Abstract
Physical activity (PA) interventions constitute a critical component of cardiovascular disease (CVD) risk reduction programs. Objective mobile health (mHealth) software applications (apps) and wearable activity monitors (WAMs) can advance both assessment and integration of PA counseling in clinical settings and support community-based PA interventions. The use of mHealth technology for CVD risk reduction is promising, but integration into routine clinical care and population health management has proven challenging. The increasing diversity of available technologies and the lack of a comprehensive guiding framework are key barriers for standardizing data collection and integration. This paper reviews the validity, utility and feasibility of implementing mHealth technology in clinical settings and proposes an organizational framework to support PA assessment, counseling and referrals to community resources for CVD risk reduction interventions. This integration framework can be adapted to different clinical population needs. It should also be refined as technologies and regulations advance under an evolving health care system landscape in the United States and globally.
Keywords: Mobile health, Physical activity, Cardiovascular disease, Clinical counseling, Population health management
There have been numerous recommendations for incorporating clinical counseling on physical activity (PA) and referrals to community-based resources to support lifestyle change for cardiovascular disease (CVD) risk reduction.1–3 Simple screening tools have been advocated to facilitate evaluation, tracking and referrals to community-based PA programs. The clinical utility of these simple screening tools to identify inactive individuals and their integration feasibility has been supported in the literature.4,5 However, as with other self-report tools, their criterion validity is limited.6,7 More accurate, objective indicators of PA can help improve PA counseling, facilitate monitoring and enable more effective evaluation of outcomes.
Objective mobile health (mHealth) software applications (apps) and wearable activity monitors (WAMs) provide opportunities to advance both assessment and integration of PA counseling in clinical settings and support community-based PA interventions. There are close to 400 WAMs in the marketplace and an estimated 100,000 mHealth apps on iTunes and Google Play.8 A recent survey showed that 58% of smartphone users have downloaded a fitness or health app.9 According to the International Data Corporation (IDC), a total of 45.7 million “wearables” were sold in 2015, up 133% from the 19.6 million units in 2014. By 2019, total volumes are predicted to reach 126 million units.10 Despite the growing popularity and penetration of consumer-oriented mHealth apps and WAMs, few of them collect and report data in a manner consistent with evidence-based public health guidelines for aerobic and muscle-strengthening PA.11,12 The use of mHealth technology for CVD risk reduction has shown promise, but it has proven challenging to institutionalize its use in routine clinical care and population health management.13 There are a variety of reasons for this, but a key barrier is the lack of a comprehensive guiding framework. If mHealth integration is not performed properly, it could further burden overworked clinicians. This paper will review the validity, utility and feasibility of implementing mHealth technology in clinical settings and suggests an organizational framework to support PA assessment, counseling and interventions for CVD risk reduction.
Issues around integration of mHealth into the clinical care workflow
There are many issues and challenges that must be overcome to develop and implement mHealth technology in clinical settings. We searched MEDLINE, PubMed, Web of Science and World Wide Web from inception to December 2015 and selected relevant studies focused on mHealth in relationship to PA assessment, counseling and interventions for CVD risk reduction to include in this review. Specific considerations include privacy and security, validity, clinical utility, clinical integration and behavior change. Each of these topics are discussed below while Figs 1 and 2 present a framework for the meaningful integration of mHealth technology to guide CVD risk reduction interventions in health care settings and population health management. Suggested roles to ensure meaningful mHealth data acquisition, use and integration for the different stakeholders involved in the proposed framework are presented in Table 1.
Fig 1.
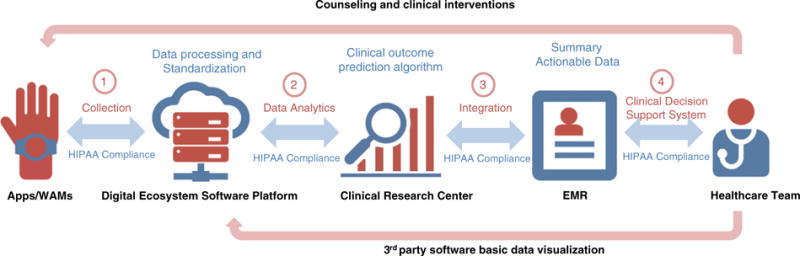
mHealth Integration Framework. The proposed model provides a framework for collecting valuable physical activity (PA) data from mobile health (mHealth) applications (apps) and wearable activity monitors (WAMs) to be analyzed for meaningful use and summarized into actionable metrics to guide PA assessment and counseling in healthcare settings. The communication between each entity is compliant with HIPAA/HITECH regulations to assure privacy and security of healthcare data. The first step of the model is the collection of real-time PA data from the patient through apps and WAMs. The data will be downloaded, processed and standardized by the proposed digital ecosystem platform. The data analytic provides a standardized format to integrate key summary data into the electronic medical record (EMR) or make it available to healthcare providers via 3rd party software. The integration step has multi-dimensional purposes, which can be specified by the healthcare team according to the clinical need and specialty. Data visualization needs to provide meaningful use and actionable metrics to guide patient care.
Fig 2.
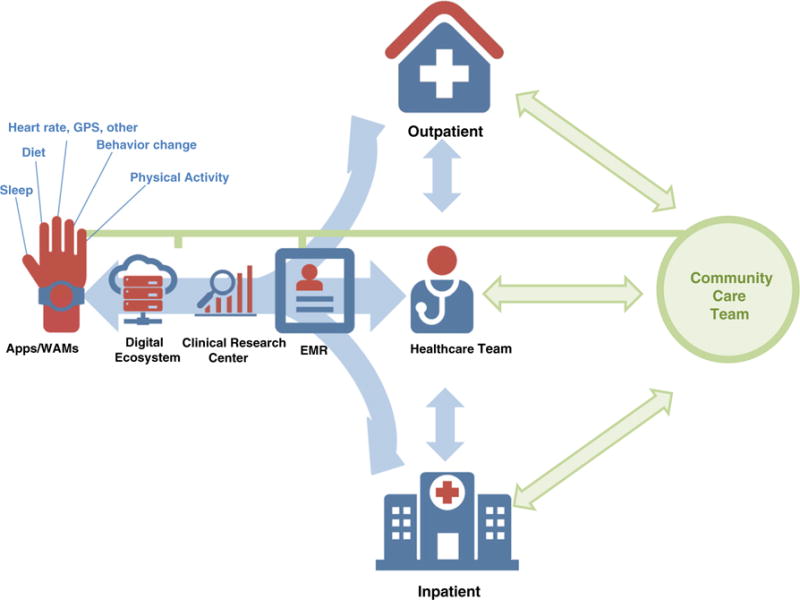
Case study—inpatient vs. outpatient models. The following case study presents the integration of mobile health (mHealth) data into the inpatient and outpatient settings. The initial steps for data collection and analytics are similar to those in Fig 1 for the proposed mHealth integration model. The summarized and processed data can be provided for different stakeholders in the clinical or community care team based on pre-specified use-case scenarios. For example, the inpatient setting will require short term monitoring of patient’s state by collecting data on physical activity (PA) to implement acute interventions for faster recovery and improvement [i.e., integrating early ambulation markers into early recovery after surgery (ERAS) protocols for improved outcomes]. The data are specified and analyzed based on the patient’s clinical status and PA needs. Once the patient is discharged, the outpatient provider can continue to monitor PA and health status of the patient through the integrated mHealth system. For prevention-oriented outpatient care (preventive cardiology, lifestyle medicine, and primary care clinics) the mHealth integration model follows the same initial flow. In this model, after the healthcare provider initiates PA assessment and counseling, a referral to the community care team (i.e., certified fitness professionals) is recommended. These teams deliver evidence-based interventions and work closely with each patient or small groups of patients based on their clinical needs and goals. However, the challenge is to track and analyze not only PA data but also behavioral change precursors that will lead to the adoption of improved PA and lifestyle and reduced CVD risk. The community care team is able to use mHealth data for increased engagement and real-time monitoring in order to implement clinician’s recommendations and relay summary behavior change outcome data as the clinician follows-up with the patient.
Table 1.
Suggested roles for different stakeholders to improve implementation and integration of physical activity data via mHealth technology to support CDV risk reduction.
| Stakeholders | Security/Privacy | Clinical Utility | Integration into Clinical and Community Care Workflow | EMR Integration |
|---|---|---|---|---|
| Clinicians/Community care team | HIPAA/HITECH compliance | Provide guidance and support to patients to adopt mHealth technology | Utilize data to guide assessment, counseling, interventions and follow-up | Collaborate on developing detailed use-case scenarios for mHealth integration and data use; data access must be approved by patient |
| Health systems | As above | Establish data analysis processes as required to ensure clinically actionable data | Collaborate on developing detailed use-case scenarios for mHealth integration and data use, plus implement customized approaches for different clinical departments and community care referral networks | Support integration efforts once summary actionable data is proven clinically useful to improve patient care, customized for different clinical departments and community care referral networks |
| Researchers | As above | Rapid assessment of validity and efficacy of mHealth technologies | Collaborate on developing detailed use-case scenarios for mHealth integration and data use; assess real-life effectiveness of mHealth technologies | Evaluate integration efforts |
| App/WAM developers | As above, plus FDA evaluation if apps/WAMs are classified as regulated mobile medical devices | mHealth technology must be valid, reliable; include evidence-based behavior change strategies | Include data collection/reporting features consistent with evidence-based PA recommendations | Open API available and constantly updated to enable integration efforts |
| Digital health ecosystem platforms | As above | Ensure a secure and simple to use platform for data download and standardization | Collaborate with researchers, clinicians and health systems to ensure appropriate analytics and data summarization protocols are in place to make data actionable and feasible for integration | Integration through secure and private network and platform |
| EMR platforms | As above | N/A | Data analytics and compliant for clinical use | Collaborate with app/WAM developers, digital health ecosystem platforms and health systems to streamline integration efforts of key summary actionable data |
| Regulators | Surveillance of HIPAA/HITECH compliance | HIPAA compliance certification of apps/WAMs if used in clinical practice | Assure patient privacy and secure use of data within clinical and research content, as approved by patient | HIPAA compliance |
Abbreviations: mHealth: mobile health; EMR: electronic medical records; App: software applications; WAM: wearable activity monitors; HIPAA: Health Insurance Portability and Accountability Act; HITECH: Health Information Technology for Economic and Clinical Health Act.
Privacy and security of mHealth technology
Mobile health technology has shown the potential to optimize the management of CVD and other chronic diseases by empowering patients through better health self-monitoring and education.14–17 However, several challenges are present when patient’s data are utilized and monitored in the healthcare setting. A common issue is the privacy and security of health information. A robust framework and guidelines are already established and utilized in software development for electronic medical record (EMR) systems.18 For example, the Health Insurance Portability and Accountability Act (HIPAA) guidelines need to be observed when handling healthcare data. HIPAA provides patients with rights to access health information and restrict its use and disclosures. In addition, the Health Information Technology for Economic and Clinical Health (HITECH) act was created to increase the scope of privacy and security protections under HIPAA and to enforce legal liability for non-compliance.19
The HIPAA and HITECH regulations are typically not applicable to consumer-oriented mHealth apps for self-monitoring.20 HIPAA compliance is necessary only if protected health information that identifies individuals in healthcare settings is used. Recently some mHealth software platforms designed for data aggregation and analytics have implemented steps to de-identify health information and therefore are typically not considered to be subject to HIPAA rules.21,22
Some research and consumer-oriented WAMs or mHealth apps may not be HIPAA/HITECH compliant unless the technology is designed for the physician or healthcare organization to directly receive the information.20,23 However, mHealth apps and WAMs can still be subject to the Federal Trade Commission (FTC) jurisdiction regarding access and storage of information on devices.24 The FTC Act provides the commission the authority to monitor and enforce its regulation on products marketed to consumers. This also applies to developers and manufacturers to assure compliance and avoid unfair practices or deceptive information about the device or product. The agency also provides safety regulations to prevent harm toward consumers that could potentially be caused by mHealth apps and WAMs.24,25 Finally, the Federal Drug Administration (FDA) also plays a role in regulating equipment or software used in treating or diagnosing diseases. Devices, including software, classified under this “medical category” are subject to FDA regulations included in the FDA Safety and Innovation Act. Developers of FDA-approved devices are required to provide registration, manufacturing and surveillance practices and typically have a proven record of safety and effectiveness.26
Validity of wearable activity monitors
An advantage of sensors and WAMs is that they provide an objective estimate of PA. The technology to monitor PA with accelerometry-based devices has been available for 25 years, but the explosion of consumer-oriented WAMs is a relatively new phenomenon. A key to the popularity of these devices has been their ability to directly link data to Web and mobile technology to provide direct access to PA patterns as well as estimates of sleep and energy expenditure. Surprisingly, these devices are released with little consideration for the accuracy of the estimates. An evaluation of popular WAMs revealed error rates ranging from 17 to 18%. However, a follow-up study demonstrated that the error rates were influenced to a large degree on a cancellation of over and underestimation from various activities.27,28 Another study actually validated the accuracy of ten popular PA apps and WAMs showing an overall similar tracking step count. However the difference was largely noticed in WAMs compared to apps.29 WAMs were also inaccurate in categorizing PA data during a full 24-hour day as shown in another study of 9 devices.30 These challenges are not unique to consumer-oriented WAMs as their overall accuracy appears to be on par with some established research-grade monitors. However, the error is considerable and points to the need for continued refinement. A challenge is that research has demonstrated that different strategies may be needed to assess free living PA and to distinguish sedentary behavior.31 Different methods and algorithms are also needed to process and interpret accelerometer data when placed on different locations such as the waist and hip.32,33 A recent study demonstrated potential for detecting accelerometer location and this may facilitate the development of more agnostic methods for wearable sensor applications.34
In recent years, there has been a movement toward the processing of “raw” accelerometer data within smartphone apps and WAMs. This offers advantages for the use of new PA pattern recognition approaches for activity classification that rely on high sampling rates, as well as implementation of low-power, dedicated “motion chips” to minimize impact on the device’s battery life.35,36 Standardization of the output PA measures from the various accelerometry-based WAMs has been a goal within the research community for years, but would be particularly important for broader adoption in clinical mHealth programs. The adoption of standardized input and output variables would also facilitate integration with EMR systems and associated cloud-based applications needed for clinical integration. Standardized methods may also aid clinician and patient acceptance, as well as the privacy/security considerations described earlier. Organizations, such as Shimmer or openmhealth.org,37,38 can help this effort by promoting guideline-based mHealth data schemas and developing tools for data aggregation and visualizing within the clinical workflow. It is expected that the accuracy of WAMs will continue to improve and that there will eventually be a hybridization between consumer- and research-grade monitors to enable improved accuracy and functionality. Adoption and utilization will increase dramatically when clinicians can trust sensor data just as they trust biomarker data from standardized labs. Utility and integration issues are discussed in the next sections.
Clinical utility
A critical challenge for integrating mHealth apps and WAMs to assess PA as part of routine care is the applicability of such data for clinical decision-making. Several studies in the areas of smoking cessation,39 diabetes40 and weight loss41 have shown a missing link between evidence-based clinical guidelines and mHealth apps and WAMs. This poses a critical issue regarding the meaningful use of integrating PA data generated by mHealth technology to guide behavioral change and lifestyle counseling for CVD risk reduction.42,43 If PA data are not collected, analyzed and reported in a manner that is consistent with these guidelines, then clinicians and community care professionals delivering PA counseling and interventions will not be able to ascertain the primary outcome: progress toward meeting the federal PA guidelines for health.44,45 This is particularly important as the optimal dose (volume, intensity, duration) of PA varies for different CVD risk factors and cardiometabolic disease endpoints.46–48 In addition, the PA prescription and interventions will vary according to patient intrinsic factors such as age, medications, and baseline functional and fitness status.49–51
Another consideration for clinical utility is whether the WAMs or apps can provide appropriate feedback and guidance to facilitate behavior change. To systematically reduce CVD risk factors, it is essential to help patients change the underlying contributing lifestyle behaviors. A review of common mHealth apps for tracking and promoting PA revealed that on average they include only 5 out of 23 behavior change techniques, such as performance feedback, self-monitoring, social support, rewards and specific goal setting.52 Another study revealed that paid apps were significantly associated with provision of behavior change techniques and evidence-based interventions compared to free ones.53 In addition, a behavior theory content survey, including 20 interventions shared by four key behavioral models/theories (health belief model, theory of planned behavior, transtheoretical model, and social cognitive theory) was recently used to rate 14 apps for type 2 diabetes self-management. Each app scored less than 50% of the total possible score for all four behavioral theories.54 These studies underscore the general consensus in the literature that current mHealth apps and WAMs are not likely to be effective unless accompanied by provision of face-to-face or online coaching and/or counseling by personnel with behavior change expertise. Better integration of behavior change theory features and data collection in mHealth apps and WAMs would address this gap and directly facilitate clinical PA behavior change programming.
EMR and clinical workflow integration
Another barrier for the adoption of mHealth apps and WAMs is the lack of integration of PA data into EMRs, clinical workflow and referrals to the community care team to support lifestyle interventions. These integrated software platforms will be bound by HIPAA and HITECH rules since patient health information would be directly incorporated into the EMR. Security is also a major issue for mHealth apps as they are used on portable devices that may have increased risk for data breach by unauthorized entities. Encryption and authentication of mHealth apps and WAMs to be used in clinical settings are security measures that need to be widely implemented to avoid unauthorized access.55,56 An additional consideration is the hacking risk to a healthcare organization when their Web services (method of communication between mHealth devices over a network) are widely exposed to enable mHealth application integration. These concerns should be addressed and followed by the healthcare system’s IT departments in coordination with EMR software developers.
Several frameworks have been proposed but there is currently no standard platform to integrate objective PA data from mHealth apps or WAMs directly into EMRs.57 This is probably influenced by the limited number of them that meet validity, security, privacy, and clinical utility and feasibility standards and are therefore good candidates for EMR and clinical workflow integration. Larger technology companies are better positioned to work on these integration efforts given their advanced resources.58–60 For example, one of the largest health care EMR software companies, Epic, has started integrating Apple’s HealthKit.61 This EMR integration will allow healthcare providers to review objective PA and additional data collected through iPhones, Apple Watch, and other apps/WAMs that link to the Apple HealthKit ecosystem, to support clinical counseling and monitor lifestyle interventions. Other health systems will begin integrating HealthKit data into their Cerner EMR in 2015.62 However, closed approaches that may only benefit patients with access to selected mHealth apps/WAMs, in selected health systems, running on selected EMRs, have the potential to perpetuate health disparities and the “digital access” divide.63,64 There is a real potential for mHealth technology to accentuate, rather than close, health behavior gaps between higher income, more educated populations who already engage in many positive health behaviors and lower income and education populations that engage in less PA, smoke more, have poorer diets, and utilize preventive health services less frequently. Therefore, special emphasis should be placed on the design and integration of mHealth technology to support behavior change in at-risk and vulnerable populations.
A framework for improving integration is essential, ideally using open application program interfaces (APIs) that are compliant with privacy and security regulations. It is also critical to establish the adequate amount of biometric, PA and behavior change data to integrate into the EMR and clinical/community care intervention workflows. First, protocols for app- and WAM-generated data extraction and cleaning need to be standardized (normalizing units of measure, time-stamping, ascertainment of non-wear time, etc.). Most consumer-oriented apps and WAMs use a “black-box” algorithm approach for basic data analytics and thresholds to define activity type, volume and intensity, which they deem “proprietary”. Therefore validation studies against reference research-grade monitors are recommended and critical to guide integration efforts. Furthermore, data output and visualization approaches vary widely and are tailored more toward the consumer than the care team. Therefore, for purposes of clinical integration, a basic analytic step is often required to process the data and present, at a minimum, metrics to ascertain compliance with Federal PA guidelines for CVD risk reduction (i.e., minutes of moderate-to-vigorous PA per week). Other metrics, such as steps, distance, and resting heart rate can become secondary information.44 Amounts of light PA versus sedentary time, while considered important, also require more thorough validation and integration into PA guidelines. If apps and WAMs are not offering this type of clinically actionable data, then it is recommended that they at least enable raw data download as part of their open API, for appropriate stakeholders to conduct basic analytics to derive the desired metrics.
An alternative approach is to bypass direct integration of mHealth app and WAM data into EMRs and instead “extract” the data via separate and secured, cloud-based software applications for data standardization, basic analytics and summarization that can then be accessed by clinicians and the community care team (i.e., certified fitness professionals delivering PA programs). WAM data and subjective assessments from patients tend to generate high volume, high variety and high velocity data that are likely to overcome traditional relational database management systems (RDMS) and require newer technologies and “big-data” analytic frameworks to separate meaningful clinical data from “noise”. Once analyzed, appropriate follow-up metrics can be integrated into EMRs, either manually by care coordinators or other office personnel, or via customized API integration efforts undertaken by health care systems in coordination with EMR companies. These digital health ecosystems serve several purposes—they help standardize the integration of normalized, actionable data from various consumer-oriented apps and WAMs into the EMR and clinical workflow. This framework can enable health systems and clinicians to integrate valuable objective PA data without interfering with the patient’s app or WAM preference, essentially a bring your own device (B.Y.O.D.) approach. It also simplifies the amount of work IT departments in health systems and clinicians/community care providers have to devote to data download and standardization while streamlining further data analytics and integration efforts.21,22 Fig 1 presents a model for the meaningful integration of mHealth data to guide CVD risk reduction interventions for clinical and population health management settings.
Behavior change programming
Systems and structures for behavior change programming are essential to fully realize the potential of mHealth technology. While coaching and brief counseling can be provided directly in the clinical setting, there are also options for referral to community care providers that are well-positioned to provide behavior change programming.65 It is recommended that health systems looking to integrate objective PA data into clinical workflow and EMRs develop comprehensive use-case scenarios as a blueprint to guide integration efforts. These use-case scenarios should include details regarding the type of data needed to support care processes, clinical and community-based personnel (clinical, patient coordination, navigation, community referral networks) and standardized interventions that are already in place or need to be established to best utilize the PA information to be collected/integrated. These use-case scenarios should also be specific for different clinical populations (e.g., CVD prevention clinic, early mobility after cardiac surgery, cardiac rehabilitation programs, primary care, pediatric obesity clinic), since the type of raw data, analytics and integration needs will be different for all these subpopulations. Fig 2 presents a summary case study for the integration of mHealth data for inpatient vs. outpatient settings.
Although basic, actionable metrics to ascertain compliance with PA recommendations should be the center of integration efforts, more detailed analytics are often needed to identify factors associated with specific CVD risk reduction outcomes in specific clinical sub-populations and to develop predictive algorithms to guide interventions. This process requires pulling together different sources of data (WAMs, apps, health risk assessments, patient surveys, intervention attendance, face-to-face and virtual engagement, behavior change, biometric and other clinical data in the EMR or the health system’s clinical data warehouse) to effectively guide community-based programming and clinical care. However, as important as data are, the availability of too many data points would likely be overwhelming for both patients and the care team, especially if they are not actionable or lack predictive validity for CVD and behavior change outcomes. Therefore, summary metrics need to be presented with sufficient granularity and specificity to be actionable and guide PA counseling and referrals to community-based interventions.
The Center for Medicare and Medicaid Services (CMS) established incentive payments for eligible healthcare providers and health systems that utilize digital health information technology (HIT). There has been an acceleration of HIT implementation in the United States with the passage of the American Recovery and Reinvestment Act (ARRA), the HITECH framework and the Patient Protection and Affordable Care Act of 2010 (ACA),66,67 which all encourage the use and adoption of HIPAA-compliant HIT, EMRs and medical technology to improve and monitor quality and efficiency in health care. HITECH established the framework for the implementation of HIT and meaningful use criteria. Medicaid- or Medicare-eligible professionals must demonstrate meaningful use to receive financial incentives by meeting certain patient volume and guidelines established by CMS.68,69
As the move from volume to value-based care accelerates, there will be greater need for implementation of clinical-community linkages to deliver evidence-based CVD prevention and management interventions. These links will require, and benefit from, integrated PA monitoring frameworks to provide objective data on outcomes change and improved patient engagement and follow-up. In this scenario apps and WAMs provide an opportunity to screen for inactivity and guide interventions among patients with CVD risk factors, as the U.S. Preventive Services Task Force (USPSTF) recommends (Grade B) provision of intensive lifestyle and behavioral interventions on PA and diet for these patients.70 In addition, the proposed CMS guidance for Stage 3 Meaningful Use specifically indicates “Patient-generated health data or data from a non-clinical setting be incorporated into the certified EHR technology for more than 15 percent of all unique patients seen by the provider or discharged by the eligible hospital or inpatient or emergency department during the EHR reporting period” as part of the measures to document patient engagement.71,72
Evidence on the use of mHealth technology and CVD risk reduction
Several interventions have shown promising results in promoting PA through digital tracking and personalized feedback.13,17,73–75 The mActive study used real-time smartphone PA tracking and an automated text-based intervention, showing increased steps/day among patients enrolled in the texting feedback system compared to those without the texting component.76 The SMART MOVE randomized trial revealed significant increases in PA with the implementation of a mobile application, the Accupedo-Pro Pedometer app, in primary care settings. This app provided tracking of steps and feedback in addition to visualizing targeted goals and information on PA benefits.77 Another study showed promising results in weight loss and increased PA after a 12-week mHealth intervention addressing healthy lifestyle changes.74 Similar findings were observed in the (TEXT ME) trial that showed the positive impact of lifestyle-focused text messaging on the reduction of blood pressure, smoking and cholesterol in patients with coronary heart disease.78 In another study, the SenseWear armband was used to accurately track daily energy expenditure and sleep in order to provide meaningful lifestyle changes. The intervention was effective in promoting weight loss in overweight and obese patients.79 A study also supported the advantages of using WAMs to self-monitor health over long periods of time for the prevention of CVD.80 Similar findings were observed in populations new to smartphone technology with an intervention focused on promoting PA in elderly patients through different motivational and behavioral methods. Positive results were noticed after 8 weeks in the majority of the participants.81 Sustained weight and hemoglobin A1c reduction after 2 years were also shown among seniors utilizing Omada Health’s online Diabetes Prevention Program.82
The growing number of published studies summarizing the results of mHealth interventions has facilitated new literature reviews on this topic. A recent review showed promising results for mHealth apps to reduce CVD risk factors by promoting lifestyle changes including smoking cessation, diet and PA.73 Another review suggested that WAMs are associated with increased PA by an estimated 26% over baseline, leading to decreased blood pressure and weight.83 Furthermore, the latest scientific statement by the American Health Association indicated that multiple mHealth studies have shown a positive impact on CVD prevention through promotion of PA and healthy diet. However, the majority of the studies included in these reviews were based on text messaging services or Web-based methods as their mHealth intervention, with only a few studies incorporating newer smartphone apps or WAMs.(13,84,85 Given the delay from initial study design to ultimate publication, this constitutes another challenge for the scientific validation of ever-evolving mHealth technologies. As the number of studies accumulates, future systematic reviews and meta-analysis of mHealth interventions using apps and WAMs are warranted.
Future trends in objective PA monitoring
The future of WAM devices is progressing, with advances in simplicity, reliability and automation. Devices will continue to become smaller and more seamlessly integrated into jewelry and clothing, facilitating continuous tracking of PA patterns and other biometric and health outcomes. One direction is for “smart clothing” that can integrate data collection and analysis. AiQ clothing and OMsignal are some of the companies producing biometric garments with the ability to measure vital signs, PA and energy expenditure.86,87 A company called Sensoria introduced smart socks infused with pressure sensors for real-time PA tracking while monitoring foot-landing technique during running.88 Another company, Citiyzen Sciences specializes in smart fabrics with embedded sensors with the ability to monitor PA, heart rate, temperature and location.89 Another direction of design is personalizing WAMs to increase adherence and compliance. For example startups such as Misfit which was recently acquired by the Fossil fashion group, incorporates its WAM technology into jewelry and accessories, while other companies like MOTA introduced a smart ring with the ability to track PA, plus social media and texts.90,91 A movement toward developing invisible and implantable wearables is also evident. A new concept by NewDealDesign is called “Project Underskin”, a sub-dermal tattoo with the ability to track PA and interact with the environment.92
In addition to these innovations in design for form, developers will aim to enhance the security and user experience for the consumers. The focus will be on allowing the consumer to have control over the distribution and integration of the collected data. The user will be informed and have more options for how the information is used and shared. Devices will also provide additional functionality with data on heart rate, temperature and stress/mood status available (with enhanced accuracy) by using GPS tracking and advanced sensors.93,94 The increased personal use and availability of drones equipped with high-definition cameras and other sensors that can follow individuals while doing different activities might also offer opportunities for tracking individual and population-based PA patterns as regulations are refined.95
Conclusion and recommendations
Mobile health is a valuable technology that can help implement clinical guidelines and recommended behavior change strategies to improve the quality of care and health status of patients with CVD risk.1 The rise and rapid evolution of mHealth technology has the potential to guide personalized medicine interventions and strengthen the relationship between patients and healthcare providers, increasing engagement and compliance with healthy lifestyle changes.
Multiple PA stakeholders, including healthcare and community providers, researchers, software developers, digital health ecosystems, EMRs and regulators, have important roles in ensuring meaningful mHealth data acquisition, use and integration (Table 1). We recommend these stakeholders work collaboratively to address privacy/security concerns and standardize frameworks to ensure reliability, validity and utility for PA promotion and CVD risk reduction applications in clinical and community settings, as well as population health management and public health advancement. App- and WAM-development teams should consistently include healthcare providers, plus behavior change and PA experts, to provide better context and functionality to improve clinical applicability of mHealth technology. Researchers can help standardize and advance this field by performing rapid validation, efficacy and effectiveness studies, while also exploring feasibility, adoption and patient-centered outcomes. Moving forward, it will be important for mHealth developers and integration efforts to ensure compliance with HIPAA/HITECH legislation, to assure health information privacy. The patient should also be allowed the option to release or withhold specific mHealth data within the integration system. Secure and HIPPA-compliant digital health ecosystems can help simplify data extraction from a growing list of medical devices, from clinical-grade to consumer-oriented mHealth apps and WAMs, and offer standardized data for the additional analytics required to identify critical summary metrics and make this information actionable for patients, clinicians and community care professionals. This integration framework can be adapted to different clinical population needs. It should also be refined as technologies and regulations advance under an evolving health care system landscape in the United States and globally.
Abbreviations and Acronyms
- Apps
applications
- CMS
Center for Medicare and Medicaid services
- CVD
cardiovascular disease
- FTC
Federal Trade Commission
- HIPAA
Health Insurance Portability and Accountability Act
- HITECH
Health Information Technology for Economic and Clinical Health
- IDC
International Data Corporation
- EMR
electronic medical record
- Mhealth
mobile health
- PA
physical activity
- USPSTF
U.S. Preventive Services Task Force
- WAMS
wearable activity monitors
Footnotes
Statement of conflicts of interest/disclosures
Dr. Lobelo is on the advisory board of the American College of Sports Medicine’ Exercise is Medicine initiative; Dr. McConnell is currently on leave from Stanford and employed by Verily Life Sciences.
References
- 1.LeFevre ML, U.S. Preventive Services Task Force Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2014;161(8):587–593. doi: 10.7326/M14-1796. [DOI] [PubMed] [Google Scholar]
- 2.Sallis R, Franklin B, Joy L, et al. Strategies for promoting physical activity in clinical practice. Prog Cardiovasc Dis. 2015;57(4):375–386. doi: 10.1016/j.pcad.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Arena R, Harrington RA, Després J-P. A message from modern-day healthcare to physical activity and fitness: welcome home! Prog Cardiovasc Dis. 2015;57(4):293–295. doi: 10.1016/j.pcad.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44(11):2071–2076. doi: 10.1249/MSS.0b013e3182630ec1. [DOI] [PubMed] [Google Scholar]
- 5.Grant RW, Schmittdiel JA, Neugebauer RS, et al. Exercise as a vital sign: a quasi-experimental analysis of a health system intervention to collect patient-reported exercise levels. J Gen Intern Med. 2014;29(2):341–348. doi: 10.1007/s11606-013-2693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball TJ, Joy EA, Goh TL, et al. Validity of two brief primary care physical activity questionnaires with accelerometry in clinic staff. Prim Health Care Res Dev. 2015;16(01):100–108. doi: 10.1017/S1463423613000479. [DOI] [PubMed] [Google Scholar]
- 7.Helmerhorst HJ, Brage S, Warren J, et al. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int J Behau Nutr Phys Act. 2012;9:103. doi: 10.1186/1479-5868-9-103. [1–55] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wearable Tech market. [cited 2015 5 November]; Available from: http://vandrico.com/wearables2015.
- 9.Krebs P, Duncan DT. Health App Use Among US Mobile Phone Owners: a national survey. JMIR Mhealth Uhealth. 2015;3(4):e101. doi: 10.2196/mhealth.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liamas R. Worldwide wearables market forecast to grow 173.3% in 2015 with 72.1 million units to be shipped, according to IDC. 2015 [cited 2015 11 November]; Available from: http://www.idc.com/getdoc.jsp?containerId=prUS25696715.
- 11.mHealth App Developer Economics. 2014 [cited 2015 28 October]; Available from: http://mhealtheconomics.com/mhealth-developer-economics-report/2014.
- 12.Knight E, Stuckey MI, Prapavessis H, et al. Public health guidelines for physical activity: is there an app for that? A review of android and apple app stores. JMIR Mhealth Uhealth. 2015;3(2):e43. doi: 10.2196/mhealth.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke LE, Ma J, Azar KM, et al. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2015;132(12):1157–1213. doi: 10.1161/CIR.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Gayar O, Timsina P, Nawar N, et al. Mobile applications for diabetes self-management: status and potential. J Diabetes Sci Technol. 2013;7(1):247–262. doi: 10.1177/193229681300700130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurling R, Catt M, Boni MD, et al. Using internet and mobile phone technology to deliver an automated physical activity program: randomized controlled trial. J Med Internet Res. 2007;9(2):e7. doi: 10.2196/jmir.9.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cadmus-Bertram LA, Marcus BH, Patterson RE, et al. Randomized trial of a Fitbit-based physical activity intervention for women. Am J Prev Med. 2015;49(3):414–418. doi: 10.1016/j.amepre.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner-McGrievy GM, Beets MW, Moore JB, et al. Comparison of traditional versus mobile app self-monitoring of physical activity and dietary intake among overweight adults participating in an mHealth weight loss program. J Am Med Inform Assoc. 2013;20(3):513–518. doi: 10.1136/amiajnl-2012-001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Office of the National Coordinator for Health Information Technology. Guide to privacy and security of electronic health information. [cited 2015 19 October]; Available from: https://www.healthit.gov/providers-professionals/guide-privacy-and-security-electronic-health-information2015.
- 19.U.S. Department of Health and Human Services. HITECH Act Enforcement Interim Final Rule. [cited 2015 10 October]; Available from: http://www.hhs.gov/ocr/privacy/hipaa/administrative/enforcementrule/hitechenforcementifr.html2009.
- 20.Martinez-Perez B, de la Torre-Diez I, Lopez-Coronado M. Privacy and security in mobile health apps: a review and recommendations. J Med Syst. 2015;39(1):181. doi: 10.1007/s10916-014-0181-3. [DOI] [PubMed] [Google Scholar]
- 21.The road ahead in connected health. [cited 2015 29 October]; Available from: https://validic.com2015.
- 22.Tactio Health Group. [cited 2015 9 November]; Available from: http://www.tactiohealth.com/2015.
- 23.Yang YT, Silverman RD. Mobile health applications: the patchwork of legal and liability issues suggests strategies to improve oversight. Health Aff. 2014;33(2):222–227. doi: 10.1377/hlthaff.2013.0958. [DOI] [PubMed] [Google Scholar]
- 24.Federal Trade Commission. Complying with the FTC’s Health Breach Notification Rule. [cited 2015 12 October]; Available from: https://www.ftc.gov/tips-advice/business-center/guidance/complying-ftcs-health-breach-notification-rule2010.
- 25.mHealth Laws and Regulations. [cited 2015 29 October]; Available from: http://cchpca.org/mhealth-laws-and-regulations2014.
- 26.Food and Drug Administration Safety and Innovation Act (FDASIA) [cited 2015 29 October]; Available from: http://www.fda.gov/RegulatoryInformation/Legislation/SignificantAmendmentstotheFDCAct/FDASIA/ucm20027187.htm2012.
- 27.Bai Y, Welk GJ, Nam YH, et al. Comparison of consumer and research monitors under semistructured settings. Med Sci Sports Exerc. 2015 doi: 10.1249/MSS.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Kim Y, Welk GJ. Validity of consumer-based physical activity monitors. Med Sci Sports Exerc. 2014;46(9):1840–1848. doi: 10.1249/MSS.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 29.Case MA, Burwick HA, Volpp KG, et al. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313(6):625–626. doi: 10.1001/jama.2014.17841. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberger ME, Buman MP, Haskell WL, et al. 24 hours of sleep, sedentary behavior, and physical activity with nine wearable devices. Med Sci Sports Exerc. 2016;48(3):457–465. doi: 10.1249/MSS.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y, Welk GJ. Criterion validity of competing accelerometry-based activity monitoring devices. Med Sci Sports Exerc. 2015;47(11):2456–2463. doi: 10.1249/MSS.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 32.Rowlands AV, Yates T, Olds TS, et al. Sedentary sphere: wrist-worn accelerometer-brand independent posture classification. Med Sci Sports Exerc. 2015 Nov 10; doi: 10.1249/MSS.0000000000000813. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Kim Y, Lee JM, Peters BP, et al. Examination of different accelerometer cut-points for assessing sedentary behaviors in children. PLoS One. 2014;9(4):e90630. doi: 10.1371/journal.pone.0090630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannini A, Sabatini AM, Intille SS. Accelerometry-based recognition of the placement sites of a wearable sensor. Pervasive Mob Comput. 2015;21:62–74. doi: 10.1016/j.pmcj.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai J, He B, Shou H, et al. Normalization and extraction of interpretable metrics from raw accelerometry data. Biostatistics. 2013:kxt029. doi: 10.1093/biostatistics/kxt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vähä-Ypyä H, Vasankari T, Husu P, et al. Validation of cut-points for evaluating the intensity of physical activity with accelerometry-based mean amplitude deviation (MAD) PLoS One. 2015;10(8):e0134813. doi: 10.1371/journal.pone.0134813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimmer. [cited 2015 December]; Available from: http://www.getshimmer.co/2015.
- 38.Open mHealth. [cited 2015 1 December]; Available from: http://www.openmhealth.org/2015.
- 39.Abroms LC, Lee Westmaas J, Bontemps-Jones J, et al. A content analysis of popular smartphone apps for smoking cessation. Am J Prev Med. 2013;45(6):732–736. doi: 10.1016/j.amepre.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chomutare T, Fernandez-Luque L, Arsand E, et al. Features of mobile diabetes applications: review of the literature and analysis of current applications compared against evidence-based guidelines. J Med Internet Res. 2011;13(3):e65. doi: 10.2196/jmir.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagoto S, Schneider K, Jojic M, et al. Evidence-based strategies in weight-loss mobile apps. Am J Prev Med. 2013;45(5):576–582. doi: 10.1016/j.amepre.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 42.Cowan LT, Van Wagenen SA, Brown BA, et al. Apps of steel: are exercise apps providing consumers with realistic expectations?: a content analysis of exercise apps for presence of behavior change theory. Health Educ Behav. 2013;40(2):133–139. doi: 10.1177/1090198112452126. [DOI] [PubMed] [Google Scholar]
- 43.Payne HE, Lister C, West JH, et al. Behavioral functionality of mobile apps in health interventions: a systematic review of the literature. JMIR Mhealth Uhealth. 2015;3(1):e20. doi: 10.2196/mhealth.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2008 [cited 2015 10 October]; Available from: http://www.health.gov/paguidelines2015.
- 45.Pratt M, Perez LG, Goenka S, et al. Can population levels of physical activity be increased? Global evidence and experience. Prog Cardiovasc Dis. 2015;57(4):356–367. doi: 10.1016/j.pcad.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med. 2009;43(1):1–2. [PubMed] [Google Scholar]
- 47.Sattelmair J, Pertman J, Ding EL, et al. Dose response between physical activity and risk of coronary heart disease a meta-analysis. Circulation. 2011;124(7):789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeFina LF, Haskell WL, Willis BL, et al. Physical activity versus cardiorespiratory fitness: two (partly) distinct components of cardiovascular health? Prog Cardiovasc Dis. 2015;57(4):324–329. doi: 10.1016/j.pcad.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25_PA):2960–2984. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Myers J, McAuley P, Lavie CJ, et al. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57(4):306–314. doi: 10.1016/j.pcad.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Carlson SA, Fulton JE, Pratt M, et al. Inadequate physical activity and health care expenditures in the United States. Prog Cardiovasc Dis. 2015;57(4):315–323. doi: 10.1016/j.pcad.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Middelweerd A, Mollee JS, van der Wal CN, et al. Apps to promote physical activity among adults: a review and content analysis. Int J Behav Nutr Phys Act. 2014;11:97. doi: 10.1186/s12966-014-0097-9. [1–9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Direito A, Dale LP, Shields E, et al. Do physical activity and dietary smartphone applications incorporate evidence-based behaviour change techniques? BMC Public Health. 2014;14:646. doi: 10.1186/1471-2458-14-646. [1–7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hale K, Capra S, Bauer J. A framework to assist health professionals in recommending high-quality apps for supporting chronic disease self-management: illustrative assessment of type 2 diabetes apps. JMIR Mhealth Uhealth. 2015;3(3):e87. doi: 10.2196/mhealth.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arora S, Yttri J, Nilse W. Privacy and security in mobile health (mHealth) research. Alcohol Res. 2014;36(1):143–151. doi: 10.35946/arcr.v36.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He D, Naveed M, Gunter CA, et al. Security concerns in android mHealth apps. AMIA Annu Symp Proc. 2014;2014:645–654. [PMC free article] [PubMed] [Google Scholar]
- 57.Marceglia S, Fontelo P, Rossi E, et al. A standards-based architecture proposal for integrating patient mHealth apps to electronic health record systems. Appl Clin Inform. 2015;6(3):488–505. doi: 10.4338/ACI-2014-12-RA-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Apple Health Kit. [cited 2015 18 November]; Available from: https://developer.apple.com/healthkit/2015.
- 59.Samsung S Health. [cited 2015 18 November]; Available from: http://shealth.samsung.com/2015.
- 60.Google Fit. [cited 2015 18 November]; Available from: https://fit.google.com2015.
- 61.Versel N. Apple’s HealthKit Connects With Mayo and Epic, but don’t call it revolutionary. [cited 2015 10 October]; Available from: http://www.forbes.com/sites/neilversel/2014/06/03/apples-healthkit-connects-with-mayo-and-epic-but-dont-call-it-revolutionary/2014.
- 62.Pennic F. Cerner Launches Apple Watch App, Push HealthKit Data to Cerner Millennium. [cited 2015 1 December]; Available from: http://hitconsultant.net/2015/04/10/cerner-launches-apple-watch-app/2015.
- 63.Yamin CK, Emani S, Williams DH, et al. The digital divide in adoption and use of a personal health record. Arch Intern Med. 2011;171(6):568–574. doi: 10.1001/archinternmed.2011.34. [DOI] [PubMed] [Google Scholar]
- 64.Lustria MLA, Smith SA, Hinnant CC. Exploring digital divides: an examination of eHealth technology use in health information seeking, communication and personal health information management in the USA. Health Inform J. 2011;17(3):224–243. doi: 10.1177/1460458211414843. [DOI] [PubMed] [Google Scholar]
- 65.Lobelo F, Stoutenberg M, Hutber A. The Exercise is Medicine Global Health Initiative: a 2014 update. Br J Sports Med. 2014;48(22):1627–1633. doi: 10.1136/bjsports-2013-093080. [DOI] [PubMed] [Google Scholar]
- 66.American Recovery and Reinvestment Act of 2009. [cited 2015 15 November]; Available from: https://www.congress.gov/bill/111th-congress/house-bill/12009.
- 67.Patient Protection and Affordable Care Act. [cited 2015 15 November]; Available from: https://www.congress.gov/bill/111th-congress/house-bill/35902009.
- 68.Blumenthal D. Stimulating the adoption of health information technology. N Engl J Med. 2009;360(15):1477–1479. doi: 10.1056/NEJMp0901592. [DOI] [PubMed] [Google Scholar]
- 69.Electronic health records (EHR) incentive programs. 2015 [cited 2015 15 November] [Google Scholar]
- 70.LeFevre ML. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2014;161(8):587–593. doi: 10.7326/M14-1796. [DOI] [PubMed] [Google Scholar]
- 71.D.o H.a H Services, editor. Medicare and Medicaid Programs. Electronic Health Record Incentive Program. Centers for Medicare and Medicaid Services; 2015. p. 16732. [Google Scholar]
- 72.The flowchart depicting ONC’s Federal Advisory Committee process for developing recommendations. [cited 2015 15 November]; Available from: https://www.healthit.gov/FACAS/2015.
- 73.Urrea B, Misra S, Plante TB, et al. Mobile health initiatives to improve outcomes in primary prevention of cardiovascular disease. Curr Treat Options Cardiovasc Med. 2015;17(12):59. doi: 10.1007/s11936-015-0417-7. [1–12] [DOI] [PubMed] [Google Scholar]
- 74.Hebden L, Cook A, van der Ploeg HP, et al. A mobile health intervention for weight management among young adults: a pilot randomised controlled trial. J Hum Nutr Diet. 2014;27(4):322–332. doi: 10.1111/jhn.12155. [DOI] [PubMed] [Google Scholar]
- 75.Kirwan M, Duncan MJ, Vandelanotte C, et al. Using smartphone technology to monitor physical activity in the 10,000 Steps program: a matched case-control trial. J Med Internet Res. 2012;14(2):e55. doi: 10.2196/jmir.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin SS, Feldman DI, Blumenthal RS, et al. mActive: a randomized clinical trial of an automated mHealth intervention for physical activity promotion. J Am Heart Assoc. 2015;4(11) doi: 10.1161/JAHA.115.002239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glynn LG, Hayes PS, Casey M, et al. Effectiveness of a smartphone application to promote physical activity in primary care: the SMART MOVE randomised controlled trial. Br J Gen Pract. 2014;64(624):e384–e391. doi: 10.3399/bjgp14X680461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chow CK, Redfern J, Hillis GS, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314(12):1255–1263. doi: 10.1001/jama.2015.10945. [DOI] [PubMed] [Google Scholar]
- 79.Shuger SL, Barry VW, Sui X, et al. Electronic feedback in a diet-and physical activity-based lifestyle intervention for weight loss: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:41. doi: 10.1186/1479-5868-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meyer J, Hein A. Live long and prosper: potentials of low-cost consumer devices for the prevention of cardiovascular diseases. Medicine. 2013;2(2):e7. doi: 10.2196/med20.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.King AC, Hekler EB, Grieco LA, et al. Harnessing different motivational frames via mobile phones to promote daily physical activity and reduce sedentary behavior in aging adults. PLoS One. 2013;8(4):e62613. doi: 10.1371/journal.pone.0062613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sepah SC, Jiang L, Peters AL. Long-term outcomes of a Web-based diabetes prevention program: 2-year results of a single-arm longitudinal study. J Med Internet Res. 2015;17(4):e92. doi: 10.2196/jmir.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 84.Widmer RJ, Collins NM, Collins CS, et al. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc. 2015;90(4):469–480. doi: 10.1016/j.mayocp.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Richards J, Thorogood M, Hillsdon M, et al. Face-to-face versus remote and web 2.0 interventions for promoting physical activity. Cochrane Database Syst Rev. 2013;9:CD010393. doi: 10.1002/14651858.CD010393.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.OMsignal Inc. OM Signal. [cited 2015 12 October]; Available from: http://www.omsignal.com/2015.
- 87.Smart Clothing AiQ. AiQ Smart. [cited 2015 12 October]; Available from: http://www.aiqsmartclothing.com/2015.
- 88.Sensoria Smart Socks. [cited 2015 27 October]; Available from: http://www.sensoriafitness.com/2015.
- 89.Sciences, C. [cited 2015 27 October]; Available from: http://www.cityzensciences.fr/en/2015.
- 90.Graziano D. Fossil to buy fitness band maker Misfit for $260 million. [cited 2015 18 November]; Available from: http://www.cnet.com/news/fossil-group-to-buy-misfit-for-260-mil]ion/2015.
- 91.Misfit Inc. MisFit. [cited 2015 12 October]; Available from: http://misfit.com/2015.
- 92.Medeiros J. How Gadi Amit is putting the future of wearables under your skin. [cited 2015 28 October]; Available from: http://www.wired.co.uk/magazine/archive/2015/10/features/redesigning-your-body2015.
- 93.Stein S. The end of fitness bands? Wearable tech feels ready to move forward. [cited 2015 10 October]; Available from: http://www.cnet.com/news/end-of-fitness-bands-future-after-nike-fuelband/2014.
- 94.Kelion L. CES 2015: wearable tech becomes invisible. [cited 2015 10-October]; Available from: http://www.bbc.com/news/technology-307277512015.
- 95.Coldewey D. Meet Lily, a camera drone that follows you automatically. [cited 2015 9 November]; Available from: http://www.nbcnews.com/tech/gadgets/meet-lily-camera-drone-follows-you-automatically-n3578112015.


