Abstract
Multiple drug resistance (MDR) and metastasis are two major factors that contribute to the failure of cancer treatment. However, the relationship between MDR and metastasis has not been characterized. Additionally, the role of the E3 ubiquitin ligase Cbl-b in metastasis of MDR gastric and breast cancer is not well known. In the present study, we found that MDR gastric and breast cancer cells possess a typical mesenchymal phenotype and enhanced cell migration capacity. Additionally, Cbl-b is poorly expressed in MDR gastric and breast cancer cells. In MDR gastric adenocarcinoma tissues, gastric cancer patients with low Cbl-b expression were more likely to have tumor invasion (P = .016) and lymph node metastasis (P = .007). Moreover, overexpression of Cbl-b reduced cell migration in MDR cell cultures both in vitro and in vivo. Cbl-b overexpression also prevented EMT by inducing ubiquitination and degradation of EGFR, leading to inhibition of the EGFR-ERK/Akt-miR-200c-ZEB1 axis. However, further overexpression of EGFR on a background of Cbl-b overexpression restored both the mesenchymal phenotype and cell migration capacity of MDR gastric and breast cancer cells. These results suggest that Cbl-b is an important factor for maintenance of the epithelial phenotype and inhibition of cell migration in MDR gastric and breast cancer cells.
Introduction
Multiple drug resistance (MDR) and metastasis are two major factors that contribute to the failure of cancer treatment [1], [2]. However, there is no conclusive evidence demonstrating an association between MDR and tumor metastasis or that MDR and metastasis regulate each other.
Previous studies have shown that MDR breast cancer cells are more metastatic than their parental cells, possibly because of the loss of E-cadherin expression [3]. The E-cadherin transcription repressors slug, twist, and ZEB1/2 are overexpressed in MDR breast cancer cells [4]. In cancer cells, the loss of E-cadherin results in epithelial-mesenchymal transition (EMT) and contributes to increased invasion and metastasis [5], [6]. Another important characteristic of tumor cells is their ability to express growth factor receptors, such as epidermal growth factor receptor (EGFR), which contribute to tumor progression, invasion, and metastasis [7]. EGFR signaling can mediate EMT in hepatocellular carcinoma [8]. Moreover, betacellulin can induce ovarian cancer cell migration via slug-dependent downregulation of E-cadherin via the EGFR signaling pathway [9]. However, whether the enhanced tumor metastasis observed in MDR gastric and breast cancer cells results from EGFR signaling pathway-regulated EMT remains unclear.
Because the activation of receptor tyrosine kinases can lead to tumor invasion and metastasis [10], activation of the receptor tyrosine kinase degradation system has become a new strategy for the inhibition of tumor metastasis. One recent study has shown that Casitas B-lineage lymphoma (Cbl) family members downregulated EGFR expression via their E3 ubiquitin ligase activity [11]. Another study showed that c-Cbl–mediated ubiquitination and downregulation of EGFR promoted tumor growth in colorectal cancer cells [12]. Our previous studies demonstrated that Cbl-b reversed drug resistance by both preventing the transporter functions of P-glycoprotein (P-gp) by inhibiting its translocation into caveolae and inhibiting EGFR downstream PI3K/Akt signaling [13], [14]. Another recent study from our laboratory showed that Cbl-b could repress IGF-1–mediated EMT through microRNA-200c–regulated ZEB2 in gastric cancer cells [15]. However, these studies did not determine whether Cbl-b could regulate the invasion and metastasis of MDR cancer cells. Because Cbl-b is expressed at low levels in MDR gastric cancer cells [14], we investigated whether this low expression of Cbl-b relieved inhibition of the EGFR pathway and thereby promoted EMT and tumor metastasis.
In our study, we demonstrate that Cbl-b maintains an epithelial phenotype by promoting ubiquitination and degradation of EGFR, thereby preventing the EGFR-ERK/Akt-miR-200c-ZEB1 axis and preventing tumor invasion and metastasis in MDR gastric and breast cancer cells.
Materials and Methods
Patients and Tissue Samples
Two hundred eight-two surgically resected gastric cancer cases were examined at the Department of Pathology, The First Hospital of China Medical University, between 1 January 2005 and 31 December 2005. No patient had received preoperative chemotherapy or radiotherapy. Age, sex, differentiation, depth of invasion, lymph node metastasis, and tumor node metastasis (TNM) stage were evaluated by reviewing medical charts and pathology records. This study was approved by the Human Ethics Review Committee of the First Hospital of China Medical University. Written informed consents were obtained from participants in accordance with the Declaration of Helsinki and its later revision. Tissue array methods and immunohistochemical staining were performed as previously described [13], [14].
Immunohistochemistry
Immunohistochemical staining for Cbl-b and P-gp was performed using a streptavidin-peroxidase procedure as described previously [13]. Antibodies against Cbl-b and P-gp were from Santa Cruz Biotechnology (Santa Cruz, CA). Immunostaining was considered positive when the tumor mass occupied more than 10% of the cross-sectional core area and when 10% or more of the neoplastic cells were stained.
Cell Culture
Gastric cancer SGC7901 cells and breast cancer MCF-7 cells were purchased from the Academy of Military Medical Science (Beijing, China). The adriamycin-resistant variant of SGC7901 (SGC7901/Adr) was kindly provided by the Fourth Military Medical University (Xi’an, China). The adriamycin-resistant variant of MCF-7 (MCF-7/Adr) was established by selecting for resistant colonies by culturing the parent cell line in 0.2 μg/ml of adriamycin. The drug-containing medium was changed weekly, and colonies that had propagated from single cells were selected. Clonal cell lines expanded from such a colony were subsequently selected in 1 μg/ml of adriamycin.
Transfections of Cbl-b WT and EGFR WT Plasmid
Transfections of Cbl-b WT and EGFR WT plasmid were performed as described previously [13], [14]. SGC7901/Adr and MCF-7/Adr cells were seeded at 4 × 105 cells/well in six-well plates overnight and then transfected by Lipofectamine 2000 reagent with pcDNA 3.1 plasmid including full-length Cbl-b or EGFR. pcDNA 3.1 vector served as the negative control. After transfections for 48 hours, the expression of Cbl-b and EGFR was evaluated by Western blot.
Western Blot and Immunoprecipitation
Western blot and immunoprecipitation were performed as described previously [15], [16], [17]. For immunoprecipitation, cell lysates were mixed with the indicated primary antibody and protein A-sepharose beads at 4°C overnight. The immunoprecipitated proteins were eluted by heat treatment at 100°C with 2× sampling buffer. Total cell proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The membranes were blocked with 5% skimmed milk in Tris-buffered saline Tween-20 buffer overnight. Various primary antibodies were as follows: Cbl-b, P-gp, p-ERK, ERK (Santa Cruz, USA); ZEB1, ZEB2, snail, slug, twist1, twist2 (Abcam, England); p-EGFR, EGFR, E-cadherin, Vimentin, p-Akt, Akt, (Cell Signaling Technology, USA). Then, the membrane was incubated with the secondary antibodies for 30 minutes at room temperature. Finally, proteins on the membranes were visualized by an enhanced chemiluminescence reagent in the electrophoresis gel imaging analysis system.
Immunofluorescence
SGC7901/Adr and MCF-7/Adr cells were seeded at 4 × 105 cells/well in Lab-Tek chamber slides overnight. Then, the cells were transfected with Cbl-b plasmid or empty vector for 48 hours and fixed in 3.3% paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked with 5% bovine serum albumin, and then incubated with anti–E-cadherin and anti-Vimentin antibody for 60 minutes at room temperature in the dark. Then, Alexa Fluor 546 or Alexa Fluor 488 IgG (Invitrogen, USA) was added in blocking solution for 30 minutes at room temperature in the dark. After mounting with the Slow Fade Antifade Kit, the cells were visualized by fluorescence microscopy (Olympus, Japan).
Transwell Migration
Migration assay was performed using Boyden chambers and polycarbonate inserts with 8-μm pore size membranes. The cells were seeded at 1 × 104 cells/well. After incubation for 24 hours, nonmigrated cells were removed from the upper surface of the chamber. The migrated cells on the lower surface of the chamber were stained using the Wright-Giemsa method. The migrated cells were counted under bright-field microscope.
Wound Healing Assay
SGC7901/Adr and MCF-7/Adr cells were seeded at 4 × 105 cells/well in six-well plates overnight. Then, the cells were transfected with Cbl-b plasmid or empty vector for 48 hours. The cells grew to nearly 100% confluence in six-well plates. Then, the cell-free line was manually created by scratching the confluent cell monolayers using a 200-μl pipette tip. We randomly choose five scratched fields. The images were captured through bright-field microscope.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Referring to our previous method [15], relative expression of microRNA was calculated via the comparative cycle threshold method, and the expression of U6 small nuclear RNA was used as reference. The forward primer for miR-200c was 5′-ACACTCCAGCTGGGTAATACTG CCGGGTAA-3′. The PCR conditions were 10 minutes at 95°C followed by 45 cycles at 95°C for 15 seconds and 58°C for 34 seconds.
Tumor Metastasis In Vivo
Female BALB/c nude mice, 4 to 6 weeks of age, were from the National Laboratory Animal Center (Shanghai, China). Cbl-b WT transfected SGC7901/Adr cells (1 × 106) in 200 μl were injected by tail vein for in vivo experimental metastasis analysis. The lung metastasis of the transfected SGC7901/Adr xenograft was observed under 3′-[18F] fluoro-3′-deoxythymidine and 2-deoxy-2-[18F] fluoro-d-glucose (Radio-pharmacy Unit, TU Munchen). Imaging was conducted using a micro–positron emission tomography (PET) system (Inveon, SIEMENS Preclinical Solutions). Tumors were fixed and stained with hematoxylin and eosin (H&E) for further pathological confirmation.
Statistical Analysis
All the presented data were expressed as the mean ± SD, and representative results were from three independent experiments. Statistical analysis was carried out using SPSS 18.0 software. Statistical comparisons were calculated by Student's two-tailed t test. P < .05 was considered statistically significant.
Results
Cell Migration Is Enhanced in MDR Gastric and Breast Cancer Cells with a Mesenchymal Phenotype
We first examined cell migration in MDR and parental gastric cancer and breast cancer cells. Our results showed that cell migration was increased in MDR gastric cancer SGC7901/Adr and breast cancer MCF-7/Adr cells compared with their parental SGC7901 and MCF-7 cells (Figure 1A). Moreover, SGC7901 and MCF-7 cells exhibited typical epithelial phenotypes with tight junctions, whereas SGC7901/Adr and MCF-7/Adr cells adopted an elongated and spindle-shaped mesenchymal phenotype (Figure 1B). Consistent with the mesenchymal phenotype, both SGC7901/Adr and MCF-7/Adr cells expressed high levels of the mesenchymal marker Vimentin and low levels of the epithelial marker E-cadherin (Figure 1C). Cbl-b was also expressed at low levels in SGC7901/Adr and MCF-7/Adr cells compared with parental cells (Figure 1C). These results suggest that enhanced migration of MDR gastric and breast cancer cells likely results from EMT and that Cbl-b may regulate cell migration.
Figure 1.
Cell migration was increased in MDR gastric and breast cancer cells. (A) SGC7901/Adr and MCF7/Adr, and their parental SGC7901 and MCF7 cells on Transwell membranes were monitored at 24 hours (upper). The migration ability was further quantified (lower). *P < .05. (B) The resistant and parental cells were cultured overnight. Photos were taken at ×20 magnification. (C) The expression of proteins was detected by Western blot.
Cbl-b Expression Negatively Correlates with Tumor Invasion and Metastasis in MDR Gastric Cancer Patients
The emergence of P-gp mediated MDR [14]. Immunohistochemical analysis of 282 gastric adenocarcinoma tissues revealed P-gp expression in 37.6% (106/282) and Cbl-b expression in 63.1% (178/282). Representative pictures for positive and negative P-gp or Cbl-b expression were shown in Figure 2A. In P-gp–negative gastric adenocarcinoma tissues, Cbl-b had no significant influence on TNM (P > .05) and lymph node metastasis (P > .05) of gastric cancer patients. However, in P-gp–positive gastric adenocarcinoma tissues, gastric cancer patients expressing Cbl-b were in T1 and T2 invasion (P = .016), exhibit no lymph node metastasis (P = .007), and early-stage TNM (P = .008) (Table 1). These data indicate that Cbl-b expression negatively correlates with tumor invasion and metastasis in MDR gastric cancer patients.
Figure 2.
Overexpression of Cbl-b inhibited MDR cancer cell migration in vitro and in vivo. (A) H&E staining P-gp or Cbl-b of adenocarcinoma tissues of gastric cancer patients was shown (20×). (B) Wound closure was monitored at 24 hours after the cells were transfected with Cbl-b WT and empty vector plasmid for 48 hours (upper). The wound closure was further quantified (lower). *P < .05. (C) The cells on Transwell membranes were monitored at 24 hours after Cbl-b WT transfection for 48 hours (upper). The migration ability was further quantified (lower). *P < .05. (D) The treated SGC7901/Adr cells were injected into mice and observed by the [18F] FDG luciferase signals. The images were obtained using micro-PET at 21 days after cell injection. (E) H&E staining of metastatic tumor colonies in the lungs were shown (40×). The numbers of metastatic tumor colonies in the lungs of nude mice injected with Cbl-b WT-transfected cells were monitored. Compared with those injected with the control cells, *P < .05.
Table 1.
Relationship between Cbl-b Expression and the Clinical Pathological Parameters in MDR Gastric Cancer Patients
| Clinical Pathological Parameters | Number | Gastric Cancer |
P | |
|---|---|---|---|---|
| Cbl-b Positive (n = 68) | Cbl-b Negative (n = 38) | |||
| Age (years) | ||||
| ≤60 | 48 | 34 | 14 | |
| >60 | 58 | 34 | 24 | .192⁎ |
| Sex | ||||
| Male | 60 | 36 | 24 | |
| Female | 46 | 32 | 14 | .309⁎ |
| Differentiation | ||||
| Well/moderate | 40 | 28 | 12 | |
| Poor | 66 | 40 | 26 | .328⁎ |
| Depth of invasion | ||||
| T1 + T2 | 32 | 26 | 6 | |
| T3 + T4 | 74 | 42 | 32 | .016⁎ |
| LN metastasis | ||||
| No | 34 | 28 | 6 | |
| Yes | 72 | 40 | 32 | .007⁎ |
| TNM stage | ||||
| I + II | 37 | 30 | 7 | |
| III + IV | 69 | 38 | 31 | .008⁎ |
LN, lymph node.
Two-sided P value; values shown in bold were statistically significant.
Chi-squared test.
Overexpression of Cbl-b Inhibits MDR Cancer Cell Migration In Vitro and In Vivo
We further investigated Cbl-b–mediated inhibition of MDR cancer cell migration using wound healing assays. We transiently transfected SGC7901/Adr and MCF-7/Adr cells with Cbl-b wild-type (WT) plasmid and found that wound gaps were significantly larger in Cbl-b WT-transfected SGC7901/Adr and MCF-7/Adr cells compared with control cells transfected with empty vector plasmid (Figure 2B). Furthermore, overexpression of Cbl-b inhibited the cell migration ability of both MDR cancer cell lines (Figure 2C). However, we did not observe any difference in cell viability at 24 hours between the cells transfected with control plasmid and Cbl-b WT (100% vs. 93.67 ± 4.50% for SGC7901/Adr, 100% vs. 94.33 ± 3.92% for MCF-7/Adr, respectively; P > .05).
We next examined whether Cbl-b could inhibit tumor metastasis in vivo. We injected SGC7901/Adr cells with or without ectopic expression of Cbl-b into the tail vein of mice and then sacrificed the animals 21 days later for lung tissue examination. Results from [18F] FDG micro-PET assays revealed that mice injected with Cbl-b WT cells had significantly reduced metastatic lung tissue compared with the control (Figure 2D). Consistent with these findings, H&E staining showed significantly decreased numbers of metastatic tumor colonies in the lungs of nude mice injected with Cbl-b WT-transfected cells compared with those injected with control cells (Figure 2E). These data suggest that Cbl-b impaired cell migration in MDR gastric and breast cancer cells.
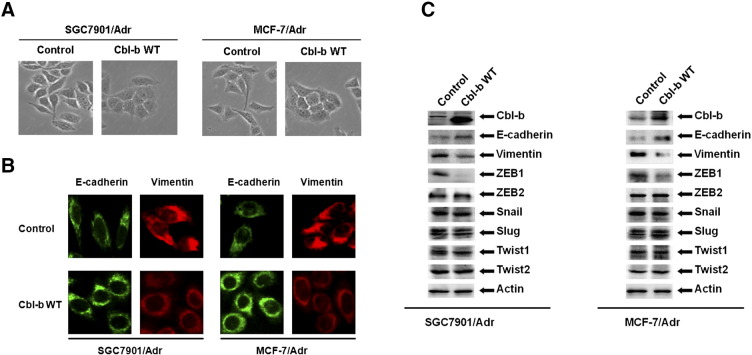
Overexpression of Cbl-b Reversed the Mesenchymal Phenotype of MDR Gastric and Breast Cancer Cells
We further investigated whether Cbl-b–mediated inhibition of cell migration was associated with EMT by transfecting SGC7901/Adr and MCF-7/Adr cells with Cbl-b WT or control plasmids. Cbl-b WT-transfected cells adopted a typical epithelial-like morphology with tight junctions (Figure 3A). The increase of E-cadherin and the decrease of Vimentin in Cbl-b WT-transfected cells were detected by immunofluorescence (Figure 3B) and Western blotting (Figure 3C). Downregulation of transcription factor ZEB1 was also detected in Cbl-b WT-transfected cells (Figure 3C). Our data indicate that Cbl-b maintains the epithelial phenotype in MDR gastric and breast cancer cells.
Figure 3.
Overexpression of Cbl-b recovered the mesenchymal phenotype in MDR gastric and breast cancer cells. SGC7901/Adr and MCF7/Adr cells were transfected with Cbl-b WT and empty vector plasmid for 48 hours. (A) The cells were cultured overnight. Photos were taken at ×20 magnification. (B) The cells were stained with antibodies to E-cadherin (green) and Vimentin (red). Images were captured by fluorescence microscopy at ×40 magnification. (C) The expression of proteins was detected by Western blot.
Overexpression of Cbl-b Represses the Mesenchymal Phenotype by Inducing Ubiquitination and Degradation of EGFR and Inhibiting the ERK/Akt-miR-200c-ZEB1 Axis
We then further explored the mechanism of Cbl-b–mediated repression of the mesenchymal phenotype in MDR cancer cells. Overexpression of Cbl-b reduced total EGFR protein level and inhibited EGFR and downstream ERK/Akt activation in SGC7901/Adr and MCF-7/Adr cells (Figure 4A). Importantly, Cbl-b WT transfection promoted Cbl-b binding to EGFR (Figure 4B) and the ubiquitination and degradation of EGFR (Figure 4C). Given that ZEB1 was downregulated in Cbl-b WT-transfected cells (Figure 3C) and miR-200c reversed EMT by targeting ZEB1 [18], we measured miR-200c and found a significant increase in Cbl-b WT-transfected cells compared to control cells (Figure 5A). Additionally, treatment of SGC7901/Adr and MCF-7/Adr cells with 25 μM LY294002 (PI3K/Akt inhibitor) and 20 μM PD98059 (ERK inhibitor) increased miR-200c expression (Figure 5B). Moreover, ZEB1 expression was decreased following treatment with LY294002 and PD98059 (Figure 5C). These findings suggest that overexpression of Cbl-b repressed the mesenchymal phenotype of MDR gastric and breast cancer cells through the ubiquitination and degradation of EGFR and preventing the ERK/Akt-miR-200c-ZEB1 axis.
Figure 4.
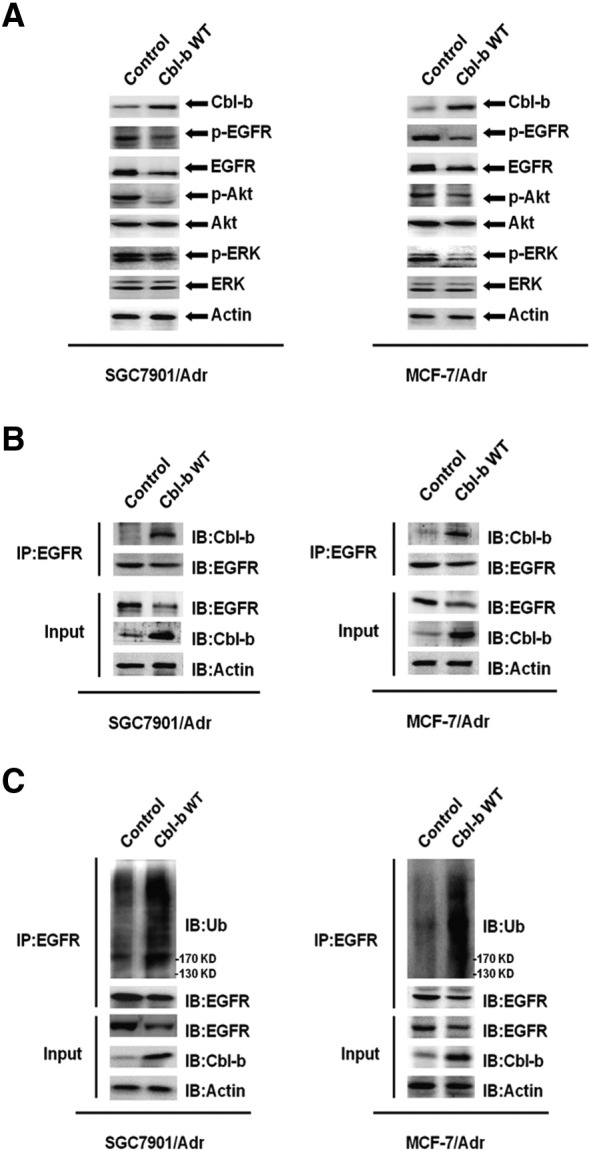
Overexpression of Cbl-b promoted the ubiquitination and degradation of EGFR and EGFR pathway inactivation. SGC7901/Adr and MCF-7/Adr cells were transfected with Cbl-b WT and empty vector plasmid for 48 hours. (A) EGFR and downstream ERK/Akt were analyzed by Western blot. (B) The interaction of Cbl-b and EGFR was detected by immunosedimentation and Western blot. (C) The ubiquitination of EGFR was detected by immunosedimentation and Western blot.
Figure 5.
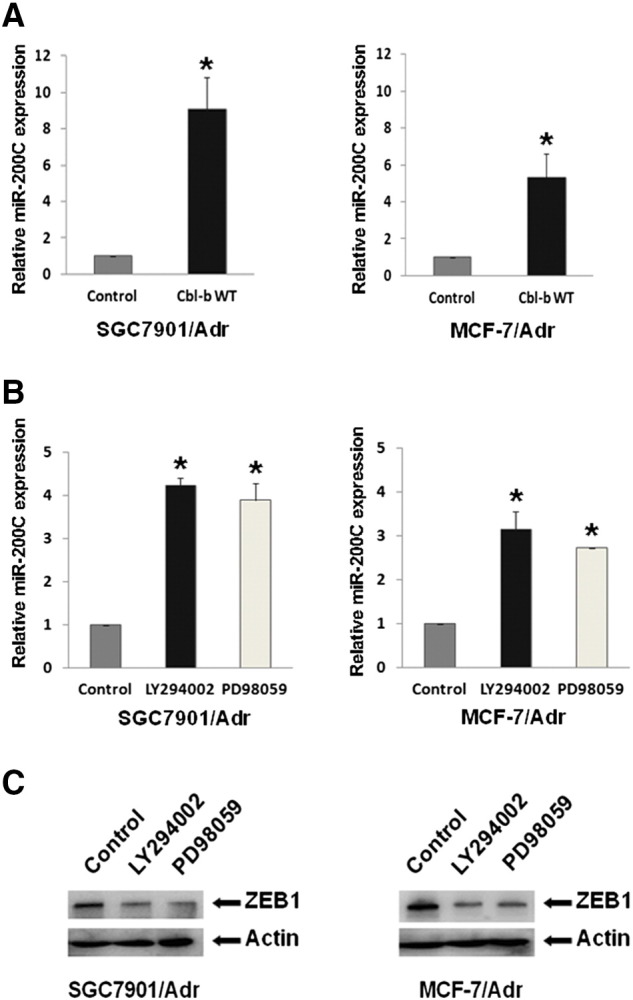
Overexpression of Cbl-b repressed the mesenchymal phenotype by the inhibition of ERK/Akt-miR-200c-ZEB1 axis. (A) SGC7901/Adr and MCF-7/Adr cells were transfected with Cbl-b WT and empty vector plasmid for 48 hours. The relative level of miR-200c was analyzed by qRT-PCR. *P < .05. (B) SGC7901/Adr and MCF-7/Adr cells were treated with LY294002 (25 μM) or PD98059 (20 μM) for 24 hours. The relative level of miR-200c was analyzed by qRT-PCR. *P < .05. (C) The expression of proteins was detected by Western blot.
Simultaneous Overexpression of EGFR and Cbl-b Restores the Mesenchymal Phenotype and Cell Migration Capacity in MDR Gastric and Breast Cancer Cells
We finally investigated whether Cbl-b overexpression represses the mesenchymal phenotype through inhibition of EGFR by transiently transfecting SGC7901/Adr and MCF-7/Adr cells with both EGFR WT and Cbl-b WT plasmids. Overexpression of EGFR on a background of Cbl-b overexpression restored EGFR expression and facilitated activation of EGFR and ERK/Akt compared with Cbl-b overexpression alone (Figure 6A). Consistent with these findings, upregulation of Vimentin and ZEB1 and downregulation of E-cadherin were also detected in co-transfected cells (Figure 6A). Moreover, the increase in miR-200c levels was partially reversed in EGFR WT and Cbl-b WT co-transfected cells compared with cells transfected with Cbl-b WT alone (Figure 6B). Finally, the simultaneous overexpression of both EGFR and Cbl-b partially recovered the cell migration capacity (Figure 6C). These results suggest that Cbl-b reversed the mesenchymal phenotype and prevented cell migration by inhibition of the EGFR-ERK/Akt-miR-200c-ZEB1 axis.
Figure 6.
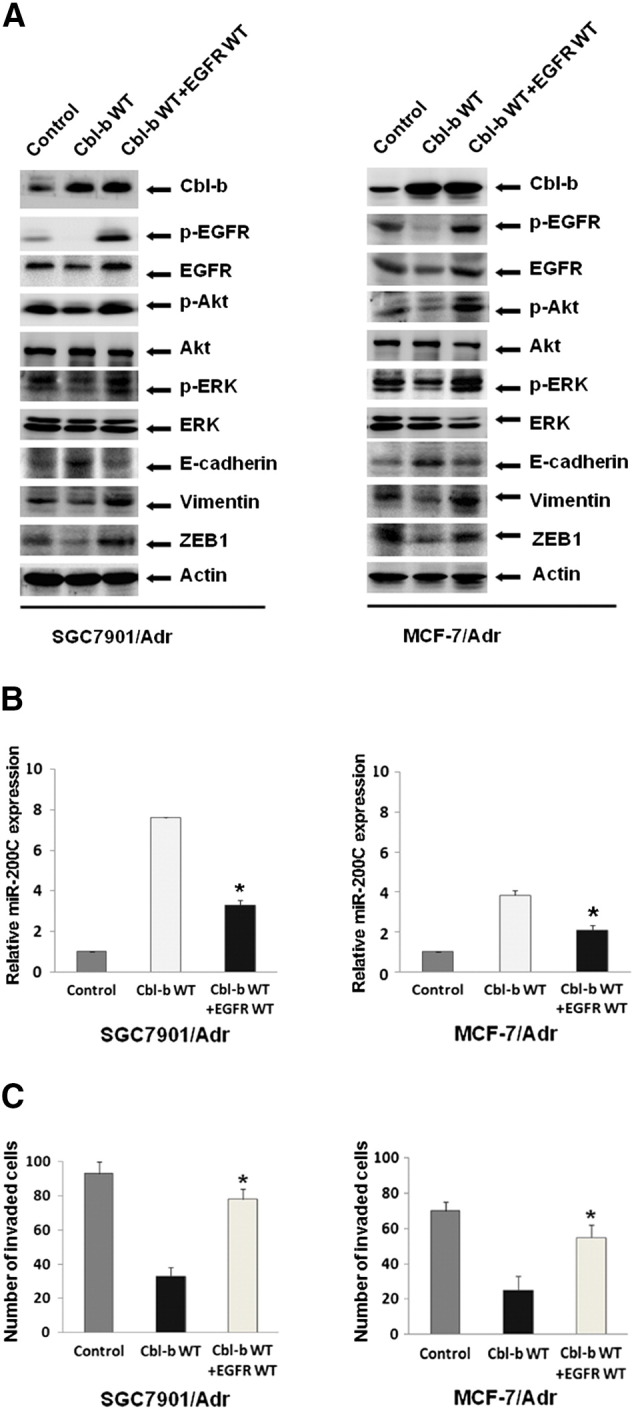
Simultaneous overexpression of EGFR and Cbl-b restored the mesenchymal phenotype and cell migration capacity. SGC7901/Adr and MCF-7/Adr cells were transfected with both EGFR WT and Cbl-b WT plasmids for 48 hours. (A) The expression of proteins was detected by Western blot. (B) The relative level of miR-200c was analyzed by qRT-PCR. *P < .05. (C) The cell migration ability was quantified at 24 hours after EGFR WT and Cbl-b WT transfection for 48 hours. *P < .05.
Discussion
Drug-induced MDR1 expression enables cancer cells to become resistant to multiple drugs, leading to the failure of antitumor treatment. Recent studies have shown that the MDR-positive phenotype correlates with a more aggressive mesenchymal phenotype and poor prognosis [19], [20]. Our current study identifies an enhanced capacity for cell migration in MDR gastric cancer and breast cancer cells compared with their parental cells. These MDR cancer cells also exhibit a typical mesenchymal phenotype accompanied by low Cbl-b expression. Interestingly, previous studies from our laboratory showed that Cbl-b could reverse drug resistance in MDR cancer cells [13], [14]. Additionally, Cbl-b improves the prognosis of RANK-positive breast cancer through the inhibition of cell migration and metastasis induced by RANKL [21]. However, whether Cbl-b regulates tumor invasion and metastasis in MDR gastric and breast cancer cells is unknown until now. Our present clinical data suggest that MDR gastric cancer patients with low levels of Cbl-b expression are more likely to have tumor invasion and lymph node metastasis. Consistent with these findings, we demonstrated that overexpression of Cbl-b prevented cell migration of MDR gastric and breast cancer cells both in vitro and in vivo. Our results indicate that Cbl-b is an important inhibitor of tumor invasion and metastasis in MDR gastric and breast cancer cells.
We next investigated the mechanism by which Cbl-b regulates tumor invasion and metastasis in MDR gastric and breast cancer cells. Emerging evidence suggests that EMT is a vital event in tumor invasion and metastasis [22], [23]. Previous studies including our own have shown that the transcription factors ZEB1/2 can down-regulate the expression of E-cadherin and promote EMT, whereas the miR-200 family can inhibit ZEB1/2 expression [15], [24], [25]. Furthermore, overexpression of EGFR increases levels of ZEB1/2 and promotes TGF-β–induced EMT [26]. Notably, the Cbl family proteins serve as negative regulators of many receptor tyrosine kinases such as EGFR. In breast cancer cells, ERβ1 inhibits EMT by enhancing the EGFR-c-Cbl interaction, thereby inducing EGFR degradation [27]. Another study showed that miR-675 binding to c-Cbl and Cbl-b mRNA increased the stability of EGFR and enhanced cell migration [28]. Moreover, ionizing radiation-inducible miR-30e promoted glioma cell invasion through EGFR stabilization by targeting Cbl-b [29]. However, whether Cbl-b regulates tumor invasion and metastasis by targeting EGFR in MDR gastric and breast cancer cells has not been previously reported. We have shown that overexpression of Cbl-b promotes an interaction between Cbl-b and EGFR, leading to the ubiquitination and degradation of EGFR. Moreover, Cbl-b overexpression inhibits the EGFR-ERK/Akt-miR-200c-ZEB1 axis and further represses the mesenchymal phenotype. However, further overexpression of EGFR on a background of Cbl-b overexpression can relieve Cbl-b–mediated EGFR degradation and restore EGFR expression, mesenchymal phenotype, and cell migration capacity in MDR gastric and breast cancer cells. Therefore, EGFR is a key target of Cbl-b in the regulation of tumor invasion and metastasis in MDR gastric and breast cancer cells.
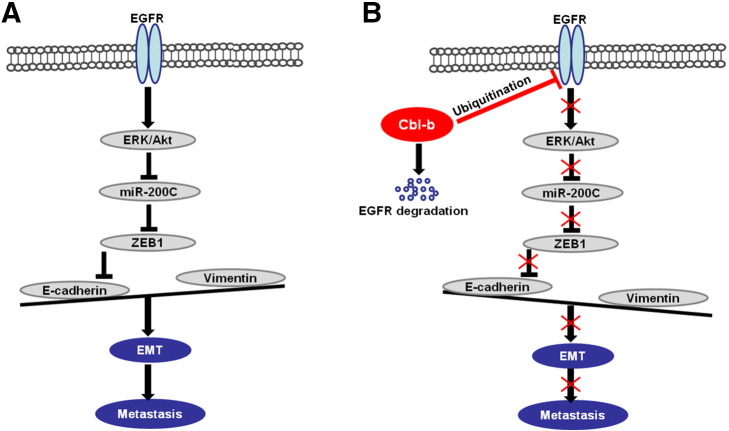
In summary, our study demonstrates that Cbl-b maintains the epithelial phenotype and prevents cell migration by inhibition of the EGFR-ERK/Akt-miR-200c-ZEB1 axis in MDR gastric and breast cancer cells (Figure 7). Cbl-b may also be a potential prognostic indicator for the invasion and metastasis of MDR cancers.
Figure 7.
Schematic representation of the proposed model. (A) The E3 ubiquitin ligase Cbl-b is expressed at low levels in high-invasive MDR gastric cancer and breast cancer cells. EGFR-activated ERK/Akt enhanced the expression of the E-cadherin transcription repressor ZEB1 through the downregulation of miR-200c. Downregulation of E-cadherin led to EMT and tumor metastasis. (B) Overexpression of Cbl-b inhibited EGFR and the downstream ERK/Akt signal by the ubiquitination and degradation of EGFR. Inactivation of the EGFR pathway decreased the expression of the E-cadherin transcription repressor ZEB1 through the upregulation of miR-200c. E-cadherin is upregulated, and EMT and tumor metastasis are repressed.
Ethics Approval and Consent to Participate
Our study is approved by the Human Ethics Review Committee of the First Hospital of China Medical University. All patients agreed to participate in our study.
Consent for Publication
We certify that no portion of this manuscript has been previously published. And authors listed have approved to submit this manuscript to the journal.
Conflict of Interest
The authors have no conflicts of interest to declare.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (no. 81673025, 81270036, 81372546, 81572374, 31300743);Program for Liaoning Excellent Talents in University (no. LR2014023); Project for clinical ability construction of Chinese Medicine, Science and Technology Plan Project of Liaoning Province (no. 2014021069, 2015020457, 2014226033, 2014225013); and National Natural Science Foundation–Outstanding Youth Foundation Training Project of China Medical University (no. YQ20160002). The authors thank Jian Gao and Lu Yao (Experiment Technology Center of China Medical University) for kindly providing technical support.
Footnotes
Conflict of interest statement: All authors disclose no potential conflicts of interest (including employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding) that are relevant to the manuscript.
Contributor Information
Xiujuan Qu, Email: xiujuanqu@yahoo.com.
Yunpeng Liu, Email: cmuliuyunpeng@hotmail.com.
References
- 1.Alizadeh AM, Shiri S, Farsinejad S. Metastasis review: from bench to bedside. Tumour Biol. 2014;35:8483–8523. doi: 10.1007/s13277-014-2421-z. [DOI] [PubMed] [Google Scholar]
- 2.Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347:159–166. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Lu L, Zhou D, Jiang X, Song K, Li K, Ding W. Loss of E-cadherin in multidrug resistant breast cancer cell line MCF-7/Adr: possible implication in the enhanced invasive ability. Eur Rev Med Pharmacol Sci. 2012;16:1271–1279. [PubMed] [Google Scholar]
- 4.Tsou SH, Chen TM, Hsiao HT, Chen YH. A critical dose of doxorubicin is required to alter the gene expression profiles in MCF-7 cells acquiring multidrug resistance. PLoS One. 2015;10:e0116747. doi: 10.1371/journal.pone.0116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galván JA, Zlobec I, Wartenberg M, Lugli A, Gloor B, Perren A, Karamitopoulou E. Expression of E-cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour-budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Br J Cancer. 2015;112:1944–1950. doi: 10.1038/bjc.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Yu H, Shen Y, Hong J, Xia Q, Zhou F, Liu X. The contribution of TGF-β in epithelial-mesenchymal transition (EMT): down-regulation of E-cadherin via snail. Neoplasma. 2015;62:1–15. doi: 10.4149/neo_2015_002. [DOI] [PubMed] [Google Scholar]
- 7.Ovcaricek T, Cufer T, Kern I, Sodja E, Sadikov A. Efficacy of tyrosine kinase inhibitors in routine clinical practice: epidermal growth factor mutations and their implications. J Cancer Res Ther. 2013;9:261–266. doi: 10.4103/0973-1482.113379. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZC, Gao Q, Shi JY, Guo WJ, Yang LX, Liu XY, Liu LZ, Ma LJ, Duan M, Zhao YJ. Protein tyrosine phosphatase receptor S acts as a metastatic suppressor in hepatocellular carcinoma by control of epithermal growth factor receptor–induced epithelial-mesenchymal transition. Hepatology. 2015;62:1201–1214. doi: 10.1002/hep.27911. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Klausen C, Qiu X, Cheng JC, Chang HM, Leung PC. Betacellulin induces Slug-mediated down-regulation of E-cadherin and cell migration in ovarian cancer cells. Oncotarget. 2016;7:28881–28890. doi: 10.18632/oncotarget.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau SK, Shields DJ, Murphy EA, Desgrosellier JS, Anand S, Huang M, Kato S, Lim ST, Weis SM, Stupack DG. EGFR-mediated carcinoma cell metastasis mediated by integrin αvβ5 depends on activation of c-Src and cleavage of MUC1. PLoS One. 2012;7:e36753. doi: 10.1371/journal.pone.0036753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennock S, Wang Z. A tale of two Cbls: interplay of c-Cbl and Cbl-b in epidermal growth factor receptor downregulation. Mol Cell Biol. 2008;28:3020–3037. doi: 10.1128/MCB.01809-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao S, Zheng P, Wu H, Song LM, Ying XF, Xing C, Li Y, Xiao ZQ, Zhou XN, Shen T. Erbin interacts with c-Cbl and promotes tumourigenesis and tumour growth in colorectal cancer by preventing c-Cbl-mediated ubiquitination and down-regulation of EGFR. J Pathol. 2015;236:65–77. doi: 10.1002/path.4502. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Qu X, Teng Y, Li Z, Xu L, Liu J, Ma Y, Fan Y, Li C, Liu S. Cbl-b inhibits P-gp transporter function by preventing its translocation into caveolae in multiple drug-resistant gastric and breast cancers. Oncotarget. 2015;6:6737–6748. doi: 10.18632/oncotarget.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Qu X, Hu X, Yang X, Hou K, Teng Y, Zhang J, Sada K, Liu Y. Reversal of P-glycoprotein–mediated multi-drug resistance by the E3 ubiquitin ligase Cbl-b in human gastric adenocarcinoma cells. J Pathol. 2009;218:248–255. doi: 10.1002/path.2533. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Xu L, Li C, Zhao L, Ma Y, Zheng H, Li Z, Zhang Y, Wang R, Liu Y. Ubiquitin ligase Cbl-b represses IGF-I-induced epithelial mesenchymal transition via ZEB2 and microRNA-200c regulation in gastric cancer cells. Mol Cancer. 2014;13:136. doi: 10.1186/1476-4598-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Zhang Y, Liu J, Qu J, Hu X, Zhang F, Zheng H, Qu X, Liu Y. TRAIL-activated EGFR by Cbl-b–regulated EGFR redistribution in lipid rafts antagonises TRAIL-induced apoptosis in gastric cancer cells. Eur J Cancer. 2012;48:3288–3299. doi: 10.1016/j.ejca.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Hu X, Qu X, Hou K, Zheng H, Liu Y. Cetuximab enhances TRAIL-induced gastric cancer cell apoptosis by promoting DISC formation in lipid rafts. Biochem Biophys Res Commun. 2013;439:285–290. doi: 10.1016/j.bbrc.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 18.Bai WD, Ye XM, Zhang MY, Zhu HY, Xi WJ, Huang X, Zhao J, Gu B, Zheng GX, Yang AG. MiR-200c suppresses TGF-β signaling and counteracts trastuzumab resistance and metastasis by targeting ZNF217 and ZEB1 in breast cancer. Int J Cancer. 2014;135:1356–1368. doi: 10.1002/ijc.28782. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Zhang H, Wang Z, Yu M, Tian R, Ji W, Yang Y, Niu R. P-glycoprotein associates with Anxa2 and promotes invasion in multidrug resistant breast cancer cells. Biochem Pharmacol. 2014;87:292–302. doi: 10.1016/j.bcp.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Saxena M, Stephens MA, Pathak H, Rangarajan A. Rangarajan, Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011;2:e179. doi: 10.1038/cddis.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Teng Y, Fan Y, Wang Y, Li W, Shi J, Ma Y, Li C, Shi X, Qu X. The E3 ubiquitin ligase Cbl-b improves the prognosis of RANK positive breast cancer patients by inhibiting RANKL-induced cell migration and metastasis. Oncotarget. 2015;6:22918–22933. doi: 10.18632/oncotarget.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rateitschak K, Kaderali L, Wolkenhauer O, Jaster R. Autocrine TGF-β/ZEB/microRNA-200 signal transduction drives epithelial-mesenchymal transition: Kinetic models predict minimal drug dose to inhibit metastasis. Cell Signal. 2016;28:861–870. doi: 10.1016/j.cellsig.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Yang G, Liang Y, Zheng T, Song R, Wang J, Shi H, Sun B, Xie C, Li Y, Han J. FCN2 inhibits epithelial-mesenchymal transition-induced metastasis of hepatocellular carcinoma via TGF-β/Smad signaling. Cancer Lett. 2016;378:80–86. doi: 10.1016/j.canlet.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, Ding Q, Sakoda M, Iino S, Ishigami S. Epithelial-mesenchymal transition and mesenchymal-epithelial transition via regulation of ZEB-1 and ZEB-2 expression in pancreatic cancer. J Surg Oncol. 2012;105:655–661. doi: 10.1002/jso.23020. [DOI] [PubMed] [Google Scholar]
- 25.Shen A, Lin W, Chen Y, Liu L, Chen H, Zhuang Q, Lin J, Sferra TJ, Peng J. Pien Tze Huang inhibits metastasis of human colorectal carcinoma cells via modulation of TGF-β1/ZEB/miR-200 signaling network. Int J Oncol. 2015;46:685–690. doi: 10.3892/ijo.2014.2772. [DOI] [PubMed] [Google Scholar]
- 26.Ohashi S, Natsuizaka M, Wong GS, Michaylira CZ, Grugan KD, Stairs DB, Kalabis J, Vega ME, Kalman RA, Nakagawa M. Epidermal growth factor receptor and mutant p53 expand an esophageal cellular subpopulation capable of epithelial-to-mesenchymal transition through ZEB transcription factors. Cancer Res. 2010;70:4174–4184. doi: 10.1158/0008-5472.CAN-09-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas C, Rajapaksa G, Nikolos F, Hao R, Katchy A, McCollum CW, Bondesson M, Quinlan P, Thompson A, Krishnamurthy S. ERbeta1 represses basal breast cancer epithelial to mesenchymal transition by destabilizing EGFR. Breast Cancer Res. 2012;14:R148. doi: 10.1186/bcr3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vennin C, Spruyt N, Dahmani F, Julien S, Bertucci F, Finetti P, Chassat T, Bourette RP, Le Bourhis X, Adriaenssens E. H19 non coding RNA-derived miR-675 enhances tumorigenesis and metastasis of breast cancer cells by downregulating c-Cbl and Cbl-b. Oncotarget. 2015;6:29209–29223. doi: 10.18632/oncotarget.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwak SY, Kim BY, Ahn HJ, Yoo JO, Kim J, Bae IH, Han YH. Ionizing radiation-inducible miR-30e promotes glioma cell invasion through EGFR stabilization by directly targeting CBL-B. FEBS J. 2015;282:1512–1525. doi: 10.1111/febs.13238. [DOI] [PubMed] [Google Scholar]