Abstract
Importance
Cardiac biomarker testing is not routinely indicated in the emergency department (ED) because of low utility and potential downstream harms from false-positive results. However, current rates of testing are unknown.
Objective
To determine the use of cardiac biomarker testing overall, as well as stratified by disposition status and selected characteristics.
Design, Setting, and Participants
Retrospective study of ED visits by adults (≥18 years old) selected from the 2009 and 2010 National Hospital Ambulatory Medical Care Survey, a probability sample of ED visits in the United States.
Exposures
Selected patient, visit, and ED characteristics.
Main Outcomes and Measures
Receipt of cardiac biomarker testing during the ED visit.
Results
Of 44 448 ED visits, cardiac biomarkers were tested in 16.9% of visits, representing 28.6 million visits. Biomarker testing occurred in 8.2% of visits in the absence of acute coronary syndrome (ACS)-related symptoms, representing 8.5 million visits, almost one-third of all visits with biomarker testing. Among individuals subsequently hospitalized, cardiac biomarkers were tested in 47.0% of all visits. In this group, biomarkers were tested in 35.4% of visits despite the absence of ACS-related symptoms. Among all ED visits, the number of other tests or services performed was the strongest predictor of biomarker testing independent of symptoms of ACS. Compared with 0 to 5 other tests or services performed, more than 10 other tests or services performed was associated with 59.55 (95% CI, 39.23-90.40) times the odds of biomarker testing. The adjusted probabilities of biomarker testing if 0 to 5, 6 to 10, or more than 10 other tests or services performed were 6.3%, 34.3%, and 62.3%, respectively.
Conclusions and Relevance
Cardiac biomarker testing in the ED is common even among those without symptoms suggestive of ACS. Cardiac biomarker testing is also frequently used during visits with a high volume of other tests or services independent of the clinical presentation. More attention is needed to develop strategies for appropriate use of cardiac biomarkers.
Acute coronary syndrome (ACS) is a leading cause of death in the United States and accounts for 625 000 hospital discharges annually.1 Cardiac biomarkers have emerged as a powerful tool to rapidly detect myocardial necrosis, which is a hallmark of ACS but can also occur in various other illnesses. Increasingly sensitive assays for cardiac biomarkers have enabled their use in the emergency department (ED) for early diagnosis of ACS, critical to the timely initiation of potentially life-saving evidence-based therapies.2
Although nontraumatic chest pain (a symptom classically associated with suspected ACS) is one of the most common reasons for a visit to the ED, cardiac biomarker testing is not routinely recommended in all patients in the ED.3,4 In individuals with low suspicion of ACS (eg, those without the typical symptom of chest pain, present in more than 90% of patients with ACS), an elevated cardiac troponin level is far more likely to represent a false-positive test result than a true type 1 myocardial infarction due to atherosclerotic plaque rupture.5-8 Therefore, even with highly sensitive assays, the decision to test for cardiac biomarkers must be considered in the context of the clinical presentation.9
The use of cardiac biomarker testing in a population with a low probability of ACS is likely to result in substantial downstream harms from false-positive test results, including patient anxiety, inappropriate additional testing, and unnecessary treatment.5 Individuals with an abnormal cardiac biomarker test result in the ED are more likely to be hospitalized; therefore, potential downstream harms owing to false-positive test results are disproportionately more likely to affect individuals subsequently hospitalized than those discharged from the ED.10-12 The potential extent of harms from false-positive test results cannot be estimated given that the current rates of cardiac biomarker testing are unknown. In this study, we examined a nationally representative sample of ED visits by adults to estimate (1) the use of cardiac biomarker testing in the ED overall and stratified by disposition status given that the ACS incidence is considerably different between individuals discharged from the ED and those subsequently hospitalized,8 (2) the use of testing given selected patient and visit characteristics, and (3) the adjusted probability of testing by selected characteristics.
Methods
Study Design
This study was deemed exempt from review by the UT Southwestern Medical Center institutional review board, and informed consent was waived. We analyzed data from the 2009 and 2010 National Hospital Ambulatory Medical Care Survey (NHAMCS), an annual nationally representative sample of visits to EDs of noninstitutional general and short-stay hospitals in the United States, excluding federal, military, and Veterans Administration hospitals.13 The NHAMCS was designed by the National Center for Health Statistics to collect data on the use and provision of ambulatory care services in hospital EDs. The survey uses a 4-stage probability design. Trained hospital staff collect data for each visit, with oversight from US Census Bureau field representatives during a randomly assigned 4-week reporting period each year. All medical coding is subject to a 2-way 10% independent verification procedure for quality control, and discrepancies and illegible entries are reviewed and adjudicated centrally at the National Center for Health Statistics.14,15 Data from the NHAMCS are derived by a multistage estimation procedure that produces unbiased national estimates.16
Study Population
We included all ED visits by adults 18 years or older in 2009 and 2010. We excluded visits if an individual had been seen in the same ED within the past 72 hours, had been discharged from any hospital within the past 7 days, had been seen as a follow-up visit, had been transferred to another facility, had left against medical advice, had an unclear disposition, or was dead on arrival or died in the ED. Hospitalization included observation stays and visits for all internal medicine and non– internal medicine conditions (eg, surgery, trauma, obstetrics, gynecology, and psychiatry).
Outcomes
The primary outcome was whether cardiac biomarker testing occurred during the ED visit, defined as cardiac biomarker tests ordered or performed in the ED and recorded as a check box on the NHAMCS data collection form. Cardiac biomarkers included creatine kinase–MB fraction, troponin I, and troponin T.
Characteristics
We defined cardiovascular comorbidities as the presence of any of the following 4 preexisting chronic conditions: cerebrovascular disease, congestive heart failure, kidney disease requiring dialysis, or diabetes mellitus, as indicated by a check box on the NHAMCS data collection form.14,15 These were determined independent of the reason for the visit and the ED provider's diagnosis fields.
Hospital staff abstracted up to 3 of the patient-reported problems, symptoms, or other reasons for a visit as free text.14,15 These fields were coded centrally by the NHAMCS personnel using a standard reason for visit classification (RVC).17,18 Using this system, we classified individuals as having potentially typical symptoms, atypical symptoms, or no symptoms of ACS. We defined typical symptoms of ACS as the presence of 1 or more RVC fields coded as heart pain or chest pain (RVCs 1050 and 1265, respectively). We defined atypical symptoms of ACS as the absence of chest pain but the presence of 1 or more RVC fields coded as nausea (RVC 1525), vomiting (RVC 1530), heartburn or indigestion (RVC 1535), abdominal pain (excluding lower abdominal pain [RVCs 1545.0, 1545.1, and 1545.3]), palpitations (RVC 1260), other symptoms of the heart (RVC 1270), shortness of breath (RVC 1415), dyspnea (RVC 1420), wheezing (RVC 1425), breathing problems (RVC 1430), general malaise (RVC 1025), fainting (RVC 1030), fluid abnormality (RVC 1035.0), edema (RVC 1035.1), diaphoresis (RVC 1035.2), vertigo or dizziness (RVC 1225), jaw pain (RVC 1055.4), neck pain (RVC 1900.1), or arm pain (RVC 1945.1). We intentionally defined atypical symptoms of ACS broadly to capture all individuals with even the slightest suspicion of ACS.
We defined abnormal vital signs as a composite measure with the presence of 1 or more of the following initial vital signs as recorded in triage: heart rate greater than 100 beats/min or less than 60 beats/min, systolic blood pressure greater than 160 mm Hg or lessthan90 mm Hg, diastolic blood pressure greater than 100mm Hg or less than60mmHg, respiratory rate greater than 20 breaths/min or less than 10 breaths/min, and oxygen saturation less than 90%. Tests or services performed in the ED included blood tests, imaging studies, and miscellaneous tests (eg, urine studies) that were ordered or provided at any time during the ED visit. Panel tests were counted as a single test. For example, a visit with a complete blood cell count and a comprehensive metabolic panel (electrolytes, glucose, kidney function, and liver function panel) equates to 5 tests or services. To avoid endogeneity of our predictor-outcome relationship, cardiac biomarkers and electrocardiograms were not included in the number of tests or services performed.
Up to 3 ED provider's diagnoses were separately recorded for each visit as free text and then coded centrally by the NHAMCS using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). We further classified ICD-9-CM diagnoses using the Agency for Healthcare Research and Quality (AHRQ) Clinical Classifications Software (CCS).19 We defined a diagnosis of cardiovascular disease as the presence of 1 or more ED provider's diagnoses mapped to the AHRQ CCS diagnoses 96 through 118, a modified version of the AHRQ CCS disease of the circulatory system category.
Statistical Analysis
We analyzed the data using statistical software (STATA version 12.0; StataCorp LP). All analyses used patient visit weight, strata, and primary sampling unit design variables provided by the NHAMCS using the svy package to account for the complex survey design and to reflect national estimates.13-16 We used F tests for descriptive statistics. Weighted percentages and the absolute number of visits with cardiac biomarker testing were estimated for the overall ED population and stratified by disposition status (subsequently hospitalized vs treat and release) and by selected patient and visit characteristics.
Predictors of cardiac biomarker testing were estimated using logistic regression, adjusting for selected patient, visit, and ED characteristics. Model fit was assessed using an extension of the Hosmer-Lemeshow goodness-of-fit test appropriate for survey-weighted data.20 From the logistic regression model, we estimated the mean predicted probability of bio-marker testing in the ED using the predictive margins command in STATA.21,22 Predictive margins estimate the mean probability of cardiac biomarker testing if every visit in the sample had a selected characteristic fixed at a particular value, even if the value differs from what it really is. For example, the predicted margins for symptoms of ACS estimate the adjusted mean probability of biomarker testing if all visits in the population were by individuals who had no symptoms of ACS, atypical symptoms of ACS, and chest pain, respectively, while holding all other characteristics constant. This strategy enables an examination of the influence of a selected characteristic on the mean predicted probability of biomarker testing, while controlling for different covariate distributions in the sample.
Results
Study Population and Characteristics
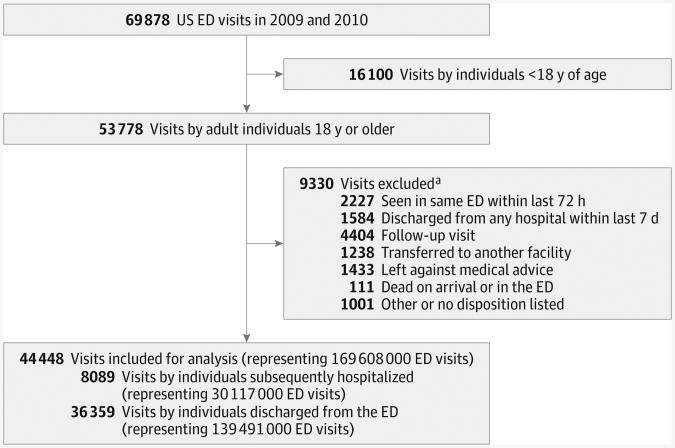
During 2009 and 2010, the NHAMCS collected data on 53 778 ED visits by adults. Of these, 9330 visits were excluded (16.4% of weighted visits), resulting in 44 448 visits included in this study (Figure). Based on the design of the survey, this sample of included visits represents 169.6 million (95% CI, 152.2-187.0 million) ED visits in the United States during the study period. Of these included visits, individuals subsequently hospitalized accounted for 8089 observed visits (17.8% of weighted visits), representing 30.1 million (95% CI, 26.2-34.0 million) ED visits, and individuals treated and released from the ED accounted for36 359 observed visits (82.2% of weighted visits), representing 139.5 million (95% CI, 125.1-153.9 million) ED visits nationally. Of all ED visits, 8.8% were among individuals with chest pain, 30.1% were among individuals with a potential atypical symptom of ACS, and 61.1%wereamongindividu-als with no symptoms of ACS. One-quarter of visits (25.9%) had more than 5 other tests or services performed during the visit, and two-thirds of visits (66.5%) were unrelated to an injury or poisoning (Table 1). Compared with treat-and-release visits, ED visits by individuals subsequently hospitalized were significantly more likely to be among individuals who were older, had a cardiovascular comorbidity (a symptom suggestive of ACS), and had a cardiovascular visit diagnosis (P < .01 for all).
Figure. Study Flow Diagram.
ED indicates emergency department.
a Not mutually exclusive.
Table 1. Characteristics of ED Visits in the United States,2009-2010a.
| Variable | Weighted % (SE) | ||
|---|---|---|---|
| All ED Visits (Unweighted N = 44 448) | Individuals Subsequently Hospitalized (Unweighted n = 8089) | Individuals Discharged From the ED (Unweighted n = 36 359) | |
| Demographic and Clinical Characteristics | |||
| Age, y | |||
| 18-29 | 27.1 (0.3) | 8.5 (0.4) | 31.2 (0.4) |
| 30-49 | 33.9 (0.3) | 22.0 (0.6) | 36.4 (0.3) |
| 50-64 | 19.6 (0.3) | 25.7 (0.6) | 18.3 (0.3) |
| ≥65 | 19.4 (0.3) | 43.9 (0.8) | 14.1 (0.4) |
| Female sex | 58.2 (0.4) | 54.8 (0.8) | 58.9 (0.4) |
| Race/ethnicity | |||
| White, non-Hispanic | 64.7 (1.6) | 68.8 (1.8) | 63.8 (1.7) |
| Black, non-Hispanic | 21.1 (1.5) | 18.8 (1.4) | 21.6 (1.6) |
| Other | 14.2 (1.1) | 12.5 (1.4) | 14.5 (1.1) |
| Primary payer | |||
| Private insurance | 31.4 (0.6) | 24.7 (0.9) | 32.8 (0.7) |
| Medicare | 22.3 (0.4) | 45.5 (0.9) | 17.3 (0.5) |
| Medicaid | 18.5 (0.7) | 14.2 (0.7) | 19.4 (0.8) |
| Other or unknown | 27.8 (0.8) | 15.5 (0.7) | 30.5 (0.9) |
| Cardiovascular comorbidities, No. | |||
| 0 | 84.3 (0.5) | 63.2 (1.0) | 88.9 (0.3) |
| 1 | 12.9 (0.4) | 27.6 (0.7) | 9.7 (0.3) |
| 2-4 | 2.8 (0.2) | 9.3 (0.6) | 1.4 (0.1) |
| Visit Characteristics | |||
| Symptom of ACS | |||
| None | 61.1 (0.5) | 42.0 (0.9) | 65.3 (0.5) |
| Atypical | 30.1 (0.4) | 39.8 (0.8) | 28.0 (0.4) |
| Chest pain | 8.8 (0.2) | 18.2 (0.7) | 6.8 (0.2) |
| Visit related to an injury or poisoning | |||
| No | 66.5 (0.5) | 79.3 (0.6) | 63.7 (0.5) |
| Yes | 33.5 (0.5) | 20.7 (0.6) | 36.3 (0.5) |
| Abnormal vital signs | 46.1 (0.5) | 62.9 (0.8) | 42.4 (0.5) |
| Glasgow Coma Scale score <14 | 2.1 (0.8) | 4.0 (0.9) | 1.7 (0.8) |
| Moderate or severe pain | 51.2 (1.0) | 37.6 (1.1) | 54.1 (75.5) |
| Disposition status | |||
| Treat and release | 82.2 (0.7) | NA | 100.0 (0.0) |
| Non–critical care unit | 15.6 (0.6) | 88.0 (0.6) | NA |
| Critical care unit | 2.1 (0.1) | 12.0 (0.6) | NA |
| Other tests or services in the ED, No. | |||
| 0-5 | 74.1 (1.2) | 36.3 (2.0) | 82.2 (0.9) |
| 6-10 | 24.1 (1.1) | 56.4 (1.8) | 17.1 (0.9) |
| >10 | 1.8 (0.2) | 7.3 (0.7) | 0.7 (0.1) |
| Emergent status by triage | 12.3 (0.5) | 28.1 (1.1) | 8.9 (0.5) |
| Duration of ED visit >2 h | 67.9 (1.4) | 90.2 (0.8) | 63.0 (1.4) |
| Cardiovascular visit diagnosis | 13.5 (0.4) | 36.0 (1.0) | 8.7 (0.3) |
| ED Characteristics | |||
| Region | |||
| Northeast | 19.3 (1.5) | 23.4 (2.1) | 18.5 (1.4) |
| Midwest | 22.5 (2.5) | 24.6 (3.2) | 22.0 (2.5) |
| South | 39.4 (2.7) | 34.9 (3.3) | 40.3 (2.7) |
| West | 18.8 (1.6) | 17.2 (2.0) | 19.2 (1.6) |
| Nonmetropolitan area | 18.2 (4.8) | 12.4 (3.8) | 19.5 (5.1) |
| Hospital owner | |||
| Nonprofit | 76.1 (2.4) | 82.0 (2.2) | 74.9 (2.5) |
| Government | 14.4 (2.0) | 11.5 (1.8) | 15.0 (2.1) |
| Proprietary | 9.5 (1.8) | 6.5 (1.5) | 10.2 (1.9) |
Abbreviations: ACS, acute coronary syndrome; ED, emergency department; NA, not applicable.
Percentages and estimated number of visits shown are weighted to reflect the complex survey design. All estimates are considered reliable (SE is <30% of the estimate) except for the estimates for Glasgow Coma Scale less than 14 for all ED visits (38% SE) and for individuals discharged from the ED (47% SE). All characteristics were statistically different between visits by individuals subsequently hospitalized and individuals discharged from the ED (P < .01).
Observed Use of Cardiac Biomarker Testing
Overall, cardiac biomarker testing occurred in 16.9% (95% CI, 15.2%-18.7%) of all ED visits, representing 28.6 million visits during the 2-year study period (Table 2). Stratified by disposition status, biomarker testing occurred in 47.0% (95% CI, 43.3%-50.8%) of visits by individuals subsequently hospitalized, representing 14.2 million visits, and in 10.4% (95% CI, 9.1%-11.8%) of treat-and-release visits, representing 14.4 million visits.
Table 2. Use of Cardiac Biomarker Testing in the ED by Selected Characteristicsa.
| Characteristic | All ED Visits | Individuals Subsequently Hospitalized | Individuals Discharged From the ED | |||
|---|---|---|---|---|---|---|
| Unadjusted % (95% CI) | No. (95% CI) of Visits With Testing, Million | Unadjusted % (95% CI) | No. (95% CI) of Visits With Testing, Million | Unadjusted % (95% CI) | No. (95% CI) of Visits With Testing, Million | |
| Overall | 16.9 (15.2-18.7) | 28.6 (24.4-32.9) | 47.0 (43.3-50.8) | 14.2 (12.0-16.3) | 10.4 (9.1-11.8) | 14.4 (12.1-16.8) |
| Cardiovascular comorbidities, No. | ||||||
| 0 | 13.4 (11.8-15.1) | 19.1 (16.1-22.1) | 41.6 (37.5-45.7) | 7.9 (6.7-9.2) | 9.0 (7.8-10.4) | 11.2 (9.3-13.1) |
| 1 | 33.3 (30.4-36.2) | 7.3 (6.1-8.5) | 54.7 (50.3-58.9) | 4.5 (3.7-5.3) | 20.2 (17.8-22.8) | 2.7 (2.2-3.3) |
| 2-4 | 47.0 (42.0-52.0) | 2.2 (1.8-2.6) | 61.8 (55.4-67.8) | 1.7 (1.4-2.1) | 25.3 (21.0-30.2) | 0.5 (0.4-0.6) |
| Symptom of ACS | ||||||
| None | 8.2 (7.1-9.5) | 8.5 (7.0-10.0) | 35.4 (31.6-39.3) | 4.5 (3.7-5.2) | 4.5 (3.7-5.3) | 4.1 (3.2-4.9) |
| Atypical | 24.2 (21.7-26.9) | 12.4 (10.5-14.2) | 47.9 (43.6-52.3) | 5.7 (4.9-6.6) | 17.0 (14.8-19.4) | 6.6 (5.5-7.7) |
| Chest pain | 51.7 (47.2-56.2) | 7.7 (6.5-9.0) | 72.0 (68.9-77.4) | 4.0 (3.2-4.7) | 39.9 (35.7-44.2) | 3.8 (3.1-4.4) |
| Visit related to an injury or poisoning | ||||||
| No | 21.6 (19.4-24.0) | 24.4 (20.7-28.0) | 49.8 (45.8-53.9) | 11.9 (10.0-13.8) | 14.0 (12.3-15.9) | 12.5 (10.4-14.5) |
| Yes | 7.5 (6.6-8.5) | 4.2 (3.6-4.9) | 36.3 (32.1-40.8) | 2.3 (1.9-2.6) | 3.9 (3.3-4.6) | 2.0 (1.6-2.4) |
| Cardiovascular visit diagnosis | ||||||
| No | 11.5 (10.2-13.0) | 16.9 (14.2-19.5) | 36.2 (32.8-39.7) | 7.0 (5.9-8.0) | 7.8 (6.7-9.0) | 9.9 (8.2-11.6) |
| Yes | 51.2 (46.8-55.5) | 11.7 (9.9-13.6) | 66.4 (60.9-71.5) | 7.2 (6.0-8.4) | 37.5 (33.6-41.7) | 4.5 (3.7-5.4) |
| Other tests or services in the ED, No. | ||||||
| 0-5 | 4.5 (3.8-5.2) | 5.6 (4.5-6.7) | 18.9 (15.7-22.7) | 2.1 (1.6-2.5) | 3.1 (2.6-3.7) | 3.5 (2.8-4.3) |
| 6-10 | 50.3 (46.4-54.2) | 20.6 (17.2-23.9) | 60.8 (56.5-65.0) | 10.3 (8.7-12.0) | 42.8 (38.5-47.3) | 10.2 (8.4-10.1) |
| >10 | 78.1 (71.6-83.4) | 2.4 (1.8-3.1) | 80.5 (74.8-85.2) | 1.8 (1.3-2.2) | 72.4 (58.4-83.0) | 0.7 (0.4-1.0) |
Abbreviations: ACS, acute coronary syndrome; ED, emergency department.
Percentages and estimated number of visits shown are weighted to reflect the complex survey design.
Overall, biomarkers were tested in 8.2% (95% CI, 7.1%-9.5%) of visits in the absence of ACS-related symptoms, representing 8.5 million visits, almost one-third of all visits with biomarker testing (Table 2). In contrast, among all ED visits with biomarker testing, chest pain was estimated to be present in only 7.7 million visits, or approximately one-quarter of all visits with biomarker testing. In addition, 27.4% of all visits with biomarker testing did not also have an electrocardiogram ordered, discordant with guidelines for diagnostic testing for suspected ACS. During visits with 6 to 10 other tests or services performed, 50.3% of individuals also had biomarker testing. Among visits by individuals subsequently hospitalized, bio-marker testing occurred in 35.4% (95% CI, 31.6%-39.3%) of visits by individuals with no symptoms suggestive of ACS, representing 4.5 million visits; in 36.3% (95% CI, 32.1%-40.8%) of ED visits for an injury or poisoning, representing 2.3 million visits; and in 80.5% (95% CI, 74.8%-85.2%) of visits with more than 10 other tests or services performed, representing 1.8 million visits. Similar patterns of cardiac biomarker testing were observed among treat-and-release visits, but with lower rates of testing.
Predictors of Cardiac Biomarker Testing
In our adjusted analysis, age, symptoms of ACS, disposition status, ED provider's diagnosis of cardiovascular disease, visit duration, and the number of other tests or services performed in the ED were strongly associated with testing for cardiac bio-markers during the visit (Table 3). The number of other tests or services performed was by far the strongest predictor of bio-marker testing. Compared with a visit with 0 to 5 other tests or services performed in the ED, a visit with more than 10 other tests or services performed was associated with 59.55 (95% CI, 39.23-90.40) times the odds of biomarker testing. Disposition status was also a strong predictor. Compared with those discharged from the ED, visits by individuals subsequently hospitalized to a non–critical care unit were associated with 1.52 (95% CI, 1.32-1.73) times the odds of biomarker testing.
Table 3. Predictors of Receiving Cardiac Biomarker Testing in the ED.
| Characteristic | Odds Ratio (95% CI) | |
|---|---|---|
| Unadjusted | Adjusteda | |
| Age, per 10 y | 1.43 (1.40-1.47) | 1.22 (1.17-1.27) |
| Female sex | 0.95 (0.87-1.03) | 0.90 (0.81-1.01) |
| Race/ethnicity | ||
| White, non-Hispanic | 1 [Reference] | 1 [Reference] |
| Black, non-Hispanic | 0.90 (0.77-1.06) | 1.09 (0.89-1.34) |
| Other | 0.89 (0.74-1.07) | 1.01 (0.85-1.20) |
| Primary payer | ||
| Private insurance | 1 [Reference] | 1 [Reference] |
| Medicare | 2.44 (2.17-2.75) | 0.99 (0.81-1.20) |
| Medicaid | 0.78 (0.66-0.91) | 0.94 (0.79-1.13) |
| Other or unknown | 0.61 (0.51-0.74) | 0.83 (0.67-1.01) |
| Cardiovascular comorbidities, No. | ||
| 0 | 1 [Reference] | 1 [Reference] |
| 1 | 3.23 (2.90-3.59) | 1.14 (0.98-1.32) |
| 2-4 | 5.74 (4.69-7.02) | 1.11 (0.82-1.50) |
| Symptom of ACS | ||
| None | 1 [Reference] | 1 [Reference] |
| Atypical | 3.56 (3.21-3.96) | 1.79 (1.57-2.03) |
| Chest pain | 11.95 (10.08-14.17) | 5.62 (4.47-7.05) |
| Visit related to an injury or poisoning | 0.29 (0.27-0.32) | 0.64 (0.58-0.71) |
| Abnormal vital signs | 1.76 (1.62-1.90) | 1.02 (0.91-1.14) |
| Glasgow Coma Scale score <14 | 1.40 (0.83-2.34) | 0.83 (0.52-1.35) |
| Moderate or severe pain | 0.56 (0.51-0.62) | 0.67 (0.60-0.75) |
| Disposition status | ||
| Treat and release | 1 [Reference] | 1 [Reference] |
| Non–critical care unit | 7.32 (6.45-8.30) | 1.52 (1.32-1.73) |
| Critical care unit | 11.14 (8.95-13.85) | 1.71 (1.32-2.21) |
| Cardiovascular visit diagnosis | 8.07 (6.99-9.31) | 2.59 (2.15-3.11) |
| Other tests or services in the ED, No. | ||
| 0-5 | 1 [Reference] | 1 [Reference] |
| 6-10 | 21.70 (18.22-25.83) | 13.65 (11.41-16.32) |
| >10 | 76.20 (53.39-108.75) | 59.55 (39.23-90.40) |
| Emergent status by triage | 3.00 (2.54-3.54) | 1.06 (0.88-1.29) |
| Duration of ED visit >2 h | 5.98 (5.03-7.11) | 1.64 (1.38-1.95) |
| Region | ||
| Northeast | 1 [Reference] | 1 [Reference] |
| Midwest | 1.01 (0.72-1.41) | 1.16 (0.73-1.85) |
| South | 0.92 (0.68-1.25) | 1.07 (0.74-1.54) |
| West | 1.13 (0.77-1.65) | 1.67 (0.98-2.83) |
| Nonmetropolitan area | 0.66 (0.49-0.89) | 1.12 (0.78-1.63) |
| Hospital owner | ||
| Nonprofit | 1 [Reference] | 1 [Reference] |
| Government | 0.79 (0.61-1.02) | 0.98 (0.72-1.34) |
| Proprietary | 0.55 (0.34-0.88) | 0.74 (0.41-1.33) |
Abbreviations: ACS, acute coronary syndrome; ED, emergency department.
Weighted multivariable logistic regression model adjusted for the covariates listed above, accounting for the complex survey design.
Adjusted Probability of Cardiac Biomarker Testing by Selected Characteristics
Among all ED visits, the adjusted probabilities of biomarker testing if 0 to 5, 6 to 10, and more than 10 other tests or services were performed were 6.3% (95% CI, 5.4%-7.3%), 34.3% (95% CI, 30.6%-37.9%), and 62.3% (95% CI, 54.0%-70.6%), respectively (Table 4). In the absence of ACS-related symptoms, the adjusted probability of biomarker testing was 13.0% (95% CI, 11.2%-14.7%). Among visits by individuals subsequently hospitalized, 38.2% (95% CI, 34.2%-42.2%) of visits by individuals seen without a symptom of ACS and 41.6% (95% CI, 37.6%-45.5%) of visits related to an injury or poisoning were estimated to have biomarker testing. Even among treat-and-release visits, 7.5% (95% CI, 6.2%-8.8%) of visits by individuals seen without a symptom of ACS, 28.6% (95% CI, 24.8%-32.3%) of visits with 6 to 10 other tests or services performed, and 57.7% (95% CI, 48.5%-66.9%) of visits with more than 10 other tests or services performed were estimated to have biomarker testing, adjusting for patient, visit, and ED characteristics.
Table 4. Adjusted Probability of Cardiac Biomarker Testing in the ED by Selected Characteristicsa.
| Characteristic | % (95% CI) | ||
|---|---|---|---|
| All ED Visits | Individuals Subsequently Hospitalized | Individuals Discharged From the ED | |
| Mean probability of testing overall | 16.9 (15.1-18.6) | 47.0 (43.3-50.8) | 10.3 (9.0-11.7) |
| Age | |||
| 10 y Younger than median | 14.0 (12.1-15.8) | 43.3 (39.2-47.4) | 8.4 (7.1-9.7) |
| Median | 15.5 (13.6-17.3) | 46.4 (42.5-50.4) | 9.5 (8.1-10.8) |
| 10 y Older than median | 17.0 (15.2-18.9) | 49.6 (45.7-53.4) | 10.7 (9.3-12.1) |
| Symptom of ACS | |||
| None | 13.0 (11.2-14.7) | 38.2 (34.2-42.2) | 7.5 (6.2-8.8) |
| Atypical | 17.4 (15.6-19.3) | 47.5 (43.6-51.5) | 11.0 (9.5-12.4) |
| Chest pain | 28.8 (25.8-31.8) | 65.1 (60.4-69.8) | 20.9 (18.2-23.6) |
| Visit related to an injury or poisoning | |||
| No | 17.6 (15.7-19.4) | 48.4 (44.6-52.3) | 10.9 (9.5-12.3) |
| Yes | 14.3 (12.6-16.0) | 41.6 (37.6-45.5) | 8.4 (7.2-9.6) |
| Cardiovascular visit diagnosis | |||
| No | 15.0 (13.3-16.8) | 41.6 (37.8-45.5) | 9.3 (8.0-10.6) |
| Yes | 23.3 (20.6-25.9) | 56.7 (52.2-61.2) | 16.1 (13.9-18.2) |
| Other tests or services in the ED, No. | |||
| 0-5 | 6.3 (5.4-7.3) | 16.6 (13.8-19.4) | 4.1 (3.5-4.8) |
| 6-10 | 34.3 (30.6-37.9) | 60.6 (56.6-64.8) | 28.6 (24.8-32.3) |
| >10 | 62.3 (54.0-70.6) | 83.8 (79.0-88.6) | 57.7 (48.5-66.9) |
Abbreviations: ACS, acute coronary
syndrome; ED, emergency department.
Estimates obtained from a weighted multivariable logistic regression model adjusted for the covariates listed above plus race/ethnicity, primary payer, cardiovascular comorbidity count, Glasgow Coma Scale score, pain level, triage status, duration of ED visit, and ED characteristics (region, metropolitan status, and hospital owner), accounting for the complex survey design.
Discussion
In this national study, we identified high rates of cardiac bio-marker testing even among individuals without clinical presentations suggestive of ACS. Furthermore, biomarker testing was highly prevalent during visits with a high volume of other tests or services rendered during the visit independent of the clinical presentation.
The high rate of testing among individuals with low suspicion of ACS is concerning because of the potential increase in health care costs and downstream harms owing to false-positive results. Hospitals with higher noninvasive cardiac imaging rates have higher rates of hospitalization and angiography, which may also be true for centers and health care providers with higher rates of cardiac biomarker testing.23 Potential consequences of unwarranted biomarker testing include patient anxiety, diagnostic red herrings, unnecessary cardiology consultations, and inappropriate subsequent testing and treatment.6,24 These potential harms owing to false-positive results are more likely to affect subsequently hospitalized patients because individuals with abnormal biomarker test results are more likely to be hospitalized.10-12 Our study describing the national use of cardiac biomarker testing in the ED is the first step in gaining a better understanding of these potential harms.
Among 28.6 million visits with biomarker testing, our findings that tests occurred in at least 8.5 million visits despite the absence of symptoms of ACS represent instances of potentially inappropriate testing. This is likely a conservative estimate because biomarker testing was also likely unwarranted in a large proportion of those with potential atypical symptoms of ACS. We intentionally defined atypical symptoms broadly to capture all individuals who may have had even the slightest suspicion of ACS. However, prior literature suggests that, among all individuals deemed to have a symptom suggestive of acute cardiac ischemia, approximately 75% had chest pain, while only 25% were seen in the ED with atypical symptoms.25 Extrapolating this proportion to our study population, which included bio-marker testing in 7.7 million visits by individuals seen with chest pain, we estimate that only 2.6 million of the visits we classified as occurring among individuals with atypical symptoms were likely to have a clinical presentation truly suggestive of ACS. In total, cardiac biomarkers may have been appropriately tested in 10.3 million visits among individuals with symptoms suggestive of ACS. The remainder (18.3 million, or approximately two-thirds of the 28.6 million ED visits with biomarker testing) represents visits where testing may have occurred among individuals in the absence of any clinical suspicion of ACS.
The high rates of testing in a population without suspicion of ACS are particularly concerning in the context of the impending adoption of highly sensitive cardiac biomarker assays in the United States, which yield more false-positive test results.6 For example, if we assume a hypothetical 2% prevalence of ACS in the 8.5 million visits among individuals with no symptoms of ACS in our study population (likely an overestimate given that this was the observed risk among a low-probability group of individuals with symptoms suggestive of ACS) and assume that all biomarker testing ordered in this population had test characteristics equivalent to those of the highly sensitive troponin T assay (95% sensitivity and 80% specificity), 1.7 million individuals would have a false-positive biomarker test result (eg, an elevated biomarker test result in the absence of confirmed ACS).26,27 In other words, even this highly sensitive bio-marker test would have only an 8.8% positive predictive value in this low-risk population. Given that the actual prevalence of ACS among those with no symptoms suggestive of ACS is likely to be less than 2%, we estimate that the actual number of false-positive results is higher and the positive predictive value of cardiac biomarkers among extremely low-risk populations is even lower than this example suggests. Further research is needed to examine the false-positive testing burden and subsequent testing, consultations, and treatment that may follow.
Our findings highlight several potential reasons why health care providers may perform biomarker testing even in the absence of symptoms suggestive of ACS. We found that increasing patient age and disposition status were significant predictors of biomarker testing, which suggests that testing occurs disproportionately among individuals perceived as being sicker, irrespective of the presence of symptoms suggestive of ACS. We also found that the number of other tests or services performed during the visit was by far the strongest predictor of whether cardiac biomarkers were also tested during the visit independent of the presence of symptoms of ACS. These findings suggest that cardiac bio-marker testing may be a consequence of overall high-volume testing behavior. There are a multitude of potential reasons for high-volume testing, including diagnostic uncertainty, defensive medicine, fee-for-service payment models, time pressure, perceived patient reassurance, and practice culture.28-30 However, these results should be interpreted cautiously. The strong association between high-volume testing and obtaining cardiac biomarkers may be the result of other factors not accounted for in the NHAMCS data, including the presence of other classic risk factors for ACS (eg, smoking), comorbidities (eg, coronary artery disease), physical examination findings (eg, Levine sign31), and test results (eg, abnormal electrocardiogram findings). Nonetheless, current practice guidelines suggest that, in the absence of symptoms suggestive of ACS, the presence of these other risk factors should not be heavily weighted in the decision to test for cardiac biomarkers in the ED.32
This study had certain limitations. First, our findings are likely conservative estimates of the use of biomarker testing because our estimates are derived from testing that occurred only during the ED visit and do not capture subsequent testing that may have occurred during the hospitalization. Also, the NHAMCS data may underreport tests performed in theED.33 Second, this study is focused on the use of cardiac biomarker testing only with respect to suspected ACS. Although evidence suggests the use of cardiac biomarker testing for risk stratification and prognostication of other conditions (eg, pulmonary embolism, stroke, congestive heart failure, sepsis, and chronic kidney disease),4 current expert consensus recommends testing for cardiac biomarkers only if clinically indicated for suspected ACS.4 Third, the NHAMCS data do not include granular clinical data (eg, the nature of the chest pain) that would allow for potential ascertainment of the appropriateness of biomarker testing at the individual visit level. However, based on certain features (eg, symptoms of ACS), we were able to reasonably identify visits with low suspicion of ACS. Fourth, results of the cardiac biomarker tests ordered were unknown, prohibiting a direct evaluation of the false-positive burden of overtesting. However, one retrospective analysis of consecutive hospitalized patients with abnormal troponin values at a single medical center found a false-positive rate of almost 80%, among whom almost half of the patients had neither chest pain nor ischemic changes on an electrocardiogram suggestive of ACS.34 Fifth, symptoms of ACS and a cardiovascular visit diagnosis may be underreported because the NHAMCS data recorded up to 3 RVC fields and 3 ED provider's diagnosis fields.
Conclusions
In 2009 and 2010, cardiac biomarker testing occurred in 16.9% of all ED visits in the United States and in 47.0% of visits by individuals subsequently hospitalized for any reason. Of 28.6 million ED visits with cardiac biomarker testing during this period, almost one-third were among individuals without symptoms suggestive of ACS, and only approximately one-quarter were among individuals with chest pain. Cardiac biomarkers were also frequently tested during visits with a high volume of other tests or services independent of the clinical presentation. Given the high use of biomarker testing in populations with a low probability of ACS, the potential downstream costs and harms owing to false-positive test results may be considerable. More attention is needed to better characterize these harms and to develop strategies for targeted and appropriate use of cardiac biomarkers in this setting.
Footnotes
Author Contributions: Dr Makam had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: All authors.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Makam.
Study supervision: Nguyen.
Conflict of Interest Disclosures: None reported.
Additional Contributions: Lei Xuan, PhD, Division of Outcomes and Health Services Research, UT Southwestern Medical Center, assisted with the Agency for Healthcare Research and Quality Clinical Classifications Software. She was not compensated for her work.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JL, Adams CD, Antman EM, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction); American College of Emergency Physicians; Society for Cardiovascular Angiography andInterventions; Society of Thoracic Surgeons; American Association of Cardiovascular and Pulmonary Rehabilitation; Society for Academic Emergency Medicine. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine [published correction appears in J Am Coll Cardiol. 2008;51(9):974] J Am Coll Cardiol. 2007;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;43:1–8. [PubMed] [Google Scholar]
- 4.Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012;60(23):2427–2463. doi: 10.1016/j.jacc.2012.08.969. [DOI] [PubMed] [Google Scholar]
- 5.de Lemos JA. Increasingly sensitive assays for cardiac troponins: a review. JAMA. 2013;309(21):2262–2269. doi: 10.1001/jama.2013.5809. [DOI] [PubMed] [Google Scholar]
- 6.de Lemos JA, Morrow DA, deFilippi CR. Highly sensitive troponin assays and the cardiology community: a love/hate relationship? Clin Chem. 2011;57(6):826–829. doi: 10.1373/clinchem.2011.163758. [DOI] [PubMed] [Google Scholar]
- 7.Brieger D, Eagle KA, Goodman SG, et al. GRACE Investigators. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the Global Registry of Acute Coronary Events. Chest. 2004;126(2):461–469. doi: 10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- 8.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163–1170. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 9.Diamond GA, Kaul S. How would the Reverend Bayes interpret high-sensitivity troponin? Circulation. 2010;121(10):1172–1175. doi: 10.1161/CIR.0b013e3181d839e8. [DOI] [PubMed] [Google Scholar]
- 10.Bandstein N, Ljung R, Johansson M, Holzmann MJ. Undetectable highsensitivity cardiac troponinT level in the emergency department and risk ofmyocardial infarction. J Am Coll Cardiol. 2014;63(23):2569–2578. doi: 10.1016/j.jacc.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Lindsell CJ, Anantharaman V, Diercks D, et al. EMCREG-International i*trACS Investigators. The Internet Tracking Registry of Acute Coronary Syndromes (i*trACS): a multicenter registry ofpatients with suspicion of acute coronary syndromes reported using the standardized reporting guidelines for emergency department chest pain studies. Ann Emerg Med. 2006;48(6):666–677. e1–e9. doi: 10.1016/j.annemergmed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Swinkels BM, Sonke GS, Muller HP, Peters RH. Prevalence and clinical significance of an elevated cardiac troponin I in patients presenting to the emergency department without chest pain. Eur J Intern Med. 2006;17(2):92–95. doi: 10.1016/j.ejim.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. [Accessed February 28, 2014];NHAMCS scope and sample design. http://www.cdc.gov/nchs/ahcd/ahcd_scope.htm#nhamcs_scope.
- 14.National Center for Health Statistics. [Accessed February 28, 2014];2009 NHAMCS micro-data file documentation. ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHAMCS/doc09.pdf.
- 15.National Center for Health Statistics. [Accessed February 28, 2014];2010 NHAMCS micro-data file documentation. ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHAMCS/doc2010.pdf.
- 16.Centers for Disease Control and Prevention. [Accessed February 28, 2014];NHAMCS estimation procedures. http://www.cdc.gov/nchs/ahcd/ahcd_estimation_procedures.htm#nhamcs_procedures.
- 17.Schneider D. An ambulatory care classification system: design, development and evaluation. Health Serv Res. 1979;14(1):77–87. [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider D, Appleton L, McLemore T. A reason for visit classification for ambulatory care. Vital Health Stat 2. 1979;78:i–vi. 1–63. [PubMed] [Google Scholar]
- 19.Agency for Healthcare Research and Quality. [Accessed February 28, 2014];Clinical Classifications Software (CCS) for ICD-9-CM. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
- 20.Archer KJ, Lemeshow S. Goodness-of-fit test for a logistic regression model fitted using survey sample data. Stata J. 2006;6(1):97–105. [Google Scholar]
- 21.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 22.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308–331. [Google Scholar]
- 23.Safavi KC, Li SX, Dharmarajan K, et al. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174(4):546–553. doi: 10.1001/jamainternmed.2013.14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fye WB. Troponin trumps common sense. J Am Coll Cardiol. 2006;48(11):2357–2359. doi: 10.1016/j.jacc.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Selker HP, Beshansky JR, Griffith JL, et al. Use of the acute cardiac ischemia time–insensitive predictive instrument (ACI-TIPI) to assist with triage of patients with chest pain or other symptoms suggestive of acute cardiac ischemia: a multicenter, controlled clinical trial. Ann Intern Med. 1998;129(11):845–855. doi: 10.7326/0003-4819-129-11_part_1-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 27.Selker HP, Griffith JL, D'Agostino RB. A tool for judging coronary care unit admission appropriateness, valid for both real-time and retrospective use: a time-insensitive predictive instrument (TIPI) for acute cardiac ischemia: a multicenter study. Med Care. 1991;29(7):610–627. doi: 10.1097/00005650-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood) 2010;29(9):1569–1577. doi: 10.1377/hlthaff.2009.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolfe A, Burton C. Reassurance after diagnostic testing with a low pretest probability of serious disease: systematic review and meta-analysis. JAMA Intern Med. 2013;173(6):407–416. doi: 10.1001/jamainternmed.2013.2762. [DOI] [PubMed] [Google Scholar]
- 30.van der Weijden T, van Bokhoven MA, Dinant GJ, van Hasselt CM, Grol RP. Understanding laboratory testing in diagnostic uncertainty: a qualitative study in general practice. Br J Gen Pract. 2002;52(485):974–980. [PMC free article] [PubMed] [Google Scholar]
- 31.Marcus GM, Cohen J, Varosy PD, et al. The utility of gestures in patients with chest discomfort. Am J Med. 2007;120(1):83–89. doi: 10.1016/j.amjmed.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 32.Amsterdam EA, Kirk JD, Bluemke DA, et al. American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122(17):1756–1776. doi: 10.1161/CIR.0b013e3181ec61df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaig LF, Burt CW. Understanding and interpreting the National Hospital Ambulatory Medical Care Survey: key questions and answers. Ann Emerg Med. 2012;60(6):716, e1. doi: 10.1016/j.annemergmed.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Nallet O, Arbaoui S, Grenier A, Michaud P, Safrano G, Sergent J. Troponin in emergency department: an overused test for patient screening without clinical suspicion of acute coronary syndrome?. Arch Cardiovasc Dis; Proceedings from the XXIst European Days of the French Society of Cardiology; January 12-15, 2011; Paris, France. 2011. p. 109. Abstract 324. [Google Scholar]