Abstract
Tissue stiffness is tightly controlled under normal conditions, but changes with disease. In cancer, tumors often tend to be stiffer than the surrounding uninvolved tissue, yet the cells themselves soften. Within the past decade, and particularly in the last few years, there is increasing evidence that the stiffness of the extracellular matrix modulates cancer and stromal cell mechanics and function, influencing such disease hallmarks as angiogenesis, migration, and metastasis. This review briefly summarizes recent studies that investigate how cancer cells and fibrosis-relevant stromal cells respond to ECM stiffness, the possible sensing appendages and signaling mechanisms involved, and the emergence of novel substrates — including substrates with scar-like fractal heterogeneity — that mimic the in vivo mechanical environment of the cancer cell.
Introduction
The fact that tumors are often stiffer than the surrounding uninvolved tissue has been known for as long as the disease has been identified. The rigid nature of tumors is the basis for using palpation as a diagnostic method in soft tissues like breast and abdomen, and more recently, as the basis for high-resolution detection of small lesions by MRI elastography [1•,2•,3•] or ultrasound [4•]. These clinical observations, together with in vitro experiments which demonstrate that stiffness-sensing by cancer and stromal cells influence cell survival and proliferation, opened the door for many investigations that employ novel biocompatible materials with tunable viscoelastic properties. These in vitro systems have the potential to elucidate the mechanical and molecular mechanisms by which cells detect changes in their environment and transduce physical signals to the biochemical signals that control their function, biochemistry, and gene expression.
Mechanotransduction of physical cues to initiate intracellular signaling pathways has recently been documented in many cancer types and a wide range of effects have been observed, ranging from acute changes such as activation of ion channels or protein kinases to long-term changes in cell phenotype that require initiation of gene transcription and protein production. This review summarizes some recent studies that use materials of tunable rigidity to identify the mechanosensing ability of cancer cells, how substrate stiffness affects some of the cancer hallmarks, and the possible mechanisms involved. New materials that mimic the viscoelasticity of normal and cancerous tissues are also highlighted.
Effects of mechanics on proliferation and apoptosis
Several studies demonstrate that mechanotransduction by cancer cells might be significantly blunted compared to normal cells, but others show the opposite. An important pioneering study by Wang et al. demonstrates that whereas normal NIH 3T3 fibroblasts are highly dependent on a rigid substrate for DNA synthesis and decreased apoptosis, cells transformed with the H-ras oncogene lose their stiffness-sensing ability [5]. More recently, a number of cancer cell types and Ha-RasV12-transformed cells (pancreatic, breast, and kidney) exhibit stiffness insensitivity, as measured by DNA synthesis rate and cell stiffness that is unaffected by the underlying substrate rigidity [6••]. This may be associated with decreased caveolin-1 (cav1), which has an inhibitory function in cell proliferation through the extracellular signal-regulated kinase 1/2, phosphoinositide 3-kinase (PI3K) or β-catenin-T-cell factor/lymphoid enhancer factor pathways, as well as a regulatory function in focal adhesion and integrin-mediated actin remodeling. When Cav1 is overexpressed or re-expressed in Ha-RasV12 transformed cells, stiffness sensing is restored; when knocked down, cells soften, their stiffness is not dependent upon substrate rigidity, and they are able to grow on soft substrates. Additionally, tumor-initiating cells or cancer stem cells are largely insensitive to stiffness with respect to spreading, migration, and proliferation, but can regain their stiffness response when myosin-dependent contractility is increased [7••]. However, the generalization that cancer cell proliferation is stiffness-independent cannot be applied to all cancer types. For example, SK-N-DZ neuroblastoma cells preferentially proliferate on softer substrates [8].
Concomitant with deregulated proliferation, the suppression of apoptosis is also necessary for the expansion and invasion of cancer cells and is influenced by substrate stiffness. As seen in TGF-β1-treated normal murine mammary gland epithelial cells and Madin-Darby canine kidney epithelial cells, soft gels induce transforming growth factor-β1 (TGF-β1) mediated apoptosis, nuclear fragmentation, and caspase activity [9]. In contrast, stiff gels trigger epithelial–mesenchymal transition (EMT) characterized by elongated morphology, delocalization of epithelial junctional markers zonula occludens-1 and E-cadherin, as well as increased N-cadherin, α-smooth muscle actin (α-SMA), and Snai1 (a TGF-β-mediated transcription factor that represses E-cadherin expression and can induce EMT). Interestingly, as substrate stiffness increases, there is a switch around 1–8 kPa where caspase-3 activity decreases and Snai1 expression increases. Various genetic and pharmacologic experiments suggest the involvement of focal adhesion kinase (FAK) and PI3K/ Akt signaling in the stiffness-sensitive apoptotic and EMT switch [9]. Regulation of EMT by substrate stiffness is supported by numerous studies [10–13].
Effects of mechanics on angiogenesis
Angiogenesis is essential for tumor growth. Since the pioneering studies of Folkman, a number of proteins and factors that promote and inhibit angiogenesis have been identified. However, knowledge of how cellular mechanics influences new vessel growth is limited. Likely, cell-generated contractile forces are needed for capillary sprouting, as shown in a 3D co-culture model with human umbilical vein endothelial cells (HUVEC) and normal human lung fibroblast, where angiogenesis was inhibited by fibrin density and even more so with the addition of myosin inhibitors [14].
Possibly the most well-studied growth factor involved in the initiation and regulation of angiogenesis is vascular endothelial growth factor (VEGF). The biochemical events that stimulate VEGF expression have been well studied, but not mechanical cues, although there exists evidence that environmental cues such as hypoxia and acidosis can drive VEGF expression. When seeded onto stiff collagen I-coated polyacrylamide gels representative of rigid, cirrhotic liver tissue, highly metastatic hepatocellular carcinoma (HCC) MHCC97H cells and lowly metastatic Hep3B cells upregulate their VEGF expression and phosphorylation levels of PI3K and Akt [15•]. When the integrin β1 is blocked in both HCC cell lines using a specific monoclonal antibody, VEGF expression and PI3K and Akt phosphorylation are lower compared to control. This result suggests that integrin β1 has a mechanosensing role in HCC cells and can mediate VEGF expression through the PI3K/Akt pathway.
A splice variant of the ECM ligand fibronectin (FN) that includes the extra domain-B (EDB-FN) is upregulated in tumors and may promote angiogenesis [16••]. When endothelial cells are seeded onto hard polyacrylamide gels, total FN and EDB-FN protein as well as pro-angiogenic PKC βII expression is increased and anti-angiogenic VEGF 165b expression is decreased compared to soft gels. The Rho/Rho-associated kinase (ROCK) pathway appears to mediate expression of EDB-FN, suggesting a mechanism by which matrix stiffness can affect angiogenesis.
Effects of mechanics on metastasis
Studies of breast cancer cells show that a compliant ECM is non-conducive to tumor cell invasion [17]. Paradoxically, some tumor cores are less stiff than the periphery, presumably because they contain mainly cancer cells and little ECM. This feature has been observed in not only breast carcinoma [18], but also prostate cancer tissue (on the micro-scale, but not on the macro-scale) [19]. The seemingly contradictory observation that tumors are stiff grossly, but soft on the micro-scale or in their core, might be explained by the stiffness of the individual cells. Lin et al. showed that cancer cells are softer than their normal counterparts across several cell types, including breast, bladder, cervix, pancreas, and transformed cells [6••]. One caveat is that these measurements were made from cells on glass, with the exception of cervical cells (accessible in situ). Softer cells are associated with increased motility and tumor invasiveness, and the development from radial growth phase to invasive vertical growth phase to metastasis is characterized by decreased cell stiffness, likely allowing cells to move through gaps within tissues and vessels [18].
The stiffness and integrity of endothelial cells and the vascular wall are also important factors in the context of extravasation. Infiltration of breast cancer cells onto pulmonary artery endothelial cells causes the activation of myosin light chain kinase and myosin II, followed by subsequent contractility of the endothelial cells, as studied in a co-culture model with MDA-MB-231 (breast cancer) and endothelial cells [20]. As a result, the ECM softens and the cytoskeleton is rearranged, leading to degradation of the endothelial cell layer which aids extravasation. Tumor cell contractility is needed for extravasation, as inhibition of ROCK and MLCK-mediated traction forces diminishes invasion through endothelial cells. However, actin polymerization and myosin II-mediated contractility are not required for cancer cell migration, as MDA-MB-231 metastatic cells can still travel toward a chemoattractant through narrow channels.
In culture, induction of a malignant phenotype does not necessarily require a pathologically stiff ECM. A number of colon and prostate cancer cells undergo a transition from an adhesive epithelial to a rounded dissociated phenotype on soft substrates that is reminiscent of metastasis, and these rounded cells express genes that are characteristic of cancer cell metastasis, migration, and proliferation [21]. Similarly, melanoma tumor-repopulating cells (TRCs) exhibit cell softening, histone 3 lysine residue 9 demethylation, and Sox2 gene expression — all of which are promoted in compliant 3D fibrin matrices, but not stiff ones [22]. Together, these studies suggest that TRCs or metastatic cells may lie dormant in stiff environments, but function optimally in softer ones, and such a switch may serve as a driving force for cancer metastasis.
Stiffened matrices as a model for tumors
The change in mechanical properties during breast cancer is relatively well-studied, and tissue stiffening associated with malignancy has been correlated with increased collagen deposition and the formation of linear patterns of collagen fibers [23•]. Also associated with high-risk breast cancer and metastasis is increased FN expression (reportedly threefold), which in normal tissue is low (~1%) [24–27]. As an arguable model for breast cancer associated stromal cells, 3T3-L1 preadipocytes produce a more rigid FN network with decreased porosity and increased fiber diameter when preconditioned with soluble tumor factors compared to controls [28]. These changes in FN are accompanied by decreased cellular adhesion and increased VEGF levels, suggesting a role for FN in migration, angiogenesis and growth of breast tumor.
A few studies have also shown that the ECM can be stiffened through crosslinking mechanisms, such as the non-enzymatic formation of advanced glycation end-products (AGE) and lysyl oxidase. In a prostate epithelial cell acini 3D model, AGE-dependent crosslinking of two major components, collagen IV and laminin, stiffens the basal lamina matrix and induces malignant transformation characterized by loss of cell polarity, loss of cell–cell junctions, and luminal infiltration [29].
Mechanisms of mechanosensing by cancer cells
Most studies of mechanosensing in cancer cells focus on the role of integrins, cadherins and other transmembrane protein complexes that link cells to essentially solid material, either other cells or the ECM. Interestingly, primary cilia have been found on the MG63 human osteosarcoma cell line and on HeLa cells [30], suggesting that cells can mechanosense fluid from their dorsal surface. This finding is consistent with the report of a connection between the overexpression of polycystin 1 and 2, proteins present in the plasma membrane and cilia, and negative clinical outcomes and invasiveness in colorectal cancer [31].
The focal adhesion protein vinculin, a membrane-cytoskeletal protein involved in cell spreading and stability of focal adhesions, is upregulated in primary invasive human cancers [32]. Nonmalignant mammary MCF10A spheroids implanted into soft collagen/recombinant basement membrane gels keep their structure, as shown by spherical acini, intact adherens junctions, and tissue polarity, whereas in stiff gels, basal polarity and cell–cell junctions are disrupted. HA-ras MCF10AT premalignant mammary spheroids keep some tissue polarity within soft gels, but the structures are completely compromised in stiff gels. Together, these results show the dependence of invasion and malignancy on matrix stiffness, and furthermore, this is a result of integrin-mediated FAK signaling, changes in vinculin function, and induction of Akt signaling [32].
Transcriptional changes elicited by matrix stiffness
The Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) transcriptional regulators are the major downstream effectors of the Hippo pathway and are recognized as oncogenes. Increased substrate stiffness upregulates YAP in a lung cancer cell line, suggesting a role for YAP/TAZ and the Hippo pathway in lung cancer cell growth [33].
Twist1 is a transcription factor that has been implicated in cell differentiation as well as cancer metastasis. With respect to matrix stiffness, it has been shown that substrate rigidity drives translocation of Twist1 to the nucleus, inducing EMT, tumor invasion, and metastasis, with changes to collagen, MMPs, and lysyl oxidases [34•].
New materials and approaches for mechanobiology research
Recently, an area of intense interest is the development and characterization of 3D matrices with tunable physical properties as models for cell biology and also as test platforms for drugs and toxins. Soft and stiff alginate scaffolds with different RGD concentrations have been used as platforms to test the cytotoxic response of glioblastoma cells to various compounds. Both substrate rigidity and cell–matrix adhesions have an effect on cellular toxicity; cells are more sensitive to toxins when seeded onto soft substrates with stiffness similar to brain and these effects are lost when integrin binding is pharmacologically inhibited [35].
Polyethylene glycol diacrylate (PEGDA) hydrogels with compressive moduli between 2 and 70 kPa are used to encapsulate cancer stem cells and evaluate their optimal matrix stiffness for growth without any confounding environmental factors [36]. The optimal stiffness for cell survival and proliferation as well as YAP/TAZ expression is dependent upon the tissue of origin, that is, 5 kPa for breast, 25 kPa for colorectal and gastric, and 50 kPa for bone.
Instead of using chemically inert and non-physiological polyacrylamide gels, hydrogels made from crosslinked networks of biopolymers such as hyaluronic acid (HA) have been used to study cancer cell behavior to stiffness. HA, a component of the ECM is increased in many cancers [37••,38], including ovarian [39], non-small-cell lung adenocarcinomas [40], prostate cancer [41,42], gastric and colorectal [43,44], bladder [45], breast [46], and head and neck [47]. In many cases, in vitro HA is methylated to allow crosslinking, with the consequence of losing the ability to activate HA receptors such as CD44 that have been implicated in cancer [48]. Nonetheless, HA hydrogels elicit cellular behavior different from polyacrylamide or tissue culture plastic. For example, HT1080 fibrosarcoma cells encapsulated in HA recovered from hypoxic stress, but cells cultured on tissue culture plastic did not [37••]. Networks of HA that more closely resemble the native glycosaminoglycan can be made when the chain is sparsely modified with sulfhydryl groups and then crosslinked by oxidation or PEGDA. When HA gels are coupled to FN, proliferation of a number of cell types — including neonatal ventricular rat myocytes, human mesenchymal stem cells (hMSC), 3T3 fibroblasts and HUVECs — can be strongly enhanced even on soft substrates (200 Pa), which, if made of polyacrylamide, would halt proliferation [49].
In addition to variations of 2D hydrogel substrates, numerous methods are being developed to study how physical signals affect cancer cells in vitro. Such methods include production of pillar arrays that impede cell migration by trapping nuclei [50], simplified microfluidic methods that apply pressure to single cells [51–53], patterned type I collagen micro-tracks that mimic the paths by which cancer cells move in vivo [54], and optical tracking microrheology to measure the very soft pericellular matrix which changes in the tumor environment [55]. These more sophisticated techniques will hopefully provide greater insight into how cancer cells sense their mechanical environment.
Fibrosis models for cell culture: heterogeneous structure with homogeneous ligand
Although HA modifications for covalent crosslinking can at least sometimes inhibit normal binding to cell receptors (Figure 1a) [43], soft gels of crosslinked HA can also uniquely allow reorganization of some matrix macromolecules such as fibronectin into fiber-like regions (Figure 1b) [30]. Such observations of matrix heterogeneity are often anecdotal, but certainly raise questions about the effects of non-homogeneous gels on cells. Decoupling the effects of ligand density, which can certainly be non-homogeneous and lead to haptotaxis, from the effects of non-homogeneous compliance are also key to elucidating mechanosensing processes used by adherent cells. A heterogeneous matrix is a particularly distinctive feature of fibrosis, which is frequently associated with solid tumors [13]. Fibrosis also results from acute injury, such as a heart attack [56], as well as chronic diseases such as liver cirrhosis or muscular dystrophy [57], and it is often referred to as a scar. A scar forms locally in most or all tissues of higher animals and is compositionally characterized by an abundance of crosslinked collagen-I fibers heterogeneously distributed within a fibrotic tissue. A scar tends to be locally stiff and long-lasting [56,57]. Focusing on the cancer context, cancer cells respond to matrix stiffness, which results from increased collagen and crosslinking (fibrosis). However, where does increased collagen come from?
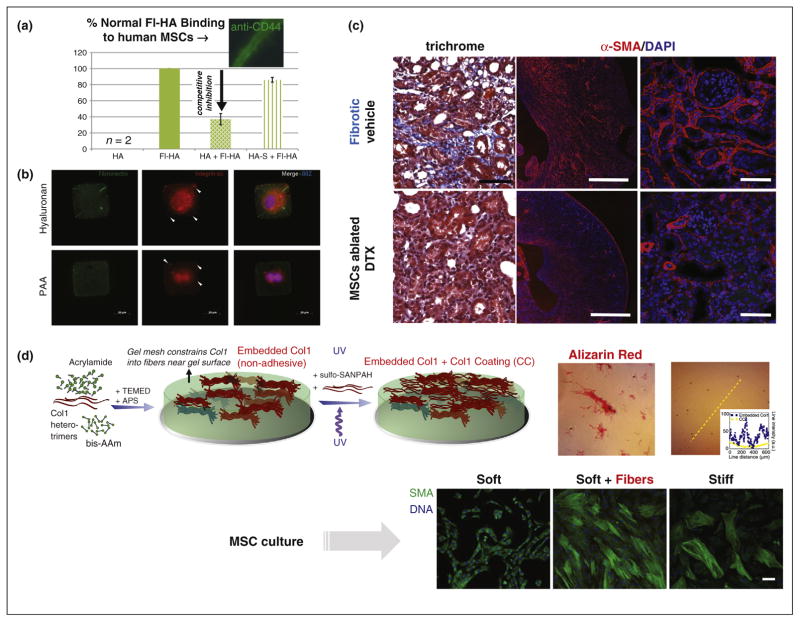
Figure 1.
(a) Modifications of hyaluronic acid (HA) can affect its activity. Thiol-modified HA (HA-S) is not biologically active compared to native HA [64]. Bar graph shows extent of binding of fluorescently labeled HA (Fl-HA) to human MSCs, which is significantly reduced by adding HA as a competing partner, but not upon adding HA-S with its chemical modification. (b) Fibronectin (FN) streaks seen on soft HA substrates. Myocytes were cultured on (1000 μm2) square FN micro-patterned 300 Pa HA and 30 kPa PAA substrates for a period of 72 hours. Myocytes were unable to migrate out of the micropatterns even after attachment to HA substrates for a 3 day period. Arrowheads indicate formation of integrin α5 clusters. FN streaks seen on soft HA substrates are due to micro-contact printing on soft substrates and existed before cell plating [49]. (c) Mesenchymal stem cells (MSCs) are perivascular cells in nearly all tissues and have a major role in fibrosis. Ablation of Gli1+ MSCs by diphtheria toxin, DTX, reduced severity of kidney fibrosis as demonstrated by trichrome staining and immunostaining for α-SMA and quantification of interstitial fibrosis. Scale bars, left two panels, 500 μm; others, 50 μm [58]. (d) Scar in a dish reveals key role of matrix rigidity, even if heterogeneous. Scar-like islands of collagen-I are heterogeneously entrapped at the subsurface of the soft hydrogel. The heterogeneity of the MMMS was confirmed by both immunofluorescence and staining with the histochemical dye, Sirius Red. Human MSCs cultured on conventional homogeneous gels and separately on MMMS [57].
Scar matrix seems to be made largely de novo, and a major role in the development of organ fibrosis has recently been ascribed to ubiquitous MSCs, which reside in perivascular niches of many organs including heart, liver, kidney, lung, and bone marrow [58]. MSCs have been well-known for decades to proliferate and to differentiate toward multiple tissue lineages (e.g. fat, bone), but genetic lineage tracing recently demonstrated that tissue-resident MSCs (specifically the Gli1+ MSCs), rather than circulating MSCs, proliferate after organ injury to generate myofibroblast-like cells typical in scars. In mouse models, genetic ablation of these cells ameliorates fibrosis (Figure 1c), and after induced heart failure, the heart also maintains ejection fraction. To better understand and perhaps control the sensitivity of human MSCs and other cell types to matrix heterogeneity and fibrosis, new reductionist culture models with scar-like heterogeneity are thus needed.
To clarify the effects of non-homogeneous matrix stiffness on cells, one recently developed approach for making minimal matrix models of scars (MMMS) entails mixing soluble collagen-I subunits with acrylamide monomers plus bis-acrylamide crosslinker and then polymerizing the mix into a gel [57]. Upon initiation of polymerization, collagen-I fibers phase separate from pre-gelation clusters of polyacrylamide, leading to highly branched fractal fiber bundles that segregate as islands heterogeneously entrapped at the subsurface of the hydrogel (Figure 1d). Importantly, collagen in the subsurface fiber bundles is not accessible for cell adhesion. A uniform over-coating of matrix ligand is therefore provided for cell attachment. Formation of this type of model scar could be viewed as a diffusion limited cluster aggregation process, with fractal sizes that could be easily controlled by varying the concentration of collagen-I. With the proper mixing ratios, a surface coverage of collagen fiber bundles of ~30% approximates the extent of fibrosis seen, for example, in muscle cross sections [59,60].
Differences in mechanoresponses have been observed when MSCs are cultured in parallel on homogeneous gels and MMMS. A key marker of fibrosis and scarring is the stress fiber associated protein α-SMA, and although α-SMA is not unique to scarring, its expression increases with contractility [61]. α-SMA increases in vivo in hepatic stellate cells (i.e. liver MSCs) in parallel with stiffening of toxin-injured liver, but preceding the detection of fibrotic collagen [62]. Despite the soft-stiff heterogeneity of MMMS gels, MSCs greatly increase expression of α-SMA compared to homogeneously low expression in MSCs on polyacrylamide gels that lack the fiber islands (Figure 1d). Interestingly, α-SMA expression was more homogeneous between cells on MMMS than seen for cells on homogeneously stiff gels. This has been explained by identification of a transcription factor, NKX2.5, that is, a strongly cooperative repressor of α-SMA, which exits the nucleus on stiff substrates (Figure 1d). While many applications might be considered to clarify scarring responses of cells, the effect of other cell types especially cancer cells could be especially interesting. As 80% of hepatocellular carcinomas occurs against a background of cirrhosis and draws increasing attention [63], MMMS could be a promising model system to study the mechanotransduction of HCCs in cirrhotic liver.
Conclusions
Whereas the biochemical signaling events involved in cancer progression have been largely investigated, there is still much to be learned about the mechanical cues provided by the ECM, its stiffness, other environmental factors, and their subsequent effects on cell function and behavior. Tumors are generally stiff, but can have soft cores consisting of cells that are softer than their normal counterparts. At first glance, this may seem contradictory, but it is hypothesized that cancer cells need to generate traction forces on stiff substrates to migrate, but also need to be soft to weave through tight spaces and extravasate or metastasize.
Generalizations made about cancer and mechanics cannot be applied to every cell type or to every cancer hallmark. Broadly, however, cancer cells often appear to have reduced mechanosensitivity, and substrate stiffness has less of an effect on spreading, migration, and proliferation. Apoptosis is preferentially promoted on soft matrices, but EMT and angiogenesis occur on hard matrices. Through pharmacological and genetic manipulations, many studies have implicated the involvement of Ras, FAK and PI3K/ Akt signaling.
A variety of platforms, such as polyacrylamide or biopolymer gels with tunable stiffness, have been routinely used to study how cancer cells respond to their underlying stiffness as measured by, but not limited to, cell spread area, proliferation, migration, invasion, and apoptosis. While these 2D substrates provide a simple method to elucidate the effects of stiffness on cellular behavior, new approaches consisting of 3D scaffolds, micropillars, microfluidic devices, and heterogeneous matrices which mimic the tumor environment will likely provide greater insight into the role of mechanotransduction in cancer.
Acknowledgments
The authors would like to acknowledge funding from the National Institutes of Health R01 EB017753 and the Physical Sciences Oncology Center at the University of Pennsylvania, National Institutes of Health U54 CA193417.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1•.Streitberger KJ, Reiss-Zimmermann M, Freimann FB, Bayerl S, Guo J, Arlt F, Wuerfel J, Braun J, Hoffmann KT, Sack I. High-resolution mechanical imaging of glioblastoma by multifrequency magnetic resonance elastography. PLOS ONE. 2014;9:e110588. doi: 10.1371/journal.pone.0110588. This article along with Refs. [2•,3•,4•] displays the emerging precision in both length scale and elastic modulus differences that can be detected in vivo. These developing methods show how mechanobiology can lead to diagnostic and therapeutic applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Poterucha JT, Johnson JN, Qureshi MY, O’Leary PW, Kamath PS, Lennon RJ, Bonnichsen CR, Young PM, Venkatesh SK, Ehman RL, et al. Magnetic resonance elastography: a novel technique for the detection of hepatic fibrosis and hepatocellular carcinoma after the Fontan operation. Mayo Clin Proc. 2015;90:882–894. doi: 10.1016/j.mayocp.2015.04.020. See annotation to Ref. [1•] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Weis JA, Flint KM, Sanchez V, Yankeelov TE, Miga MI. Assessing the accuracy and reproducibility of modality independent elastography in a murine model of breast cancer. J Med Imaging (Bellingham) 2015;2:036001. doi: 10.1117/1.JMI.2.3.036001. See annotation to Ref. [1•] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Zaleska-Dorobisz U, Kaczorowski K, Pawlus A, Puchalska A, Inglot M. Ultrasound elastography — review of techniques and its clinical applications. Adv Clin Exp Med. 2014;23:645–655. doi: 10.17219/acem/26301. See annotation to Ref. [1•] [DOI] [PubMed] [Google Scholar]
- 5.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 6••.Lin HH, Lin HK, Lin IH, Chiou YW, Chen HW, Liu CY, Harn HI, Chiu WT, Wang YK, Shen MR, et al. Mechanical phenotype of cancer cells: cell softening and loss of stiffness sensing. Oncotarget. 2015;6:20946–20958. doi: 10.18632/oncotarget.4173. This article demonstrates that cancer cells are softer than normal counterparts using AFM and lose stiffness sensing with respect to proliferation, likely through cav1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Wong SY, Ulrich TA, Deleyrolle LP, MacKay JL, Lin JM, Martuscello RT, Jundi MA, Reynolds BA, Kumar S. Constitutive activation of myosin-dependent contractility sensitizes glioma tumor-initiating cells to mechanical inputs and reduces tissue invasion. Cancer Res. 2015;75:1113–1122. doi: 10.1158/0008-5472.CAN-13-3426. This study demonstrates the stiffness-insensitivity of TIC in spreading, migration, and proliferation and suggests that decreased myosin-dependent contractile forces are involved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam WA, Cao L, Umesh V, Keung AJ, Sen S, Kumar S. Extracellular matrix rigidity modulates neuroblastoma cell differentiation and N-myc expression. Mol Cancer. 2010:9. doi: 10.1186/1476-4598-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-beta1-induced apoptosis and epithelial–mesenchymal transition. Mol Biol Cell. 2012;23:781–791. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasrollahi S, Pathak A. Topographic confinement of epithelial clusters induces epithelial-to-mesenchymal transition in compliant matrices. Sci Rep. 2016;6:18831. doi: 10.1038/srep18831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGrail DJ, Kieu QM, Dawson MR. The malignancy of metastatic ovarian cancer cells is increased on soft matrices through a mechanosensitive Rho-ROCK pathway. J Cell Sci. 2014;127:2621–2626. doi: 10.1242/jcs.144378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K, Chen QK, Lui C, Cichon MA, Radisky DC, Nelson CM. Matrix compliance regulates Rac1b localization, NADPH oxidase assembly, and epithelial–mesenchymal transition. Mol Biol Cell. 2012;23:4097–4108. doi: 10.1091/mbc.E12-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kniazeva E, Putnam AJ. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. Am J Physiol Cell Physiol. 2009;297:C179–C187. doi: 10.1152/ajpcell.00018.2009. [DOI] [PubMed] [Google Scholar]
- 15•.Dong Y, Xie X, Wang Z, Hu C, Zheng Q, Wang Y, Chen R, Xue T, Chen J, Gao D, et al. Increasing matrix stiffness upregulates vascular endothelial growth factor expression in hepatocellular carcinoma cells mediated by integrin beta1. Biochem Biophys Res Commun. 2014;444:427–432. doi: 10.1016/j.bbrc.2014.01.079. This study uses collagen I-coated polyacrylamide gels to evaluate VEGF expression in HCC cells. The role of integrin β1 is evaluated. [DOI] [PubMed] [Google Scholar]
- 16••.Bordeleau F, Califano JP, Negron Abril YL, Mason BN, LaValley DJ, Shin SJ, Weiss RS, Reinhart-King CA. Tissue stiffness regulates serine/arginine-rich protein-mediated splicing of the extra domain B-fibronectin isoform in tumors. Proc Natl Acad Sci U S A. 2015;112:8314–8319. doi: 10.1073/pnas.1505421112. The authors investigate the effects of substrate stiffness on EDB-FN expression, which likely involves ROCK and PI3K/Akt pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weder G, Hendriks-Balk MC, Smajda R, Rimoldi D, Liley M, Heinzelmann H, Meister A, Mariotti A. Increased plasticity of the stiffness of melanoma cells correlates with their acquisition of metastatic properties. Nanomedicine. 2014;10:141–148. doi: 10.1016/j.nano.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Wang J, Liu Y, Zong H, Che X, Zheng W, Chen F, Zhu Z, Yang D, Song X. Alterations in mechanical properties are associated with prostate cancer progression. Med Oncol. 2014;31:876. doi: 10.1007/s12032-014-0876-9. [DOI] [PubMed] [Google Scholar]
- 20.Stroka KM, Konstantopoulos K. Physical biology in cancer. 4. Physical cues guide tumor cell adhesion and migration. Am J Physiol Cell Physiol. 2014;306:C98–C109. doi: 10.1152/ajpcell.00289.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang X, Kuhlenschmidt TB, Li Q, Ali S, Lezmi S, Chen H, Pires-Alves M, Laegreid WW, Saif TA, Kuhlenschmidt MS. A mechanically-induced colon cancer cell population shows increased metastatic potential. Mol Cancer. 2014;13:131. doi: 10.1186/1476-4598-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan Y, Tajik A, Chen J, Jia Q, Chowdhury F, Wang L, Chen J, Zhang S, Hong Y, Yi H, et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat Commun. 2014;5:4619. doi: 10.1038/ncomms5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES, Weaver VM. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 2015;7:1120–1134. doi: 10.1039/c5ib00040h. This paper associates breast cancer progression and metastasis with stroma stiffness and collagen linearization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Z, Qutaish M, Han Z, Schur RM, Liu Y, Wilson DL, Lu ZR. MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat Commun. 2015;6:7984. doi: 10.1038/ncomms8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Garcia B, Eiro N, Marin L, Gonzalez-Reyes S, Gonzalez LO, Lamelas ML, Vizoso FJ. Expression and prognostic significance of fibronectin and matrix metalloproteases in breast cancer metastasis. Histopathology. 2014;64:512–522. doi: 10.1111/his.12300. [DOI] [PubMed] [Google Scholar]
- 26.Suer S, Baloglu H, Gungor Z, Sonmez H, Kokoglu E. The distribution of tissue fibronectin and sialic acid in human breast cancer. Cancer Biochem Biophys. 1998;16:63–70. [PubMed] [Google Scholar]
- 27.Yao ES, Zhang H, Chen YY, Lee B, Chew K, Moore D, Park C. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007;67:659–664. doi: 10.1158/0008-5472.CAN-06-2768. [DOI] [PubMed] [Google Scholar]
- 28.Wang K, Andresen Eguiluz RC, Wu F, Seo BR, Fischbach C, Gourdon D. Stiffening and unfolding of early deposited-fibronectin increase proangiogenic factor secretion by breast cancer-associated stromal cells. Biomaterials. 2015;54:63–71. doi: 10.1016/j.biomaterials.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Teja M, Gronau JH, Breit C, Zhang YZ, Minamidate A, Caley MP, McCarthy A, Cox TR, Erler JT, Gaughan L, et al. AGE-modified basement membrane cooperates with Endo180 to promote epithelial cell invasiveness and decrease prostate cancer survival. J Pathol. 2015;235:581–592. doi: 10.1002/path.4485. [DOI] [PubMed] [Google Scholar]
- 30.Kowal TJ, Falk MM. Primary cilia found on HeLa and other cancer cells. Cell Biol Int. 2015;39:1341–1347. doi: 10.1002/cbin.10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gargalionis AN, Korkolopoulou P, Farmaki E, Piperi C, Dalagiorgou G, Adamopoulos C, Levidou G, Saetta A, Fragkou P, Tsioli P, et al. Polycystin-1 and polycystin-2 are involved in the acquisition of aggressive phenotypes in colorectal cancer. Int J Cancer. 2015;136:1515–1527. doi: 10.1002/ijc.29140. [DOI] [PubMed] [Google Scholar]
- 32.Rubashkin MG, Cassereau L, Bainer R, DuFort CC, Yui Y, Ou G, Paszek MJ, Davidson MW, Chen YY, Weaver VM. Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3,4,5)-triphosphate. Cancer Res. 2014;74:4597–4611. doi: 10.1158/0008-5472.CAN-13-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan Y, Zhong W, Ma G, Zhang B, Tian H. Yes-associated protein regulates the growth of human non-small cell lung cancer in response to matrix stiffness. Mol Med Rep. 2015;11:4267–4272. doi: 10.3892/mmr.2015.3231. [DOI] [PubMed] [Google Scholar]
- 34•.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, et al. Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. The TWIST1-G3BP2 may be a mechanotransduction pathway that drives EMT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zustiak SP, Dadhwal S, Medina C, Steczina S, Chehreghanianzabi Y, Ashraf A, Asuri P. Three-dimensional matrix stiffness and adhesive ligands affect cancer cell response to toxins. Biotechnol Bioeng. 2015 doi: 10.1002/bit.25709. [DOI] [PubMed] [Google Scholar]
- 36.Jabbari E, Sarvestani SK, Daneshian L, Moeinzadeh S. Optimum 3D matrix stiffness for maintenance of cancer stem cells is dependent on tissue origin of cancer cells. PLOS ONE. 2015;10:e0132377. doi: 10.1371/journal.pone.0132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Shen YI, Abaci HE, Krupsi Y, Weng LC, Burdick JA, Gerecht S. Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting. Biomater Sci. 2014;2:655–665. doi: 10.1039/C3BM60274E. The authors use 3D HA hydrogels of varying stiffness to demonstrate how hypoxia and substrate stiffness influence endothelial sprouting and invasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 39.Ween MP, Oehler MK, Ricciardelli C. Role of versican, hyaluronan and CD44 in ovarian cancer metastasis. Int J Mol Sci. 2011;12:1009–1029. doi: 10.3390/ijms12021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pirinen R, Tammi R, Tammi M, Hirvikoski P, Parkkinen JJ, Johansson R, Bohm J, Hollmen S, Kosma VM. Prognostic value of hyaluronan expression in non-small-cell lung cancer: increased stromal expression indicates unfavorable outcome in patients with adenocarcinoma. Int J Cancer. 2001;95:12–17. doi: 10.1002/1097-0215(20010120)95:1<12::aid-ijc1002>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 41.Posey JT, Soloway MS, Ekici S, Sofer M, Civantos F, Duncan RC, Lokeshwar VB. Evaluation of the prognostic potential of hyaluronic acid and hyaluronidase (HYAL1) for prostate cancer. Cancer Res. 2003;63:2638–2644. [PubMed] [Google Scholar]
- 42.Lipponen P, Aaltomaa S, Tammi R, Tammi M, Agren U, Kosma VM. High stromal hyaluronan level is associated with poor differentiation and metastasis in prostate cancer. Eur J Cancer. 2001;37:849–856. doi: 10.1016/s0959-8049(00)00448-2. [DOI] [PubMed] [Google Scholar]
- 43.Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Agren U, Alhava E, Kosma VM. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res. 1998;58:342–347. [PubMed] [Google Scholar]
- 44.Setala LP, Tammi MI, Tammi RH, Eskelinen MJ, Lipponen PK, Agren UM, Parkkinen J, Alhava EM, Kosma VM. Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br J Cancer. 1999;79:1133–1138. doi: 10.1038/sj.bjc.6690180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lokeshwar VB, Schroeder GL, Selzer MG, Hautmann SH, Posey JT, Duncan RC, Watson R, Rose L, Markowitz S, Soloway MS. Bladder tumor markers for monitoring recurrence and screening comparison of hyaluronic acid-hyaluronidase and BTA-Stat tests. Cancer. 2002;95:61–72. doi: 10.1002/cncr.10652. [DOI] [PubMed] [Google Scholar]
- 46.Delpech B, Chevallier B, Reinhardt N, Julien JP, Duval C, Maingonnat C, Bastit P, Asselain B. Serum hyaluronan (hyaluronic acid) in breast cancer patients. Int J Cancer. 1990;46:388–390. doi: 10.1002/ijc.2910460309. [DOI] [PubMed] [Google Scholar]
- 47.Franzmann EJ, Schroeder GL, Goodwin WJ, Weed DT, Fisher P, Lokeshwar VB. Expression of tumor markers hyaluronic acid and hyaluronidase (HYAL1) in head and neck tumors. Int J Cancer. 2003;106:438–445. doi: 10.1002/ijc.11252. [DOI] [PubMed] [Google Scholar]
- 48.Kim Y, Kumar S. CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. Mol Cancer Res. 2014;12:1416–1429. doi: 10.1158/1541-7786.MCR-13-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, Raz-Ben Aroush D, Galie PA, Pogoda K, Bucki R, et al. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2014;35:71–82. doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagayama K, Hamaji Y, Sato Y, Matsumoto T. Mechanical trapping of the nucleus on micropillared surfaces inhibits the proliferation of vascular smooth muscle cells but not cervical cancer HeLa cells. J Biomech. 2015;48:1796–1803. doi: 10.1016/j.jbiomech.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Lee LM, Liu AP. A microfluidic pipette array for mechanophenotyping of cancer cells and mechanical gating of mechanosensitive channels. Lab Chip. 2015;15:264–273. doi: 10.1039/c4lc01218f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song HH, Park KM, Gerecht S. Hydrogels to model 3D in vitro microenvironment of tumor vascularization. Adv Drug Deliv Rev. 2014;79–80:19–29. doi: 10.1016/j.addr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci U S A. 2015;112:214–219. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carey SP, Rahman A, Kraning-Rush CM, Romero B, Somasegar S, Torre OM, Williams RM, Reinhart-King CA. Comparative mechanisms of cancer cell migration through 3D matrix and physiological microtracks. Am J Physiol Cell Physiol. 2015;308:C436–C447. doi: 10.1152/ajpcell.00225.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nijenhuis N, Mizuno D, Spaan JA, Schmidt CF. High-resolution microrheology in the pericellular matrix of prostate cancer cells. J R Soc Interface. 2012;9:1733–1744. doi: 10.1098/rsif.2011.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 57.Dingal PC, Bradshaw AM, Cho S, Raab M, Buxboim A, Swift J, Discher DE. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat Mater. 2015;14:951–960. doi: 10.1038/nmat4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am J Physiol Cell Physiol. 2014;306:C889–C898. doi: 10.1152/ajpcell.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olsen AL, Bloomer SA, Chan EP, Gaca MD, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G110–G118. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis GL, Dempster J, Meler JD, Orr DW, Walberg MW, Brown B, Berger BD, O’Connor JK, Goldstein RM. Hepatocellular carcinoma: management of an increasingly common problem. Proc (Bayl Univ Med Cent) 2008;21:266–280. doi: 10.1080/08998280.2008.11928410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rehfeldt F, Brown AE, Raab M, Cai S, Zajac AL, Zemel A, Discher DE. Hyaluronic acid matrices show matrix stiffness in 2D and 3D dictates cytoskeletal order and myosin-II phosphorylation within stem cells. Integr Biol (Camb) 2012;4:422–430. doi: 10.1039/c2ib00150k. [DOI] [PubMed] [Google Scholar]