Summary
The multi-domain scaffolding protein Scribble (Scrib) organizes key signaling complexes to specify basolateral cell polarity and suppress aberrant growth. In many human cancers, genetically normal Scrib mislocalizes from cell–cell junctions to the cytosol, correlating with enhanced growth signaling and malignancy. Here we confirm that expression of the epithelial-to-mesenchymal transcription factor (EMT-TF) Snail in benign epithelial cells leads to Scrib displacement from the plasma membrane, mimicking the mislocalization observed in aggressive cancers. Upon further examination, Snail promotes a transcriptional program that targets genes in the palmitoylation cycle, repressing many protein acyl transferases and elevating expression and activity of protein acyl thioesterase 2 (APT2). APT2 isoform-selective inhibition or knockdown rescued Scrib membrane localization and palmitoylation while attenuating MEK activation. Overall, inhibiting APT2 restores balance to the Scrib palmitoylation cycle, promoting membrane re-localization and growth attenuation. These findings emphasize the importance of S-palmitoylation as a post-translational gatekeeper of cell polarity-mediated tumor suppression.
In Brief
The cell polarity tumor suppressor Scribble re-localizes from lateral membranes to the cytosol in epithelial cancers. Hernandez, et.al. demonstrate that inhibitors of the protein de-palmitoylase APT2 rescue Scribble membrane localization and restore tumor suppressor properties in Snail-expressing cells.
Introduction
The polarity protein Scrib was first identified in Drosophila as a multi-functional tumor suppressor regulating epithelial apical-basolateral polarity, junctional integrity, proliferation, and metastasis (Bilder and Perrimon, 2000; Gateff, 1978; Pagliarini and Xu, 2003). Scrib C-terminal PDZ domains coordinate multiple protein-protein interactions (Ivarsson et al., 2014; Wang et al., 2014), which establish a polarity module that mutually antagonizes the basolateral diffusion of apical determinants such as Crumbs, aPKC, and Par proteins (Grifoni et al., 2007; Grzeschik et al., 2010; Humbert et al., 2008). Dozens of Scrib interacting proteins have been reported (Anastas et al., 2012; Wang et al., 2014), including APC (Takizawa et al., 2006), Rac1 (Boczonadi et al., 2014), β-catenin (Gujral et al., 2013), ERK (Nagasaka et al., 2010), PTEN (Feigin et al., 2014), and β2/3-spectrins (Boeda and Etienne-Manneville, 2015), highlighting the role of Scrib as a hub for crosstalk between many signaling pathways.
Scrib localizes to the basolateral membrane in polarized cells (Dow et al., 2003), yet is mislocalized to the cytosol in most epithelial cancers. This mislocalization correlates with disrupted cell polarity, enhanced growth signaling, and transformation (Elsum and Humbert, 2013; Feigin et al., 2014; Pearson et al., 2011). Furthermore, Scrib deletion or mislocalization cooperates with oncogenic Ras or Myc to promote tumorigenesis (Wu et al., 2010; Zhan et al., 2008). While Scrib is not widely mutated in cancers, its mislocalization and amplification are clinically correlated with high-grade cervical carcinomas (Nakagawa and Huibregtse, 2000; Nakagawa et al., 2004), as well as malignant colon (Gardiol et al., 2006), prostate, breast cancers (Pearson et al., 2011; Zhan et al., 2008), and other epithelial cancers (Vaira et al., 2011). Several classes of viruses employ mechanisms to bypass Scrib repression. The papilloma viral protein E6 binds Scrib directly, targeting it for ubiquination and degradation (Nakagawa and Huibregtse, 2000). Similarly, the retroviral protein Tax and the avian influenza virus protein NS1 each re-localize Scrib to the cytosol (Arpin-Andre and Mesnard, 2007; Liu et al., 2010). In addition, Scrib+/− heterozygous male mice develop prostate hyperplasia (Pearson et al., 2011), and MMTV-driven conditional knockout of Scrib in female mice induces breast hyperplasia and widespread tumor formation (Feigin et al., 2014). Overall, Scrib mislocalization is highly correlated with poor survival in human cancers (Pearson et al., 2011).
Scrib also plays a direct role in growth signaling, where it recruits PP1α away from Shoc2 to decrease Raf activation (Young et al., 2013). However, cytosolic Scrib recruits PTEN away from the plasma membrane to induce over-activation of the Akt/mTOR/S6K signaling pathway (Feigin et al., 2014). Cells engineered to over-express the EMT-TF Snail redistribute Scrib from the membrane to the cytosol (Cordenonsi et al., 2011), mimicking the cytosolic localization observed in cancers. Importantly, Scrib fusion to a C-terminal CAAX prenylation motif rescues plasma membrane localization and reduces levels of activated TAZ, the central transducer of the Hippo signaling pathway. Without its plasma membrane localization, Scrib no longer represses Ras/MAPK-driven cell invasion and EMT phenotypes, yet retains the ability to suppresses anchorage-independent growth (Elsum and Humbert, 2013).
Despite the critical role for Scrib membrane localization, little is known about how Scrib re-localizes to the cytosol during malignancy. We previously identified Scrib as palmitoylated in MCF10A human epithelial cells (Hernandez et al., 2016), as well as the most dynamic and enzymatically regulated palmitoylated protein in highly aggressive T-cell hybridoma cells (Martin et al., 2012). Indeed, the SwissPalm database lists Scrib as one of the more detectable palmitoylated proteins in mammalian cells, returning confident annotations in 12 of 25 reported mammalian S-palmitoylation proteomics datasets (Blanc et al., 2015). In addition, Scrib Cys4 and Cys10 are conserved throughout multicellular organisms, and required for Scrib palmitoylation (Chen et al., 2016). Thus, the observed rapid palmitoylation dynamics suggest that Scrib participates in an active palmitoylation cycle similar to that of palmitoylated Ras (Grecco et al., 2011; Rocks et al., 2010), where zDHHC protein acyl transferases (PATs) add and acyl protein thioesterases (APTs) remove S-palmitoyl groups from Scrib. By metabolic pulse-chase labeling with bioorthogonal alkynyl fatty acid analogues, we previously reported that treatment with the generic serine lipase inhibitor hexadecylfluorophosphonate (HDFP) completely stabilized Scrib palmitoylation. Therefore, Scrib de-palmitoylation is catalyzed by one or more HDFP-sensitive hydrolases, which include the acyl protein thioesterases APT1 (LYPLA1) and APT2 (LYPLA2). Additionally, a recent report found the protein acyl transferase zDHHC7 promotes Scrib membrane localization, further supporting a role for Scrib in a dynamic acylation cycle (Chen et al., 2016).
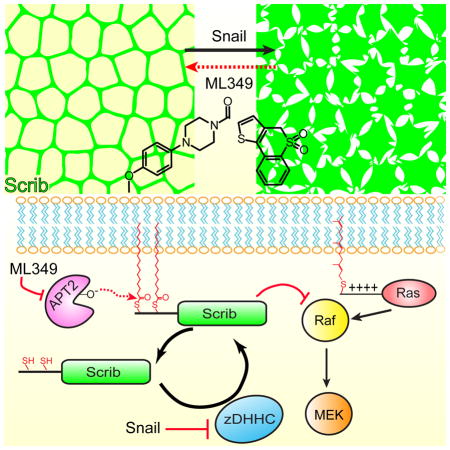
Here we demonstrate that expression of the EMT-TF Snail attenuates Scrib S-palmitoylation and recapitulates Scrib mislocalization from the plasma membrane to the cytosol. Furthermore, Snail expression led to the repression of select zDHHC PATs and increased APT2 expression, suggesting Snail initiates a transcriptional program that directly targets the S-palmitoylation cycle. When Snail over-expressing cells are treated with selective inhibitors of the de-palmitoylating enzyme APT2, Scrib localization is restored to the plasma membrane, rescuing markers of cell polarity and suppressing MAPK signaling. Altogether, APT enzymes participate in an altered S-palmitoylation cycle imbalanced by Snail expression.
Results
Snail reprograms the S-palmitoylation cycle
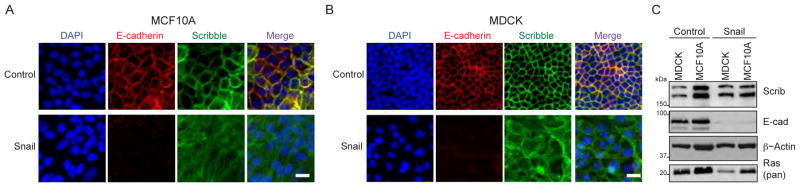
In human cancers, Scrib is predominantly mislocalized from the lateral membranes to the cytosol (Vaira et al., 2011). Since Scrib requires S-palmitoylation for membrane recruitment (Chen et al., 2016), we explored if defects in Scrib S-palmitoylation might provide a potential mechanism for mislocalization during malignancy. Polarized MDCK and MCF10A epithelial cells were transduced with the EMT-TF Snail, which transcriptionally represses the expression of cellular adhesion proteins like E-cadherin, while activating expression of antioxidant, glycolytic, and cytoskeletal remodeling enzymes (Hernandez et al., 2016). In control cell lines transduced with an empty viral vector, Scrib and E-cadherin co-localize at the plasma membrane, confirming a polarized phenotype (Qin et al., 2005). After Snail over-expression, Scrib re-localizes from the membrane to the cytosol (Figures 1A and 1B), successfully recapitulating Scrib mislocalization using a defined EMT model. Additionally, Snail over-expression led to the complete loss of E-cadherin and a marginal reduction in total Ras levels, yet no change in Scrib levels (normalized to β-actin) (Figure 1C). These findings confirm the initiation of a Snail-dependent EMT program, and provide further support that Snail contributes to mitogenic reprogramming.
Figure 1. Snail-induced EMT mislocalizes Scrib in MDCK and MCF10A cells.
(A)E-cadherin (red) co-localizes with membrane-bound Scrib (green) in MCF10A and MDCK cells. Transduction with pPGS-Snail-NEO results in complete loss of E-cadherin expression and mostly cytoplasmic re-localization of Scrib. White scale bar = 20 μm. Fluorescence imaging data is representative of 3 independent biological. (B) Immunoblots of total Scrib, E-cadherin, β–actin, and Ras in MDCK and MCF10A vector control and Snail-transformed cell lines. Representative of duplicate biological replicates.
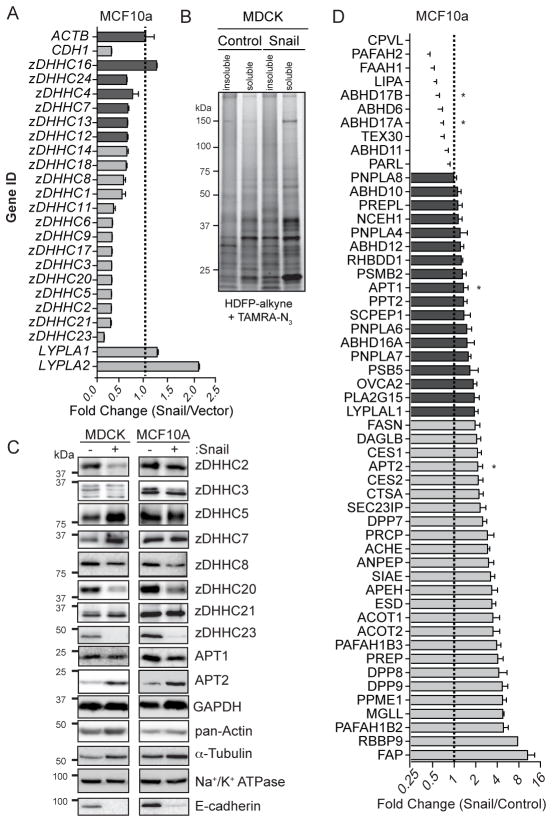
Since Scrib membrane localization requires S-palmitoylation, we hypothesized that the Snail-induced transcriptional program may perturb the Scrib S-palmitoylation cycle equilibrium by disrupting normal PAT and APT expression. To explore this question, total RNA was isolated from MCF10A control and MCF10A-Snail cells for quantitative, real-time PCR (RT-qPCR) of all 23 zDHHC PATs. RT-qPCR values were normalized to β-actin, which was previously validated by mass spectrometry to remain constant after Snail over-expression (Hernandez et al., 2016). Surprisingly, nearly all zDHHC mRNAs were significantly reduced in expression after Snail over-expression, including an 8-fold reduction in zDHHC23 expression (Figure 2A and Figure S1). This finding corroborates a recently reported transcriptional profile from Snail-transformed MCF7 cells (Mezencev et al., 2016), which reports a similar repression of zDHHC enzymes. While zDHHC7 was recently reported to participate in Scrib plasma membrane localization Scrib (Chen et al., 2016), we found zDHHC7 mRNA levels to be only marginally reduced. To further validate these findings, we profiled Snail-dependent changes in protein levels across a panel of 8 zDHHC enzymes by western blot (Figure 2C). This analysis confirmed reductions in zDHHC2, 3, 8, 20, and 23 protein levels, while zDHHC5 and 21 protein levels remained relatively unchanged. The discrepancy between mRNA levels and protein levels for zDHHC5 and zDHHC21 enzymes suggests the involvement of additional post-transcriptional regulation. For example, epidermal growth factor (EGF) signaling stabilizes zDHHC5, preventing proteasomal degradation (Li et al., 2012). Importantly, zDHHC7 levels were unchanged or elevated in Snail over-expressing cells, suggesting this model of epithelial-to-mesenchymal transition either attenuates zDHHC7 activity or bypasses zDHHC7-dependent Scrib palmitoylation. Furthermore, Snail had little effect on APT1 mRNA levels, yet doubled the levels of APT2 mRNA, potentially shifting the balance of the S-palmitoylation cycle. This is also corroborated by Oncomine expression analysis, which classifies APT2 as a significantly up-regulated gene in breast cancer (Rhodes et al., 2004). Indeed, Snail over-expression in MCF10A led to a 2-fold or greater reduction in alkynyl-fatty acid bioorthogonal enrichment for ~13% of validated S-palmitoylated proteins, which was often uncoupled from protein abundance (Hernandez et al., 2016). This targeted suppression of zDHHC enzymes demonstrates Snail functionally targets the S-palmitoylation machinery, which cooperates in disrupting cell polarity and the displacement of Scrib from the plasma membrane.
Figure 2. Snail represses zDHHC palmitoyl acyl transferases and increases APT2 activity.
(A) Snail over-expression in MCF10A reduces zDHHC mRNA levels. All mRNA levels were calculated relative to β-actin by qPCR and are represented as 2 to the power of the difference in measured CT-values between Snail and control lines (2−ΔΔCT). Dark gray bars represent gene expression changes that were not statistically different than change measured for β-actin, whereas genes represented with light gray bars were significantly reduced (p < 0.05) upon transformation with Snail. LYPLA1 and LYPLA2 are the gene names for APT1 and APT2 proteins, respectively. Data was acquired from triplicate independent cDNA isolations. (B) HDFP-alkyne labeling profile in control and Snail-expressing MDCK cells reveals a significantly enhanced signal for a low molecular weight hydrolase. (C) Immunoblot validation of qPCR expression data confirms a subset of zDHHC enzymes are expressed at lower levels, while APT2 expression is increased. (D) Snail over-expression in MCF10A cells leads to widespread changes in serine hydrolase activities. Fluorophosphonate-biotin SILAC-ABPP profile of MCF10A-control and MCF10A-Snail cells are shown. Snail-induced changes in activity levels are indicated by white bars (decrease), dark gray bars (not changing) and light gray bars (increased). Asterisks denote putative de-palmitoylases. Error bars represent standard deviations.
Next, Snail-dependent changes in de-palmitoylating enzyme activities were profiled by HDFP-alkyne, a lipase-selective serine hydrolase activity-based probe (Martin et al., 2012). After click conjugation to TAMRA-azide, active lipases were detected by in-gel fluorescence (Figure 2B). In MDCK cells, we observed a dramatic increase in the fluorophosphonate labeling of a low molecular weight hydrolase (~24 kD), corresponding exclusively to the molecular weights of both APT1 and APT2. Western blot analysis confirmed APT1 levels remained constant, while APT2 protein levels were significantly increased (Figure 2C), demonstrating a direct link between mRNA, protein abundance, and enzyme activity. Separately, Snail-dependent changes in serine hydrolases were examined in MCF10A cells using quantitative fluorophosphonate activity-based protein profiling (SILAC-ABPP) by mass spectrometry (Figure 2D and Table S1) (Adibekian et al., 2011). Over 50 hydrolases were quantitatively profiled, identifying significant increases in the extracellular endopeptidase FAP (SEPR) involved in extracellular matrix remodeling and invasion (Liu et al., 2012), as well as RBBP9, a tumor-associated hydrolase involved in TGF-β signaling (Shields et al., 2010). Monoacylglycerol lipase (MGLL), PAFAH1B2, and protein phosphatase methylesterase (PPME1) were also highly elevated, mirroring their roles in promoting cancer aggressiveness (Kohnz et al., 2015; Nomura et al., 2010; Puustinen et al., 2009). Overall, activities of the putative de-palmitoylases ABHD17A and ABHD17B (Lin and Conibear, 2015) were reduced ~2-fold, while APT1 and APT2 activity were increased 1.3 and 2-fold, respectively. Western blot analysis of APT1 levels revealed its expression to be unaffected by Snail over-expression, in agreement with both the ABPP SILAC ratio (Figure 2D) and previously reported unenriched SILAC analysis of MCF10A-Snail cells (Hernandez et al., 2016). Based on these data, increased APT2 activity and decreased zDHHC expression likely synergize to shift the S-palmitoylation cycle equilibrium for APT2-specific substrates, providing a potential mechanism for attenuated Scrib S-palmitoylation and membrane association.
APT2 inhibition rescues Scrib plasma membrane localization and S-palmitoylation
We next explored if inhibiting de-palmitoylation might restore balance to its S-palmitoylation cycle and rescue Scrib membrane localization. Both APT1 and APT2 are annotated as protein de-palmitoylases (Tom and Martin, 2013), and are widely expressed and active across nearly all tissues (Bachovchin et al., 2010). Both enzymes are targets of several classes of covalent serine hydrolase inhibitors, including β-lactones (Dekker et al., 2010), triazole ureas (Adibekian et al., 2010c), and N-hydroxyhydantoin carbamates (Cognetta et al., 2015), as well as the reversible isoform selective piperazine amide inhibitors ML348 and ML349 (Adibekian et al., 2012; Davda and Martin, 2014; Won et al., 2016). The β-lactone inhibitor Palmostatin B is the most widely used APT inhibitor, and is primarily selective for both APT1 and APT2 at low micromolar concentrations. Treatment with Palmostatin B reportedly blocks the Ras S-palmitoylation cycle, restoring epithelial-like features to HRasG12V-transformed MDCK cells (Dekker et al., 2010). The APT1 inhibitor ML348 (Ki = 280 nM) and the APT2 inhibitor ML349 (Ki = 120 nM) were recently identified by high throughput screening using a competitive activity-based fluorescence polarization assay (Adibekian et al., 2012). Each inhibitor was validated for potency and selectivity in cell lysates, living cells, and in vivo across several mouse tissues using kinetically-tuned activity-based protein profiling (Adibekian et al., 2012). Both ML348 and ML349 maintain isoform selectivity even at concentrations approaching the inhibitor solubility (>10 μM), validating selectivity across dozens of serine hydrolases. Thus, ML348 and ML349 are validated pharmacological tools to individually probe the functional contributions of APT1 (ML348) or APT2 (ML349) in mammalian cells (Adibekian et al., 2010a, b).
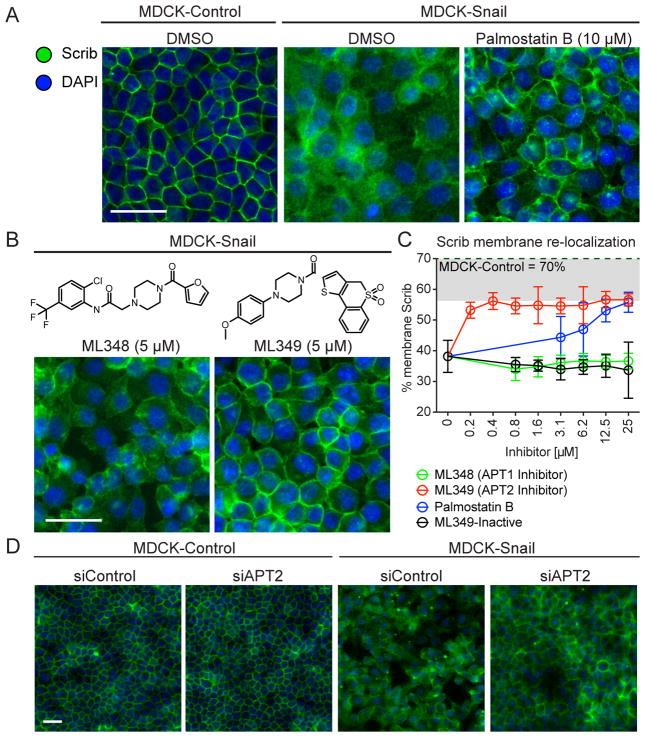
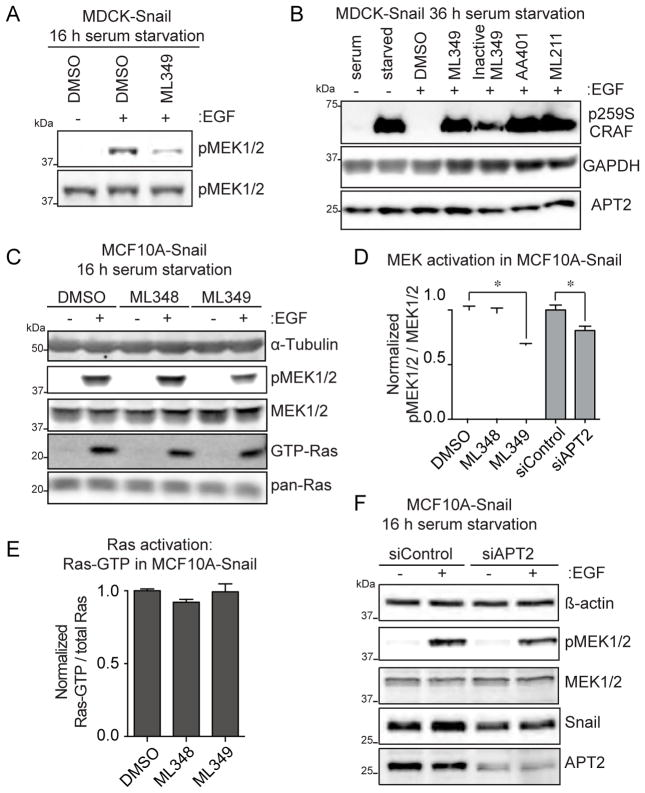
Given the observed dynamic S-palmitoylation of Scrib, we hypothesized that de-palmitoylase inhibition might enhance Scrib S-palmitoylation levels and restore plasma membrane localization in Snail-expressing cells. MDCK-Snail cells were grown in 384-well plates for high-content, automated microscopy analysis. Following various inhibitor or siRNA treatments, MDCK cells were fixed, immunostained, and imaged in 6 replicates with 5 fields of view per well, providing statistical data across >104 cells per condition. Using a custom image processing workflow (see Figure S2A and Supporting Experimental Procedures), ~5 pixel perimeter masks were assigned across all DAPI positive-cells. Since the epifluorescence resolution limits the ability to accurately segment just the plasma membrane, the assigned cell border mask more accurately defines the cell perimeter. Perimeter-localized Scrib fluorescence was divided by the total cell-derived Scrib signal, providing accurate quantitation of the fraction associated with the cell border (Z′ = 0.58). In control MDCK cells, ~70% of Scrib localized to the cell perimeter, but after Snail over-expression, perimeter localization was reduced to ~30% (Figure 3A and 3C). Palmostatin B treatment promoted Scrib membrane re-localization maximally at the highest concentration tested (25 μM), which at that concentration is sufficient to inadvertently inactivate multiple other hydrolases (Lin and Conibear, 2015). To resolve the individual contribution from each APT enzyme, cells were treated overnight with ML348 and ML349. APT2 inhibition had a striking effect on re-distributing Scrib in MDCK-Snail cells, restoring Scrib perimeter localization to nearly 60% after overnight treatment with 400 nM ML349 (Figure 3B and 3C). This inhibitor concentration is ~4-times higher than the Ki, suggesting ML349 is ready cell permeable and achieves equivalent inhibition in vitro and in living cells. ML348 had no significant effect on Scrib membrane localization, similar to an inactive ML349 derivative where the sulfone is reduced (Won et al., 2016). Importantly, Palmostatin B does not enhance Scrib perimeter localization to a higher level than ML349. Thus, Scrib regulation by Palmostatin B is entirely APT2-dependent. Furthermore, APT inhibitors had no significant effect on Scrib localization in MDCK-Control cells (Figure S2B), limiting the response to the Snail-transformed, non-polarized cells.
Figure 3. APT2 inhibition enhances Scrib perimeter localization in Snail-transformed cells.
(A–B) Representative immunofluorescence images of Scrib in MDCK-control and MDCK-Snail cells treated overnight with indicated inhibitors. (C) Dose-dependent enhancement of Scrib perimeter localization quantified by high-content automated microscopy, and processed as described in the Supporting Experimental Procedures. ML349-inactive lacks the two sulfone oxygens, which are required for APT2 inhibition in vitro. Standard deviations are shown for each condition. (D) APT2 knockdown in MDCK-Snail rescues Scrib membrane-localization. Representative images from high-content analysis of MDCK Control and Snail lines transfected with either non-targeting (siControl) or APT2 siRNA. Scale bar = 40 μm across each panel.
Next, in order to rule out off-target ML349 effects, Scrib membrane re-targeting was quantified after siRNA knockdown of either APT1 or APT2 (Figure 3D, Figure S2C–D). Again, APT2 knockdown led to the significant perimeter recruitment of Scrib to nearly the same level as MDCK control cells, whereas APT1 knockdown had no significant effect. MDCK-Control and MDCK-Snail cells were next transduced with Scrib-GFP or Δ22-Scrib-GFP (deleting the first N-terminal 22 amino acids) and treated overnight with either vehicle (DMSO) or ML349 (Figure S3). Line fluorescence intensity profiles across cell-cell junctions showed that ML349 treatment induced a 1.6-fold increase in Scrib-GFP perimeter localization in MDCK-Control cells and rescued Scrib perimeter localization in MDCK-Snail cells. Importantly, overnight ML349 treatment in MDCK-Snail cells expressing Δ22-Scrib-GFP retained diffuse localization, confirming that ML349-dependent membrane recruitment requires the first 22 amino acids of Scrib, which contains the S-palmitoylated cysteines. Based on these quantitative data, blocking APT2 activity restores full-length Scrib membrane localization in Snail-expressing cells, and provides further evidence supporting S-palmitoylation-dependent membrane recruitment.
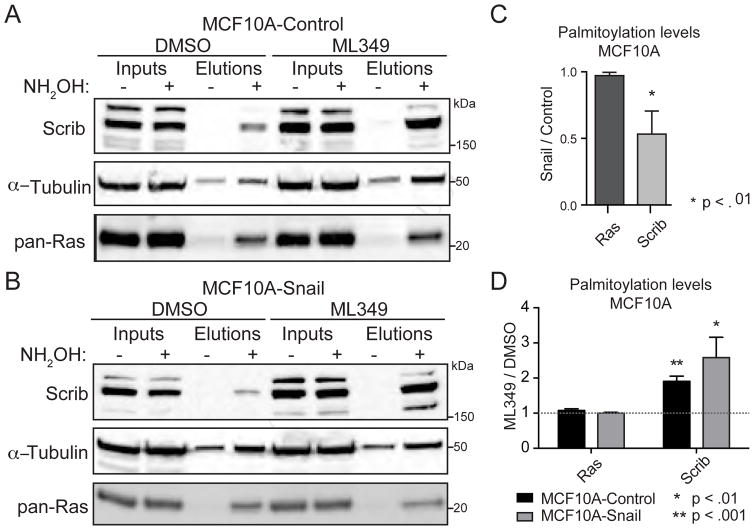
We next tested if Scrib membrane re-localization is correlated with enhanced Scrib S-palmitoylation. First, we performed metabolic labeling with the alkynyl fatty acid analogue 17-octadecynoic acid in cells treated with ML349. After copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) to Cy5-azide, we observed no detectable differences in S-palmitoylation across a short time course by in-gel fluorescence (Figure S4). This strongly supports the notion that APT2 is not a global regulator of cellular S-palmitoylation, but instead may affect only a small subset of dynamically palmitoylated proteins. In order to directly quantify steady-state Scrib S-palmitoylation levels, cells were treated overnight with ML349 and analyzed by acyl-biotin exchange (ABE). Strikingly, Scrib S-palmitoylation was enhanced several-fold by ML349 treatment, confirming that APT2 participates in a Scrib S-palmitoylation cycle in both normal and Snail-expressing cells (Figure 4A–C). Additionally, steady-state Scrib S-palmitoylation is significantly reduced upon Snail-overexpression (Figure 4D), further confirming a Snail-dependent defect in Scrib S-palmitoylation. While the total abundance of all Ras isoforms decreased upon Snail expression, steady-state Ras S-palmitoylation remained proportional and unaffected by ML349 treatment. Conversely, overexpression of APT1 or APT2 significantly reduced Ras S-palmitoylation in HEK293T cells, demonstrating that S-palmitoylated Ras isoforms are accommodated as APT substrates, but only when the enzymes are expressed well above physiological levels (Figure S5A). Ras de-palmitoylation was greater after APT2 over-expression than after APT1 over-expression, showing a 2-fold more reduction in steady-state Ras S-palmitoylation. This finding highlights an important caveat of over-expression studies, since enzyme levels regulate the balance of S-palmitoylation and de-palmitoylation and ultimately the membrane anchoring and localization of select S-palmitoylated proteins. Overall, we found that Scrib is regulated by an APT2-dependent S-palmitoylation cycle, which is necessary for efficient membrane localization.
Figure 4. APT2 inhibition increases Scrib S-palmitoylation.
(A–B) Acyl-biotin exchange analysis demonstrates that ML349 enhances Scrib S-palmitoylation, but not Ras S-palmitoylation, in MCF10A-Control and MCF10A-Snail cells. (C) Blot quantitation confirms Snail-expression reduces Scrib S-palmitoylation, but Ras S-palmitoylation is unchanged. Input-normalized data reporting standard deviations from 3 replicates are shown. (D) Blot quantitation demonstrates ML349 enhances Scrib, but not Ras, palmitoylation in both MCF10A-Control and MCF10A-Snail cells. Input-normalized, background-subtracted data with standard deviations from 3 replicates are shown. Snail-induced S-palmitoylation changes were calculated using the DMSO lanes of MCF10A-Control and MCF10A-Snail cells.
APT2 inhibition attenuates MAPK activation
Knockdown or deletion of Scrib has been widely shown to enhance MEK activation (Dow et al., 2003; Elsum and Humbert, 2013), supporting a direct role in suppressing MAPK growth signaling. Furthermore, wild-type Scrib over-expression suppresses MEK activation, yet when Scrib is mislocalized to the cytosol, this suppression is lost (Elsum and Humbert, 2013). In HEK293T cells, Scrib forms a multi-protein complex that competes away PP1 from the scaffolding Shoc2 to suppress Raf activation (Young et al., 2013). Based on these reports, we examined whether ML349-mediated Scrib relocalization correlates with MAPK suppression. First, we observed a dose-dependent reduction in both MCF10A control and Snail cell proliferation by ML349, which was significantly more robust than ML348 or the MEK inhibitor PD980859 (Figure S5B). Next, serum starved MDCK-Snail cells were incubated with ML349 and stimulated with EGF for 5 minutes, revealing a significant attenuation of MEK activation (Figure 5A). After extended serum starvation, we also observed that ML349, as well as the triazole urea dual APT1/APT2 inhibitors AA401 and ML211 (Adibekian et al., 2010c), blocked Raf Ser-259 dephosphorylation, a prerequisite for disengagement of inhibitory 14-3-3 dimers (Dhillon et al., 2007) (Figure 5B). While not as precipitous, ML349 similarly led to a partial reduction in MEK activation in MCF10A-Snail cells, independent of Ras activation (Figure 5C, 5E–F). These findings are further corroborated by partial (~70%) knockdown of APT2, leading to fractional attenuation of MEK phosphorylation, as well as a significant reduction in the levels of Snail (Figure 5D). In summary, endogenous APT2 does not affect the S-palmitoylation or activation of endogenous Ras, and supports a direct role for ML349-dependent Scrib suppression of Raf.
Figure 5. APT2 inhibition reduces EGF-dependent MAPK signaling in Snail over-expressing cells.
(A) ML349 treatment suppresses MEK1/2 activation in MDCK-Snail cells. Immunoblot analysis of cells serum-starved treated overnight with 5 μM ML349 or DMSO and briefly stimulated with EGF. (B) Several different APT inhibitors attenuate CRAF S259 dephosphorylation under extended starvation conditions in MDCK-Snail cells. Immunoblot analysis of stimulated cells starved with 5 μM of ML349 or ML349-inactive, 1 μM of AA401, or 1 μM ML211. (C) APT2 inhibition reduced MEK activation without affecting GTP-Ras levels in MCF10A-Snail cells. Active-Ras pull-downs were performed with GST-RBD. (D–E) Normalized blot quantitation of (C) with standard deviations from 3 independent replicates. Asterisks denote p value < 0.05. (F) Partial APT2 knockdown in MCF10A-Snail reduces Snail levels and EGF-dependent stimulation of pMEK1/2. Immunoblot analysis of stimulated cells pre-treated with indicated siRNAs.
Discussion
Studies in Drosophila and mice have shown that oncogenes (such as Ras and Myc) are insufficient to drive tumor formation alone, and require secondary mutations to relieve contact inhibition, abolish cell polarity, and promote cell migration (Martin-Belmonte and Perez-Moreno, 2012; Wu et al., 2010; Zhan et al., 2008). Tumors achieve this by uncoupling key growth suppressors from their site of action, and in certain instances by disrupting plasma membrane localization. In human cancers, Scrib is mislocalized to the cytosol, which leads to a cascade of changes that disrupt cell polarity and adhesion junctions, enhance growth signaling, and increase cell survival and proliferation (Doggett et al., 2011; Vaira et al., 2011; Yamanaka and Ohno, 2008). Based on sequence conservation, recurring annotations in numerous S-palmitoylation proteomics studies, and the correlated fluorescence imaging and biochemical S-palmitoylation studies presented here, we confirm that dynamic S-palmitoylation is critical for Scrib plasma membrane localization.
Upon Snail over-expression in polarized epithelial cells, we observe a complete loss of membrane-bound Scrib, recapitulating the mislocalization observed in human cancers. This correlates with repression of select zDHHC palmitoyl acyl transferases, and manifests in the significant attenuation of S-palmitoylation (Hernandez et al., 2016). After induction of EMT by Snail, reduced zDHHC activity likely leads to an imbalanced S-palmitoylation cycle that culminates in reduced membrane organization and polarity. This imbalance is further exacerbated by Snail-mediated induction of APT2 expression and activity. All together, Snail promotes a shift in the S-palmitoylation cycle, reducing palmitoyl transferases and increasing acyl protein thioesterase expression.
We previously demonstrated the non-selective lipase inhibitor HDFP blocks the de-palmitoylation of a subset of palmitoylated proteins important in growth regulation, including small GTPases (N-Ras, H-Ras), G-proteins (Gαs, Gα13), and LAP family proteins (Erbin and Scrib) (Martin et al., 2012). More selective S-palmitoylation cycle inhibitors should allow targeted disruption of the sub-cellular trafficking and cellular distribution of these important growth regulators (Hernandez et al., 2013; Tom and Martin, 2013). Therefore, we examined a series of acyl protein thioesterase inhibitors using a combination of biochemical S-palmitoylation profiling and high-content image analyses. Through these experiments, we found that Scrib is hyper-palmitoylated after APT2 inhibition, confirming its participation in a dynamic S-palmitoylation cycle. Interestingly, Scrib S-palmitoylation is enhanced in both normal and Snail-expressing cells, implying S-palmitoylation is not the sole factor driving membrane localization in normal cells. While Ras GTPases use a S-palmitoylation cycle to drive dynamic plasma membrane association, multiple Scrib protein-protein interactions are likely to trap Scrib at the lateral membrane perimeter in normal cells. After disruption of lateral membrane polarization, Scrib membrane association appears more reliant on S-palmitoylation, especially since ML349 is highly effective at driving membrane re-localization. Furthermore, while ML349 re-localizes Scrib to the cell perimeter, cells do not completely regain polarity, as E-cadherin expression remains repressed. Collectively, our findings argue that S-palmitoylation is necessary, but not sufficient for Scrib membrane localization, and suggests that polarized membrane trafficking and organization require additional cellular components.
While APT1 or other thioesterases may regulate Ras S-palmitoylation, we found that selective APT2 inhibition dramatically enhances Scrib, but not Ras, S-palmitoylation in Snail-over-expression models. In contrast, Palmostatin B is reported to attenuate MAPK activation in Ras-transformed MDCK cells (Zimmermann et al., 2013), yet APT1/2 knockdown has no effect on Ras S-palmitoylation dynamics (Lin and Conibear, 2015). Among several off-target hydrolases, Palmostatin B was found to also inactivate the uncharacterized ABHD17 family of 3 membrane-associated hydrolases in mice and 7 in humans. Triple ABHD17A, B, and C knockdown affected Ras S-palmitoylation dynamics, suggesting Palmostatin B acts independently of APT enzymes (Lin and Conibear, 2015). However, we find that Snail expression reduces levels of active ABHD17 enzymes with no corresponding change in total palmitoylated Ras. Rather, our data suggest APT2 inhibition can affect MAPK activation indirectly by recruiting Scrib to plasma membrane, which in turn leads to Raf suppression. This notion is further supported by the observed reduction in Snail levels following APT2 knockdown, since Snail levels are ultimately regulated downstream of MAPK signaling. Importantly, this effect is cell-line dependent, since ML349 attenuation of MEK activation is more robust in MDCK cells than MCF10A cells. Indeed, ML348 and ML349 have little effect on NRAS or BRAF mutant melanoma cell lines (Vujic et al., 2016), highlighting the context-dependent role for APT enzymes in cell polarity and growth signaling. Looking forward, ongoing advances in metabolic labeling and pulse-chase strategies open new opportunities to profile APT2 substrates by chemical proteomics, which will likely benefit from analysis in defined models of cell polarity.
In conclusion, these data highlight Scrib S-palmitoylation as a post-translational switch in the progression and initiation of epithelial cancers. We demonstrate that reduced Scrib S-palmitoylation leads to increased cytoplasmic localization and loss of MAPK repression. These data align with emerging evidence that scaffolding proteins, such as Scrib, tune how cells respond to growth signals in normal and malignant cells. In addition, ML349 restores balance to the S-palmitoylation cycle in Snail-expressing cells, functionally rescuing Scrib tumor suppressor properties. We anticipate these findings will impact on understanding how membrane anchors support cell polarity, and provide novel routes for therapeutic rescue of polarity-mediated tumor suppression in cancer.
Significance
In epithelial cells, maintenance of apical-basolateral polarity gradients by distinct molecular complexes ensures appropriate contact inhibition and spatially defined proliferation. In a global S-palmitoylation proteomic screen, we previously identified the polarity scaffolding protein Scrib as a substrate of cellular de-palmitoylases. Scrib acts to organize cell polarity gradients and suppress growth signals at the plasma membrane, but is widely re-localized to the cytosol in aggressive cancers. When the EMT-TF Snail is over-expressed, Scrib is re-localized to the cytosol and is less palmitoylated, likely driven by transcriptional attenuation of palmitoyl acyl transferases. Treatment with the isoform-selective, competitive APT2 inhibitor ML349 relocalizes Scrib to the plasma membrane in malignant cells, elevates Scrib S-palmitoylation, and restores MAPK suppression independent of Ras. These findings provide evidence that S-palmitoylation is specifically disrupted after Snail over-expression, which reduces the membrane association of key polarity tumor suppressors and promotes enhanced growth signaling. By blocking Scrib de-palmitoylation, balance is restored to the Scrib S-palmitoylation cycle to re-establish Scrib membrane localization. Overall, these studies outline a novel post-translational mechanism controlling oncogenic pathways in Snail-transformed cells by changing sub-cellular compartmentalization of the scaffolding protein Scrib.
Experimental Procedures
Cell culture, transfection, and stable lines
The human Scribble P305L gene (AddGene, plasmid #24601) was reverted back to wild-type by site-directed mutagenesis. C-terminally GFP-tagged Scrib variants were sub-cloned into a modified pCLNCX (Imgenex). MDCK cells, MCF10A cells, and pPGS-hSNAIL-fl-FLAG were a generous gift from Dr. Benjamin Margolis (University of Michigan). MDCK were grown in complete medium consisting of DMEM (Thermo) supplemented 10% fetal bovine serum (FBS). MCF10A cells were grown in DMEM/F12 (Thermo) with supplements as previously described (Debnath et al., 2003). Cell lines were transduced with retrovirus at low passage number with either empty pPGS-NEO or pPGS-hSnail-NEO vectors, and selected for with 0.6 mg/mL G418 for several passages. Cell lines were validated by immunoblotting for Snail and E-Cadherin, as well by observing signature morphological features of EMT by light microscopy. FugeneHD (Roche) was used for transient transfections following the manufacturer’s recommended guidelines. For siRNA knockdowns, predesigned siRNA pools (Dharmacon, ON-TARGETplus, L-010007-00-0005, L-009256-00-0005 and D-001810-10-05) were transfected into 50% confluent MCF10A and MDCK cells. Briefly, 125 nM of the siRNA pool was mixed with 1.25% v/v (DharmaFECT) in a total of 800 μL of Opti-MEM (Gibco) for 5 minutes, and diluted 5-fold into growth media before adding to a 6 cm dish of sub-confluent cells. Experiments were performed 48 hours post-transfection.
Epifluorescence microscopy
Cells were grown on glass coverslips in 12-well plates and treated with different inhibitors overnight. Confluent cells were fixed with ice-cold methanol for 10 minutes, washed 1 min with ice-cold acetone, and then washed with DPBS. Glass coverslips were then incubated with SuperBlock (Thermo) for 15 minutes, and then incubated overnight with 1:200 Goat-anti-Scribble (SCBT, sc1149) and 1:250 mouse-anti-E-cadherin (BD Bioscience, 610181) at 4°C in 0.3% v/v Triton X-100/PBS (PBST). Primary antibodies removed and membranes were washed 3 times with PBST, followed by incubation for 1 hour with species-matched 1:500 AlexaFluor secondary antibodies (Life Technologies) in PBST. Coverslips stained with DAPI, washed, and mounted onto slides with Fluoromount (Sigma). Images were acquired with a Nikon Eclipse TE2000-S inverted epifluorescence microscope and analyzed with ImageJ (NIH).
Quantitative RT-PCR measurements
Total RNA was extracted from MCF10A-Control and MCF10A-Snail cell lines using Trizol reagent (Invitrogen) and reverse transcribed using iScriptTM Advanced cDNA Synthesis Kit (Bio-Rad). Equal volumes of cDNAs were loaded onto a pre-loaded custom 96-well qPCR plate (Bio-Rad) and mixed with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) in triplicate biological replicates, each with three technical replicates using a StepOne Plus instrument (Applied Biosystems). All cycle threshold (CT) values were normalized to β-actin. The relative expression level for each gene of interest was calculated using the 2−ΔΔ-CT method (Debnath et al., 2003)
Serine hydrolase activity-based protein profiling
MCF10A-control and MCF10A-Snail cells were passaged more than six times in SILAC-DMEM-F12 (Thermo) supplemented with 100 μg/mL of either [12C6] [14N2]-lysine and [12C6] [14N4]-arginine (LIGHT condition) or [13C6] [15N2]-lysine and [13C6] [15N4]-arginine (HEAVY condition), and 5% v/v dialyzed horse serum (Valley Biomedical), 20 ng/mL recombinant human EGF (Shenandoah Biotechnology), 0.5 mg/L cortisol (Sigma), 0.1 mg/L Cholera toxin (Sigma), 10 mg/L insulin (Gibco) and 1x penicillin-streptomycin (Gibco). Cell pellets were sonicated in DPBS and centrifuged at 100,000 × g for 30 minutes to separate soluble and insoluble proteomes. After normalizing protein concentrations to 2 mg/mL, reciprocal isotopic proteome pairs of MCF10A-Control and MCF10A-Snail proteomes were combined in 1:1 molar ratio and reacted with 5 μM FP-PEG-biotin for 1 hour at room temperature. The reaction mixture was then extracted with chloroform-methanol to remove unreacted probe, re-solubilized in 1 mL of 10 g/L SDS in DPBS, reduced with 10 mM TCEP (Sigma), alkylated with 20 mM iodoacetamide (Sigma), diluted 10-fold in DPBS, and incubated with streptavidin-agarose for 1 hour. The beads were then washed extensively with 2% SDS/PBS, 6 M Urea/PBS, and PBS using a multi-port vacuum manifold. The final beads were digested with 2 μg trypsin/LysC (Promega) overnight in 2 M urea/PBS. The following day, eluted peptides were purified by solid phase extraction using an Oasis μElution plate (Waters). Tryptic peptide digests were separated using a Waters NanoAcquity UPLC system equipped with a 5 μm Symmetry C18 (180 μm × 20 mm) trap column and a 1.8 μm high-strength silica (HSS-T3) analytical column (75 μm × 150 mm) heated to 35 °C coupled to a picotip emitter (New Objective). Tryptic peptides were loaded onto the trap column over 3 min, followed by analytical separation over a 90 min gradient (3% ACN to 40% ACN over 90 min). Peptides were analyzed using a Waters Synapt G2S HDMS time-of-flight mass spectrometer with ion mobility separation and data independent fragmentation algorithms. The data were corrected in post-acquisition analysis using the doubly charged monoisotopic ion of [Glu1]-fibrinopeptide B (m/z = 785.8426) collected every 30 s from a separate calibrant fluidics source coupled to a tapertip emitter (New Objective). LC–MS spectra were collected in continuum mode and searched using the ProteinLynx Global SERVER version 3.0.2 (Waters) against the reviewed human reference with a 1% false discovery rate using a reversed database. By using in-house Python scripts, all data from technical and biological replicates were merged by removing any precursors greater than ± 10 ppm in mass difference from the calculated theoretical mass.
EGF stimulation assays
For MEK and RAF phospho-blots, cells were plated at 25–50% confluency in 6 cm-diameter plates with complete medium modified with dialyzed sera and appropriate supplements as necessary. After 24 to 48 hours, the cells (~80% confluency) were washed with DPBS and cultured overnight (10 – 14 h) or for an extended period (24–36 h) in supplement-free growth medium spiked with a given concentration of compound to give a final 0.1% v/v DMSO. The following day, cells were treated with either vehicle (DPBS) or 20 ng/mL human recombinant EGF (Shenandoah) for 5 minutes, washed with DPBS, and immediately lysed in Laemmli sample buffer for SDS-PAGE and western blot analysis. For GST-RBD pull-downs, the same initial procedure was followed up to the lysis step. Cells were sonicated in ice-cold lysis buffer containing 50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 5 mM MgCl2 and 5% glycerol. Whole cell lysates were clarified by centrifugation at 20,000 × g at 4° C for 15 min. Input samples were set aside and 500 μg of cleared lysate was incubated with 80 μg of purified GST-Raf-RBD (bacterially expressed from pGEX-2T plasmid, Addgene) in 700 μL final volume on a rotisserie at 4° C for 30 min. 50 μL of glutathione sepharose 4B beads (GE Life Science) was added to the cell lysate GST-RBD mixture and incubated for an additional 1.5 h at 4° C. The beads were washed 4x in lysis buffer and 2x in DPBS, and then boiled at 90° C in Laemmli SDS-PAGE sample buffer for 5 minutes for gel-based analysis.
Western blot analyses
Frozen cell pellets were sonicated and quantified using the DC protein assay (Bio-Rad). Generally, 15 – 25 μg of whole cell lysate was loaded in each well, separated using 8% or 12% SDS-PAGE, transferred to a methanol-activated PVDF membrane (Millipore), and blocked with 5% non-fat milk or 5% BSA (Fisher) in Tris-buffered saline with 0.5% (v/v) Tween-20 (TBST). Primary antibody solutions were prepared in Odyssey Blocking Buffer (Li-Cor) according to manufacturer’s recommendations and incubated at 4° C overnight. The following species-matched secondary antibodies were used and diluted to indicated concentrations in TBST: goat-anti-rabbit HRP (Thermo-Fisher, 32460) and goat-anti-mouse HRP both at 1:500 (Thermo-Fisher, 32430), donkey-anti-mouse 647 at 1:1000 (Life Technologies, A31571), Goat-anti-rabbit secondary near-IR 700 antibody at 1:5000 (Azure AC2135), Goat-anti-mouse secondary near-IR 800 antibody at 1:5000 (Azure AC2128). Blots were incubated at room temperature for one hour, and imaged using an Azure c600. Acquired images were processed for publication and protein band densitometry measurements were perform on ImageJ software (NIH). See Supporting Experimental Procedures for commercial antibody sources and dilutions.
Acyl-Biotin Exchange
Cell pellets were lysed in 4% w/v SDS in PBS (4SB) and adjusted to 2 mg/mL. The sample was then alkylated with 50 mM N-ethyl maleimide for 2 hours at room temperature, followed by chloroform/methanol precipitation, cold methanol washes, and resuspension in the same volume of 4SB. Samples were split in half, and boiled at 95° C for 5 minutes in the presence of either neutralized 0.5 M NH2OH or 0.5 M NaCl. Samples were immediately chloroform/methanol precipitated and resuspended the same volume of 2% SDS (w/v) in DPBS. The lysates were mixed for 90 minutes with 200 μM biotin-PEG-maleimide (Click Chemistry Tools) to capture any liberated thiols. Unreacted biotin-PEG-maleimide was removed by chloroform/methanol precipitation, and samples were resuspended in 1% SDS (w/v) in DPBS and quantified, setting aside a small aliquot as an input control. The remaining sample was then combined with 20 μL of streptavidin agarose (Millipore) in 0.25% SDS/DPBS for 90 minutes. Beads were washed 4-times in 0.2% SDS/DPBS and 2-times in DPBS, and then boiled in SDS sample loading buffer for 10 minutes for analysis by SDS-PAGE.
High-content fluorescence imaging microscopy
Cells were fixed with cold methanol/acetone or with 4% paraformaldehyde in 1% Triton X-100/PBS and immunostained. Automated imaging was performed on 5 regions per well using a Molecular Devices ImageXpress Micro XLS High-Content Analysis System and custom ImageJ macros (see Supporting Experimental Procedures).
Supplementary Material
Highlights.
Scribble S-palmitoylation is reduced after over-expression of Snail
Snail over-expression reduces levels of select zDHHC protein acyl transferases
After Snail overexpression, APT2 inhibition rescues Scribble localization
MEK activation is attenuated following APT2 inhibition in Snail-expressing cells
Acknowledgments
We would like to thank James Carolan, Carol Fierke, Paul Jenkins, Martha Larsen, Anna Mapp, and Benjamin Margolis (University of Michigan) for reagents, technical support, and advice. Financial support for these studies was provided by the National Institutes of Health R00 CA151460, DP2 GM114848, the American Cancer Society PF-13-177-01 (J.L.H.), the American Heart Association 14POST20420040 (J.D.M.), and the University of Michigan.
Footnotes
Author Contributions
J.L.H. and B.R.M. conceived the study. J.L.H., D.D, and B.R.M designed all experiments. J.L.H performed fluorescence imaging and S-palmitoylation assays. J.L.H. and D.D. performed growth factor stimulations, western blots, mass spectrometry and data analysis. M.C.S.K. assisted with siRNA imaging experiments. J.D.M. provided mass spectrometry support. S.J.W. assisted with qPCR experiments. M.G., A.I.C., C.M.B., and S.C.P. provided molecular and cell biology support. J.L.H., D.D., and B.R.M wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibekian A, Martin BR, Chang JW, Hsu KL, Tsuboi K, Bachovchin DA, Speers AE, Brown SJ, Spicer T, Fernandez-Vega V, et al. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010a. Characterization of a Selective, Reversible Inhibitor of Lysophospholipase 1 (LYPLA1) [PubMed] [Google Scholar]
- Adibekian A, Martin BR, Chang JW, Hsu KL, Tsuboi K, Bachovchin DA, Speers AE, Brown SJ, Spicer T, Fernandez-Vega V, et al. Characterization of a Selective, Reversible Inhibitor of Lysophospholipase 2 (LYPLA2). Probe Reports from the NIH Molecular Libraries Program; Bethesda (MD). 2010b. [PubMed] [Google Scholar]
- Adibekian A, Martin BR, Chang JW, Hsu KL, Tsuboi K, Bachovchin DA, Speers AE, Brown SJ, Spicer T, Fernandez-Vega V, et al. Confirming target engagement for reversible inhibitors in vivo by kinetically tuned activity-based probes. J Am Chem Soc. 2012;134:10345–10348. doi: 10.1021/ja303400u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibekian A, Martin BR, Speers AE, Brown SJ, Spicer T, Fernandez-Vega V, Ferguson J, Cravatt BF, Hodder P, Rosen H. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010c. Optimization and characterization of a triazole urea dual inhibitor for lysophospholipase 1 (LYPLA1) and lysophospholipase 2 (LYPLA2) [PubMed] [Google Scholar]
- Adibekian A, Martin BR, Wang C, Hsu KL, Bachovchin DA, Niessen S, Hoover H, Cravatt BF. Click-generated triazole ureas as ultrapotent in vivo-active serine hydrolase inhibitors. Nat Chem Biol. 2011;7:469–478. doi: 10.1038/nchembio.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastas JN, Biechele TL, Robitaille M, Muster J, Allison KH, Angers S, Moon RT. A protein complex of SCRIB, NOS1AP and VANGL1 regulates cell polarity and migration, and is associated with breast cancer progression. Oncogene. 2012;31:3696–3708. doi: 10.1038/onc.2011.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpin-Andre C, Mesnard JM. The PDZ domain-binding motif of the human T cell leukemia virus type 1 tax protein induces mislocalization of the tumor suppressor hScrib in T cells. The Journal of biological chemistry. 2007;282:33132–33141. doi: 10.1074/jbc.M702279200. [DOI] [PubMed] [Google Scholar]
- Bachovchin DA, Ji T, Li W, Simon GM, Blankman JL, Adibekian A, Hoover H, Niessen S, Cravatt BF. Superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus-library screening. Proc Natl Acad Sci U S A. 2010;107:20941–20946. doi: 10.1073/pnas.1011663107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Blanc M, David F, Abrami L, Migliozzi D, Armand F, Burgi J, van der Goot FG. SwissPalm: Protein Palmitoylation database. F1000Res. 2015;4:261. doi: 10.12688/f1000research.6464.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczonadi V, Gillespie R, Keenan I, Ramsbottom SA, Donald-Wilson C, Al Nazer M, Humbert P, Schwarz RJ, Chaudhry B, Henderson DJ. Scrib:Rac1 interactions are required for the morphogenesis of the ventricular myocardium. Cardiovascular Research. 2014;104:103–115. doi: 10.1093/cvr/cvu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeda B, Etienne-Manneville S. Spectrin binding motifs regulate Scribble cortical dynamics and polarity function. Elife. 2015;4 doi: 10.7554/eLife.04726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Zheng B, DeRan M, Jarugumilli GK, Fu J, Brooks YS, Wu X. ZDHHC7-mediated S-palmitoylation of Scribble regulates cell polarity. Nat Chem Biol. 2016 doi: 10.1038/nchembio.2119. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognetta AB, 3rd, Niphakis MJ, Lee HC, Martini ML, Hulce JJ, Cravatt BF. Selective N-Hydroxyhydantoin Carbamate Inhibitors of Mammalian Serine Hydrolases. Chem Biol. 2015;22:928–937. doi: 10.1016/j.chembiol.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Davda D, Martin BR. Acyl protein thioesterase inhibitors as probes of dynamic S-palmitoylation. Medchemcomm. 2014;5:268–276. doi: 10.1039/C3MD00333G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Dekker FJ, Rocks O, Vartak N, Menninger S, Hedberg C, Balamurugan R, Wetzel S, Renner S, Gerauer M, Scholermann B, et al. Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat Chem Biol. 2010;6:449–456. doi: 10.1038/nchembio.362. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, von Kriegsheim A, Grindlay J, Kolch W. Phosphatase and Feedback Regulation of Raf-1 Signaling. Cell Cycle. 2007;6:3–7. doi: 10.4161/cc.6.1.3593. [DOI] [PubMed] [Google Scholar]
- Doggett K, Grusche FA, Richardson HE, Brumby AM. Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling. BMC Dev Biol. 2011;11:57. doi: 10.1186/1471-213X-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow LE, Brumby AM, Muratore R, Coombe ML, Sedelies KA, Trapani JA, Russell SM, Richardson HE, Humbert PO. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene. 2003;22:9225–9230. doi: 10.1038/sj.onc.1207154. [DOI] [PubMed] [Google Scholar]
- Elsum IA, Humbert PO. Localization, not important in all tumor-suppressing properties: a lesson learnt from scribble. Cells Tissues Organs. 2013;198:1–11. doi: 10.1159/000348423. [DOI] [PubMed] [Google Scholar]
- Feigin ME, Akshinthala SD, Araki K, Rosenberg AZ, Muthuswamy LB, Martin B, Lehmann BD, Berman HK, Pietenpol JA, Cardiff RD, et al. Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with Basal breast cancer. Cancer research. 2014;74:3180–3194. doi: 10.1158/0008-5472.CAN-13-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol D, Zacchi A, Petrera F, Stanta G, Banks L. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int J Cancer. 2006;119:1285–1290. doi: 10.1002/ijc.21982. [DOI] [PubMed] [Google Scholar]
- Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200:1448–1459. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- Grecco HE, Schmick M, Bastiaens PI. Signaling from the living plasma membrane. Cell. 2011;144:897–909. doi: 10.1016/j.cell.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Grifoni D, Garoia F, Bellosta P, Parisi F, De Biase D, Collina G, Strand D, Cavicchi S, Pession A. aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene. 2007;26:5960–5965. doi: 10.1038/sj.onc.1210389. [DOI] [PubMed] [Google Scholar]
- Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20:573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Gujral TS, Karp ES, Chan M, Chang BH, MacBeath G. Family-wide investigation of PDZ domain-mediated protein-protein interactions implicates beta-catenin in maintaining the integrity of tight junctions. Chem Biol. 2013;20:816–827. doi: 10.1016/j.chembiol.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JL, Davda D, Majmudar JD, Won SJ, Prakash A, Choi AI, Martin BR. Correlated S-palmitoylation profiling of Snail-induced epithelial to mesenchymal transition. Molecular BioSystems. 2016 doi: 10.1039/c6mb00019c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JL, Majmudar JD, Martin BR. Profiling and inhibiting reversible palmitoylation. Current Opinion in Chemical Biology. 2013;17:20–26. doi: 10.1016/j.cbpa.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- Ivarsson Y, Arnold R, McLaughlin M, Nim S, Joshi R, Ray D, Liu B, Teyra J, Pawson T, Moffat J, et al. Large-scale interaction profiling of PDZ domains through proteomic peptide-phage display using human and viral phage peptidomes. Proc Natl Acad Sci U S A. 2014;111:2542–2547. doi: 10.1073/pnas.1312296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnz RA, Mulvihill MM, Chang JW, Hsu KL, Sorrentino A, Cravatt BF, Bandyopadhyay S, Goga A, Nomura DK. Activity-Based Protein Profiling of Oncogene-Driven Changes in Metabolism Reveals Broad Dysregulation of PAFAH1B2 and 1B3 in Cancer. ACS Chem Biol. 2015;10:1624–1630. doi: 10.1021/acschembio.5b00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Martin BR, Cravatt BF, Hofmann SL. DHHC5 protein palmitoylates flotillin-2 and is rapidly degraded on induction of neuronal differentiation in cultured cells. The Journal of biological chemistry. 2012;287:523–530. doi: 10.1074/jbc.M111.306183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DT, Conibear E. ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. Elife. 2015;4:e11306. doi: 10.7554/eLife.11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Golebiewski L, Dow EC, Krug RM, Javier RT, Rice AP. The ESEV PDZ-binding motif of the avian influenza A virus NS1 protein protects infected cells from apoptosis by directly targeting Scribble. Journal of virology. 2010;84:11164–11174. doi: 10.1128/JVI.01278-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Li H, Liu L, Yu J, Ren X. Fibroblast activation protein: A potential therapeutic target in cancer. Cancer Biol Ther. 2012;13:123–129. doi: 10.4161/cbt.13.3.18696. [DOI] [PubMed] [Google Scholar]
- Martin BR, Wang C, Adibekian A, Tully SE, Cravatt BF. Global profiling of dynamic protein palmitoylation. Nat Methods. 2012;9:84–89. doi: 10.1038/nmeth.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2012;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- Mezencev R, Matyunina LV, Jabbari N, McDonald JF. Snail-induced epithelial-to-mesenchymal transition of MCF-7 breast cancer cells: systems analysis of molecular changes and their effect on radiation and drug sensitivity. BMC Cancer. 2016;16:1–21. doi: 10.1186/s12885-016-2274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaka K, Pim D, Massimi P, Thomas M, Tomaic V, Subbaiah VK, Kranjec C, Nakagawa S, Yano T, Taketani Y, et al. The cell polarity regulator hScrib controls ERK activation through a KIM site-dependent interaction. Oncogene. 2010;29:5311–5321. doi: 10.1038/onc.2010.265. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol. 2000;20:8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Yano T, Nakagawa K, Takizawa S, Suzuki Y, Yasugi T, Huibregtse JM, Taketani Y. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br J Cancer. 2004;90:194–199. doi: 10.1038/sj.bjc.6601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302:1227–1231. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- Pearson HB, Perez-Mancera PA, Dow LE, Ryan A, Tennstedt P, Bogani D, Elsum I, Greenfield A, Tuveson DA, Simon R, et al. SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. The Journal of Clinical Investigation. 2011;121:4257–4267. doi: 10.1172/JCI58509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puustinen P, Junttila MR, Vanhatupa S, Sablina AA, Hector ME, Teittinen K, Raheem O, Ketola K, Lin S, Kast J, et al. PME-1 protects extracellular signal-regulated kinase pathway activity from protein phosphatase 2A-mediated inactivation in human malignant glioma. Cancer research. 2009;69:2870–2877. doi: 10.1158/0008-5472.CAN-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: A Cancer Microarray Database and Integrated Data-Mining Platform. Neoplasia (New York, NY) 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocks O, Gerauer M, Vartak N, Koch S, Huang ZP, Pechlivanis M, Kuhlmann J, Brunsveld L, Chandra A, Ellinger B, et al. The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell. 2010;141:458–471. doi: 10.1016/j.cell.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Shields DJ, Niessen S, Murphy EA, Mielgo A, Desgrosellier JS, Lau SK, Barnes LA, Lesperance J, Bouvet M, Tarin D, et al. RBBP9: a tumor-associated serine hydrolase activity required for pancreatic neoplasia. Proc Natl Acad Sci U S A. 2010;107:2189–2194. doi: 10.1073/pnas.0911646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa S, Nagasaka K, Nakagawa S, Yano T, Nakagawa K, Yasugi T, Takeuchi T, Kanda T, Huibregtse JM, Akiyama T, et al. Human scribble, a novel tumor suppressor identified as a target of high-risk HPV E6 for ubiquitin-mediated degradation, interacts with adenomatous polyposis coli. Genes Cells. 2006;11:453–464. doi: 10.1111/j.1365-2443.2006.00954.x. [DOI] [PubMed] [Google Scholar]
- Tom CT, Martin BR. Fat chance! Getting a grip on a slippery modification. ACS Chem Biol. 2013;8:46–57. doi: 10.1021/cb300607e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira V, Faversani A, Dohi T, Maggioni M, Nosotti M, Tosi D, Altieri DC, Bosari S. Aberrant Overexpression of the Cell Polarity Module Scribble in Human Cancer. The American Journal of Pathology. 2011;178:2478–2483. doi: 10.1016/j.ajpath.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujic I, Sanlorenzo M, Esteve-Puig R, Vujic M, Kwong A, Tsumura A, Murphy R, Moy A, Posch C, Monshi B, et al. Acyl protein thioesterase 1 and 2 (APT-1, APT-2) inhibitors palmostatin B, ML348 and ML349 have different effects on NRAS mutant melanoma cells. 2016 doi: 10.18632/oncotarget.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li X, Huang J, Feng L, Dolinta KG, Chen J. Defining the Protein–Protein Interaction Network of the Human Hippo Pathway. Molecular & Cellular Proteomics. 2014;13:119–131. doi: 10.1074/mcp.M113.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won SJ, Davda D, Labby KJ, Hwang SY, Pricer R, Majmudar JD, Armacost KA, Rodriguez LA, Rodriguez CL, Chong FS, et al. Molecular Mechanism for Isoform-Selective Inhibition of Acyl Protein Thioesterases 1 and 2 (APT1 and APT2) ACS Chem Biol. 2016 doi: 10.1021/acschembio.6b00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463:545–548. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- Young LC, Hartig N, Munoz-Alegre M, Oses-Prieto JA, Durdu S, Bender S, Vijayakumar V, Vietri Rudan M, Gewinner C, Henderson S, et al. An MRAS, SHOC2, and SCRIB complex coordinates ERK pathway activation with polarity and tumorigenic growth. Mol Cell. 2013;52:679–692. doi: 10.1016/j.molcel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann TJ, Bürger M, Tashiro E, Kondoh Y, Martinez NE, Görmer K, Rosin-Steiner S, Shimizu T, Ozaki S, Mikoshiba K, et al. Boron-Based Inhibitors of Acyl Protein Thioesterases 1 and 2. ChemBioChem. 2013;14:115–122. doi: 10.1002/cbic.201200571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.