Abstract
Remote Ischemic Conditioning (RIC), induced by brief cycles of ischemia and reperfusion, protects vital organs from a prolonged ischemic insult. While several biochemical mediators have been implicated in RIC's mechanism of action, it remains unclear whether the localization or “dose” of RIC affects the extent of protective signaling. In this randomized crossover study of healthy individuals, we tested whether the number of cycles of RIC and its localization (arm versus thigh) determines biochemical signaling and cytoprotection. Subjects received either arm or thigh RIC and then were crossed over to receive RIC in the other extremity. Blood flow, tissue perfusion, concentrations of the circulating protective mediator nitrite, and platelet mitochondrial function were measured after each RIC cycle. We found that plasma nitrite concentration peaked after the first RIC cycle and remained elevated throughout RIC. This plasma nitrite conferred cytoprotection in an in vitro myocyte model of hypoxia/reoxygenation. Notably, though plasma nitrite returned to baseline at 24 h, RIC conditioned plasma still mediated protection. Additionally, no difference in endpoints between RIC in thigh versus arm was found. These data demonstrate that localization and “dose” of RIC does not affect cytoprotection and further elucidate the mechanisms by which nitrite contributes to RIC-dependent protection.
1. Introduction
Remote ischemic conditioning (RIC) refers to brief sub-lethal ischemia applied to an area remote from the organ being targeted for protection from a future (preconditioning) or past (postconditioning) ischemic insult. In practical terms this is usually achieved in human subjects using a blood pressure cuff placed on an extremity and inflated above systolic blood pressure (often 200 mm Hg), rendering the extremity ischemic for a period of 5 min prior to deflation (which permits reperfusion) [1], [2]. RIC was first shown to be cardioprotective in 1993 [3] and subsequent studies have confirmed that other organs such as brain [4], [5] and liver [6] can be similarly protected. RIC is attractive from a clinical perspective as it is minimally invasive compared to traditional ischemic preconditioning, which involves targeting sublethal ischemia to the organ intended for protection, and carries minimal risk and discomfort [7]. While RIC has shown promise in preliminary clinical studies with surrogate endpoints [1], [2], [8] and possible survival benefits [9], [10], other recent large randomized clinical trials have yielded disappointing neutral results [11], [12], [13], [14].
The inconsistences in the benefit of RIC between trials may derive from a lack of understanding of how best to “dose” RIC. The commonly used protocol of 5 min inflation-deflation of a blood pressure cuff placed on an upper extremity repeated for 3–4 cycles has been adopted in clinical studies [11], [12], [13], [14] without formal testing of the optimal dose or location in humans. Thus, it is unknown whether some or all patients would benefit from more or less than 3–4 cycles of RIC. Further, it remains unknown whether RIC applied to an upper (arm) versus lower (thigh) extremity would alter the required dose or efficacy of RIC due to considerable differences in vascular and soft tissue mass in the arm versus leg.
One reason for the absence of a human dose titration of RIC is likely due to the lack of understanding of the precise mechanisms that underlie RIC-mediated protection [15], [16], [17]. Though a number of mechanisms have been proposed including neural and autonomic signaling [18], [19], studies demonstrating that organ protection can be transferred from a RIC-treated animal to an untreated animal by the simple transfer of blood suggest that humoral factors are likely imperative to protection [20], [21]. In this regard, Rassaf and colleagues recently demonstrated that nitrite, the one electron oxidation product of nitric oxide (NO), was significantly increased in the blood after RIC and necessary and sufficient to confer cardioprotection when applied to an ex vivo heart model of ischemia/reperfusion [20]. This production of nitrite was dependent on the reactive hyperemia (RH) that ensues during the reperfusion phase of RIC and due to oxidation of nitric oxide derived from vascular endothelial nitric oxide synthase [20]. Notably, prior studies have shown that nitrite mimics the effects of RIC and mediates cytoprotection in a number of ischemia/reperfusion models, and this is due to its inhibitory effect on mitochondrial respiration in the target organ [20], [22], [23], [24], [25]. Thus, the extent of RH and the resulting concentration of plasma nitrite generated represent a quantifiable parameter to measure the “dose” of RIC.
Here we measure RH and plasma nitrite as physiological and biochemical signaling mediators of RIC and use these parameters to quantify the “dose” of RIC delivered using sequential cycles of arm versus thigh RIC. We sought to determine whether RIC applied to a larger endothelial bed (thigh) would generate more nitrite than a smaller bed (arm) and whether consecutive cycles of RIC would further increase production of protective plasma nitrite. We measured platelet mitochondrial respiration in each subject as a target of the action of nitrite as well as utilized an in vitro cultured myocyte model of hypoxia-reoxygenation to demonstrate cytoprotection. We discuss our results in the context of further understanding RIC-dependent biochemical signaling as well as determining how to optimize RIC dosing in the clinic.
2. Methods
2.1. Human subject selection
The study was approved by and in accordance with the University of Pittsburgh Institutional Review Board and informed consent was obtained from each subject. Inclusion criteria included any healthy individual over 18 years of age. Prisoners, pregnant females, history of cardiovascular disease, circulation abnormalities, renal disease, liver disease, and anyone currently taking any medication were excluded. The subjects were not allowed to eat, consume caffeine or alcohol, use nicotine or exercise for 12 h prior to the study.
2.2. Study design
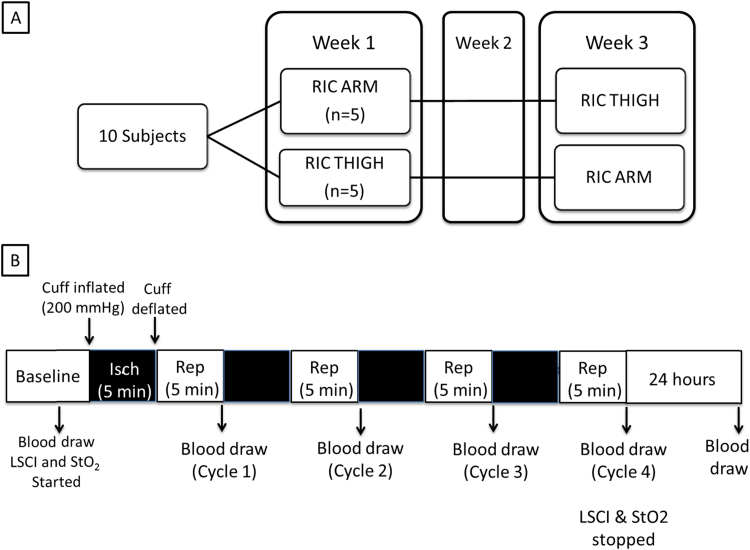
The study was performed in the University of Pittsburgh Applied Physiology Lab. Subjects were randomized to receive RIC applied to the right forearm or right thigh during week 1, with the procedure repeated on the other extremity two weeks later (Fig. 1A). After a baseline blood pressure measurement, the subjects were allowed to get comfortably seated and a blood pressure cuff was placed either on the right forearm or right thigh. An 18 gauge intravenous (IV) catheter was placed in the left antecubital vein (ie contralateral side from the RIC extremity) and was used for all subsequent blood draws. The extremity being used for RIC was secured to the contralateral extremity using cushions and tape assuring the subject was comfortable. This was to minimize movement and permit simultaneous imaging of both ipsilateral and contralateral extremities by laser speckle contrast imaging (LSCI; Perimed AB, Jarfalla, Sweden) throughout the RIC protocol. The LSCI camera was positioned 20 cm over the dorsal surface of the feet or palmar surface of the hands for the thigh and arm occlusion, respectively. Tissue oximetry (StO2; Inspectra 650, Hutchinson Technologies, Hutchinson, Minnesota) pads where placed on both thenar eminences or both halluces for the thigh and arm occlusion, respectively. Stable baseline recordings were collected using the LSCI and StO2 devices for one minute prior to the first cuff occlusion during which time a baseline blood draw (14 ml) was collected (Fig. 1B). RIC was initiated by inflating the blood pressure cuff to 200 mmHg to cause vaso-occlusion. This was at least 50 mm Hg greater than all subjects’ systolic blood pressure, measured at the beginning of each study day. The blood pressure cuff was maintained at 200 mmHg for 5 min with continuous monitoring of pressure through an in-line manometer. After 5 min the cuff was rapidly released permitting reperfusion, which in all cases could be verified visually by witnessed hyperemia. Additional blood samples were obtained four minutes after cuff release. LSCI and StO2 recording continued until at least 5 min after the final cuff release. The subjects returned 24 h later for the final blood draw (Fig. 1B).
Fig. 1.
Experimental Design. (A) Subjects (n=10) were randomized to have remote ischemic conditioning (RIC) applied to their forearm (n=5) or thigh (n=5) followed by a 2 week washout period before RIC was applied to the other extremity. (B) The study consisted of a baseline period followed by RIC (4 cycles of 5 min cuff inflation to induce ischemia and 5 min reperfusion following rapid deflation). Blood flow (LSCI) and tissue oximetry (Near Infrared Spectroscopy) measurements were begun 1 min prior to RIC initiation and ended 5 min after the last RIC cycle. Blood was obtained at baseline, 4 min into reperfusion (i.e. 1 min before next cycle) and at 24 h.
2.3. Whole blood and plasma nitrite measurements
For plasma and whole blood nitrite measurements, blood was collected in EDTA. Whole blood (1 ml) was immediately mixed with nitrite preservation solution (0.8 mol/L ferricyanide, 10 mmol/L N-ethylmaleimide, and 1% NP-40) at a 4:1 ratio and frozen for nitrite analysis as previously described [26]. The remaining blood (4 ml) was centrifuged within 5 min at 1000×g, 5 min, 4 °C to obtain plasma. Plasma was immediately separated from the RBC pellet and frozen for nitrite analysis. Plasma and whole blood nitrite concentrations were measured by tri-iodide based reductive chemiluminescence as previously described [26].
2.4. Platelet isolation and mitochondrial analysis
At each time point blood (8 ml) was collected in citrate (CPT BD Vacutainer) and processed within one hour of collection. Platelet rich plasma and platelets were isolated by differential centrifugation as previously described [27], [28] and outlined in Supplemental Methods. Oxygen consumption rate (OCR) was measured in isolated platelets (50×106/well) by Seahorse Extracellular Flux analysis as previously described [27], [29]. A bioenergetic profile was generated by measuring basal OCR followed by OCR in the presence of oligomycin A (2.5 µmol/L), FCCP (0.7 µmol/L), and rotenone (15 µmol/L). Values reported are rotenone subtracted reflective of mitochondrial specific OCR. In separate experiments platelets were incubated with MitoSOX (5 µmol/L; 10 min; Invitrogen, Carlsbad, CA) and mitochondrial superoxide generation quantified as previously described [27], [29].
2.5. Tissue oximetry
Two InSpectra Tissue Spectrometers Model 650 were used, each with its own probe as previously described [30]. The pads were placed over each thenar eminence of the subject's hands during RIC performed on the forearm, or on each hallux, when RIC was performed on the thigh. Each machine was turned on at least 5 min prior to the beginning of RIC and a stable baseline verified for 1 min before experiment start. The devices recorded data throughout the study and were stopped >5 min after the final vaso-occlusion. The StO2 data (expressed as a saturation percentage from 0 to 100) was analyzed for the baseline tissue oxygenation, the change from baseline to the RH peak after each vaso-occlusion, the time from cuff deflation to RH peak after each cycle, and the nadir five minutes after each VO cycle. Measurements were obtained simultaneously in the extremity ipsilateral and contralateral to the RIC. Since RH was not noted contralaterally, the “peak” and “nadir” corresponded in time to the ipsilateral side. All values are expressed as percent StO2 relative to baseline (100%). The StO2 measurements ranged from 5% to 98%, thus never reaching the limits of detection (0–100%).
2.6. Laser speckle contrast imaging (LSCI)
The PeriCam PSI LSCI system was used as previously described [31]. LSCI involves a near infrared (780 nm) laser, which is refracted based on movement, forming a random ‘speckled’ pattern. Changes in the speckled pattern correspond to red blood cell movement, thus allowing a relative measurement of blood flow rate [32]. The camera was placed 20 cm above the palms or ventral aspect of the feet. Baseline LSCI measurements were obtained for one minute prior to vaso-occlusion and recorded continuously until >5 min after the final vaso-occlusion. We focused on a region of interest in the center of the palm or foot, obtaining average blood flow measurements throughout. Alternative regions of interest yielded similar results (data not shown). The LSCI data was analyzed in the same manner as the StO2 with data normalized to baseline blood flow. During ischemia blood flow declined to 0–10 arbitrary units (AU) confirming vaso-occlusion but all subsequent values were on an arbitrary linear scale (range of scale is 0–500 AU; values spanned 100–461 AU).
2.7. In vitro anoxia-reoxygenation model
H9c2 myocytes purchased from ATCC (Rockville, MD) were maintained in normoxia (21% O2, 5% CO2) throughout. Ischemia was simulated by subjecting cells to hypoxia, (1% O2, 5% CO2, 94% N2; 5 h), in modified Esumi buffer (137 mmol/L NaCl, 12 mmol/L KCl, 0.5 mmol/L MgCl2, 0.9 mmol/L CaCl2, 20 mmol/L HEPES; 20 mmol/L 2-Deoxy-d-Glucose (2-DG), pH 6.2) as described by previous publications [33], [34], [35], [36]. Cells were then reoxygenated in the same buffer in normoxia (21% O2, 5% CO2) for 1 h. In some cases plasma (100 µl) from subjects who underwent RIC was added to the cells during the hypoxic period.
2.8. Lactate dehydrogenase (LDH) activity
LDH activity in the media was measured spectrophotometrically by measuring the decrease in NADH at 340 nm and was expressed as a percent of total LDH in the media and lysed cells.
2.9. Statistical analysis
Statistical analyses were performed using Prism 6.05 (GraphPad Software Inc., La Jolla, CA). All data are reported as mean±standard deviation and analyzed using parametric analyses based on normal distribution. Comparisons between results over time and between groups using repeated measures 1-way ANOVA with post-hoc Sidak's comparison made between cycle 1 and 4 data. If the ANOVA was significant, paired t-tests were performed to compare baseline values to cycle 1–4 separately. All other comparisons between two groups utilized unpaired t-tests. In all cases p<0.05 was considered significant and 0.05<p<0.10 was considered indicative of a trend.
3. Results
Ten healthy subjects (eight males and two females) were enrolled and completed the study. Subject demographics are outlined in Table 1. All subjects underwent RIC administered to the thigh and arm (Fig. 1). The only adverse effect reported in the context of the study was mild to moderate pain during ischemia and early reperfusion (during RH), and this was experienced by all participants. All subjects reported that pain during thigh RIC was greater than arm RIC and that the perception of pain diminished with each subsequent cycle. None of the participants requested that the procedure be stopped due to the pain severity and all subjects reported resolution of pain shortly after the last RIC cycle.
Table 1.
Subject characteristics (n=10).
| Age | 34.5 (26.5–38.75) |
| Gender, male | 8 (80%) |
| SBP | 118 (114–122) |
| DBP | 78 (68–80) |
| Lactate | 0.6 (0.58–0.65) |
| Hemoglobin | 16.7 (14.83–17.15) |
| Hematocrit | 44.5 (40.75–47) |
Values represent median (interquartile range) or n.
3.1. RIC causes reactive hyperemia measured by blood flow and tissue oxygen saturation
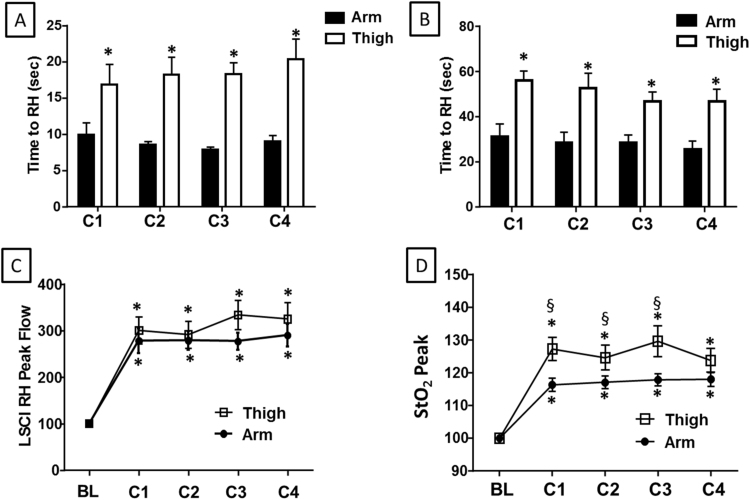
We first compared blood flow and tissue oxygen saturation after each cycle of RIC administered to the arm versus the thigh. Consistent with prior studies, RH was observed after each RIC cycle in both the arm and thigh (when measured by LSCI) and tissue oxygenation (measured by StO2) was also significantly increased. Notably, RH was observed earlier after cuff deflation (initiation of reperfusion) when measured by LSCI compared to StO2 in both extremities (all p<0.01; Fig. 2A–B). Additionally, comparison of RIC in each extremity showed that RH peaked significantly earlier following arm RIC compared to thigh when measured by either LSCI or StO2 (Fig. 2A–B). Despite an earlier peak with arm RIC, blood flow measured by LSCI was comparable in the forearm (278±85% of baseline) versus the thigh (300±92% of baseline; Fig. 2C) at the peak RH (after cycle 1). Tissue oxygen delivery measured by StO2 after RIC in the forearm (116±6% of baseline) was slightly lower than that generated by RIC delivered to the thigh (127±11% of baseline; p=0.013; Fig. 2D). The blood flow and StO2 nadirs generally showed non-significant trends towards being increased after the first RIC cycle with little variation over subsequent cycles (Supplemental Fig. 1A–B). In the case of the StO2 nadir after thigh RIC, these differences were small (7% absolute increase) but significant (Supplemental Fig. 1C–D). Blood flow and StO2 measured in the extremity contralateral to that used for RIC did not change throughout the period of observation (data not shown).
Fig. 2.
RIC to the arm or thigh causes reactive hyperemia (RH) and increased tissue oxygen delivery by StO2. The time to (A) peak blood flow (measured by LSCI) and (B) peak tissue oxygen saturation after RIC applied to either the arm (black bars) or thigh (white bars). Peak (C) blood flow and (D) tissue oxygenation after each cycle of RIC (C1-C4) applied to the arm (closed circles) or thigh (open squares). All measurements are normalized to the baseline (BL) for the respective appendage. (A–B) *p<0.01 versus arm measurement for the same cycle. (C–D) *p<0.01 versus baseline measurement. §p<0.05 for thigh versus arm. N=10. All data are mean±SEM.
3.2. Circulating nitrite levels peak after the first cycle of RIC
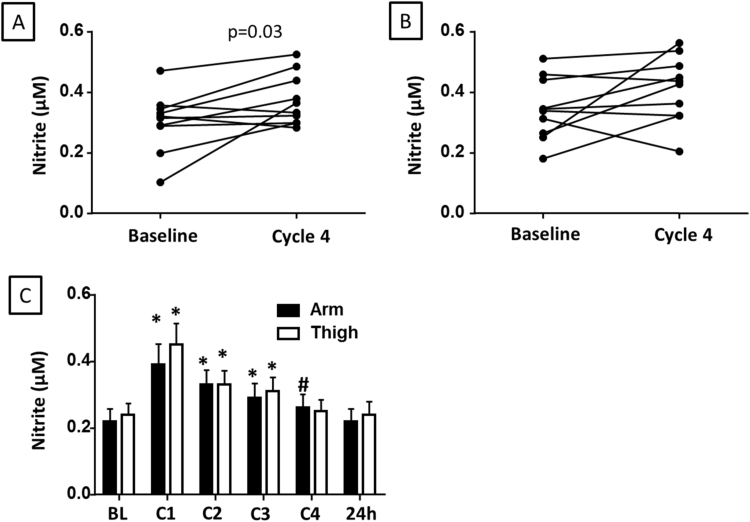
To determine whether RH observed after RIC resulted in the production of nitrite, we measured plasma nitrite at baseline and after 4 cycles of RIC. Plasma nitrite concentration at the end of 4 cycles of RIC was increased after arm or thigh RIC in eight out of ten subjects (Fig. 3A-B), with this elevation reaching significance after RIC in the arm (Fig. 3A). Notably, RIC (4 cycles) was ineffective in elevating plasma nitrite in the arm or thigh of the same two subjects. We next determined the effect of each cycle of RIC on the concentration of nitrite in platelet rich plasma (PRP). All ten subjects showed an increase in PRP nitrite after the first RIC cycle and at this peak, both arm (0.39±0.06 µmol/L) and thigh (0.20±0.06 µmol/L) PRP nitrite was significantly augmented compared to baseline (0.22±0.04 and 0.24±0.03 µmol/L respectively; Fig. 3C). Nitrite levels remained significantly elevated for each subsequent cycle, but decreased by ~10% with each cycle of RIC (Fig. 3C). The peak nitrite concentration and its rate of decrease did not significantly differ in the arm compared to thigh. In both cases, concentration was not significantly different from baseline 24 h after RIC (Fig. 3C). No significant change in whole blood nitrite was detected (data not shown).
Fig. 3.
RIC increases plasma nitrite concentration. (A–B) Plasma nitrite concentrations for each subject at baseline and after 4 cycles of RIC applied to the (A) arm or (B) thigh. (C) Nitrite concentration in the platelet rich plasma at baseline (BL) and after each cycle of RIC (C1-C4) applied to the arm (black bars) or thigh (white bars) as well as at 24 h. Data are mean±SEM. *p<0.01 and #p<0.05 compared to baseline for each appendage. N=10.
3.3. RIC inhibits platelet mitochondrial respiration
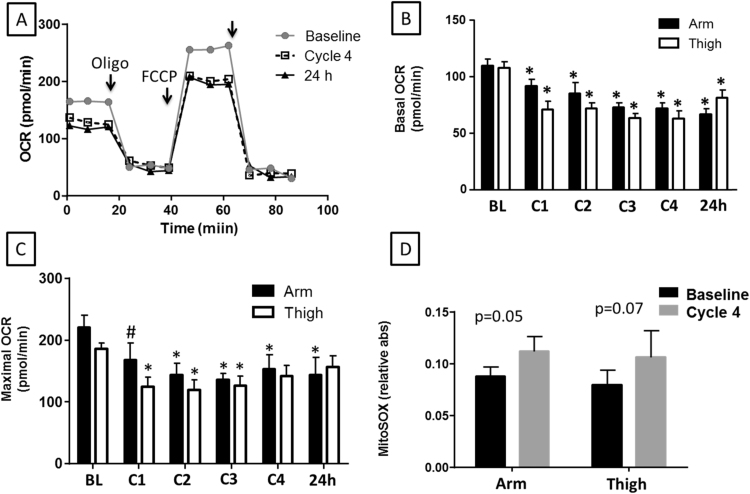
Mitochondria have been identified as a cytoprotective target of both nitrite and RIC. To determine whether RIC modulates mitochondrial function, we next measured the platelet mitochondrial respiration rate in each subject after each cycle of RIC. RIC performed in both the arm and thigh significantly inhibited platelet basal and maximal uncoupled mitochondrial respiration, but had no effect on mitochondrial proton leak (Fig. 4A). RIC performed in the arm decreased basal platelet mitochondrial OCR from baseline values of 109.3±19.7 pmol/min to 72.7±13.5 pmol/min by cycle 3 representing a maximal inhibition of 34%. Interestingly, significant inhibition was still present 24 h after RIC. RIC administered to the thigh resulted in a similar pattern of inhibition of basal OCR with maximal inhibition reaching a slightly greater level than arm (41%; Fig. 4B). Maximal uncoupled OCR was also inhibited to a similar extent by both arm and thigh RIC (Fig. 4C). However, no significant change was observed in mitochondrial proton leak (oligomycin-sensitive OCR; data not shown). This inhibition of cellular respiration was accompanied by a trend towards increased platelet mitochondrial production of superoxide that remained elevated 24 h after RIC (Fig. 4D).
Fig. 4.
RIC inhibits platelet mitochondrial respiration. (A) Representative Seahorse XF Analysis trace measuring oxygen consumption rate (OCR) in platelets from one subject at baseline (gray circles), after 4 cycles of RIC applied to the arm (open squares), and 24 h later (black triangles). For each trace, after a baseline reading, oligomycin A (2.5 µmol/L), FCCP (0.7 µmol/L) and rotenone (15 µmol/L) were added as noted by the arrows. (B-C) Quantitation of (B) basal and (C) maximal respiration rates calculated from traces similar to that shown in (A). (D) Mitochondrial superoxide production by platelets at baseline and after 4 cycles of RIC applied to the arm or thigh. (B–D) are means±SEM. *p<0.01 compared to baseline for the respective appendage. N=10.
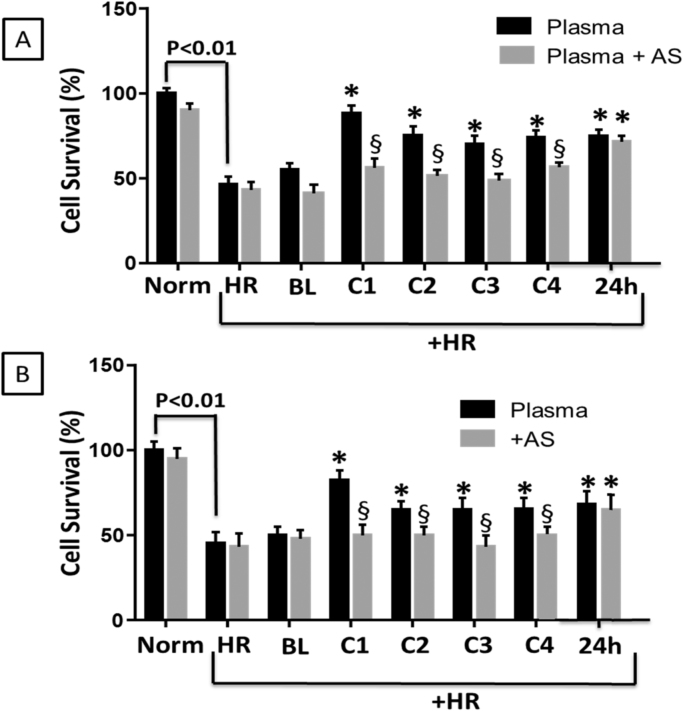
3.4. Plasma from the first cycle of RIC confers the greatest cytoprotection ex vivo
We next sought to determine the cytoprotective potency of each cycle of RIC utilizing an in vitro hypoxia-reoxygenation model (Fig. 5). In this model, cultured myocytes subjected to hypoxia (1%O2; 5 h) and subsequent reoxygenation (21% O2; 1 h) showed ~50% cell death. Treatment of the cells with PRP from each cycle of RIC (from either arm or thigh) as well as PRP collected 24 h after RIC significantly attenuated cell death. However, PRP from the first cycle of RIC showed the greatest cytoprotection (Fig. 5). To determine whether the nitrite generated by RIC was responsible for the cytoprotection, PRP samples were treated with acidified sulfanilamide to bind bioavailable nitrite. Scavenging of nitrite significantly inhibited the cytoprotective effect of PRP from the first through the fourth RIC cycle, but had no effect on cytoprotection from the 24 h sample (Fig. 5).
Fig. 5.
Plasma nitrite generated by RIC attenuates cell death in a model of hypoxia-reoxygenation. Cells were exposed to 5 h of anoxia (1% O2, 2.5 mmol/L 2-deoxyglucose, pH 6.5) to mimic ischemia, followed by reperfusion (1 h) and then cell death was measured by lactate dehydrogenase release. Panels A and B show percentage of survival in normoxic cells, those exposed to hypoxia-reoxygenation (HR) alone, or in the presence of 100 µl of plasma from subjects at baseline (BL) or after each cycle of RIC (C1-C4) or 24 h later (24 H) in the (A) arm or (B) thigh. In a second group of experiments, the plasma was first treated with acidified sulfanilamide (plasma+AS) to decrease nitrite concentration before being used to treat the cells (gray bars). All data are means±SEM. *p<0.01 compared to HR alone. §p<0.01 for plasma versus plasma+AS. N=10.
4. Discussion
In this study we compared the physiological and biochemical effects of 1–4 cycles of RIC applied to the arm versus thigh of healthy subjects. Our major finding was that physiological (RH and tissue oxygen saturation) and biochemical (plasma nitrite concentration) effects of RIC were not significantly different when RIC was applied to the arm versus thigh. Consistent with RH, we found that blood flow increases after each RIC cycle, and we extended previous studies to show that this results in a smaller but significant increase in tissue oxygen saturation compared to baseline in the ipsilateral extremity. On a biochemical level, circulating nitrite concentration increased to the greatest extent after the first cycle of RIC but remained elevated after subsequent cycles. Nitrite production was concomitant with a progressive inhibition of platelet mitochondrial respiration and a trend to increased superoxide production. Plasma taken from subjects after RIC protected myocytes from cell death in an in vitro model of hypoxia-reoxygenation and the protection for plasma from cycles 1–3 was dependent on nitrite.
Our data confirm prior studies noting that RIC results in RH [20], [37] and extend these results to demonstrate that increased blood flow results in a significant augmentation of tissue oxygen saturation. Notably, the increases in tissue oxygen saturation peaked later and were 10-fold lower in magnitude than the increase in blood flow. This may be a reflection of increased tissue oxygen consumption after ischemia which would limit the measurement of oxygen saturation or may reflect a more limited blood supply to the regions where we assessed tissue oximetry. It also must be noted that the duration of peak RH blood flow was brief with peaks lasting only several seconds and return to baseline (pre-cuff occlusion) blood flows within 120 s such that oxygen delivery is likely more reflective of the area under the blood flow curve rather than just the peak. The lack of increased blood flow in the contralateral extremity or changes in the nadir blood flow before the subsequent RIC cycle are consistent with this and imply that RIC is not causing a prolonged change in systemic blood flow, but rather having a discrete blood flow effect limited to the tissue bed undergoing ischemia and reperfusion.
It is interesting that we failed to see further augmentation of RH with multiple cycles of RIC or when RIC was applied to the thigh, which encompasses a much larger tissue/endothelial bed than the arm. These findings are consistent with those of Johnson et al. who showed in a murine model of RIC that increasing cycle number or performing RIC on two hindlimbs instead of one did not confer greater protection [38]. In our study, while blood flow did peak significantly earlier in the forearm than thigh, this may merely be a reflection of the distance from the cuff to the site of LSCI data collection. With respect to multiple cycles of RIC, it is possible that the 5 min cycle length achieves maximal dilation in healthy volunteers. Consistent with this, Raff and colleagues saw little increase in forearm blood flow (FBF) measured by plethysmography between 2 and 5 min of occlusion but noted a difference between 1 and 2 min of occlusion [39]. Notably, these authors did report an increase in FBF when the blood pressure cuff was applied to the wrist (ie forearm) versus upper arm [39], though differences in blood flow measurement methodology could account for this.
We measured RIC-induced increases in plasma nitrite of the same magnitude as previously reported by Rassaf and colleagues in healthy subjects [37]. Our results are consistent with NOS-dependent production of NO, which is oxidized to nitrite during RH [39], [40]. However, this is the first report that demonstrates that circulating nitrite concentration peaks after the first cycle of RIC and decreases with subsequent cycles. This is likely due to ischemic consumption of nitrite. Nitrite is a recognized endocrine reservoir of NO that can be reduced to bioavailable NO in conditions of hypoxia and acidosis, such as those present during ischemia [19], [23], [41], [42]. Hypoxic metabolism of nitrite results in the generation of a number of NO-dependent species including iron-nitrosylated and S-nitrosated proteins [42], which are markers of NO production and can be responsible for downstream signaling. Prior studies demonstrate that RIC-dependent NO generation is maximal after 1 min of ischemia [39]. Thus our 5 min ischemic period potentially results in a net consumption rather than augmented production of nitrite. This finding leads us to wonder whether shorter ischemic duration or fewer cycles would actually increase nitrite generation at least in subjects with “healthy” endothelium.
The consumption of nitrite has potential consequences for the number of RIC cycles required to mediate cytoprotection. Though it did not reach statistical significance, plasma collected after the first cycle of RIC showed the greatest level of cytoprotection in our in vitro model of hypoxia-reoxygenation. Interestingly, though plasma from subsequent cycles showed significant cytoprotection, this cytoprotection appeared to be less dependent on nitrite (evidenced by the decreased effect of acidified sulfanilamide). We and others have previously shown that acute nitrite-dependent cytoprotection after ischemia/reperfusion is dependent on the S-nitrosation and inhibition of complex I, which decreases respiratory rate [22], [25], [43]. Our data for the four cycles of RIC are consistent with this mechanism as we show that platelet mitochondrial respiration is progressively more inhibited with each RIC cycle. While we did not measure S-nitrosation in this study, the consumption of nitrite is consistent with the production of S-nitrosothiols.
The data demonstrating that plasma collected 24 h after RIC also mediated cytoprotection is consistent with the paradigm that RIC mediates two temporal windows of protection [44]. The acute window (minutes to hours after RIC is administered) is traditionally thought to be due to biochemical mediators, while delayed cytoprotection (hours to days after RIC) is usually attributed to changes in gene or protein expression [44]. Our data suggests the presence of plasma nitrite is required for acute protection (cycles 1–4), while nitrite does not have to be present to mediate protection 24 h after RIC is administered. We have previously shown that nitrite induces delayed protection through the production of mitochondrial ROS and subsequent upregulation of AMP Kinase, and we observe a trend to increased mitochondrial ROS production in this study. However, the protective effect observed with the plasma at 24 h could also be mediated by other factors including the release of high mobility group box-1 [45] or adenosine [46], [47].
Our data have potential implications for the interpretation of the recent neutral RIC clinical trials [13], [14]. We recently showed that subjects with comorbid endothelial dysfunction show diminished RH [31] and it is known that these subjects show minimal increases in nitrite after RIC [37]. Thus an alternative explanation for the failure to translate RIC to humans may be a failure to appreciate dosing requirements in the setting of health vs. disease. However, our data suggesting progressive mitochondrial inhibition with multiple cycles which persists at 24 h may suggest a more delayed effect could show efficacy in these subjects. Further investigation is required to elucidate the exact mechanism of RIC-dependent mitochondrial inhibition and its effect on delayed protection.
A limitation of our study is the inclusion of only healthy subjects as well as only Caucasian subjects. Prior studies suggest that blood flow regulation may be variable between subjects of different race [48], [49], [50]. It is therefore important to replicate these results in other healthy and diseased populations to fully understand the effects of RIC which will be needed to achieve the optimal dose. Additionally, our study has shown that while nitrite may stimulate signaling mediating delayed protection, its actual presence at 24 h is potentially not required. Future studies should expand on this pathway. Notwithstanding the limitations we have demonstrated that RIC increases blood flow and tissue oxygen saturation after RIC. These increases correspond with augmented plasma nitrite which is associated with mitochondrial inhibition and cytoprotection. These effects of RIC are similar regardless of extremity used and suggest that while nitrite-mediated protection is maximized after the initial cycle of RIC, significant protection is retained with up to four cycles.
Sources of funding
This work was supported by funds from the Pennsylvania Emergency Medical Foundation (FXG), K08-NS069817 (CD), the Hemophilia Center of Western Pennsylvania (SS), R01-GM113816-01 (SS) and American Heart Association grant 16GRNT27740024 (SS).
Author contributions
CD, FXG, JCR, and SS conceived, designed and directed the study. CD and SS analyzed and interpreted the data and drafted the manuscript. MT executed and analyzed physiological data. CC, GH and NK executed measurement of nitrite and mitochondrial data.
Competing financial interests
None to report.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.03.010.
Appendix A. Supplementary material
Supplementary material
References
- 1.Loukogeorgakis S.P. Remote ischemic preconditioning provides early and late protection Against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J. Am. Coll. Cardiol. 2005;46:450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy D.J. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 3.Przyklenk K., Bauer B., Ovize M., Kloner R.A., Whittaker P. Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 4.Chen G., Yang J., Lu G., Guo J., Dou Y. Limb remote ischemic post-conditioning reduces brain reperfusion injury by reversing eNOS uncoupling. Indian J. Exp. Biol. 2014;52:597–605. [PubMed] [Google Scholar]
- 5.Peng B. Remote ischemic postconditioning protects the brain from global cerebral ischemia/reperfusion injury by up-regulating endothelial nitric oxide synthase through the PI3K/Akt pathway. Brain Res. 2012;1445:92–102. doi: 10.1016/j.brainres.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Costa F.L. Combined remote ischemic perconditioning and local postconditioning on liver ischemia-reperfusion injury. J. Surg. Res. 2014;192:98–102. doi: 10.1016/j.jss.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 7.Koch S., Katsnelson M., Dong C., Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke. 2011;42:1387–1391. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarbock A. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. Jama. 2015;313:2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 9.Thielmann M. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 10.Sloth A.D. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur. Heart J. 2014;35:168–175. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 11.Hougaard K.D. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45:159–167. doi: 10.1161/STROKEAHA.113.001346. [DOI] [PubMed] [Google Scholar]
- 12.Meybohm P. Postoperative neurocognitive dysfunction in patients undergoing cardiac surgery after remote ischemic preconditioning: a double-blind randomized controlled pilot study. PLoS One. 2013;8:e64743. doi: 10.1371/journal.pone.0064743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hausenloy D.J. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. New Engl. J. Med. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 14.Meybohm P. A multicenter trial of remote ischemic preconditioning for heart surgery. New Engl. J. Med. 2015;373:1397–1407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 15.Dave K.R., Saul I., Prado R., Busto R., Perez-Pinzon M.A. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci. Lett. 2006;404:170–175. doi: 10.1016/j.neulet.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Andreka G. Remote ischaemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart. 2007;93:749–752. doi: 10.1136/hrt.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schott R.J., Rohmann S., Braun E.R., Schaper W. Ischemic preconditioning reduces infarct size in swine myocardium. Circ. Res. 1990;66:1133–1142. doi: 10.1161/01.res.66.4.1133. [DOI] [PubMed] [Google Scholar]
- 18.Lim S.Y., Yellon D.M., Hausenloy D.J. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res. Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- 19.Vinten-Johansen J., Shi W. The science and clinical translation of remote postconditioning. J. Cardiovasc. Med. 2013;14:206–213. doi: 10.2459/JCM.0b013e32835cecc6. [DOI] [PubMed] [Google Scholar]
- 20.Rassaf T. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ. Res. 2014;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 21.M. Shimizu, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clinical Science (London, England: 1979) 117, 2009, pp. 191–200. [DOI] [PubMed]
- 22.Dezfulian C. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elrod J.W., Calvert J.W., Gundewar S., Bryan N.S., Lefer D.J. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc. Natl. Acad. Sci. USA. 2008;105:11430–11435. doi: 10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiva S. Mitochondria as metabolizers and targets of nitrite. Nitric Oxide. 2010;22:64–74. doi: 10.1016/j.niox.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chouchani E.T. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacArthur P.H., Shiva S., Gladwin M.T. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Cardenes N. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood. 2014;123:2864–2872. doi: 10.1182/blood-2013-09-529420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villagra J. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu W., Cardenes N., Corey C., Erzurum S.C., Shiva S. Platelets from asthmatic individuals show less reliance on glycolysis. PLoS One. 2015;10:e0132007. doi: 10.1371/journal.pone.0132007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suffoletto B. Near-infrared spectroscopy in post-cardiac arrest patients undergoing therapeutic hypothermia. Resuscitation. 2012;83:986–990. doi: 10.1016/j.resuscitation.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Domsic R.T. Endothelial dysfunction is present only in the microvasculature and microcirculation of early diffuse systemic sclerosis patients. Clin. Exp. Rheuma. 2014;32:S-154–S-160. [PMC free article] [PubMed] [Google Scholar]
- 32.Ruaro B. Laser speckle contrast analysis: a new method to evaluate peripheral blood perfusion in systemic sclerosis patients. Ann. Rheum. Dis. 2014;73:1181–1185. doi: 10.1136/annrheumdis-2013-203514. [DOI] [PubMed] [Google Scholar]
- 33.Kamga Pride C. Nitrite activates protein kinase A in normoxia to mediate mitochondrial fusion and tolerance to ischaemia/reperfusion. Cardiovasc. Res. 2014;101:57–68. doi: 10.1093/cvr/cvt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lecour S. Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase) Circulation. 2005;112:3911–3918. doi: 10.1161/CIRCULATIONAHA.105.581058. [DOI] [PubMed] [Google Scholar]
- 35.Stephanou A. Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J. Biol. Chem. 2000;275:10002–10008. doi: 10.1074/jbc.275.14.10002. [DOI] [PubMed] [Google Scholar]
- 36.Suleman N., Somers S., Smith R., Opie L.H., Lecour S.C. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc. Res. 2008;79:127–133. doi: 10.1093/cvr/cvn067. [DOI] [PubMed] [Google Scholar]
- 37.Rassaf T. Plasma nitrite reserve and endothelial function in the human forearm circulation. Free Radic. Biol. Med. 2006;41:295–301. doi: 10.1016/j.freeradbiomed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Johnsen J. The remote ischemic preconditioning algorithm: effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res. Cardiol. 2016;111:10. doi: 10.1007/s00395-016-0529-6. [DOI] [PubMed] [Google Scholar]
- 39.Raff U. Nitric oxide and reactive hyperemia: role of location and duration of ischemia. Am. J. Hypertens. 2010;23:865–869. doi: 10.1038/ajh.2010.72. [DOI] [PubMed] [Google Scholar]
- 40.Meredith I.T. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 1996;270:H1435–H1440. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 41.Gladwin M.T. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 42.Cosby K. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 43.Shiva S. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xuan Y.T. Biphasic response of cardiac NO synthase isoforms to ischemic preconditioning in conscious rabbits. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2360–H2371. doi: 10.1152/ajpheart.2000.279.5.H2360. [DOI] [PubMed] [Google Scholar]
- 45.Izuishi K. Cutting edge: high-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J. Immunol. 2006;176:7154–7158. doi: 10.4049/jimmunol.176.12.7154. [DOI] [PubMed] [Google Scholar]
- 46.Pell T.J., Baxter G.F., Yellon D.M., Drew G.M. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am. J. Physiol. 1998;275:H1542–H1547. doi: 10.1152/ajpheart.1998.275.5.H1542. [DOI] [PubMed] [Google Scholar]
- 47.Kerendi F. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res. Cardiol. 2005;100:404–412. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- 48.Kahn D.F. Effects of black race on forearm resistance vessel function. Hypertension. 2002;40:195–201. doi: 10.1161/01.hyp.0000024571.69634.ed. [DOI] [PubMed] [Google Scholar]
- 49.Kalinowski L., Dobrucki I.T., Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 50.Morris A.A. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J. Am. Heart Assoc. 2013;2 doi: 10.1161/JAHA.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material