Abstract
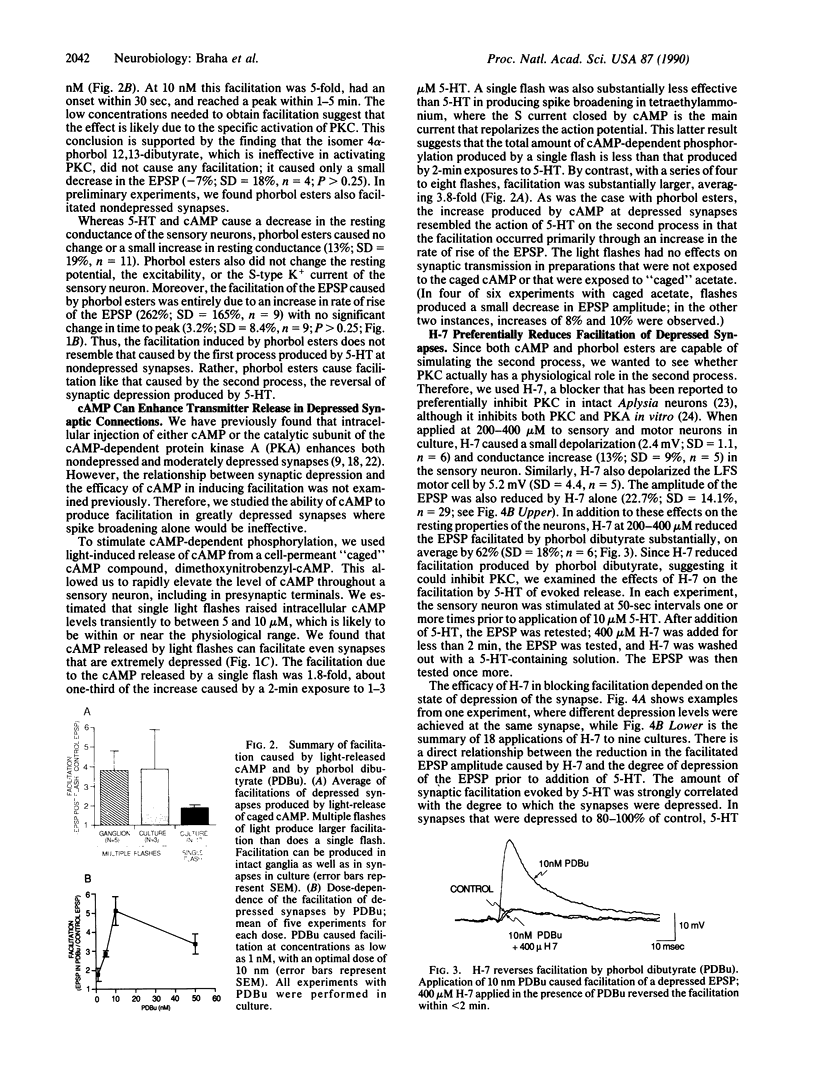
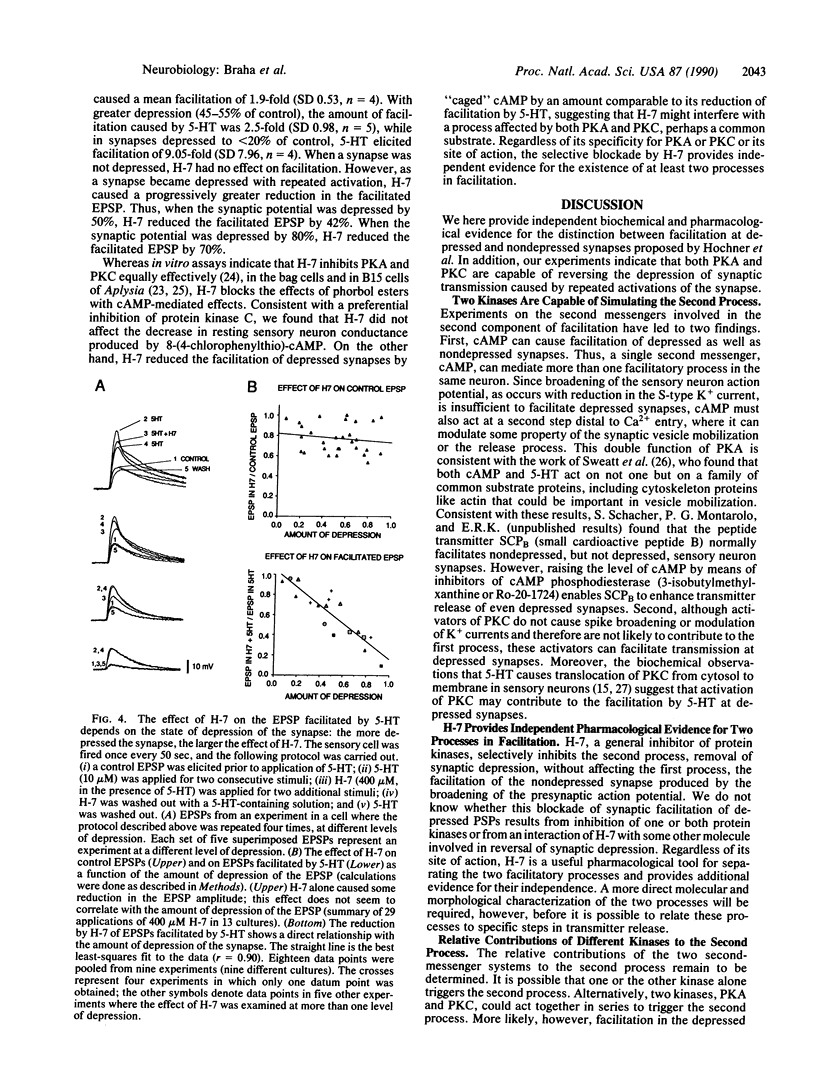
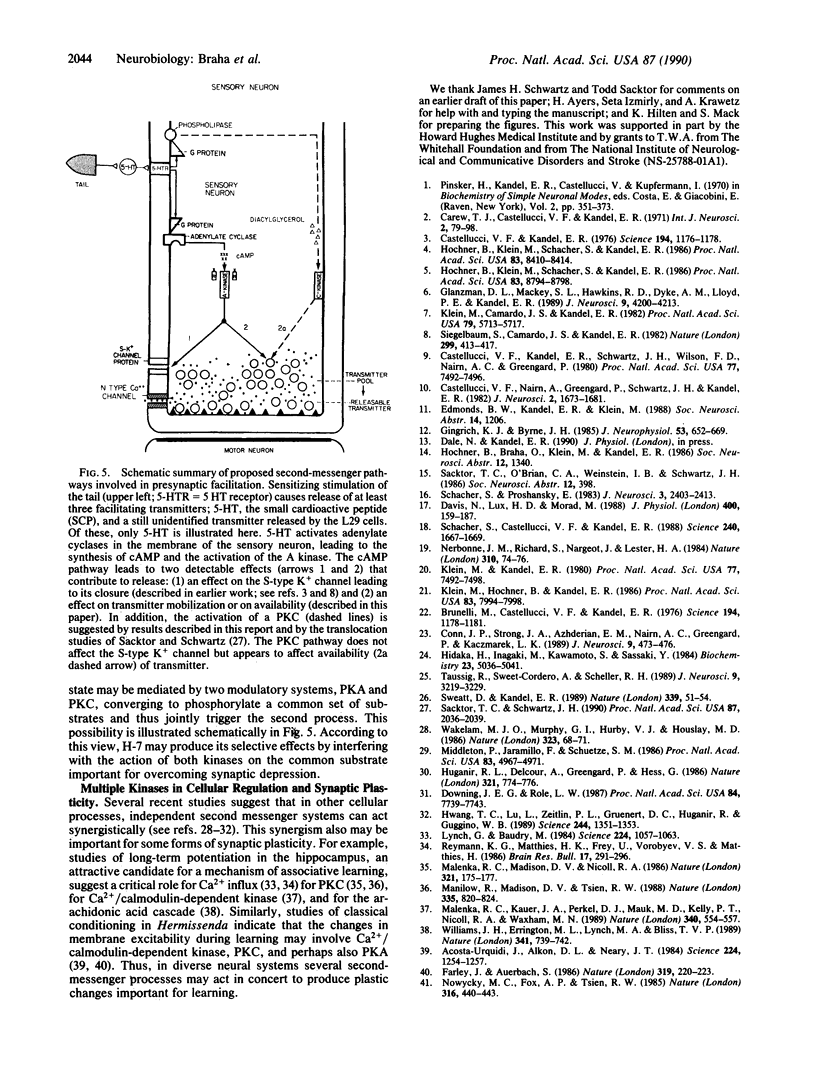
Presynaptic facilitation of transmitter release contributes to behavioral sensitization and dishabituation, two simple forms of learning in Aplysia. This enhancement of transmitter release can be simulated by the facilitatory transmitter serotonin and has been shown to result from two types of mechanisms. The first facilitating process involves broadening of the presynaptic action potential in the sensory neurons of the reflex and is maximally effective when the synapse has not been depressed by repeated stimulation, as during sensitization. The second process is independent of changes in spike duration and can enhance release even when the synapse is quite depressed, as during dishabituation. Earlier work suggests that the first process is mediated by an increase in the intracellular level of cyclic AMP in the sensory neurons. We show here that release of free cyclic AMP from a photolyzable analogue introduced into sensory neurons can enhance release even at depressed synapses, indicating that cyclic AMP can activate the second as well as the first process. In addition, we find that phorbol esters, activators of protein kinase C, enhance release at depressed synapses. This is consistent with the report in the accompanying paper [Sacktor, T. C. and Schwartz, J. H. (1990) Proc. Natl. Acad. Sci. USA 87, 2036-2039] that serotonin and sensitizing stimuli translocate protein kinase C from cytoplasm to membrane. Our findings suggest that the cyclic AMP-dependent phosphorylation system can mediate more than one facilitatory process and that both cyclic AMP-dependent kinase and protein kinase C may be involved in facilitation of depressed synapses.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta-Urquidi J., Alkon D. L., Neary J. T. Ca2+-dependent protein kinase injection in a photoreceptor mimics biophysical effects of associative learning. Science. 1984 Jun 15;224(4654):1254–1257. doi: 10.1126/science.6328653. [DOI] [PubMed] [Google Scholar]
- Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976 Dec 10;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Castellucci V. F., Kandel E. R. An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int J Neurosci. 1971 Aug;2(2):79–98. doi: 10.3109/00207457109146995. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R., Schwartz J. H., Wilson F. D., Nairn A. C., Greengard P. Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R., Schwartz J. H., Wilson F. D., Nairn A. C., Greengard P. Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Nairn A., Greengard P., Schwartz J. H., Kandel E. R. Inhibitor of adenosine 3':5'-monophosphate-dependent protein kinase blocks presynaptic facilitation in Aplysia. J Neurosci. 1982 Dec;2(12):1673–1681. doi: 10.1523/JNEUROSCI.02-12-01673.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V., Kandel E. R. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976 Dec 10;194(4270):1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Conn P. J., Strong J. A., Azhderian E. M., Nairn A. C., Greengard P., Kaczmarek L. K. Protein kinase inhibitors selectively block phorbol ester- or forskolin-induced changes in excitability of Aplysia neurons. J Neurosci. 1989 Feb;9(2):473–479. doi: 10.1523/JNEUROSCI.09-02-00473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. W., Lux H. D., Morad M. Site and mechanism of activation of proton-induced sodium current in chick dorsal root ganglion neurones. J Physiol. 1988 Jun;400:159–187. doi: 10.1113/jphysiol.1988.sp017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. E., Role L. W. Activators of protein kinase C enhance acetylcholine receptor desensitization in sympathetic ganglion neurons. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7739–7743. doi: 10.1073/pnas.84.21.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley J., Auerbach S. Protein kinase C activation induces conductance changes in Hermissenda photoreceptors like those seen in associative learning. Nature. 1986 Jan 16;319(6050):220–223. doi: 10.1038/319220a0. [DOI] [PubMed] [Google Scholar]
- Gingrich K. J., Byrne J. H. Simulation of synaptic depression, posttetanic potentiation, and presynaptic facilitation of synaptic potentials from sensory neurons mediating gill-withdrawal reflex in Aplysia. J Neurophysiol. 1985 Mar;53(3):652–669. doi: 10.1152/jn.1985.53.3.652. [DOI] [PubMed] [Google Scholar]
- Glanzman D. L., Mackey S. L., Hawkins R. D., Dyke A. M., Lloyd P. E., Kandel E. R. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989 Dec;9(12):4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hochner B., Klein M., Schacher S., Kandel E. R. Action-potential duration and the modulation of transmitter release from the sensory neurons of Aplysia in presynaptic facilitation and behavioral sensitization. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8410–8414. doi: 10.1073/pnas.83.21.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochner B., Klein M., Schacher S., Kandel E. R. Additional component in the cellular mechanism of presynaptic facilitation contributes to behavioral dishabituation in Aplysia. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8794–8798. doi: 10.1073/pnas.83.22.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Hochner B., Kandel E. R. Facilitatory transmitters and cAMP can modulate accommodation as well as transmitter release in Aplysia sensory neurons: Evidence for parallel processing in a single cell. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7994–7998. doi: 10.1073/pnas.83.20.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984 Jun 8;224(4653):1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., Waxham M. N. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989 Aug 17;340(6234):554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Madison D. V., Tsien R. W. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988 Oct 27;335(6193):820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- Middleton P., Jaramillo F., Schuetze S. M. Forskolin increases the rate of acetylcholine receptor desensitization at rat soleus endplates. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4967–4971. doi: 10.1073/pnas.83.13.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerbonne J. M., Richard S., Nargeot J., Lester H. A. New photoactivatable cyclic nucleotides produce intracellular jumps in cyclic AMP and cyclic GMP concentrations. Nature. 1984 Jul 5;310(5972):74–76. doi: 10.1038/310074a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Reymann K. G., Matthies H. K., Frey U., Vorobyev V. S., Matthies H. Calcium-induced long-term potentiation in the hippocampal slice: characterization of the time course and conditions. Brain Res Bull. 1986 Sep;17(3):291–296. doi: 10.1016/0361-9230(86)90234-0. [DOI] [PubMed] [Google Scholar]
- Sacktor T. C., Schwartz J. H. Sensitizing stimuli cause translocation of protein kinase C in Aplysia sensory neurons. Proc Natl Acad Sci U S A. 1990 Mar;87(5):2036–2039. doi: 10.1073/pnas.87.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S., Castellucci V. F., Kandel E. R. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science. 1988 Jun 17;240(4859):1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- Schacher S., Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci. 1983 Dec;3(12):2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Sweatt J. D., Kandel E. R. Persistent and transcriptionally-dependent increase in protein phosphorylation in long-term facilitation of Aplysia sensory neurons. Nature. 1989 May 4;339(6219):51–54. doi: 10.1038/339051a0. [DOI] [PubMed] [Google Scholar]
- Taussig R., Sweet-Cordero A., Scheller R. H. Modulation of ionic currents in Aplysia motor neuron B15 by serotonin, neuropeptides, and second messengers. J Neurosci. 1989 Sep;9(9):3218–3229. doi: 10.1523/JNEUROSCI.09-09-03218.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelam M. J., Murphy G. J., Hruby V. J., Houslay M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986 Sep 4;323(6083):68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- Williams J. H., Errington M. L., Lynch M. A., Bliss T. V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989 Oct 26;341(6244):739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]





