Abstract
3,4-Methylenedioxypyrovalerone (MDPV) is a commonly abused synthetic cathinone in the United States and is associated with dangerous side effects. MDPV is a dopamine transporter blocker that is 10-fold more potent than cocaine as a locomotor stimulant in rats. Previous in vitro and in vivo metabolism studies identified 3,4-dihydroxypyrovalerone (3,4-catechol-PV) and 4-hydroxy-3-methoxypyrovalerone (4-OH-3-MeO-PV) as the two primary MDPV metabolites. This study examined MDPV pharmacokinetics and metabolism, along with associated pharmacodynamic effects in rats receiving 0.5, 1.0 and 2.0 mg/kg subcutaneous (s.c.) MDPV. Blood was collected by an indwelling jugular catheter before dosing and at 10, 20, 30, 60, 120, 240 and 480 minutes thereafter. Plasma specimens were analyzed by liquid chromatography coupled to high-resolution tandem mass spectrometry. Maximum concentrations (Cmax) and area-under-the-curve (AUC) for MDPV and two metabolites increased proportionally with administered dose, showing linear pharmacokinetics. MDPV exhibited the highest Cmax at all doses (74.2–271.3 μg/l) and 4-OH-3-MeOH-PV the highest AUC (11 366–47 724 minutes per μg/l), being the predominant metabolite. MDPV time to Cmax (Tmax) was 12.9–18.6 minutes, while 3,4-catechol-PV and 4-OH-3-MeO-PV peaked later with Tmax 188.6–240 minutes after s.c. dosing. Horizontal locomotor activity (HLA) and stereotypy correlated positively with plasma MDPV concentrations, while HLA correlated negatively with MDPV metabolites. These results suggest that the parent compound mediates motor stimulation after systemic MDPV administration, but additionally, metabolites may be inhibitory, may not be active or may not pass the blood brain barrier.
Keywords: bath salts, designer drugs, MDPV, metabolites, PK/PD, synthetic cathinones
INTRODUCTION
3,4-Methylenedioxypyrovalerone (MDPV) is a designer drug of the synthetic cathinone family. Synthetic cathinones, also known as ‘legal highs’, ‘plant food’, ‘bath salts’ or ‘research chemicals’ are sold as alternatives to illicit drugs such as 3,4-methylenedioxymeth-amphetamine (MDMA), amphetamines or cocaine. MDPV was first synthesized in 1969 and evaluated for the treatment of chronic fatigue. Drug development was stopped during clinical trials because of MDPV’s adverse effects (Kesha et al. 2013). Starting in Europe in 2005, and in the United States in 2008, MDPV became one of the most abused synthetic cathinones, because of powerful psychostimulant effects (Gunderson et al. 2013). However, several deleterious effects were reported including agitation, paranoia, tachycardia and even death (Kyle et al. 2011; Spiller et al. 2011; Borek & Holstege 2012). Consequently, MDPV is now classified as a schedule I drug based on the legislation enacted in 2012 (Drug Enforcement Administration (DEA), Department of Justice 2013).
Recent pre-clinical studies characterized acute behavioral effects of experimenter-administered MDPV (Meltzer et al. 2006; Aarde et al. 2013; Baumann et al. 2013; Cameron et al. 2013; Fantegrossi et al. 2013; Gatch, Taylor & Forster 2013; Simmler et al. 2013; Gregg & Rawls 2014; Marusich et al. 2014; Watterson et al. 2014). Utilizing in vitro transporter assays, Baumann et al. demonstrated that MDPV is a potent monoamine transporter blocker, similar to cocaine (Baumann et al. 2013). In contrast to cocaine, MDPV is selective for nor-epinephrine and dopamine transporters, with little serotonin transporter activity. In rats, MDPV is at least 10-fold more potent than cocaine as a locomotor stimulant (Baumann et al. 2013; Gatch et al. 2013; Gregg & Rawls 2014). Previous studies also showed that locomotor activity induced by stimulants, including MDPV is linked to the extracellular dopamine concentration in brain reward pathways (Pennartz, Groenewegen & Lopes da Silva 1994; Ikemoto 2002; Zolkowska et al. 2009; Baumann et al. 2011, 2013).
MDPV metabolism was studied in vitro using human liver microsomes, and in vivo in rats and humans (Meyer et al. 2010; Strano-Rossi et al. 2010). These studies showed that MDPV is metabolized to 3,4-dihydroxypyrovalerone (3,4-catechol-PV) by O-demethylenation of the 3,4-methylenedioxy ring, and to 4-hydroxy-3-methoxy-pyrovalerone (4-OH-3-MeO-PV) by O-methylation of 3,4-catechol-PV prior to urinary excretion. Using recombinant human cytochrome P450 (CYP) enzymes, the isoforms involved in MDPV biotransformation are CYP1A2, CYP2D6 and CYP2C19 for the first metabolic step, and catechol-O-methyltransferase (COMT) for the O-methylation (Meyer et al. 2010; Strano-Rossi et al. 2010). Both phase I metabolites, 3,4-catechol-PV and 4-OH-3-MeO-PV, are further metabolized by phase 2 conjugation; in human and rat urine only glucuronides are found, but in human liver microsomes, sulfates are also detected (Meyer et al. 2010; Strano-Rossi et al. 2010).
Currently, there are no data available with regard to MDPV pharmacokinetics in rats or humans. Knowledge of MDPV pharmacokinetics could provide clues to the drug’s mechanism of action. Additionally, pharmacokinetic data with MDPV in animal models can aid forensic toxicologists in interpreting findings from human subjects exposed to MDPV. To obtain useful pharmacokinetic data in humans, controlled administration studies are needed. However, these studies can only be performed after acquisition of pre-clinical toxicity data. Thus, the aim of this study was to evaluate for the first time the pharmacokinetic profiles for MDPV and its metabolites after subcutaneous (s.c.) MDPV administration to rats, and examine the relationships between MDPV’s behavioral effects and MDPV pharmacokinetics.’
MATERIALS AND METHODS
Chemicals and materials
MDPV and MDPV-d8 were obtained from Cayman Chemical Company (Ann Arbor, MI, USA) and Cerilliant (Round Rock, TX, USA). 3,4-Catechol-PV and 4-OH-3-MeO-PV were synthesized and purified by the Drug Design and Synthesis Section of the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP), Baltimore, MD, USA (Anizan et al. 2014). Formic acid, methanol, LC-MS grade acetonitrile (ACN) and water, ethylenediaminetetraacetic acid (EDTA) and sodium metabisulfite (SMBS) were acquired from Fisher Scientific (Fair Lawn, NJ, USA). 4-Methylcatechol and sodium hydroxide were purchased from Sigma (St Louis, MO, USA) and JT Baker (Phillipsburg, NJ, USA), respectively. β-glucuronidase from Red Abalone was obtained from Kura Biotec (Culver City, CA, USA). Water for EDTA, 4-methylcatechol and SMBS solution preparation was purified-in-house by an ELGA Purelab Ultra Analytic purifier (Siemens Water Technologies, Lowell, MA, USA).
Animals and surgery
Male Sprague-Dawley rats weighing 300–400 g were housed under conditions of controlled temperature (22 ± 2°C) and humidity (45 ± 5%) with food and water freely available. Rats were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and procedures were carried out in accordance with the Animal Care and Use Committee of the NIDA IRP. Lights were on from 7:00 am to 7:00 pm and experiments were carried out between 9:00 am and 2:00 pm. After 2 weeks of acclimation to the vivarium facilities, rats were anesthetized with pentobarbital sodium (60 mg/kg, intraperitoneal) and indwelling catheters made of Silastic tubing (Dow Corning, Midland, MI, USA) were implanted into the jugular vein as described previously (Baumann et al. 2008; Concheiro et al. 2013). In brief, the proximal end of the catheter was inserted into the right jugular vein and advanced to the atrium, with the distal end exteriorized on the nape and plugged with a metal stylet. Rats were single-housed postoperatively and allowed 1 week to recover from surgery. Catheter patency was checked only once, on the day prior to an experiment, using 48 IU/ml heparin saline. Only rats with patent catheters were included in the experiments. Of the 30 rats that received surgically implanted jugular catheters, 28 had patent catheters and were included in blood sampling experiments (i.e., N = 7 rats/group for four groups)
Blood sampling collection
The night before an experiment, rats were moved to the testing room. Each rat was placed into its own Plexiglass arena (see later), and allowed to acclimate to the surroundings overnight. The next morning, polyethylene extension tubes (30 cm) were filled with sterile saline, connected to intravenous catheters, and threaded outside the arenas. Catheters were flushed with 0.3 ml 48 IU/ml heparin saline to facilitate blood withdrawal. Rats were attached to a tethering device that allowed free movement within the arena. Groups of seven rats received sterile saline (zero-dose control), 0.5, 1.0 or 2.0 mg/kg MDPV in a volume of 1 ml/kg by the s.c. route; injections were administered posterior to the shoulder blades. Blood specimens (0.3 ml) were withdrawn before (t = 0) and at 10, 20, 30, 60, 120, 240 and 480 minutes after treatment. An equal volume of sterile saline was infused after each blood withdrawal to maintain volume and osmotic homeostasis. Blood was collected into 1-ml disposable tuberculin syringes, transferred to 1.5-ml plastic tubes on ice, and spun for 10 minutes at 1500 r.p.m.; plasma was decanted and SMBS (5 μl of 250 mM) was added before storage at −80°C.
Locomotor activity testing in rats
During the overnight acclimation period, each rat was housed within a square Plexiglass arena (43 cm length × 43 cm width × 43 cm height) equipped with an activity monitoring system (Tru Scan, Coulbourn Instruments, Whitehall, PA, USA). A sensor ring lined with photobeams spaced 2.54 cm apart was positioned in the horizontal plane to allow for real-time monitoring of various motor parameters. During the experiment (i.e., during blood sampling), motor activity was monitored for 60 minutes before and for 480 minutes after s.c. administration of saline or MDPV. Activity was monitored in 20 minutes bins. Horizontal locomotor activity (HLA) and stereotypy were quantified separately; HLA is defined as the total distance traveled in the horizontal plane (measured in cm), whereas stereotypy is defined as the number of photobeam breaks < ± 1.5 beam spaces and back to the original point, not exceeding 2 seconds apart (measured in number of moves).
Analysis of MDPV and its metabolites in plasma
MDPV, 3,4-catechol-PV and 4-OH-3-MeO-PV were quantified in plasma using a fully validated method (Anizan et al. 2014). Briefly, 100 μl plasma was hydrolyzed, precipitated with chilled ACN (400 μl) and centrifuged; 300 μl supernatant was evaporated to dryness and reconstituted in 0.1% formic acid LC-MS grade water. Analysis was performed by liquid chromatography coupled to high-resolution tandem mass spectrometry (Q-Exactive mass spectrometer from Thermo Scientific, Fremont, CA, USA). The linear range was 0.25–1000 μg/l for all three analytes.
Data analysis and statistics
Pharmacokinetic and pharmacodynamic data were tabulated, analyzed and graphically depicted using GraphPad Prism (version 5.02; GraphPad Software, Inc., San Diego, CA, USA). Plasma pharmacokinetic data were further analyzed with Phoenix/WinNonlin (version 6.3; Pharsight, Mountain View, CA, USA) to determine non-compartmental pharmacokinetic constants including maximum concentration (Cmax), time of maximum concentration (Tmax), area-under-the-curve (AUC), last measured concentration (Clast) and elimination half-life (t1/2). At least three data points on the descending linear limb of the time–concentration curve were employed to calculate t1/2 values. To evaluate MDPV and metabolites linear pharmacokinetics, Cmax and AUCs following 0.5 mg/kg were multiplied by two and four to calculate expected AUC values for 1 and 2 mg/kg doses, respectively. The expected values were compared with observed results at each MDPV dose by unpaired t-tests. HLA and stereotypy data were evaluated by two-way ANOVA (treatment × time) followed by a Bonferroni post hoc test. In order to examine potential relationships between pharmacodynamics and pharmacokinetics of MDPV and its two metabolites, Pearson’s correlation coefficients (r) were calculated with data from all individual treated rats. P < 0.05 was considered the minimal criterion for statistical significance.
RESULTS
Pharmacokinetics of MDPV, 3,4-catechol-PV and 4-OH-3-MeO-PV
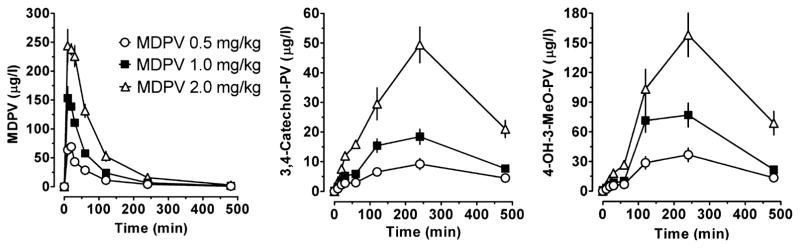
Pharmacokinetic profiles of plasma MDPV, 3,4-catechol-PV and 4-OH-3-MeO-PV after s.c. administration of 0.5, 1.0 and 2.0 mg/kg MDPV are presented in Fig. 1; pharmacokinetic parameters derived from these data are summarized in Table 1.
Figure 1.
Time–concentration profiles for 3,4-methylenedioxypyrovalerone (MDPV), 3,4-dihydroxypyrovalerone (3,4-Catechol-PV) and 4-hydroxy-3-methoxy-pyrovalerone (4-OH-3-MeO-PV) in rats after receiving subcutaneous injections of 0.5, 1.0 and 2.0 mg/kg MDPV. Data are mean ± standard error of the mean (SEM), N = 7 rats/dose
Table 1.
Pharmacokinetic constants (mean ± SD) for plasma MDPV, 3,4-catechol-PV and 4-OH-3-MeO-PV after subcutaneous administration of 0.5, 1.0 and 2.0 mg/kg to seven rats in each dosing group
| Dose (mg/kg) | Cmax (μg/l) | Tmax (min) | AUC (min·μg/l) | t1/2 (min) | Clast (μg/L) | |
|---|---|---|---|---|---|---|
| MPDV | 0.5 | 74.2 ± 18.7 | 15.7 ± 5.3 | 5393 ± 1129 | 97.9 ± 29.1 | 0.9 ± 0.4 |
| 1.0 | 165 ± 36 | 12.9 ± 4.9 | 11 277 ± 1177 | 77.8 ± 11.6 | 1.3 ± 0.6 | |
| 2.0 | 271 ± 39.4 | 18.6 ± 10.7 | 23 163 ± 4477 | 83.8 ± 32.2 | 2.6 ± 1.7 | |
| 3,4-catechol-PV | 0.5 | 9.9 ± 4.3 | 240 ± 120 | 3025 ± 1145 | N/Aa | 4.5 ± 0.8 |
| 1.0 | 20.1 ± 5.1 | 206 ± 58.6 | 6048 ± 1599 | N/Aa | 7.7 ± 2.8 | |
| 2.0 | 50.6 ± 14.8 | 257 ± 108 | 15 141 ± 3014 | N/Aa | 21.1 ± 7.7 | |
| 4-OH-3-MeO-PV | 0.5 | 41.5 ± 15.4 | 206 ± 58.6 | 11 366 ± 3968 | N/Aa | 13.5 ± 4.3 |
| 1.0 | 87.6 ± 22.9 | 189 ± 64.1 | 23 585 ± 7968 | N/Aa | 21.7 ± 5.4 | |
| 2.0 | 165 ± 52.9 | 206 ± 58.6 | 47 724 ± 15 085 | N/Aa | 68.8 ± 31.9 |
Not available (N/A) because of too few data points in the terminal elimination curve for accurate assessment.
Cmax = maximum concentration, Tmax = time of maximum concentration, AUC = area-under the curve, t1/2 = half-life and Clast = last measured concentration; MDPV = 3,4-methylenedioxypyrovalerone; 3,4-catechol-PV = 3,4-dihydroxypyrovalerone; 4-OH-3-MeO-PV = 4-hydroxy-3-methoxy-pyrovalerone.
Mean (±standard deviation) MDPV Cmax were 72.4 ± 18.7 μg/l, 164.7 ± 36 μg/l and 271.3 ± 39.4 μg/l for 0.5, 1 and 2 mg/kg, respectively. Tmax was 15.7 ± 5.3 minutes after 0.5 Tmg/kg, 12.9 ± 4.9 minutes after 1 mg/kg and 18.6 ± 10.7 minutes after 2 mg/kg MDPV, and t1/2 ranged from 77.8 ± 11.6 to 97.9 ± 29.1 minutes. At the end of the study, 480 minutes after dosing, MDPV was present, but at low concentrations, between 0.9 ± 0.4 μg/l (0.5 mg/kg) and 2.6 ± 1.7 μg/l (2 mg/kg). MDPV AUC were 5393 ± 1129, 11 277 ± 1177 and 23 163 ± 4477 minutes·μg/l after 0.5, 1 and 2 mg/kg, respectively.
Compared with MDPV, 3,4-catechol-PV Cmax were lower and ranged from 9.9 ± 4.3 to 50.6 ± 14.8 μg/l depending on the dose. 3,4-catechol-PV peaked later than MDPV with Tmax of 240 ± 1 minutes for 0.5 mg/kg, 205.7 ± 58.6 minutes for 1 mg/kg and 257.1 ± 108 minutes for the 2 mg/kg dose. 3,4-catechol-PV showed the lowest AUCs from 3025 ± 1145 to 15 141 ± 3014 minutes·μg/l. The kinetic profile of 4-OH-3-MeO-PV was similar to 3,4-catechol-PV, but with 4-fold higher Cmax and AUC values. For 0.5 mg/kg dose, Cmax was 41.5 ± 15.4 μg/l and AUC was 11 366 ± 3968 minutes μg/l, and for 2 mg/kg dose, Cmax was 164.5 ± 52.9 μg/l and AUC was 47 724 ± 15 085 minutes μg/l. The half-life of the two MPDV metabolites could not be determined because of the lack of sufficient data points to characterize the descending linear limb of the time–concentration curve.
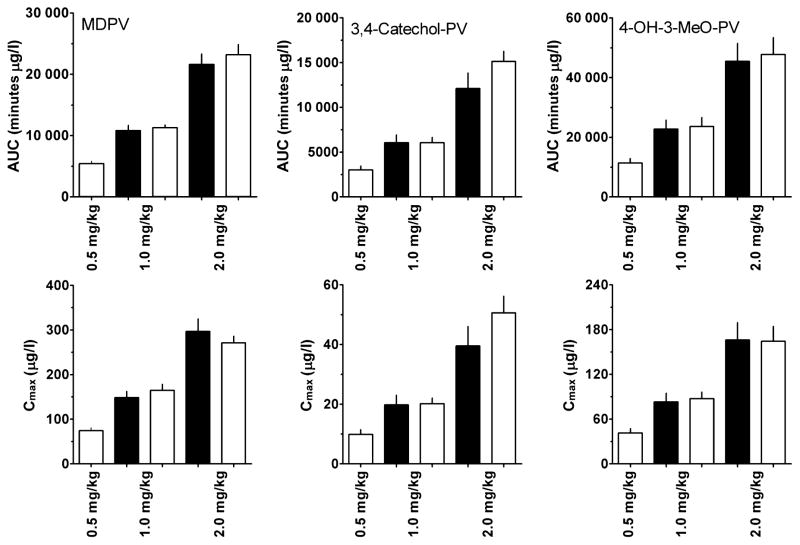
The linearities of MDPV and metabolites’ concentrations were investigated by comparing the theoretical and experimental Cmax and AUC. If analyte pharmacokinetics were linear, Cmax and AUC at 1 and 2 mg/kg should be two- and fourfold higher than those at 0.5 mg/kg. Figure 2 shows that all three compounds exhibited linear pharmacokinetics; there were no significant differences between theoretical and experimental AUCs or Cmax.
Figure 2.
Theoretical (black bars) vs. observed (open bars) areas-under-the-curve (AUCs) and maximum concentrations (Cmax) for 3,4-methylenedioxypyrovalerone (MDPV), 3,4-dihydroxypyrovalerone (3,4-catechol-PV) and 4-hydroxy-3-methoxy-pyrovalerone (4-OH-3-MeO-PV). Theoretical AUC and Cmax for 1 and 2 mg/kg doses are calculated by multiplying by 2 and 4 the observed AUC and Cmax for 0.5 mg/kg dose. Data are means ± standard error (SEM), N = 7 rats/dose
Pharmacodynamic effects
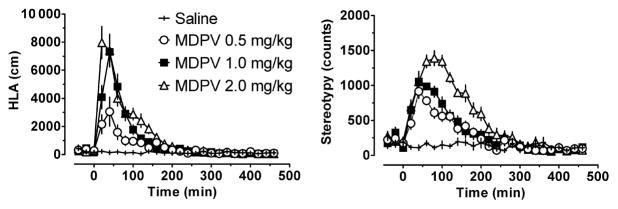
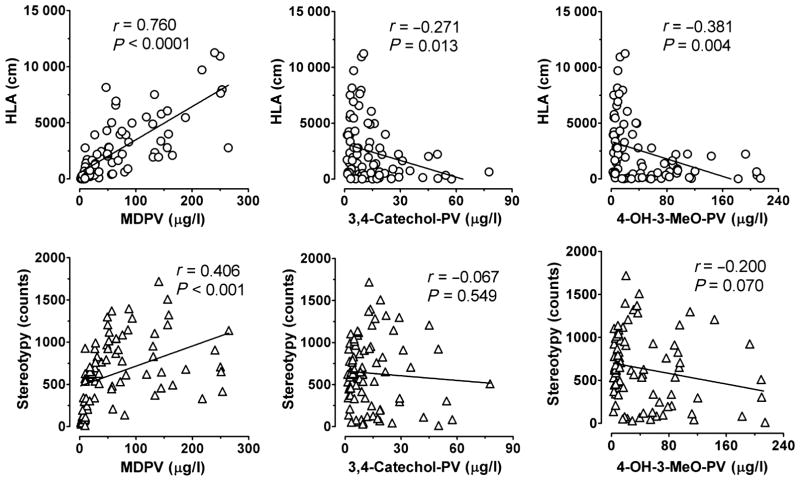
Two locomotor endpoints, HLA and stereotypy, were monitored for 20 minutes bins before and after dosing (Fig. 3). HLA increased immediately after s.c. dosing, with maxima reached within 20–40 minutes. HLA increased in a dose-dependent manner (F3, 624 = 81.51, P < 0.0001), with a MDPV dose × time interaction (F75,624 = 11.85, P < 0.0001). For 60 (0.5 mg/kg), 100 (1 mg/kg) and 140 minutes (2 mg/kg) after dosing, rats had significantly higher HLA than after saline. After 0.5 mg/kg MDPV, HLA was significantly lower for 80 and 180 minutes compared with the 1 and 2 mg/kg doses, respectively; however, after 1 and 2 mg/kg, HLA was similar except 20 minutes after dosing (P < 0.001). Correlations between HLA or stereotypy and plasma concentrations of MDPV, 3,4-catechol-PV and 4-OH-3-MeO-PV were investigated (Fig. 4). HLA was significantly and positively correlated with MDPV plasma concentrations with correlation coefficients (r) of 0.76 (P < 0.0001). A significant, but negative correlation was observed between HLA and MDPV metabolites with correlation coefficients of −0.27 to −0.38 depending upon the metabolite (P < 0.015).
Figure 3.
Time–effect curves for horizontal locomotor activity (HLA) and stereotypy in rats after receiving subcutaneous injections of saline or 0.5, 1.0 and 2.0 mg/kg MDPV. Results are total for each 20 minutes bin and are expressed as means ± standard error of the mean (SEM), N = 7 rats/dose
Figure 4.
Correlation between plasma 3,4-methylenedioxypyrovalerone (MDPV), 3,4-dihydroxypyrovalerone (3,4-catechol-PV) or 4-hydroxy-3-methoxy-pyrovalerone (4-OH-3-MeO-PV) and horizontal locomotor activity (HLA) or stereotypy after rats received subcutaneous injection of 0.5, 1.0 or 2.0 mg/kg MDPV. Pearson’s correlation coefficient ‘r’ was calculated for each data set. N = 21 rats
Stereotypy also increased after dosing, peaking at 40 (0.5 and 1.0 mg/kg doses) or 80 minutes (2 mg/kg). Stereotypy was affected by dose (F3, 624 = 144.8, P < 0.0001) and there was a dose × time interaction (F75, 624 = 10.18, P < 0.0001). For at least 120 minutes (0.5 mg/kg) and up to 240 minutes (2 mg/kg) after dosing, MPDV-treated rats had significantly higher stereotypy scores than saline-treated animals. Rats dosed with 0.5 and 1.0 mg/kg s.c. MDPV had similar stereotypy at all times, but 80 minutes (P < 0.05); however, 2 mg/kg produced significantly higher stereotypy between 60 and 200 minutes after dosing. Stereotypy was significantly and positively correlated with MDPV plasma concentrations (r = 0.41, P = 0.001), but did not correlate with metabolites.
DISCUSSION
Here, we report the first pharmacokinetic profiles for MDPV and its phase I metabolites, 3,4-catechol-PV and 4-OH-3-MeO-PV. Significant relationships were noted between MDPV, its metabolites and locomotor measures. In order to explore the effect of dose on pharmacokinetics and pharmacodynamics, three different s.c. doses of MDPV were administrated to rats. After dosing, MDPV Cmax occurred within 20 minutes and the values achieved after each dose were proportional to the dose administered. In vivo metabolism studies in rats and humans show that MDPV undergoes O-demethylenation of the methylenedioxy functional group, followed by O-methylation of the intermediate di-hydroxy meta-bolite (Meyer et al. 2010; Strano-Rossi et al. 2010). This is an established biotransformation pathway for amphetamine-like compounds containing a methylenedioxy group like MDMA (Maurer et al. 2000; de la Torre et al. 2004; Huestis et al. 2007; Kolbrich et al. 2008; Hartman et al. 2013) and methylone (Kamata et al. 2006; Mueller & Rentsch 2012; Pedersen et al. 2013).
After s.c. MDPV, its two main metabolites, 3,4-catechol-PV and 4-OH-3-MeO-PV, had later Tmax than MDPV. It is also important to note that 3,4-catechol-PV concentrations had a significantly lower AUC than 4-OH-3-MeO-PV and MDPV, confirming that 3,4-catechol-PV is an intermediate metabolite. Similar results to those shown here for MDPV were observed previously for the dihydroxy MDMA metabolite, (±)-3,4 dihydroxymeth-amphetamine (HHMA) and the hydroxymethoxy metabolite, (±)-4-hydroxy-3-methoxymethamphetamine (HMMA) after s.c. 2.5, 5.0 and 10.0 mg/kg MDMA to rats (Concheiro et al. 2013).
The relatively short half-life of MDPV (77.8–97.9 minutes) explains why at the end of the experiment, 8 hours after dosing, MDPV is present at low concentrations equivalent to 1% of the Cmax. In contrast, 3,4-catechol-PV and 4-OH-3-MeO-PV concentrations were approximately 40% of their Cmax 8 hours after dosing. The findings demonstrate that MDPV metabolites are present much longer than MDPV in the systemic circulation; these results indicate that for drug testing, targeting the metabolites could be more useful than targeting MDPV to extend the window of detection. The determination of half-life for the two metabolites was not possible because only two data points were on the descending linear limb of the time–concentration curve. To better characterize the pharmacokinetics of these metabolites, longer collection times are necessary.
We clearly demonstrated that MDPV, 3,4-catechol-PV and 4-OH-3-MeO-PV concentrations were linear with respect to dose administered, suggesting that at the doses tested here, MDPV does not inhibit its own metabolism. The linear pharmacokinetics for MDPV contrasts with the non-linear accumulation and metabolic inhibition associated with MDMA (Chu et al. 1996; Baumann et al. 2009; Concheiro et al. 2013). A pattern of non-linear kinetics was observed for methylone, another synthetic cathinone, in rats given high 15 or 30 mg/kg oral doses (Lopez-Arnau et al. 2013). Our results with 3,4-catechol-PV and 4-OH-3MeO-PV are different from those obtained for HHMA and HMMA after s.c. MDMA administration of 2.5, 5.0 and 10.0 mg/kg (Concheiro et al. 2013); however, in the present study, doses of MPDV were fivefold lower because of the higher potency of MDPV compared with MDMA. It is possible that MDPV might display non-linear kinetics at higher doses, and this hypothesis warrants further investigation.
We explored locomotor behavior after s.c. MDPV dosing and the relationship to MDPV and metabolites plasma concentrations to better understand the link between MDPV pharmacodynamics and pharmacokinetics. MDPV administration produced an immediate effect on behavior. HLA rapidly increased and reached its maximum by 60 minutes after dosing, with significant differences between saline and MDPV-treated rats for 60–140 minutes. These results confirm previous findings demonstrating that HLA rapidly increased after dosing and in a dose-dependent manner in rats and mice (Baumann et al. 2013; Fantegrossi et al. 2013; Gatch et al. 2013; Marusich et al. 2014). Other studies with mice investigated higher 10 and 30 mg/kg MDPV doses, and documented that at these high doses HLA did not increase, and actually decreased (Fantegrossi et al. 2013; Gatch et al. 2013).
In our experiments, MDPV-induced stereotypy time profiles were similar to those of HLA, but lasted slightly longer, in accordance with previous studies in which rats received 0.5–5.6 mg/kg MDPV (Aarde et al. 2013). The 2 mg/kg dose used here produced greater stereotypy than the 1.0 or 0.5 mg/kg doses, which produced similar effects. At the 2-mg/kg dose, rats tended to engage in more repetitive movements (i.e., stereotypy), such as head-bobbing or sniffing, instead of walking in their cages, perhaps explaining the suppression of forward locomotor behavior mentioned earlier at MDPV doses of 10 or 30 mg/kg in mice (Fantegrossi et al. 2013; Gatch et al. 2013).
In addition, we also observed a positive correlation between MDPV plasma concentrations and HLA or stereotypy, indicating that MDPV is an active compound able to cross the blood–brain barrier. However, the correlation between MDPV concentrations and stereotypy was weaker than with HLA; perhaps this can be explained by the differences between maximum MDPV concentration and maximum measured stereotypy as seen in Fig. 5. A major advantage of our work is that pharmacokinetic and motor activity parameters were collected simultaneously from the same rats. However, a limitation is that plasma collection and HLA or stereotypy measurement matched at only four time points (20, 60, 120 and 240 minutes). So, MDPV brain concentrations would provide valuable additional information to better characterize the relationship between the pharmacodynamic effects and the pharmacokinetics.
Figure 5.
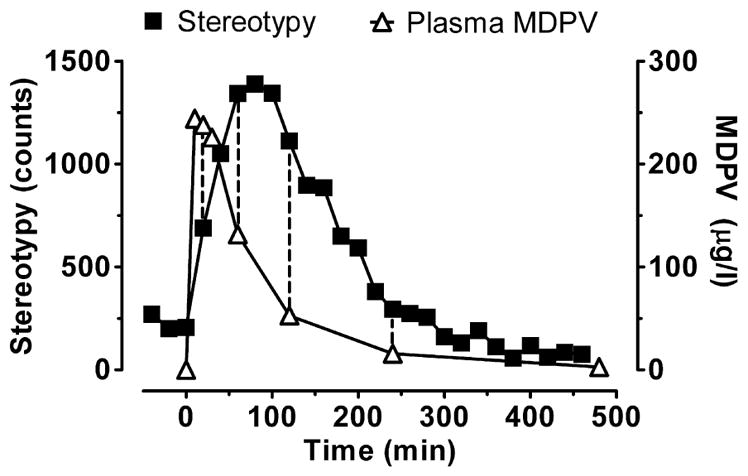
Time-plasma 3,4-methylenedioxypyrovalerone (MDPV) concentration (left axis) and time-stereotypy (right axis) profiles after rats received 2 mg/kg subcutaneous MDPV. Dashed vertical lines indicate the data points available for correlation calculation (stereotypy measurement and plasma collection matched at four time points: 20, 60, 120 and 240 minutes). Data are mean ± standard error of the mean (SEM), N = 7 rats
Finally, our data demonstrated a significant weak negative correlation between the concentration of MDPV metabolites and HLA, but no correlation was found between metabolites and stereotypy. When 3,4-catechol-PV and 4-OH-3-MeO-PV concentrations were at their maximums at about 240 minutes, the lowest HLA and stereotypy were observed. These data suggest the possibility that hydroxylated metabolites of MDPV might exert effects that suppress motor activity. Other hypotheses are that metabolites either do not pass the blood–brain barrier or are inactive. It might be predicted that hydroxylated metabolites of MDPV interact with transporters for dopamine and norepinephrine (Meltzer et al. 2006), but the present findings provide no support for this hypothesis, as the metabolites appeared to inhibit locomotor activity. Additional in vitro and in vivo experiments are needed to verify the mechanism of action for MDPV metabolites.
In conclusion, we documented linear pharmacokinetics for MDPV and its metabolites, and the 4-OH-3-MeO-PV metabolite may be a good target for drug testing as its window of detection is longer than that of the parent. MDPV concentrations were positively correlated with HLA, while its metabolites were negatively correlated with observed pharmacodynamic effects.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and by the National Institutes of Health (NIH).
Footnotes
Authors Contributions
MHB, MAH and MC participated in research design; KRL, MOB and MHB conducted experiments; SA, MC, MS and KCR contributed new reagents or analytic tools; SA and MC performed data analysis; and all authors wrote or contributed to the writing of the article.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psycho-motor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anizan S, Ellefsen K, Concheiro M, Suzuki M, Rice KC, Baumann MH, Huestis MA. 3,4-Methylenedioxypyrovalerone (MDPV) and metabolites quantification in human and rat plasma by liquid chromatography-high resolution mass spectrometry. Anal Chim Acta. 2014;827:54–63. doi: 10.1016/j.aca.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M, Zolkowska D, Kim I, Scheidweiler K, Rothman R, Huestis M. Effects of dose and route of administration on pharmacokinetics of (+or −)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos. 2009;37:2163–2170. doi: 10.1124/dmd.109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience. 2008;152:773–784. doi: 10.1016/j.neuroscience.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of ‘bath salts’ containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60:103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Jr, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T, Kumagai Y, DiStefano EW, Cho AK. Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem Pharmacol. 1996;51:789–796. doi: 10.1016/0006-2952(95)02397-6. [DOI] [PubMed] [Google Scholar]
- Concheiro M, Baumann MH, Scheidweiler KB, Rothman RB, Marrone GF, Huestis MA. Nonlinear Pharmacokinetics of ({±})3,4-methylenedioxymethamphetamine (MDMA) and its pharmacodynamic consequences in the rat. Drug Metab Dispos. 2013;42:119–125. doi: 10.1124/dmd.113.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Cami J. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration (DEA), Department of Justice. Establishment of drug codes for 26 substances. Final Rule. Fed Regist. 2013;78:664–666. [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Rawls SM. Behavioral pharmacology of designer cathinones: a review of the preclinical literature. Life Sci. 2014;97:27–30. doi: 10.1016/j.lfs.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson EW, Kirkpatrick MG, Willing LM, Holstege CP. Substituted cathinone products: a new trend in ‘bath salts’ and other designer stimulant drug use. J Addict Med. 2013;7:153–162. doi: 10.1097/ADM.0b013e31829084b7. [DOI] [PubMed] [Google Scholar]
- Hartman RL, Desrosiers NA, Barnes AJ, Yun K, Scheidweiler KB, Kolbrich-Spargo EA, Gorelick DA, Goodwin RS, Huestis MA. 3,4-Methylenedioxymethamphetamine (MDMA) and metabolites disposition in blood and plasma following controlled oral administration. Anal Bioanal Chem. 2013;406:587–599. doi: 10.1007/s00216-013-7468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, de Martinis BS, Goodwin RS, Gorelick DA. Disposition of MDMA and metabolites in human sweat following controlled MDMA administration. The International Association of Therapeutic Drug Monitoring and Clinical Toxicology Annual Meeting—2007; Nice, France. 2007. [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neuroscience. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Kamata HT, Shima N, Zaitsu K, Kamata T, Miki A, Nishikawa M, Katagi M, Tsuchihashi H. Metabolism of the recently encountered designer drug, methylone, in humans and rats. Xenobiotica. 2006;36:709–723. doi: 10.1080/00498250600780191. [DOI] [PubMed] [Google Scholar]
- Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, Doyon S, Chi P, Fowler DR. Methylenedioxypyrovalerone (‘bath salts’), related death: case report and review of the literature. J Forensic Sci. 2013;58:1654–1659. doi: 10.1111/1556-4029.12202. [DOI] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monit. 2008;30:320–332. doi: 10.1097/FTD.0b013e3181684fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle PB, Iverson RB, Gajagowni RG, Spencer L. Illicit bath salts: not for bathing. J Miss State Med Assoc. 2011;52:375–377. [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Carbo M, Pubill D, Escubedo E, Camarasa J. An integrated pharmacokinetic and pharmacodynamic study of a new drug of abuse, methylone, a synthetic cathinone sold as ‘bath salts. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:64–72. doi: 10.1016/j.pnpbp.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the ‘bath salts’ constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer HH, Bickeboeller-Friedrich J, Kraemer T, Peters FT. Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (‘Ecstasy’) Toxicol Lett. 2000;112–113:133–142. doi: 10.1016/s0378-4274(99)00207-6. [DOI] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxypyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC–MS and LC–high-resolution MS and its detectability in urine by GC–MS. J Mass Spectrom. 2010;45:1426–1442. doi: 10.1002/jms.1859. [DOI] [PubMed] [Google Scholar]
- Mueller DM, Rentsch KM. Generation of metabolites by an automated online metabolism method using human liver microsomes with subsequent identification by LC-MS(n), and metabolism of 11 cathinones. Anal Bioanal Chem. 2012;402:2141–2151. doi: 10.1007/s00216-011-5678-8. [DOI] [PubMed] [Google Scholar]
- Pedersen AJ, Reitzel LA, Johansen SS, Linnet K. In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test Anal. 2013;5:430–438. doi: 10.1002/dta.1369. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of ‘bath salts’ and ‘legal highs’ (synthetic cathinones) in the United States. Clin Toxicol (Phila) 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Strano-Rossi S, Cadwallader AB, de la Torre X, Botre F. Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MDPV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:2706–2714. doi: 10.1002/rcm.4692. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]