficient mice are unable to generate efficient antitumor T cell responses and prevent melanoma tumor growth(44) (Figure 2A). STING protein is predominantly expressed in macrophages, T cells, DCs endothelial cells and select fibroblasts and epithelial cells(27, 28, 45–47). However, in the tumor microenvironment, the main sources of IFN-β are DCs and endothelial cells(48, 49). One hypothesis to account for these observations is that CD8α+ DCs engulf necrotic tumor cells, and the tumor cell-derived DNA triggers STING signaling in DCs(44, 50–52). The resultant type I IFNs, functioning in a paracrine or autocrine manner, enhance DCs cross-presentation activity and T cell activation. Similarly, in a mouse model of de novo gliomas, CD11b+ brain-infiltrating leukocytes (BIL) are the main source of type I IFNs. Consequently, glioma-bearing mice with a single nucleotide variant (T596A) of STING that functions as a null allele and fails to produce detectable protein, showed shorter survival and lower expression levels of IFNs compared with wild-type mice. Furthermore, BILs of those mice showed increased CD11b+ Gr-1+ immature myeloid suppressor and CD25+ Foxp3+ regulatory T cells (Treg) and decreased IFNγ-producing CD8+ T cells(53). Accordingly, CD4+ and CD8+ T cells that received direct type I IFN signals showed lesser degrees of regulatory activity and increased levels of antitumor activity, respectively(53, 54).
Figure 2.
STING-driven cytokine secretion can activate adaptive immunity and prevent tumor growth
A) Tumor derived-DNA recognition by STING, leads to IFNα/β secretion by CD8α+ DC, increased cross-priming and T cell activation(44)
B) DNA damage induced by carcinogens, such as azoxymethane (AOM) or dextran sulfate sodium (DSS), triggers activation of the STING signaling pathway, resulting in IL-18 and IL-1β cytokine expression and favoring wound repair and tumor growth control(59).
4. STING in intestinal wound repair and therapy-induced anti-cancer immunity
The anticancer effects of STING activation were also demonstrated in a model of colitis-associated carcinogenesis (CAC). CAC can be experimentally induced by carcinogens and inflammatory agents such as azoxymethane (AOM) and dextran sulfate sodium (DSS)(52, 55). DNA damage induced by these agents resulted in the leakage of DNA into the cytosol and activation of the intrinsic STING pathway in intestinal cells. This event triggered wound repair cytokine expression such as IL-1β, IL-18 as well as IL-22 binding protein(56–59). This series of events contributed to tissue protection and prevented cancer development. Thus, STING signaling activation has important protective effects against colon cancer (Figure 2B).
Recent studies have shown that the STING pathway is also implicated in radiation-induced antitumor T cell responses(50). Antitumor effects of radiation were previously shown to be dependent on type I IFN signaling(60). Moreover, radiation induces cell stress and causes excess DNA breaks, indicating that nucleic acid sensing could account for the induction of type I IFNs upon radiation. Accordingly, the induction of IFN-β in tumors was reduced in the absence of STING in the host after radiation. In line with the immunogenicity of IFNs, the anticancer efficacy of radiation therapy was impaired in STING-deficient mice compared to controls, suggesting that STING-dependent cytosolic DNA sensing is critical for the therapeutic effect of radiation in vivo(50). STING was further shown to be essential for tumor-infiltrating DCs type I IFN production after radiation. The functional ability of DCs to cross-present antigen was augmented by the stimulation of irradiated-tumor cells compared to nonirradiated-tumor cells, whereas the deficiency of STING in DCs resulted in their inability to cross-prime CD8+ T cells(50). Thus a component provided by irradiated-tumor cells, presumably DNA, somehow gains access to the cytosolic DNA sensing pathway to trigger STING-dependent type I IFN induction and anticancer immunity(50).
STING is a DNA sensor located in the cytosol of the cell. This observation raises a major question concerning the DNA immunogenicity and suggests that the presence of DNA outside of the nucleus is the key danger signal for STING-dependent immune activation. As discussed above, the host immune system is able to initiate innate immune sensing of tumor DNA leading to the induction of a STING-dependent adaptive immune response against tumors. Mechanistically, it is unknown how DNA is transferred to APCs cytosol from tumor cells in order to activate the STING pathway. It was suggested that CD8α+ DCs engulf necrotic tumor cells, and the tumor cell-derived DNA triggers STING signaling in the DC(44, 50, 51). In line with this hypothesis, in vitro incubation of DCs with tumor-cell-derived DNA led to IFN-β production and DC activation via the cGAS-STING-IRF3 axis(44). To determine whether tumor-derived DNA can be transferred to host APCs within the tumor microenvironment and lead to STING activation, B16 tumor cells stained in vitro with DNA-intercalating dye DRAQ5 were implanted in vivo. The analysis of tumor infiltrating DCs revealed the presence of CD45+CD11c+ cells positive for staining with tumor-cell-derived DRAQ5. In addition, multiple tumor cell lines were also labeled with the nucleotide analog EdU prior to injection into mice. Similar to DRAQ5, EdU staining was observed on a large population of tumor-infiltrating CD45+CD11c+ cells, arguing that this is a general phenomenon. Moreover, by a co-staining approach, using the nuclear and lysosomal markers Lamin A and LAMP-1, the authors showed that the majority of the signal did not colocalize with either marker suggesting that the DNA label detected in host APCs appears to be localized in the cytosol, which would provide access to the STING pathway for engagement. Subsequently, the ectopic presence of tumor-derived DNA in the cytosol of DCs correlated with STING pathway activation illustrated by IRF-3 translocation to the nucleus and expression of IFN-β (Figure 2A)(44). Another study has shown that cGAS responds to irradiated-murine and -human tumor cells and initiates type I IFN to enhance DC cross-priming activity. Thus, DNA from irradiated-tumor cells somehow gains access to the cytosolic DNA sensing pathway to trigger STING-dependent type I IFN induction. The priming ability of DCs in response to irradiated-tumor cells was not impaired by the presence of DNase I, suggesting that DCs do not engulf free DNA fragments. By contrast, the addition of latrunculin B, an actin polymerization inhibitor, in the coculture of DCs and tumor irradiated cells led to a dramatic reduction in the ability of DCs to induce cross-priming suggesting that DNA delivery might be mediated by direct cell-cell contact. Production of IFN-β by DCs in response to irradiated-tumor cells was also greatly decreased by application of a physical barrier or an actin polymerization inhibitor. These results suggest that DNA from irradiated-tumor cells is sensed by host cGAS during a cell-cell contact-mediated process(50). Altogether, these results show that STING activation can trigger DNA-dependent anticancer immune responses.
5. STING expression shapes cancer cell immunogenicity
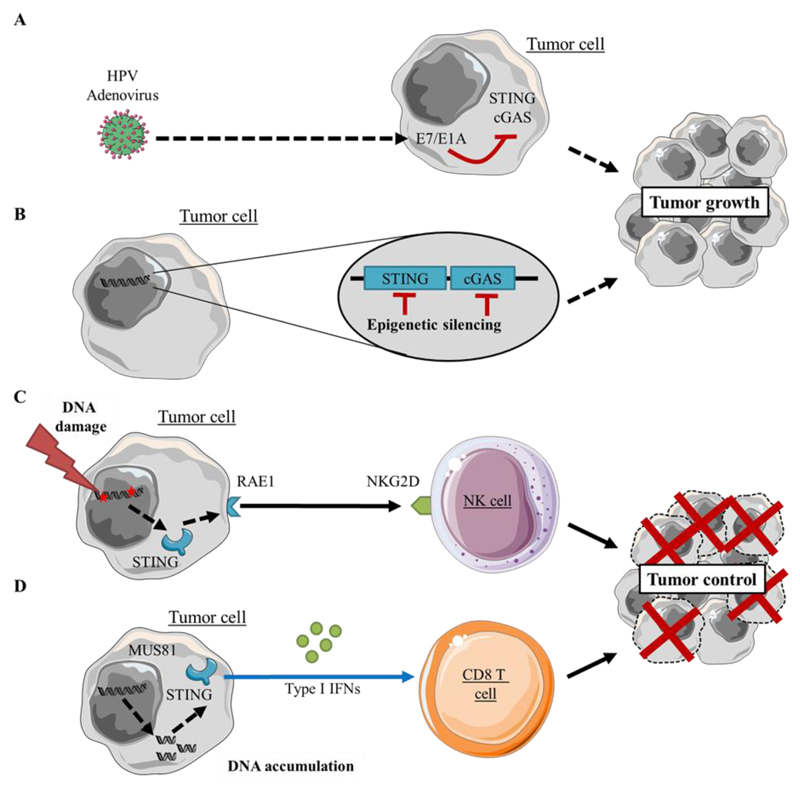
While the STING pathway has been mostly characterized in antigen presenting cells (APCs) in the tumor microenvironment, tumor cells, T cells, endothelial cells and fibroblasts all have been observed to produce type I IFN production upon stimulation with STING agonists ex vivo(61). The relevance of STING signaling in tumor cells was recently underscored. The oncogenes E7 and E1A expressed by DNA tumor viruses inhibit the cGAS-STING pathway and prevent innate immune signaling(62) (Figure 3A). Barber and colleagues also showed that STING signaling is inhibited in a wide variety of cancers. STING and/or cGAS expression is silenced through epigenetic hypermethylation processes. As a consequence of STING signaling loss, cancer cells featured impaired type I IFN secretion in response to DNA(63) (Figure 3B). Another study in lymphoma cells showed that cytosolic DNA contributes to the expression of retinoic acid early transcript 1 (RAE1) in response to DNA damage(64). The induction of RAE1 relies on a STING-dependent DNA sensor pathway involving the effector molecules TBK1 and IRF3. RAE1 is a ligand for the immunoreceptor NKG2D originally identified in natural killer cells that recognizes ligands that are upregulated on tumor cells. Expression of NKG2D ligands is activated by the DNA damage response, which is often activated constitutively in cancer cells, enabling their detection and killing by natural killer cells as a mechanism of immunosurveillance(65) (Figure 3C). The relevance of STING-driven enhancement of cancer immunogenicity was further shown in prostate cancer cells. In an elegant study, Ho et al. showed that the cleavage of genomic DNA by the endonuclease MUS81 was responsible for cytosolic DNA accumulation in prostate cancer cells, leading to their type I IFN-dependent rejection(66). These studies altogether demonstrate that STING expression by tumor cells can shape their immunogenicity and make a decisive contribution to cancer cell immunosurveillance (Figure 3D).
Figure 3.
Cell-intrinsic effect of STING activation in tumor cells
The functionality of the STING signaling pathway can be altered in tumors.
A) Oncolytic viruses like HPV or adenoviruses drive E7 or E1A oncoprotein expression. These proteins act as STING antagonists able to inhibit the STING pathway, possibly leading to their evasion from immune cells(62)
B) Spontaneous epigenetic silencing of STING signaling components is also observed in various cancer types(63)
C) Tumor DNA damage leads to Natural Killer cell activation through STING-dependent expression of RAE1(64).
D) MUS81 endonuclease induces genomic DNA cleavage and accumulation followed by STING, type I IFN and CD8 T cell dependent rejection of tumor cells(66)
6. STING in cancer immunotherapy
6.1. Pioneering studies for pharmacological use of STING agonists in immunotherapy
The first pharmacological STING agonist was initially used as an anticancer drug long before the discovery of STING. Flavone acetic acid (FAA) has potent antitumor activity against murine colon tumors (Table 1)(67). The further characterization of FAA as vascular disrupting agent led to testing its clinical potential. However, FAA failed in a phase I clinical trial and showed no activity in rat tumor models implying possible species specificity issues(68). To obtain compounds able to induce tumor hemorrhagic necrosis, the molecular structure of FAA was modified giving rise to 5,6-dimethyllxanthenone-4-acetic acid (DMXAA), which not only showed activity against a rat mammary carcinoma but also featured IFNs and TNF-dependent anticancer functions in different mouse models(69, 70). Unfortunately Phase III trials in non-small-cell lung cancer (NSCLC) patients failed to confirm the anti-tumor effect of DMXAA in humans(71). Further molecular characterization revealed that the mechanism underlying DMXAA-induced type I IFNs and TNF-α secretion and anticancer activity was STING dependent(72). Importantly, structural studies of mouse and human STING showed that only mouse STING binds and signals in response to DMXAA. This species-specific DMXAA recognition by STING likely explains the negative results observed in phase III clinical trial in humans (Table 1)(73–75).
Table 1.
| STING agonist | Co-treatment | Model | References |
|---|---|---|---|
| Flavone Acetic Acid (FAA) | - | Murine MC38 Colon model | Plowman and al, 1986, Cancer Treat Rep |
| - | Phase I Clinical and Pharmacokinetic Trial of LM985 (Flavone Acetic Acid Ester) and Flavone Acetic Acid in patients with advanced cancer | Kerr and al, 1986, Cancer Res Havlin and al, 1991, J Natl Cancer Inst | |
| DMXAA (ASA404) | - | Phase III clinical trial in patient with non–small-cell lung cancer | Lara and al, 2011, J Clin Oncol ClinicalTrials.gov Identifier: NCT00662597 |
| C-di-GMP | - | Murine 4T1 mammary model | Chandra and al, 2014, Cancer Immunol Res |
| - | Murine GL261 glioma model | Ohkuri, 2015, Oncoimmunology | |
| TRIVAX vaccine association | Murine B16 melanoma model | Wang and Celis, 2015, Cancer Immunol Immunother | |
| cGAMP | - | Murine B16 melanoma and MC38 Colon model | Demaria and al, 2015, PNAS USA |
| ionizing-radiation co-treatment | Murine MC38 Colon model | Deng and al, 2014, Immunity | |
| Alone and 5-FU co-treatment | Murine CT26 colon model | Li and al, 2016, Sci Rep | |
| Disodium dithio-(RP, RP)-[cyclic[A(2′,5′)p A(3′,5′)p]], (ML RR-S2 CDA) | - | Murine B16-F10 melanoma, 4T1 mammary and CT26 colon model | Corrales and al, 2016, Cell Rep |
| GM-CSF producing cells association (STINGVAX) | Murine B16 melanoma model | Fu and al, 2015, Sci Transl Med | |
| STINGVAX and anti-PD1 co-treatment | Murine B16 melanoma model | Fu and al, 2015, Sci Transl Med | |
| - | Phase I clinical trial in patients with advanced/metastatic solid tumors or lymphomas | ClinicalTrials.gov Identifier : NCT02675439 |
6.2. Cyclic dinucleotides (CDNs) STING agonists as potent anti-cancer agents in mice
As previously discussed, CDNs are second messengers able to activate the STING pathway, leading to type I IFNs and pro-inflammatory cytokine expression(76). In mice, cyclic diguanylate monophosphate (c-di-GMP) showed anti-tumor effects in the 4T1 metastatic breast cancer model when daily injected at low doses after immunization with an attenuated Listeria monocytogenes (LM)-based vaccine (Table 1 and Figure 4A)(77). These observations were confirmed in the GL261 glioma murine model and in the B16 melanoma bearing mice treated with c-di-GMP associated with the TRIVAX vaccine, a mix of synthetic CD8 T cell epitopes (Table 1 and Figure 4B)(77, 78). A recent study using the 1000 Human Genome Project database allowed the identification of five human STING (hSTING) alleles named WT, REF, HAQ, AQ and Q. This variability on hSTING gene does not exist in mice, explaining the divergence concerning STING activation upon different CDNs treatments. Indeed, some of these natural variants of hSTING are poorly responsive to canonical CDNs(79). Thus, bacterial-derived canonical CDNs molecules may not be suitable for clinical development(80, 81).
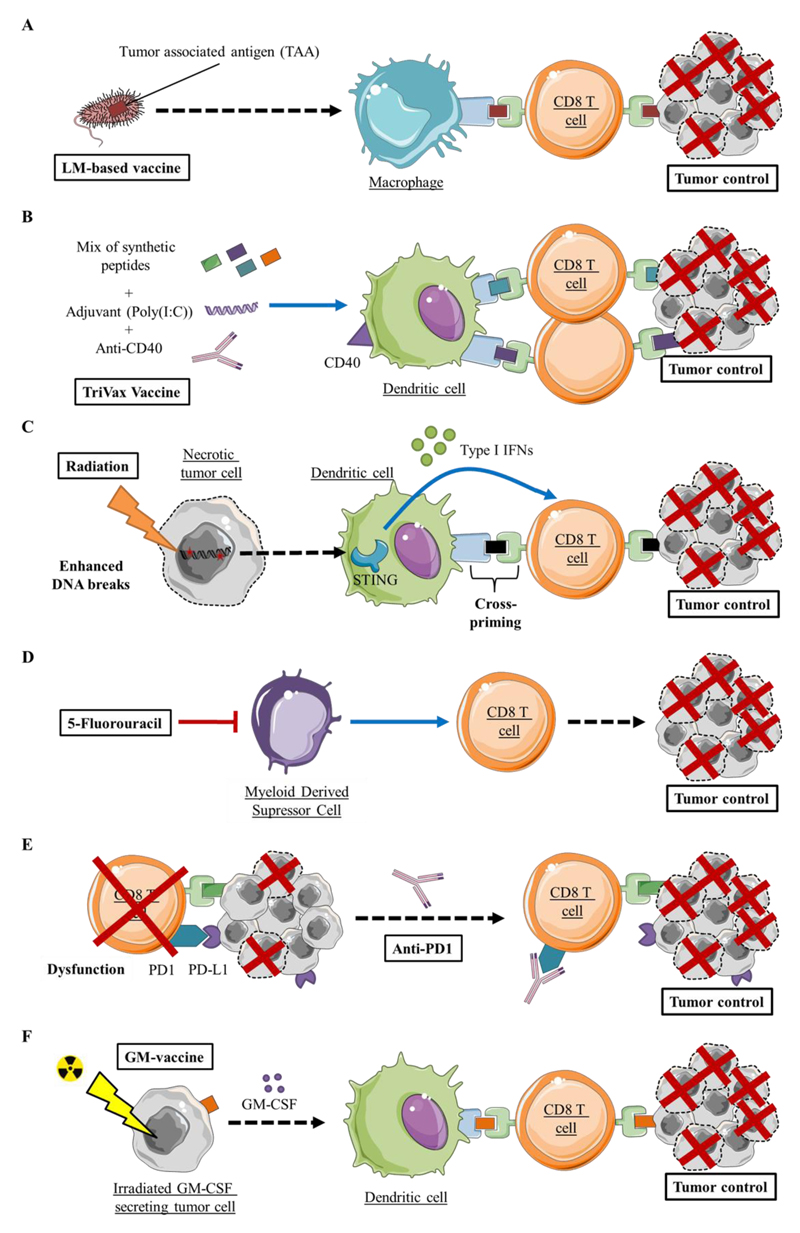
Figure 4.
Therapeutic strategies combining STING targeting with immunomodulation and anticancer therapies
The addition of STING agonists was shown to enhance the anticancer activity of the following anticancer therapies and immunomodulation strategies that elicit CD8 T cell dependent anticancer responses:
A) Vaccination using Tumor Associated Antigen expressing attenuated Listeria Monocytogenes(77)
B) Vaccination using Trivax (anti-CD40 as a co-stimulation signal, Poly(I:C) as adjuvant and peptide mix)(78)
C) Radiotherapy(50)
D) Chemotherapy, such as 5-Fluorouracil(82)
E) Checkpoint inhibitors, such as anti-PD1(88)
F) Vaccination using irradiated GM-CSF secreting tumor cells(88)
Cyclic GMP-AMP (cGAMP) is also a natural STING ligand(31). Importantly, there are hSTING variants poorly responsive to cGAMP but normally responsive to DNA and cGAS signaling. In an effort to explain this paradox, Diner and colleagues found that the cGAS product is actually a noncanonical CDN [G(2′-5′)pA(3′-5′)p], which contains a single 2′-5′ phosphodiester bond. These results indicate that hSTING variants are able to distinguish conventional (3′-5′) CDNs, mainly produced by bacteria, from the noncanonical CDNs produced by mammalian cGAS(80). Like c-di-GMP and DMXAA, studies demonstrated CD8+ T and type I IFNs dependent antitumor effect of cGAMP, in melanoma and colon cancer models in mice(48). It was also demonstrated that STING was required for type I IFN-dependent antitumor effects of ionizing-radiation with an enhanced antitumor immunity with cGAMP co-treatment(50). Intratumorally administrated cGAMP after radiation effectively reduced tumor burden compared to radiation alone in mice, showing that cGAMP treatment potentiates the effect of radiation(50) (Figure 4C). In 2016, Li et al. confirmed the potent antitumor effect of cGAMP in CT26 colon adenocarcinoma bearing mice. The antitumor activity of cGAMP relied on DC activation and CD8+ T cell cross-priming. The improved antitumor activity and the reduced toxicity of 5-FU in combination with cGAMP injection strengthened the therapeutic potential of cGAMP for applications in cancer immunotherapy(82) (Table 1 and Figure 4D). Woo and colleagues also tested the contribution of STING signaling in context of combined treatment with checkpoint inhibitors and found that the therapeutic effect of CTLA-4 and anti-PD-L1 mAbs was lost in STING-deficient mice(44). Their results suggest that the host STING pathway plays a critical role in the therapeutic efficacy of cancer immunotherapies and provide impetus to deliberately activate STING using STING agonists for treating cancer. Thus, manipulation of STING signaling can be successfully integrated in the context of combination therapies (Table 1).
In addition to its established role as a signaling adaptor in the response to cytosolic DNA, STING was proposed to function as a direct sensor able to directly bind different DNA species or CDNs(76, 83). Biotin pull-down assays using in vitro transcribed STING and different biotinylated DNA species showed that STING directly binds ssDNA and dsDNA without a requirement for accessory molecules(83). Moreover, using radiolabeled c-di-GMP32 binding assays, Burdette and colleagues shown that STING can also directly and specifically binds CDNs(76) in contrast to a study showing that c-di-GMP was detected by DDX41(84). To unveil the underlying mechanism, Parvatiyar and colleagues performed binding assays to determine the affinities of c-di-GMP for DDX41 and for STING in parallel. Physiologically, binding of c-di-GMP with endogenous DDX41 turned out to be greater than the association between c-di-GMP and endogenous STING. Accordingly, c-di-GMP bound to purified recombinant DDX41 with stronger affinity than purified recombinant STING in pulldown binding assays. These findings thus suggest that even though STING can directly bind c-di-GMP, DDX41 is the major sensor of c-di-GMP, operating upstream of STING to facilitate downstream signaling and type I IFN activation(84). In line with this work, Zhang and colleagues found that c-di-GMP bound to STING with a Kd of 1.21 μM(85). Interestingly, both natural cGAMP and synthetic 2′3′-cGAMP bound to STING with a high affinity(85). Indeed, the Kd of 2′3′-cGAMP was nearly 300 fold lower than those of c-di-GMP, 3′2′-cGAMP and 3′3′-cGAMP, and around 75 fold lower than that of 2′2′-cGAMP. In addition, unlike the binding of c-di-GMP, which is an exothermic process, the binding of natural and 2′3′-cGAMP to STING was endothermic, suggesting that the energy may be used for STING conformational change(85). The authors further showed that 2′3′- cGAMP and other cGAMP isomers are much more potent than c-di-GMP in inducing IFNβ in cells(85). Overall, these results illustrate the potential to design cGAMP isomers with a potentially enhanced ability to trigger type I IFN secretion, thereby resulting in more potent anticancer immune responses.
6.3. STING agonists for cancer treatment in humans
In 2014, Li discovered an ecto-nucleotide pyrophosphatase/phosphodiesterase enzyme named ENPP1(86). This enzyme is a 2'3'cGAMP hydrolizing protein, giving rise to a hydrolysis-resistant bisphosphothioate analog of 2′3′-cGAMP named 2′3′- cGsAsMP. This improved hSTING agonist could have higher potency for cancer therapy as vaccine adjuvant in humans(86). In the light of Li’s work a new synthetic CDN, ML RR-S2 CDA, with high stability, high anti-tumor effect and able to activate all hSTING variants, has been synthesized. This improved synthetic CDN contains, like endogenous human 2'3' cGAMP, a phosphate bridge configuration with both 2′-5′ and 3′-5′ linkages, called “mixed linkage” (ML). This ML endows this molecule with increased binding affinity to STING. Moreover, like the 2′3′- cGsAsMP, the bisphosphothioate analog of ML-CDA (ML RR-S2 CDA) is protected against ENPP1 hydrolysis and has higher ability for STING stimulation. Accordingly, this new synthetic CDNs agonist has shown potent anti-tumor efficiency in various tumor models like B16F10 melanoma, 4T1 mammary adenocarcinoma and CT26 colon carcinoma dependent on STING and CD8+ T priming. ML RR-S2 CDA also induced the establishment of long-term immune memory(87). This agonist has also been used in combination with other immunomodulatory agents. In 2015, Fu synthetized STINGVAX, a cell based cancer vaccine combining synthetic CDNs, used in Corrales study including ML RR-S2 CDA, with granulocyte-macrophage colony-stimulating factor (GM-CSF)–producing cells (Table 1 and Figure 4F). The high efficiency of this treatment in B16 melanoma bearing mice could also be increased upon neutralization of the PD-1/PD-L1 pathway(88) (Figure 4E). Similar results were obtained in a mouse model of head and neck cancer(89). A clinical trial studying the safety and efficacy of ML RR-S2 CDA (also called MIW815 or ADU-S100) in patients with advanced/metastatic solid tumors or lymphomas (ClinicalTrials.gov Identifier: NCT02675439) is actually ongoing (Table 1). Despite all this encouraging evidence showing the rationale for implementing STING targeting therapy into the clinic, further characterization of the STING pathway is necessary for the development of tailored treatments relying on the modulation of the STING pathway.
Conclusion
STING protein is essential for cytosolic DNA sensing in mammal cells. Although the precise mechanism of action of STING is currently not fully understood, STING acts as a scaffold protein for the assembly of multiprotein complexes driving type I IFN and inflammatory cytokine production. An increasing amount of evidence indicates that intratumoral STING agonists are promising cancer therapeutic agents. However, numerous questions still remain unanswered. It is still unclear how tumor derived DNA gains access to host APCs following tumor cell death. The role of STING signaling in the efficacy of other cancer therapeutics in addition to radiotherapy, including chemotherapy and kinase inhibitors, remains to be characterized. Little is also known about STING pathway regulation and the characterization of negative feedback mechanisms will facilitate the establishment of more accurate strategies to regulate the STING signaling pathway for therapeutic use. Eventually, we believe that STING axis activation and its functional consequences in different cell subsets within the tumor microenvironment need deeper characterization. CD4 and CD8 T cells actively participate in shaping antitumor immunity in the tumor microenvironment(90–92). Interestingly STING is expressed at high levels in lymphoid tissues and particularly, in T lymphocytes suggesting that STING might be an active player in T cell signaling cascades (our unpublished data and (93, 94)). Further investigation will be required to address this hypothesis, but even if we assume that STING-mediated induction of IFNs/ISG in T cells is not physiologically relevant in norm(95), it nevertheless becomes of high importance for the study of high affinity synthetic agonists of STING for their subsequent use as anticancer therapeutic agents.
Acknowledgements
The authors are supported by grants from the Fondation de France (L.A. and T.R.V.), the Association pour la recherche sur le cancer (L.A.), the Ligue Régionale contre le cancer comité grand est (L.A.), the Institut Mérieux (L.A.), the Conseil Régional de Bourgogne and FEDER (L.A.), the Agence Nationale de la Recherche [ANR-13-JSV3-0001] (L.A.) and [ANR-11-LABX-0021]. L.A. has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement N°677251).
Footnotes
Conflict of interest: Lionel Apetoh is a consultant for Roche and Merck.
References
- 1.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol. 2011;23(1):10–20. doi: 10.1016/j.coi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12(7):479–91. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 5.Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. doi: 10.1146/annurev-immunol-031210-101340. [DOI] [PubMed] [Google Scholar]
- 6.Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12(3):168–79. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubensky TW, Jr, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22(3):155–61. doi: 10.1016/j.smim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Aarntzen EH, Figdor CG, Adema GJ, Punt CJ, de Vries IJ. Dendritic cell vaccination and immune monitoring. Cancer Immunol Immunother. 2008;57(10):1559–68. doi: 10.1007/s00262-008-0553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalinski P, Muthuswamy R, Urban J. Dendritic cells in cancer immunotherapy: vaccines and combination immunotherapies. Expert Rev Vaccines. 2013;12(3):285–95. doi: 10.1586/erv.13.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuler G. Dendritic cells in cancer immunotherapy. Eur J Immunol. 2010;40(8):2123–30. doi: 10.1002/eji.201040630. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117(5):1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4(3):321–7. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speiser DE, Baumgaertner P, Voelter V, Devevre E, Barbey C, Rufer N, et al. Unmodified self antigen triggers human CD8 T cells with stronger tumor reactivity than altered antigen. Proc Natl Acad Sci U S A. 2008;105(10):3849–54. doi: 10.1073/pnas.0800080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175(9):6169–76. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 17.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434(7029):88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 18.Filipazzi P, Pilla L, Mariani L, Patuzzo R, Castelli C, Camisaschi C, et al. Limited induction of tumor cross-reactive T cells without a measurable clinical benefit in early melanoma patients vaccinated with human leukocyte antigen class I-modified peptides. Clin Cancer Res. 2012;18(23):6485–96. doi: 10.1158/1078-0432.CCR-12-1516. [DOI] [PubMed] [Google Scholar]
- 19.Melief CJ, van Hall T, Arens R, Ossendorp F, van der Burg SH. Therapeutic cancer vaccines. J Clin Invest. 2015;125(9):3401–12. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6(10):715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 21.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 23.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol. 2000;18:927–74. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 25.Krieg AM, Vollmer J. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol Rev. 2007;220:251–69. doi: 10.1111/j.1600-065X.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 26.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6(11):823–35. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–8. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–92. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15(12):760–70. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unterholzner L. The interferon response to intracellular DNA: why so many receptors? Immunobiology. 2013;218(11):1312–21. doi: 10.1016/j.imbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142–9. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–30. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abe T, Barber GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-kappaB activation through TBK1. J Virol. 2014;88(10):5328–41. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondo T, Kobayashi J, Saitoh T, Maruyama K, Ishii KJ, Barber GN, et al. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci U S A. 2013;110(8):2969–74. doi: 10.1073/pnas.1222694110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38(5):870–80. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn J, Barber GN. Self-DNA, STING-dependent signaling and the origins of autoinflammatory disease. Curr Opin Immunol. 2014;31:121–6. doi: 10.1016/j.coi.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Lemos H, Huang L, McGaha TL, Mellor AL. Cytosolic DNA sensing via the stimulator of interferon genes adaptor: Yin and Yang of immune responses to DNA. Eur J Immunol. 2014;44(10):2847–53. doi: 10.1002/eji.201344407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang L, Li L, Lemos H, Chandler PR, Pacholczyk G, Baban B, et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J Immunol. 2013;191(7):3509–13. doi: 10.4049/jimmunol.1301419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, et al. STING Promotes the Growth of Tumors Characterized by Low Antigenicity via IDO Activation. Cancer Res. 2016;76(8):2076–81. doi: 10.1158/0008-5472.CAN-15-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-driven carcinogenesis is mediated through STING. Nat Commun. 2014;5:5166. doi: 10.1038/ncomms6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6(7):722–9. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 43.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun W, Li Y, Chen L, Chen H, You F, Zhou X, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci U S A. 2009;106(21):8653–8. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29(4):538–50. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28(16):5014–26. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci U S A. 2015;112(50):15408–13. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klarquist J, Hennies CM, Lehn MA, Reboulet RA, Feau S, Janssen EM. STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J Immunol. 2014;193(12):6124–34. doi: 10.4049/jimmunol.1401869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A. 2012;109(47):19386–91. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, et al. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res. 2014;2(12):1199–208. doi: 10.1158/2326-6066.CIR-14-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun. 2011;79(2):688–94. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salcedo R, Cataisson C, Hasan U, Yuspa SH, Trinchieri G. MyD88 and its divergent toll in carcinogenesis. Trends Immunol. 2013;34(8):379–89. doi: 10.1016/j.it.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu Q, Man SM, Gurung P, Liu Z, Vogel P, Lamkanfi M, et al. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J Immunol. 2014;193(10):4779–82. doi: 10.4049/jimmunol.1402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491(7423):259–63. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207(8):1625–36. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahn J, Konno H, Barber GN. Diverse roles of STING-dependent signaling on the development of cancer. Oncogene. 2015;34(41):5302–8. doi: 10.1038/onc.2014.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–96. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corrales L, Gajewski TF. Molecular Pathways: Targeting the Stimulator of Interferon Genes (STING) in the Immunotherapy of Cancer. Clin Cancer Res. 2015;21(21):4774–9. doi: 10.1158/1078-0432.CCR-15-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau L, Gray EE, Brunette RL, Stetson DB. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science. 2015;350(6260):568–71. doi: 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
- 63.Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING Signaling in Colorectal Carcinoma Constrains DNA Damage Responses and Correlates With Tumorigenesis. Cell Rep. 2016;14(2):282–97. doi: 10.1016/j.celrep.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lam AR, Le Bert N, Ho SS, Shen YJ, Tang ML, Xiong GM, et al. RAE1 ligands for the NKG2D receptor are regulated by STING-dependent DNA sensor pathways in lymphoma. Cancer Res. 2014;74(8):2193–203. doi: 10.1158/0008-5472.CAN-13-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ho SS, Zhang WY, Tan NY, Khatoo M, Suter MA, Tripathi S, et al. The DNA Structure-Specific Endonuclease MUS81 Mediates DNA Sensor STING-Dependent Host Rejection of Prostate Cancer Cells. Immunity. 2016;44(5):1177–89. doi: 10.1016/j.immuni.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Plowman J, Narayanan VL, Dykes D, Szarvasi E, Briet P, Yoder OC, et al. Flavone acetic acid: a novel agent with preclinical antitumor activity against colon adenocarcinoma 38 in mice. Cancer Treat Rep. 1986;70(5):631–5. [PubMed] [Google Scholar]
- 68.Cummings J, Smyth JF. Flavone 8-acetic acid: our current understanding of its mechanism of action in solid tumours. Cancer Chemother Pharmacol. 1989;24(5):269–72. doi: 10.1007/BF00304756. [DOI] [PubMed] [Google Scholar]
- 69.Liu JJ, Ching LM, Goldthorpe M, Sutherland R, Baguley BC, Kirker JA, et al. Antitumour action of 5,6-dimethylxanthenone-4-acetic acid in rats bearing chemically induced primary mammary tumours. Cancer Chemother Pharmacol. 2007;59(5):661–9. doi: 10.1007/s00280-006-0321-7. [DOI] [PubMed] [Google Scholar]
- 70.Baguley BC, Ching LM. Immunomodulatory actions of xanthenone anticancer agents. BioDrugs. 1997;8(2):119–27. doi: 10.2165/00063030-199708020-00005. [DOI] [PubMed] [Google Scholar]
- 71.Lara PN, Jr, Douillard JY, Nakagawa K, von Pawel J, McKeage MJ, Albert I, et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(22):2965–71. doi: 10.1200/JCO.2011.35.0660. [DOI] [PubMed] [Google Scholar]
- 72.Prantner D, Perkins DJ, Lai W, Williams MS, Sharma S, Fitzgerald KA, et al. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J Biol Chem. 2012;287(47):39776–88. doi: 10.1074/jbc.M112.382986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao P, Ascano M, Zillinger T, Wang W, Dai P, Serganov AA, et al. Structure-function analysis of STING activation by c[G(2',5')pA(3',5')p] and targeting by antiviral DMXAA. Cell. 2013;154(4):748–62. doi: 10.1016/j.cell.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S, Li L, Maliga Z, Yin Q, Wu H, Mitchison TJ. Anticancer flavonoids are mouse-selective STING agonists. ACS Chem Biol. 2013;8(7):1396–401. doi: 10.1021/cb400264n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190(10):5216–25. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478(7370):515–8. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, et al. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res. 2014;2(9):901–10. doi: 10.1158/2326-6066.CIR-13-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Z, Celis E. STING activator c-di-GMP enhances the anti-tumor effects of peptide vaccines in melanoma-bearing mice. Cancer Immunol Immunother. 2015;64(8):1057–66. doi: 10.1007/s00262-015-1713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yi G, Brendel VP, Shu C, Li P, Palanathan S, Cheng Kao C. Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic dinucleotides. PLoS One. 2013;8(10):e77846. doi: 10.1371/journal.pone.0077846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 2013;3(5):1355–61. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, et al. Cyclic [G(2',5')pA(3',5')p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153(5):1094–107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li T, Cheng H, Yuan H, Xu Q, Shu C, Zhang Y, et al. Antitumor Activity of cGAMP via Stimulation of cGAS-cGAMP-STING-IRF3 Mediated Innate Immune Response. Sci Rep. 2016;6:19049. doi: 10.1038/srep19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abe T, Harashima A, Xia T, Konno H, Konno K, Morales A, et al. STING recognition of cytoplasmic DNA instigates cellular defense. Mol Cell. 2013;50(1):5–15. doi: 10.1016/j.molcel.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13(12):1155–61. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51(2):226–35. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L, Yin Q, Kuss P, Maliga Z, Millan JL, Wu H, et al. Hydrolysis of 2'3'-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat Chem Biol. 2014;10(12):1043–8. doi: 10.1038/nchembio.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015;11(7):1018–30. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7(283):283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moore E, Clavijo PE, Davis R, Cash H, Van Waes C, Kim Y, et al. Established T Cell-Inflamed Tumors Rejected after Adaptive Resistance Was Reversed by Combination STING Activation and PD-1 Pathway Blockade. Cancer Immunol Res. 2016;4(12):1061–1071. doi: 10.1158/2326-6066.CIR-16-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Apetoh L, Smyth MJ, Drake CG, Abastado JP, Apte RN, Ayyoub M, et al. Consensus nomenclature for CD8+ T cell phenotypes in cancer. Oncoimmunology. 2015;4(4):e998538. doi: 10.1080/2162402X.2014.998538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rivera Vargas T, Humblin E, Vegran F, Ghiringhelli F, Apetoh L. TH9 cells in anti-tumor immunity. Semin Immunopathol. 2016 doi: 10.1007/s00281-016-0599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 93.Poltorak A, Kurmyshkina O, Volkova T. Stimulator of interferon genes (STING): A “new chapter” in virus-associated cancer research. Lessons from wild-derived mouse models of innate immunity. Cytokine Growth Factor Rev. 2016;29:83–91. doi: 10.1016/j.cytogfr.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 94.Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, et al. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science. 2014;343(6169):428–32. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berg RK, Rahbek SH, Kofod-Olsen E, Holm CK, Melchjorsen J, Jensen DG, et al. T cells detect intracellular DNA but fail to induce type I IFN responses: implications for restriction of HIV replication. PLoS One. 2014;9(1):e84513. doi: 10.1371/journal.pone.0084513. [DOI] [PMC free article] [PubMed] [Google Scholar]