Synopsis
Chemical-induced depigmentation of the skin has been recognized for over 75 years, first as an occupational hazard but then extending to those using household commercial products as common as hair dyes. Since their discovery, these chemicals have been used therapeutically in patients with severe vitiligo to depigment their remaining skin and improve their appearance. The importance of recognizing this phenomenon was highlighted during an outbreak of vitiligo in Japan during the summer of 2013, when over 16,000 users of a new skin lightening cosmetic cream developed skin depigmentation at the site of contact with the cream and many in remote areas as well. Depigmenting chemicals appear to be analogs of the amino acid tyrosine that disrupt melanogenesis and result in autoimmunity and melanocyte destruction. Because chemical-induced depigmentation is clinically and histologically indistinguishable from non-chemically induced vitiligo, and because these chemicals appear to induce melanocyte autoimmunity, this phenomenon should be known as “chemical-induced vitiligo”, rather than less accurate terms that have been previously used.
Keywords: vitiligo, leukoderma, chemical, phenol, rhododendrol, monobenzone, cellular stress, autoimmunity
Introduction
Like many autoimmune diseases, vitiligo pathogenesis is influenced by genetic, stochastic, and environmental factors. This is clear from the fact that first degree relatives of patients with vitiligo have a 5–6 fold increased risk of disease and identical twins have a 23-fold increased risk, clearly implicating genetics as an important risk factor for vitiligo. However despite sharing almost all of their genes, identical twins are only 23% concordant for disease, meaning that if one has vitiligo the other will only have it 23% of the time. This clearly implicates other, non-heritable risk factors for developing vitiligo as well. Stochastic mechanisms, or the influence of random chance, likely play a role, particularly during the development of the immune system, which occurs through random recombination of T cell receptors and antibodies. This process is responsible for “building” the autoreactive cells that ultimately attack melanocytes in vitiligo. The role of stochastic factors in developing vitiligo and other autoimmune diseases is not likely to account for all of the non-genetic risk, and so many believe that factors from the environment strongly influence the likelihood of developing autoimmunity.
Vitiligo is one of the few autoimmune diseases in which environmental factors are well-known, including the depigmenting effect of the chemical monobenzyl ether of hydroquinone (MBEH) discovered by Oliver and colleagues in a tanning factory, but includes many others as well. Some have been directly implicated via topical challenge through patch testing, others through large population studies, and still others more indirectly. This chapter will summarize the chemicals that have been clearly implicated as causing or exacerbating vitiligo, as well as the mechanism by which this occurs. Recognizing these chemicals and their implications for managing vitiligo is important during patient counseling and follow up, both when thinking about disease prevention, as well as improveing therapeutic responses.
Chemicals directly implicated in inducing vitiligo
MBEH
In 1939, Oliver, Shwartz, and Warren reported a case series of workers in a leather manufacturing company who developed patchy depigmentation on their hands and arms. In fact, 50% of the workers in this factory and others who wore a particular brand of gloves developed depigmentation on skin that contacted the gloves, and several of them also had similar lesions on remote areas that did not contact the gloves. The ingredients used in manufacturing the gloves were obtained by the medical team, and each systematically applied to the workers through patch testing. Only patches containing the antioxidant MBEH induced an inflammatory response, which was then followed by depigmentation. This chemical ingredient was removed from the gloves, and workers subsequently repigmented1. Depigmentation was also reported following exposure to other products that contained MBEH, primarily by items made of rubber2. MBEH has been removed from manufacturing in the US rubber industry, although may still be in use in other countries3.
After this observation, others attempted to use MBEH as a treatment for hypermelanoses4–6, however reports of complete and irreversible depigmentation at the site of application and in remote areas limited its use7–10, and resulted in its removal from commercial products. The ability of MBEH to permanently remove skin pigment prompted Mosher, Lerner, and Fitzpatrick to test it as a topical treatment for patients with severe vitiligo. They recommended the use of MBEH in vitiligo patients who failed to respond to therapy with psoralen ultraviolet A (PUVA) and with depigmentation of over 50% of their body surface area (BSA). Their retrospective study of 18 patients who used topical MBEH revealed that 8 patients completely depigmented in 4–12 months11. Since then dermatologists have used this as a therapy in severe patients who desired it, noting also depigmentation remote from the site of application, and sparing of hair and eye color2. It is currently the only Food and Drug Association (FDA)-approved treatment for vitiligo, and details about its use will be discussed later in this issue (see P. Grimes, Depigmentation Therapy, in this issue). In addition, monomethyl ether of hydroquinone has been reported to induced depigmentation in 2 subjects12, and has been used therapeutically to depigment vitiligo patients13, 14.
Hydroquinone, a chemical structurally related to MBEH and frequently used in skin-lightening agents, has not been clearly implicated in inducing or exacerbating vitiligo when used for cosmetic purposes. Despite many cases attributed to MBEH, only two patients reportedly developed depigmentation after exposure to photographic developing solution containing hydroquinone, and in both patients the depigmentation was preceded by allergic dermatitis15, 16. However despite the use of hydroquinone creams for many years, including to “feather” the border of vitiligo lesions to make them less apparent, it results in only uniform lightening of the skin, and no cases of focal depigmentation have been reported following this method of treatment2. Thus, hydroquinone-containing topical treatments are probably safe to use in vitiligo patients who request treatment for coexisting hyperpigmentation (i.e. melasma, for example), although this should be considered on a case-by-case basis.
4-TBC
The application of a single chemical-soaked patch to the skin was also used to implicate other phenols in products that induced depigmentation in vitiligo patients. In the 1970s, a smaller percentage (4/75, ~5%) of factory workers in a tappet (valve lifters) assembly plant developed acral depigmentation due to contact with 4-tert-butylcatechol (4-TBC) present in a lubricating oil. All patients had severe inflammation prior to depigmentation at the site of contact, and 3/4 had remote depigmentation as well17. Patch testing with 4-TBC induced an inflammatory response in 3 of the 4 affected, with clear depigmentation in 1, while none out of 6 volunteers developed depigmentation17. Studies in guinea pigs confirmed the ability of 4-TBC to depigment the skin, particularly in high concentrations and in strong solvents18.
4-TBP and 4-TAP
Bajaj et al. reported the characteristics of 100 consecutive patients who presented with depigmentation under their bindi, a decorative item worn on the forehead of many Indian women, often using an adhesive resin. Seventy-three exhibited dermatitis at the site prior to depigmentation, and 34 had depigmentation remote from the site of bindi application. Upon patch testing of 15 patients with the adhesive resin, 5 had irritant reactions and 3 of those depigmented 15–60 days later19. The chemical 4-tert-butylphenol (4-TBP) was the suspected culprit based on its high content in the samples tested, as well as a number of other reports that implicated the chemical in other occupations. An additional report described perioral depigmentation in a patient following the use of lip liner that contained 4-TBP. Patch testing to the chemical was positive in this patient20. Other groups similarly implicated 4-TBP and 2,4-ditert-butylphenol (2,4-dTBP) in causing depigmentation following occupational exposures21–24, and three groups reported a variety of systemic abnormalities, including thyroid, liver, and/or splenic changes following exposure. Based on these observations, they suggested that the chemical may be capable of inducing inflammation in organs beyond the skin24–26.
Kahn reported depigmentation in hospital workers at two separate locations, 5 in one hospital and 7 in another. Both groups worked directly with germicidal detergents, although the specific detergents were different brands: 5 were exposed to O-Syl (similar to Lysol) and 7 to Ves-Phene. While the ingredients between these products varied, one contained 4-TBP and the other contained 4-tert-amylphenol (4-TAP). Both reproduced depigmentation with patch testing in all subjects, supporting the results discussed above. Three of 5 and 4 of 7 subjects experienced pruritus and erythema prior to depigmentation of the patch testing sites. Sites of contact with the detergent were affected in all workers, while 2 had involvement of remote sites as well (1 in each group). One of the hospitals discontinued use of the detergent (O-Syl), while the other did not (Ves-Phene). Two of the 5 workers who discontinued using O-Syl regained their pigment27.
An additional study from Russia implicated 4-TBP in workers who manufactured synthetic condensation resins. Interestingly, of those who were in contact with the chemical less than 2 years total, 15% were affected, while of those who were exposed for longer than 2 years, over 40% were affected28, suggesting that length of exposure influenced the incidence of disease.
Dyes
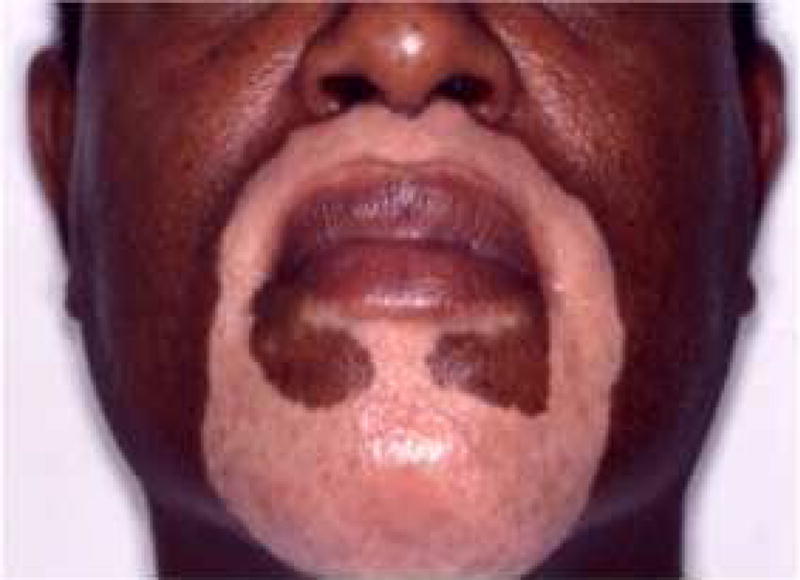
Taylor, et al. first reported the ability of hair dyes and, specifically, para-phenylenediamine (PPD) to induce depigmentation. They described 4 subjects who developed depigmentation of the scalp and hair after using hair dyes, and found that 3 of the subjects exhibited depigmentation following patch testing (2 with PPD and 1 with the hair dye itself). Three of the subjects at least partially repigmented after discontinuing use of the dyes29. Other cases implicating PPD in hair dyes have been reported as well, although not all cases confirmed depigmentation through patch testing30–34. One group published a case series of 3 subjects who developed depigmentation of the scalp that abruptly stopped at the hair line, but was independent from hair dye use. They postulated that scalp depigmentation alone did not necessarily implicate the use of hair dyes as causative35. Patients may develop hair dye-induced depigmentation on the scalp or face, depending on the site of application (Fig. 1A,B).
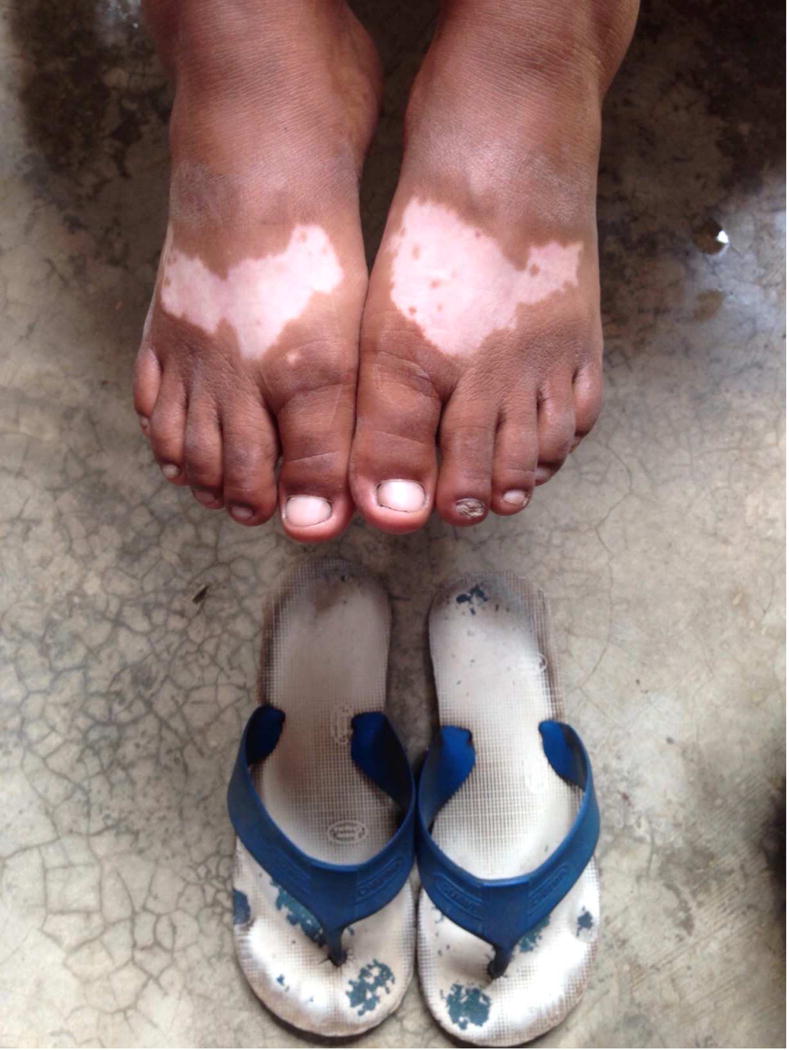
Fig. 1.
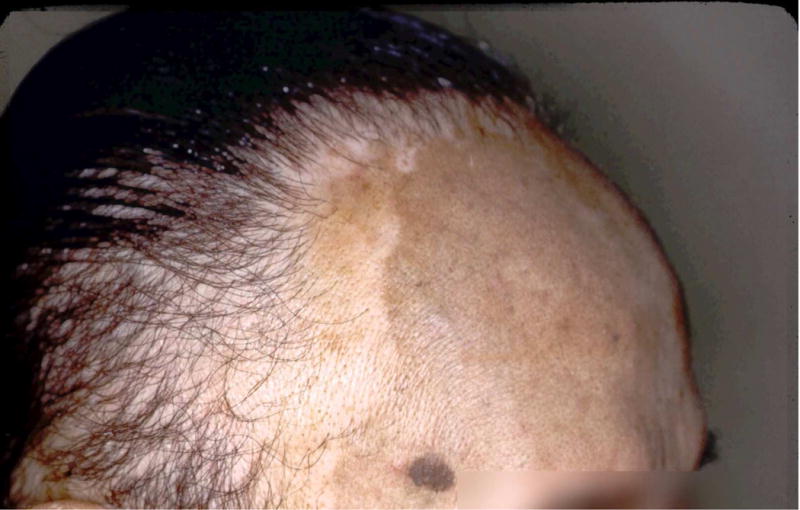
(A) Man who used hair dyes and developed scalp depigmentation at the hairline.
Courtesy of William James, MD, Philadelphia, PA.
(B) Man with depigmentation on the face after treating his goatee with a comb-in dye.
Courtesy of Pearl E. Grimes, MD, Los Angeles, CA.
(C) Depigmentation of the feet in the distribution of sandal straps.
Courtesy of Inbal Braunstein, MD, Baltimore, MD.
Alta is a red dye solution used in India as a cosmetic coloring agent for the feet. The dye has been reported to induce vitiligo at the location of application, and patch testing in one subject implicated the dye components Crocein Scarlet MOO, or brilliant crocein, and rhodamine B, or tetraethyl rhodamine. Depigmentation was preceeded by contact dermatitis in this subject, and she also developed remote depigmentation on the hands. Negative results were observed in over 20 controls who were also patch tested with the dyes. The subject also developed a similar reaction to PPD, thought to be a well-known cross-reaction between the chemicals, as the alta contained no trace of PPD. All sites depigmented approximately 6 weeks after application of the patch tests, and repigmented after 6 months36. Some have reported that depigmentation occurred following direct exposure to dyes in leather products and other clothing when in close contact with the skin, such as shoes, wallets, and sandals3, 37, 38 (Fig. 1C).
Products indirectly implicated in vitiligo induction through population-based studies
The critical importance of commercial products in the induction of vitiligo became evident in the summer of 2013 when Kanebo, a Japanese cosmetics company, was forced to recall a new skin lightening cream after over 16,000 users developed vitiligo (~2% of all users). The active ingredient was rhododendrol39, 40. A large retrospective analysis of users revealed that most experienced depigmentation only at the site of exposure to the cream, however a small percentage (5%) experienced depigmentation at remote sites as well41. Data acquired through questionnaires of affected individuals reported that following discontinuation of the cream, 7% of lesions had completely resolved, 27% had improved by more than half, 38% were improved by less than half, 25% were unchanged, and 2% had increased in size (1% could not be determined). Many patients (67%) improved even without therapy, while slightly more (77%) reported improvement when treated with standard therapies for vitiligo40. Lesions induced by rhododendrol were mostly indistinguishable from vitiligo clinically and histologically40, 41, although one report suggested that melanocytes were largely reduced in number but not always completely absent in chemical-induced lesions42. Some affected users developed eczematous reactions after using the cosmetic, and 14% reacted to rhododendrol patch testing with pruritus and erythema, although the number of those who depigmented at the location of the patch has not been reported40.
A large, prospective population-based study reported that the use of hair dyes increased the risk of developing vitiligo. This group queried a database from the Nurses Health Study, established in 1976, in which a cohort of over 68,000 participants provided information on their health conditions and exposures on a yearly basis. The study had an over 90% response rate to biennial questionnaires, and validated accurate reporting of the incidence of vitiligo. The authors found that women who started using hair dyes before the age of 30 and those who used hair dyes for over 5 years (regardless of the starting age) had a 50% higher risk of developing vitiligo43. This used an unbiased approach in a large cohort to support earlier case studies that implicated hair dyes in causing vitiligo. One caveat of the study was that it couldn’t rule out an association between early hair graying and vitiligo, which has been reported previously2, 44, as the causative factor in the use of hair dyes at a young age.
Household products thought to induce vitiligo
A variety of common household products have been reported to induce and/or worsen vitiligo in patients, however the offending chemical ingredients have not necessarily been identified27, 29, 45, 46. This has resulted in numerous lawsuits against the manufacturers, but no option for making safer products or educating patients about which ingredients to avoid. Phenol was used as the first surgical antiseptic by Joseph Lister in 186547. Due to the fact that it is inexpensive, water-soluble, mildly acidic, and highly reactive, phenol derivatives are used to produce a wide variety of common household products, including disinfectants, cosmetics, diaper creams, detergents, cosmetic dyes, adhesives, pharmaceutical drugs, and others. According to a search of the Household Products Database (U.S. Department of Health and Human Services), 44 unique phenols are used as ingredients in over 8400 household products sold by 81 distinct manufacturers48.
Outside of patch testing a specific suspected chemical as described above it is difficult, if not impossible, to definitively implicate a product as a causative initiator of vitiligo, since there are currently no ways to distinguish this from spontaneous or idiopathic vitiligo. Thus, causation by a commercial product can only be strongly inferred based on patient history, physical examination, exposures, and knowledge about the chemical content of suspected products. Ghosh and Mukhopadhyay reported the largest study of the role of exposures in patients with vitiligo. They extensively interviewed all patients with vitiligo over a 5-year period and identified 864 cases in which they strongly suspected chemically-induced disease. They reported that of these cases, 66% were thought to be initiated by the exposure, while 34% had preexisting vitiligo that was thought to be exacerbated by use of the product. Depigmentation was limited to the site of exposure in 74%, while 26% developed lesions remote from the site of contact. While pruritus was present in 22% of cases, only a minority (5%) had evidence of contact dermatitis through an eczematous eruption at the site of contact and depigmentation. Hair dye was the most commonly implicated product (27%), and this was presumably due to PPD. Other products contained 4-TBP, MBEH, and Azo dyes, previously implicated by patch testing in other studies (see above). The authors reported that a better response to therapy was observed in patients with depigmentation localized to the site of chemical contact (de novo vitiligo) than those with pre-existing vitiligo, suggesting that identifying and eliminating the chemical improved treatment responses45.
Interestingly, the authors noted the presence of “confetti macules” in the majority (89%) of their suspected chemical-induced vitiligo patients, and suggested their presence as a distinct sign of chemical-induced vitiligo45. This had previously been described by Ortonne, et al. as well2. However the presence of confetti-like depigmentation was recently reported to be a clinical sign of highly active vitiligo49. I have personally observed these macules to be widely distributed in patients with rapidly progressing disease, in areas distinct from exposure to chemicals or products (personal observations). Thus, I suspect that confetti macules are not necessarily indicative of chemical-induced vitiligo, but rather that chemical-induced vitiligo is often rapidly progressing, which is why it may be marked by the presence of confetti macules.
Other products reported to have induced vitiligo through case reports and case studies are prevalent in the literature, and include condoms, colored strings, herbal oils, detergents, footwear, hair color, dental acrylics, nylon thread, Vick’s Vaporub, compounded phenol-containing cream, a methylphenidate patch, electrocardiogram pads, synthetic leather wallets, ornamental azo dyes, cinnamon toothpaste, eye drops, rubber, and “black henna” tattoos (although the reaction was likely due to PPD in the product, as black henna is not actual henna, which is orange or red)3, 23, 29, 36, 37, 50–61. All of these reported cases occurred through topical exposures, which theoretically delivers a high concentration of the chemical to melanoyctes in their epidermal niche. It is unknown whether systemic exposures to similar agents (through diet, medications, or supplements) could result in a similar effect. If so, it could have serious implications for individuals who use future “skin-whitening candy” or other supplements, which appear to contain phenols62, 63. One study found that intramuscular injection of 4-TBP in black rabbits and dogs caused depigmentation28, while there are conflicting reports of depigmentation after feeding MBEH to guinea pigs4, 64.
Mechanism of chemical-induced vitiligo
Depigmentation from chemicals may be associated with an initial reaction consistent with allergic contact dermatitis, and so some have dismissed chemical-induced depigmentation as a nonspecific post-inflammatory change or koebner response to inflammation. However many cases occur in the absence of any apparent dermatitis, and most who experience contact dermatitis to these agents do not develop depigmentation. In fact, they typically develop post-inflammatory hyperpigmentation2. Also, many subjects with chemical-induced depigmentation also develop lesions at sites that are remote from the site of contact with the chemical46, 65. These observations are particularly apparent following the therapeutic administration of MBEH cream, which most often (>80% of patients) does not induce a contact dermatitis, and results in depigmentation of the entire body, including untreated sites. Thus, the evidence strongly supports a more complicated pathogenesis, one that involves either direct toxicity to melanocytes or subclinical inflammation that can be communicated to remote, unexposed melanocytes.
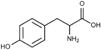
The common feature shared by offending chemicals in this category is their chemical structure, which typically includes a phenol group, composed of a benzene ring with a hydroxyl side chain. Phenols with a nonpolar side chain in the para (or 4-) position, and in particular an ether group at that position, appear to be the most potent depigmenting chemicals66. This chemical structure is shared with the amino acid tyrosine, which is the basic building block of melanin upon which tyrosinase and other melanogenic enzymes make key modifications2 (Table). Thus, offending phenols appear to act as tyrosine analogs that interfere with melanogenesis. A small number of these chemicals are not phenols (i.e. PPD), and whether these chemicals are “close enough” to phenols or are metabolized into phenols before becoming toxic is currently unknown. Early hypotheses suggested that depigmenting chemicals entering the melanogenesis pathway generated toxic metabolites that destroyed melanocytes from the inside out2, 46.
Table 1.
The amino acid tyrosine and its chemical structure along with patch test-proven vitiligo-inducing chemicals and the products in which they are used as ingredients.
| Chemical name | Structure | Commercial products | References |
|---|---|---|---|
| Tyrosine |
|
||
| Monobenzyl ether of hydroquinone (MBEH) |
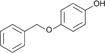
|
Benoquin® cream, rubber | 1, 7–11 |
| 4-tert-butylcatechol (4-TBC) |
|
Lubricating oil | 17 |
| 4-tert-butylphenol (4-TBP) |
|
Detergents/disinfectants, sealant/adhesive | 19–24, 27, 28 |
| 4-tert-amylphenol (4-TAP) |
|
Detergents/disinfectants | 27 |
| Para-phenylenediamine (PPD) |
|
Hair dyes, “black henna” | 29–34 |
| Brilliant crocein |
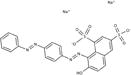
|
Alta dyes | 3, 36 |
| Rhodamine B |
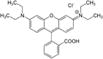
|
Alta dyes, air freshener dyes | 3, 36 |
| Rhododendrol |
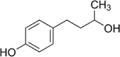
|
Skin-lightening cosmetic | 39–42 |
Initial studies found that adding these chemicals to human melanocytes cultured in vitro resulted in toxicity at high concentrations, suggesting that they could be directly toxic to melanocytes. This required either tyrosinase or tyrosinase-related protein 1 (Tyrp1), depending on the chemical studied67, 68. However this seemed too simplistic, since the chemical concentrations required to induce melanocyte death in vitro are likely much higher than what is achievable in the basal epidermis following topical exposure, since typically only a fraction of chemicals penetrate the skin. According to previous studies with phenol, ~4% of the total topical dose was ultimately absorbed through the skin, at a rate of 0.25% per hour69. When hair dyes are used on the scalp, resorcinol is systemically absorbed at 0.076% of the total applied dose70, although the highest concentration reached in the epidermis is unknown. In addition, the majority of individuals do not depigment after exposure, and a large subset of exposed subjects develop lesions remote from the site of exposure. This was particularly evident in the large population of Japanese exposed to rhododendrol – only 2% of users developed depigmentation, and about 5% of those had remote depigmentation40. Therefore, it is likely that chemicals induce depigmentation through an indirect mechanism that requires predisposition of the individual (likely genetic), and can spread to unexposed skin following an initial induction event46.
Kroll and colleagues first demonstrated that a depigmenting phenol could act to induce melanocyte death indirectly, by activating an inflammatory cascade in dendritic cells co-cultured with exposed melanocytes. They added 4-TBP to melanocytes and found that this induced the cellular stress response, resulting in the secretion of HSP70, a proinflammatory heat shock protein. This secreted protein activated dendritic cells that were co-cultured with the melanocytes, which then killed the melanocytes71. A separate study found that 4-TBP and MBEH induced reactive oxygen species (ROS) in melanocytes and also activated the unfolded protein response (UPR), a cellular stress response that results in the production of chaperone proteins (including heat shock proteins like HSP70) to aid in protein folding. A key mediator of the UPR, a protein called XBP1, induced the production of interleukin (IL)-6 and IL-8, both inflammatory cytokines. These data also suggested that vitiligo-inducing phenols indirectly caused melanocyte death by stressing the cells and inducing the secretion of proinflammatory signals.
Van den Boorn et al. found that in addition to these pathways, MBEH treatment of melanocytes initiated autophagy in the cells, a condition in which cells under significant stress begin to break down organelles and other cytoplasmic contents in order to recycle those contents and survive the assault. They observed that tyrosinase became modified by the chemical through quinone haptenation, a covalent linking of the enzyme and chemical that created a novel protein that could act as a foreign antigen to initiate an immune response. This was then secreted from the cells packaged into small nanoparticles called exosomes, which were absorbed by co-cultured dendritic cells and presented to autoreactive T cells67. More recent work in mice revealed that skin macrophages and natural killer (NK) cells activated by MBEH-stressed melanocytes through innate pattern recognition receptors may participate in melanocyte destruction as well72.
Rhododendrol treatment of human melanocytes induced tyrosinase-dependent apoptosis, activation of ER stress through the UPR, IL-8 production, and autophagy, but not ROS39, 73, 74. Interestingly, rhododendrol toxicity correlated with tyrosinase activity in human melanocyte cell lines tested from a variety of donors from different racial backgrounds, suggesting that individual variability in tyrosinase activity could at least partially be responsible for the variable response to rhododendrol73. Histology of rhododendrol-induced lesions revealed a significant reduction of melanocytes, melanin incontinence, and an increased infiltration of T cells42, 75. Patients also had more melanocyte-specific CD8+ T cells in their blood compared to controls76, suggesting that these chemically-induced melanocyte changes activated an autoimmune response that has been observed in conventional vitiligo patients as well77–79.
Thus, the pathogenicity of vitiligo-inducing phenols appears to be due to their structural similarity to the amino acid tyrosine, which is the basic building block of the pigment melanin. As a consequence, tyrosinase or other enzymes of melanogenesis engage the chemicals as they would tyrosine, but instead covalently bind to it, resulting in persistent ROS generation (in some cases), UPR activation, autophagy, and exosome production. Exosomes deliver new antigens to neighboring immune cells, which initiate inflammation and activate autoreactive T cells, so the chemical-induced stress in melanocytes initiates an autoimmune response that results in their destruction (Figure 2, reviewed in80, 81). This may explain why not all exposed individuals develop depigmentation, as genetic influences likely predispose affected subjects through more sensitive melanocytes, a predisposition toward autoimmunity, or both. It also may explain why exposed subjects can develop depigmentation remote from the site of chemical exposure, as an initiated immune response would likely target other melanocytes that are not directly stressed through chemical exposure. Sparing of the hair pigment following chemical exposure may be due to immune privilege of the follicle, meaning that it is protected from immune attack, a phenomenon observed in non-chemically induced vitiligo as well.
Fig. 2.
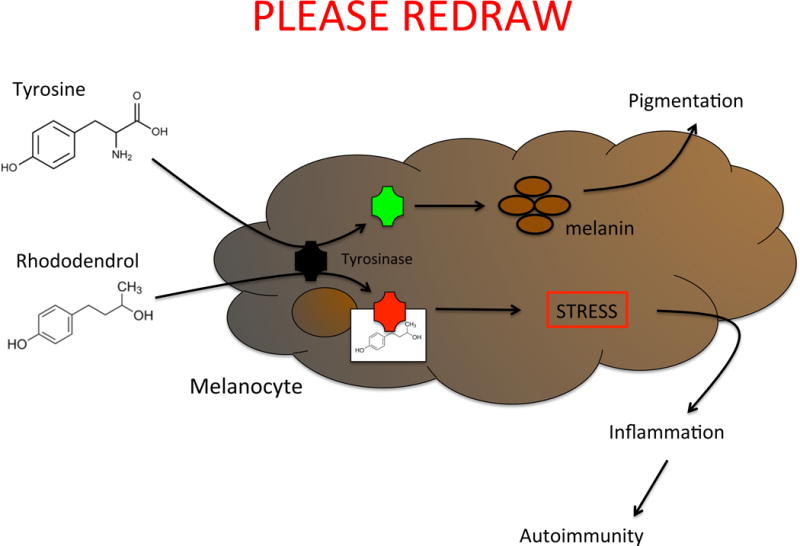
Schematic of the pathogenesis of chemical-induced vitiligo. Tyrosine is processed into melanin by the enzyme tyrosinase. Chemicals that act as tyrosine analogs interact with tyrosinase (or other melanin-producing enzymes), disrupt melanin production, and induce the cellular stress response, which leads to inflammation and autoimmune destruction of melanocytes.
Summary
Skin depigmentation following exposure to chemical phenols is indistinguishable from vitiligo2, 29, 40, 65, and appears to be due to activation of melanocyte-specific autoimmunity, as is also seen in non-chemically induced vitiligo81, 82. In fact, chemicals may simply accelerate stress pathways that are already present in healthy melanocytes, but push them above a tolerated threshold to exceed the capacity that can be appropriately managed by healthy cells, leading to autoimmune inflammation. This is likely a mechanism by which non-chemically induced vitiligo is initiated as well, but genetic influences may increase cellular stress in melanocytes or set a lower threshold for stress that can be tolerated by the immune system81.
Therefore, since chemical-induced vitiligo and nonchemically-induced vitiligo appear on the same spectrum clinically, histologically, and pathogenically, chemical-induced depigmentation should be called “chemical-induced vitiligo”, rather than other names that have been used, such as chemical leukoderma, occupational vitiligo/leukoderma, and contact vitiligo/leukoderma/depigmentation. Admittedly, it is difficult to rule out a component of non-immune direct melanocyte toxicity in subjects exposed to high concentrations of chemical, where the effect remains localized to the site of contact. However other terms are either less specific or do not apply in all cases, as “contact” implies only a local effect as well as allergic response, “leukoderma” is nonspecific and could apply to any form of hypopigmentation including post-inflammatory changes, and “occupational” does not include the large number of subjects affected at home by commercial products. In addition, these chemicals should not be considered “bleaching chemicals” when used in vitiligo, since bleaching implies denaturing of pigment proteins, and the mechanism of chemical-induced vitiligo is much more complex. The prevalence of phenols in many common household products makes this topic of significant importance, particularly in light of potential future oral skin-lightening supplements that may soon become available62, 63.
Key Points.
Chemical exposure may serve as an environmental risk factor for developing vitiligo
Chemical-induced depigmentation is indistinguishable from vitiligo, and should be considered “chemical-induced vitiligo”.
Chemical-induced vitiligo is typically localized to the site of application and may also spread to remote, unexposed locations
Monobenzyl ether of hydroquinone (MBEH) was the first chemical noted to induce depigmentation in the skin, and is now used therapeutically in vitiligo patients to complete their depigmentation
Most chemicals that induce vitiligo are phenols and act as tyrosine analogs to disrupt melanocyte function, resulting in autoimmunity
The use of phenols is widespread as ingredients in household commercial products
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Consultant for Combe, Inc.
References
- 1.Oliver EASL, Warren LH. Occupational leukoderma. JAMA. 1939;113:927–8. [Google Scholar]
- 2.Ortonne J-P, Mosher DB, Fitzpatrick TB. Vitiligo and other hypomelanoses of hair and skin. New York: Plenum Medical Book Co; 1983. [Google Scholar]
- 3.Bajaj AK, Saraswat A, Srivastav PK. Chemical leucoderma: Indian scenario, prognosis, and treatment. Indian J Dermatol. 2010;55:250–4. doi: 10.4103/0019-5154.70674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denton CR, Lerner AB, Fitzpatrick TB. Inhibition of melanin formation by chemical agents. J Invest Dermatol. 1952;18:119–35. doi: 10.1038/jid.1952.16. [DOI] [PubMed] [Google Scholar]
- 5.Lerner AB, Fitzpatrick TB. Treatment of melanin hyperpigmentation. J Am Med Assoc. 1953;152:577–82. doi: 10.1001/jama.1953.03690070011004. [DOI] [PubMed] [Google Scholar]
- 6.Kelly EW., Jr Pigmented skin lesions; treatment with monobenzyl-ether of hydroquinone. J Mich State Med Soc. 1956;55:303–4. passim. [PubMed] [Google Scholar]
- 7.Canizares O, Uribe Jaramillo F, Kerdel Vegas F. Leukomelanoderma subsequent to the application of monobenzyl ether of hydroquinone; a vitiligoid reaction observed in Colombia and Venezuela. AMA Arch Derm. 1958;77:220–3. doi: 10.1001/archderm.1958.01560020074009. [DOI] [PubMed] [Google Scholar]
- 8.Dorsey CS. Dermatitic and pigmentary reactions to monobenzyl ether of hydroquinone: report of two cases. Arch Dermatol. 1960;81:245–8. doi: 10.1001/archderm.1960.03730020081012. [DOI] [PubMed] [Google Scholar]
- 9.Ito M. Monobenzylether-hydroquinone leukomelanoderma. Tahuku Journal of Experimental Medicine. 1957;65:64. [Google Scholar]
- 10.Sidi E, Bourgeois-Spinasse J. Depigmentation resulting from the application of hydroquinone-monobenzylether. Sem Hop. 1958;34:417–20. [PubMed] [Google Scholar]
- 11.Mosher DB, Parrish JA, Fitzpatrick TB. Monobenzylether of hydroquinone. A retrospective study of treatment of 18 vitiligo patients and a review of the literature. Br J Dermatol. 1977;97:669–79. doi: 10.1111/j.1365-2133.1977.tb14275.x. [DOI] [PubMed] [Google Scholar]
- 12.Chivers CP. Two cases of occupational leucoderma following contact with hydroquinone monomethyl ether. Br J Ind Med. 1972;29:105–7. doi: 10.1136/oem.29.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Nuzzo S, Masotti A. Depigmentation therapy in vitiligo universalis with cryotherapy and 4-hydroxyanisole. Clin Exp Dermatol. 2010;35:215–6. doi: 10.1111/j.1365-2230.2009.03412.x. [DOI] [PubMed] [Google Scholar]
- 14.Njoo MD, Vodegel RM, Westerhof W. Depigmentation therapy in vitiligo universalis with topical 4-methoxyphenol and the Q-switched ruby laser. J Am Acad Dermatol. 2000;42:760–9. doi: 10.1067/mjd.2000.103813. [DOI] [PubMed] [Google Scholar]
- 15.Das M, Tandon A. Occupational vitiligo. Contact dermatitis. 1988;18:184–5. doi: 10.1111/j.1600-0536.1988.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 16.Duffield JA. Depigmentation of skin by quinol and its monobenzyl ether. Lancet. 1952;259:1164. [Google Scholar]
- 17.Gellin GA, Possick PA, Davis IH. Occupational depigmentation due to 4-tertiarybutyl catechol (TBC) J Occup Med. 1970;12:386–9. [PubMed] [Google Scholar]
- 18.Gellin GA, Maibach HI, Misiaszek MH, Ring M. Detection of environmental depigmenting substances. Contact dermatitis. 1979;5:201–13. doi: 10.1111/j.1600-0536.1979.tb04853.x. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj AK, Gupta SC, Chatterjee AK. Contact depigmentation from free para-tertiary-butylphenol in bindi adhesive. Contact dermatitis. 1990;22:99–102. doi: 10.1111/j.1600-0536.1990.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 20.Angelini E, Marinaro C, Carrozzo AM, Bianchi L, Delogu A, Giannello G, et al. Allergic contact dermatitis of the lip margins from para-tertiary-butylphenol in a lip liner. Contact dermatitis. 1993;28:146–8. doi: 10.1111/j.1600-0536.1993.tb03375.x. [DOI] [PubMed] [Google Scholar]
- 21.Malten KE, Seutter E, Hara I, Nakajima T. Occupational vitiligo due to paratertiary butylphenol and homologues. Trans St Johns Hosp Dermatol Soc. 1971;57:115–34. [PubMed] [Google Scholar]
- 22.Okmura YST. Vitiliginous lesions occurring among workers in a phenol derivative factory. Japanese Journal of Dermatology. 1962;7:617–9. [Google Scholar]
- 23.O’Malley MA, Mathias CG, Priddy M, Molina D, Grote AA, Halperin WE. Occupational vitiligo due to unsuspected presence of phenolic antioxidant byproducts in commercial bulk rubber. J Occup Med. 1988;30:512–6. [PubMed] [Google Scholar]
- 24.James O, Mayes RW, Stevenson CJ. Occupational vitiligo induced by p-tert-butylphenol, a systemic disease? Lancet. 1977;2:1217–9. doi: 10.1016/s0140-6736(77)90451-2. [DOI] [PubMed] [Google Scholar]
- 25.Rodermund OE, Wieland H. Vitiliginous depigmentation, liver and splenic lesions and struma due to occupational contact with paratertiary butylphenol–a new systemic occupational disease. Berufsdermatosen. 1975;23:193–5. [PubMed] [Google Scholar]
- 26.Goldmann PJ, Thiess AM. Occupational vitiligo caused by para-tertiary-butylphenol, a trias of vitiligo, hepatosis and struma. Hautarzt. 1976;27:155–9. [PubMed] [Google Scholar]
- 27.Kahn G. Depigmentation caused by phenolic detergent germicides. Arch Dermatol. 1970;102:177–87. [PubMed] [Google Scholar]
- 28.Babanov GP, Chumakov NN. The etiology and pathogenesis of occupational vitiligo. Vestn Dermatol Venerol. 1966;40:44–8. [PubMed] [Google Scholar]
- 29.Taylor JS, Maibach HI, Fisher AA, Bergfeld WF. Contact leukoderma associated with the use of hair colors. Cutis. 1993;52:273–80. [PubMed] [Google Scholar]
- 30.Bajaj AK, Gupta SC, Chatterjee AK, Singh KG, Basu S, Kant A. Hair dye depigmentation. Contact dermatitis. 1996;35:56–7. doi: 10.1111/j.1600-0536.1996.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 31.Brancaccio R, Cohen DE. Contact leukoderma secondary to para-phenylenediamine. Contact dermatitis. 1995;32:313. doi: 10.1111/j.1600-0536.1995.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 32.Saitta P, Cohen D, Brancaccio R. Contact leukoderma from para-phenylenediamine. Dermatitis: contact, atopic, occupational, drug. 2009;20:56–7. [PubMed] [Google Scholar]
- 33.Farsani TT, Jalian HR, Young LC. Chemical leukoderma from hair dye containing para-phenylenediamine. Dermatitis: contact, atopic, occupational, drug. 2012;23:181–2. doi: 10.1097/DER.0b013e318260d5cd. [DOI] [PubMed] [Google Scholar]
- 34.Trattner A, David M. Hair-dye-induced contact vitiligo treated by phototherapy. Contact dermatitis. 2007;56:115–6. doi: 10.1111/j.1600-0536.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- 35.Verma S, Kumar B. Contact leukoderma of the scalp or an unusual variant of vitiligo? J Dermatol. 2001;28:554–6. doi: 10.1111/j.1346-8138.2001.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 36.Bajaj AK, Pandey RK, Misra K, Chatterji AK, Tiwari A, Basu S. Contact depigmentation caused by an azo dye in alta. Contact dermatitis. 1998;38:189–93. doi: 10.1111/j.1600-0536.1998.tb05705.x. [DOI] [PubMed] [Google Scholar]
- 37.Bajaj AK, Gupta SC, Chatterjee AK. Contact depigmentation of the breast. Contact dermatitis. 1991;24:58. doi: 10.1111/j.1600-0536.1991.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 38.Bajaj AK, Gupta SC, Chatterjee AK. Footwear depigmentation. Contact dermatitis. 1996;35:117–8. doi: 10.1111/j.1600-0536.1996.tb02313.x. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki M, Kondo M, Sato K, Umeda M, Kawabata K, Takahashi Y, et al. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment Cell Melanoma Res. 2014;27:754–63. doi: 10.1111/pcmr.12269. [DOI] [PubMed] [Google Scholar]
- 40.Nishigori C, Aoyama Y, Ito A, Suzuki K, Suzuki T, Tanemura A, et al. Guide for medical professionals (i.e., dermatologists) for the management of Rhododenol-induced leukoderma. J Dermatol. 2015;42:113–28. doi: 10.1111/1346-8138.12744. [DOI] [PubMed] [Google Scholar]
- 41.Tokura Y, Fujiyama T, Ikeya S, Tatsuno K, Aoshima M, Kasuya A, et al. Biochemical, cytological, and immunological mechanisms of rhododendrol-induced leukoderma. J Dermatol Sci. 2015;77:146–9. doi: 10.1016/j.jdermsci.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Tanemura A, Yang L, Yang F, Nagata Y, Wataya-Kaneda M, Fukai K, et al. An immune pathological and ultrastructural skin analysis for rhododenol-induced leukoderma patients. J Dermatol Sci. 2015;77:185–8. doi: 10.1016/j.jdermsci.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Wu S, Li WQ, Cho E, Harris JE, Speizer F, Qureshi AA. Use of permanent hair dyes and risk of vitiligo in women. Pigment Cell Melanoma Res. 2015 doi: 10.1111/pcmr.12402. [DOI] [PubMed] [Google Scholar]
- 44.Lerner AB. Vitiligo. J Invest Dermatol. 1959;32:285–310. [PubMed] [Google Scholar]
- 45.Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinico-aetiological study of 864 cases in the perspective of a developing country. Br J Dermatol. 2009;160:40–7. doi: 10.1111/j.1365-2133.2008.08815.x. [DOI] [PubMed] [Google Scholar]
- 46.Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208–14. doi: 10.1111/j.1600-0749.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 47.Lister J. On the Antiseptic Principle in the Practice of Surgery. British medical journal. 1867;2:246–8. doi: 10.1136/bmj.2.351.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Department of Health and Human Services. Household Products Database: National Institutes of Health, National Library of Medicine, Specialized Information Services. 2013 [Google Scholar]
- 49.Sosa JJ, Currimbhoy SD, Ukoha U, Sirignano S, O’Leary R, Vandergriff T, et al. Confetti-like depigmentation: A potential sign of rapidly progressing vitiligo. J Am Acad Dermatol. 2015;73:272–5. doi: 10.1016/j.jaad.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh SK, Bandyopadhyay D. Chemical leukoderma induced by colored strings. J Am Acad Dermatol. 2009;61:909–10. doi: 10.1016/j.jaad.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh SK, Bandyopadhyay D. Chemical leukoderma induced by herbal oils. Journal of cutaneous medicine and surgery. 2010;14:310–3. doi: 10.2310/7750.2010.09075. [DOI] [PubMed] [Google Scholar]
- 52.Pandhi RK, Kumar AS. Contact leukoderma due to ‘Bindi’ and footwear. Dermatologica. 1985;170:260–2. doi: 10.1159/000249545. [DOI] [PubMed] [Google Scholar]
- 53.Kanerva L, Estlander T. Contact leukoderma caused by patch testing with dental acrylics. American journal of contact dermatitis: official journal of the American Contact Dermatitis Society. 1998;9:196–8. [PubMed] [Google Scholar]
- 54.Boyse KE, Zirwas MJ. Chemical Leukoderma Associated with Vicks VapoRub. The Journal of clinical and aesthetic dermatology. 2008;1:34–5. [PMC free article] [PubMed] [Google Scholar]
- 55.Hernandez C, Reddy SG, Barfuss A, Le Poole C. Contact leukoderma after application of a compounded phenol cream and narrowband-UVB. Eur J Dermatol. 2008;18:593–5. doi: 10.1684/ejd.2008.0481. [DOI] [PubMed] [Google Scholar]
- 56.Ghasri P, Gattu S, Saedi N, Ganesan AK. Chemical leukoderma after the application of a transdermal methylphenidate patch. J Am Acad Dermatol. 2012;66:e237–8. doi: 10.1016/j.jaad.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valsecchi R, Leghissa P, Di Landro A, Bartolozzi F, Riva M, Bancone C. Persistent leukoderma after henna tattoo. Contact dermatitis. 2007;56:108–9. doi: 10.1111/j.1600-0536.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 58.Banerjee R, Banerjee K, Datta A. Condom leukoderma. Indian journal of dermatology, venereology and leprology. 2006;72:452–3. doi: 10.4103/0378-6323.29345. [DOI] [PubMed] [Google Scholar]
- 59.Bajaj AK, Misra A, Misra K, Rastogi S. The azo dye solvent yellow 3 produces depigmentation. Contact dermatitis. 2000;42:237–8. [PubMed] [Google Scholar]
- 60.Mathias CG, Maibach HI, Conant MA. Perioral leukoderma simulating vitiligo from use of a toothpaste containing cinnamic aldehyde. Arch Dermatol. 1980;116:1172–3. [PubMed] [Google Scholar]
- 61.Suchi ST, Gupta A, Srinivasan R. Contact allergic dermatitis and periocular depigmentation after using olapatidine eye drops. Indian J Ophthalmol. 2008;56:439–40. doi: 10.4103/0301-4738.42431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O M. Skin whitening candy is coming. Timecom. 2014 [Google Scholar]
- 63.Lighter skin from within: Melagenol offers an effective natural solution for skin whitening applications. 2014 [Google Scholar]
- 64.Peck SMSH. Effect of monobenzyl hydroquinone on oxidase systems in vivo and in vitro. Journal of Investigative Dermatology. 1941;4:325–9. [Google Scholar]
- 65.Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255–8. doi: 10.4103/0019-5154.70680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riley P. Pathological disturbances of pigmentation. In: Jarrett A, editor. The Physiology and Pathophysiology of the Skin. London: Academic; 1974. [Google Scholar]
- 67.van den Boorn JG, Picavet DI, van Swieten PF, van Veen HA, Konijnenberg D, van Veelen PA, et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J Invest Dermatol. 2011;131:1240–51. doi: 10.1038/jid.2011.16. [DOI] [PubMed] [Google Scholar]
- 68.Yang F, Sarangarajan R, Le Poole IC, Medrano EE, Boissy RE. The cytotoxicity and apoptosis induced by 4-tertiary butylphenol in human melanocytes are independent of tyrosinase activity. J Invest Dermatol. 2000;114:157–64. doi: 10.1046/j.1523-1747.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 69.Feldmann RJ, Maibach HI. Absorption of some organic compounds through the skin in man. J Invest Dermatol. 1970;54:399–404. doi: 10.1111/1523-1747.ep12259184. [DOI] [PubMed] [Google Scholar]
- 70.Wolfram LJ, Maibach HI. Percutaneous penetration of hair dyes. Archives of dermatological research. 1985;277:235–41. doi: 10.1007/BF00404323. [DOI] [PubMed] [Google Scholar]
- 71.Kroll TM, Bommiasamy H, Boissy RE, Hernandez C, Nickoloff BJ, Mestril R, et al. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: relevance to vitiligo. J Invest Dermatol. 2005;124:798–806. doi: 10.1111/j.0022-202X.2005.23653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van den Boorn JG, Jakobs C, Hagen C, Renn M, Luiten RM, Melief CJ, et al. Inflammasome-Dependent Induction of Adaptive NK Cell Memory. Immunity. 2016;44:1406–21. doi: 10.1016/j.immuni.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Kasamatsu S, Hachiya A, Nakamura S, Yasuda Y, Fujimori T, Takano K, et al. Depigmentation caused by application of the active brightening material, rhododendrol, is related to tyrosinase activity at a certain threshold. J Dermatol Sci. 2014;76:16–24. doi: 10.1016/j.jdermsci.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Yang L, Yang F, Wataya-Kaneda M, Tanemura A, Tsuruta D, Katayama I. 4-(4-hydroroxyphenyl)-2-butanol (rhododendrol) activates the autophagy-lysosome pathway in melanocytes: insights into the mechanisms of rhododendrol-induced leukoderma. J Dermatol Sci. 2015;77:182–5. doi: 10.1016/j.jdermsci.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Nishioka M, Tanemura A, Yang L, Tanaka A, Arase N, Katayama I. Possible involvement of CCR4(+)CD8(+) T cells and elevated plasma CCL22 and CCL17 in patients with Rhododenol-induced leukoderma. J Dermatol Sci. 2015;77:188–90. doi: 10.1016/j.jdermsci.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 76.Fujiyama T, Ikeya S, Ito T, Tatsuno K, Aoshima M, Kasuya A, et al. Melanocyte-specific cytotoxic T lymphocytes in patients with rhododendrol-induced leukoderma. J Dermatol Sci. 2015;77:190–2. doi: 10.1016/j.jdermsci.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 77.Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. The Journal of experimental medicine. 1998;188:1203–8. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129:2220–32. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- 79.Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, et al. CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Science translational medicine. 2014;6:223ra23. doi: 10.1126/scitranslmed.3007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. Journal of leukocyte biology. 2013;94:1167–84. doi: 10.1189/jlb.0313153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harris JE. Cellular stress and innate inflammation in organ-specific autoimmunity: lessons learned from vitiligo. Immunological Reviews. 2016;269:11–25. doi: 10.1111/imr.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: danger from within. Current opinion in immunology. 2013;25:676–82. doi: 10.1016/j.coi.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


