Abstract
The transmission of Buruli ulcer (BU), caused by Mycobacterium ulcerans (MU), remains puzzling although a number of hypothesis including through bites of infected aquatic insects have been proposed. We report the results of experiments using ICR mice that give credence to our hypothesis that Acanthamoeba species may play a role in BU transmission. We cocultured MU N2 and MU 1615 which expresses red fluorescent protein (RFP) and Acanthamoeba polyphaga (AP), and confirmed infected AP by Ziehl-Neelsen (ZN) staining. We tested for viability of MU inside AP and observed strong RFP signals inside both trophozoites and cysts after 3 and 42 days of coculturing respectively. ICR mice were topically treated, either on shaved intact or shaved pinpricked rumps, with one of the following; MU N2 only (2.25 x 106 colony forming units [CFU] / ml), MU N2:AP coculture (2.96 x 104 CFU: 1.6 x 106 cells/ml), AP only (1.6 x 106 cells/ml), PYG medium and sterile distilled water. Both MU N2 only and MU N2:AP elicited reddening on day (D) 31; edema on D 45 and D 44 respectively, and ulcers on D 49 at pinpricked sites only. To ascertain infectivity and pathogenicity of MU N2 only and MU N2:AP, and compare their virulence, the standard mouse footpad inoculation method was used. MU N2:AP elicited reddening in footpads by D 3 compared to D 14 with MU N2 only of the same dose of MU N2 (2.96 x 104 CFU). ZN-stained MU were observed in both thin sectioned and homogenized lesions, and aspirates from infected sites. Viable MU N2 were recovered from cultures of the homogenates and aspirates. This study demonstrates in ICR mice MU transmission via passive infection, and shows that punctures in the skin are prerequisite for infection, and that coculturing of MU with AP enhances pathogenesis.
Introduction
Buruli ulcer (BU), a necrotic skin disease caused by Mycobacterium ulcerans, is the third most prevalent mycobacterial infection after tuberculosis and leprosy [1]. It has been reported in West Africa, Central Africa, East Africa, Australia, China, South America and Japan with West Africa being the most endemic region [2].
Although the associated risk factors have been adequately studied [2,3], the mode of transmission still remains poorly understood [2]. Two main transmission hypotheses have been expounded; direct inoculation into broken skin through contact with contaminated environmental sources [2,4] and vector-mediated transmission [4–7].
Based on the detection of M. ulcerans DNA in the environment, many agents have been speculated as possible reservoirs [2] and this has given support to the proposition that contact with environmental reservoirs is the source of transmission. However analysis of the M. ulcerans genome and pathogenic mechanisms have revealed genome reduction and intracellular niche specialization in the environment [8–10] thus indicating that biological reservoirs such as amoebae may also be likely candidates.
It has been demonstrated in laboratory studies that biting aquatic bugs (Naucoridae) fed on M. ulcerans-infested grub, could transmit M. ulcerans through bites and cause BU lesions in mice [7]. In Australia, mosquitoes have also been implicated as possible insect vectors because M. ulcerans DNA has been detected in lysates of pooled mosquitoes. Also the M. ulcerans DNA positivity rate of sampled mosquitoes correlated with BU endemicity in local communities [11]. Additionally, a laboratory-based study showed that M. ulcerans DNA was found to persist in three successive instars of mosquito [12]. A recent experimental mouse-tail infection model has shown that Anopheles notoscriptus, could transmit M. ulcerans to mice through bites and cause BU [13].
Free living amoebae (FLA) have been reported severally in literature as possible reservoirs of pathogenic mycobacteria [14–20]. The difficulty in implicating an arthropod vector for the transmission of leprosy, and the intracellular-niche-requiring character of M. leprae led to the demonstration that Acanthamoeba spp could successfully maintain viable M. leprae intracellularly [17]. This implicates Acanthamoeba spp as an important reservoir in the transmission of leprosy in nature. Similarly, we had earlier posited that Acanthamoeba spp may play an important role in BU transmission but did not support it with data [21]. However a study by Gryseels et al. [22] undermined the potential role of Acanthamoeba in BU transmission in the environment. The current study provides evidence, albeit in a laboratory model, in support of the hypothesis that Acanthamoeba spp may play a role in BU transmission. Furthermore, we also investigated whether Acanthamoeba polyphaga would enhance the virulence of M. ulcerans as reported for M. leprae [19] and M. avium [20].
Studies have demonstrated that injection of M. ulcerans into the footpad of mice [23], skin of grasscutters [24] and guinea pigs [25] results in BU, but topical application of M. ulcerans on the abraded skin of the same guinea pigs did not lead to BU [25], suggesting that deeper dermal inoculation is required for transmission. Here, we show in an ICR mouse model that passive inoculation of naked M. ulcerans via contact with punctured skin could result in BU. We also show here for the first time that the mouse model could present undermined ulcer, which is the hallmark of BU. Finally, our study demonstrates for the first time that A. polyphaga cocultured with M. ulcerans causes BU. Our study also demonstrates that coculturing A. polyphaga with M. ulcerans enhances BU pathogenesis.
Methods
Ethical considerations
The Scientific and Technical Committee of Noguchi Memorial Institute for Medical Research (NMIMR) approved the study. The study protocols and procedures received approval from the NMIMR Institutional Animal Care and Use Committee (NIACUC#: 2014-01-1N). The NIACUC is governed by the Public Health Service (PHS), Animal Welfare Act (AWA) and the United States Department of Agriculture and is guided by the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training.
Standard hygienic practices of laboratory animal maintenance were ensured. Animal usage was in accordance with Applied Research Ethics National Association and the institutional guidelines on animal research.
Animals and maintenance
Male and female outbred ICR mice produced in the Department of Animal Experimentation of the Noguchi Memorial Institute for Medical Research were used for the experiments. Forty-eight (48) mice were put into 16 groups of three mice per cage. The mice were maintained in a level two animal containment facility with a 12–12 hour light-dark cycle automatic lighting system, an ambient temperature of 23–25°C and relative humidity of 55–65%. The mice were fed daily on autoclaved feed pellets and water ad libitum.
Maintenance of Mycobacterium ulcerans and A. polyphaga cultures
The Mycobacterium ulcerans N2 strain used in this study was isolated from biopsy of a BU patient who had to undergo reparative surgical treatment at Nsawam General Hospital, Ghana in 2007. Our laboratory served as a reference laboratory that undertook confirmatory tests for BU cases that year. The M. ulcerans N2 strain was identified as an IS2404 positive organism and isolates were cultured on Lӧwenstein-Jensen (LJ) plates at 32°C for 8 weeks. The M. ulcerans 1615::RFP and A. polyphaga 1501/3b used in this study were kind donations from Heather Williamson formerly of the University of Tennessee, USA to Lydia Mosi. The A. polyphaga trophozoites were grown in PYG medium in 25 cm2 culture flask at 30°C.
Preparation of M. ulcerans suspension
Mycobacterium ulcerans (all strains) suspension [(2.25 ± 0.05) x 106 CFU/ml) was prepared from eight-week-old axenic cultures. Briefly, colonies of M. ulcerans grown on LJ slants were scraped off and introduced into 1ml sterile double distilled water in sterile universal culture bottle that contained sterile glass beads. The tightly closed bottle was placed on ice for 2 minutes and then vortexed vigorously for 20 seconds to break up the clumps of M. ulcerans. The cycle was repeated with intermittent addition of distilled water; the suspension was allowed to settle and thereafter the supernatant was decanted into sterile universal bottle. The turbidity of the supernatant was matched with freshly prepared McFarland (McF) 5 nephelometric standard and adjustments made by intermittent addition of sterile distilled water. The adjusted supernatant was used within an hour. To determine the CFU/ml of the McF 5 M. ulcerans suspension, 100 μl each of 1 in 10 dilutions (10−1 to 10−9) of McF 5 M. ulcerans suspension was inoculated on LJ slants in triplicates and incubated at 32°C for eight weeks. The average CFU/ml was determined for the 1 in 100 dilution inocula on three LJ slants.
Coculture of A. polyphaga trophozoites and M. ulcerans
Acanthamoeba polyphaga trophozoites were cultured in PYG medium at 30°C until confluence reached about 70%. The viable cell density was determined to be 1.6 ± 0.1 x 106 cells/ml. This was done by staining cells with 0.4% Trypan Blue solution (Gibco, UK) in a 1:4 ratio, and counting viable cells using a Neubauer improved Hemocytometer (Blaubrand, Germany).
An aliquot of 200μl M. ulcerans suspension [(2.25 ± 0.05) x 106 CFU/ml] was added to 15 ml of PYG medium containing 1.6 ± 0.1 x 106 cells/ml of A. polyphaga trophozoites in 25 cm2 culture flask. The coculture was incubated at 30°C with daily observation for three days for the animal infection studies, and 42 days for viability studies using red fluorescent protein expression by M. ulcerans 1615::RFP in trophozoites and cysts respectively. Culturing for 42 days without changing media stresses the trophozoites through nutrient depletion and thus makes them encyst.
Direct examination of M. ulcerans viability using fluorescence from M. ulcerans::RFP inside trophozoites and cysts of A. polyphaga
Mycobacterium ulcerans: A. polyphaga coculture (ratio = 2.96 x 104 CFU/ml: 1.6 x 106 cells/ml) was incubated for 3 days and 42 days without changing growth medium. For the three day culture, the medium was decanted and washed twice (3000 rpm for 5 minutes at 25°C) with freshly prepared PYG medium. The washed trophozoites were finally detached from the walls of the flask using 0.25% Trypsin—EDTA (1X) solution (Gibco, UK). Briefly, 1ml of the Trypsin solution was added to adhered trophozoites in the flask and incubated at 30°C for 5 minutes. Thereafter, 15ml of PYG medium was added and the flask was tapped gently to suspend the cells in solution. The cell suspension was then centrifuged at 3000rpm for 5 minutes at 25°C. The supernatant was decanted and the pelleted cells suspended in 5 ml of freshly prepared PYG medium and then 1 ml aliquots dispensed onto sterile glass cover slides. Slides were incubated for 10 minutes in a humid chamber and thereafter observed through the red channel of a fluorescent microscope (Olympus VX41, Japan). For the cysts, the 42-day old M. ulcerans: A. polyphaga coculture was harvested by centrifuging at 3000 rpm for 5 minutes at 25°C. Cysts were washed twice with sterile distilled water as described and finally centrifuged to concentrate cell pellets. The cells were then applied onto slides and similarly observed under a fluorescent microscope.
Coculture preparation for animal infection studies
After incubation, the medium was decanted and the adhered trophozoites were washed three times with freshly prepared PYG medium. Supernatant from the last wash was inoculated on LJ slants for growth of M. ulcerans and also examined for the presence of extracellular M. ulcerans using Ziehl-Neelsen (ZN) acid-fast staining method. Extracellular mycobacteria were not seen in supernatant, neither was M. ulcerans growth observed on LJ after 10 weeks of incubation. The washed trophozoites were finally detached from the walls of the flask using 0.25% Trypsin—EDTA (1X) solution (Gibco, UK) as described above. After centrifugation, the cell pellet was suspended in 5 ml of freshly prepared PYG medium. This was used immediately for the infection experiments. An aliquot of 100μl of the cell suspension was ZN-stained and examined under the microscope (1000X) to confirm the internalization of M. ulcerans in A. polyphaga trophozoites. Additionally, 2 ml aliquot of the cell suspension was treated with 2ml of 0.5% sterile SDS solution for 5 minutes to lyse the trophozoites and then washed three times with sterile distilled water by centrifuging at 3000 rpm for 5 minutes. The lysate was again reconstituted in 2ml of sterile distilled water. An aliquot of 1 ml of the cell lysate was then inoculated on LJ slants and incubated at 32°C to confirm growth of intracellular M. ulcerans.
Animal infection experiments
Forty-eight (48) ICR mice were placed under two treatment groups; A and B. In group A, 9 mice divided equally into three groups, were shaved on their rumps and the area disinfected with 70% ethanol. The rumps were then superficially pinpricked (10 pinpricks at a depth of about 1.5mm) using a sterile 27-gauge TERUMO needle (TERUMO Corporation, Japan) over an area of 1cm2. The three groups of mice (3 mice each) were then infected by topical application of 50 μl of each of the following inocula: i) 2.25 x 106 CFU/ml M. ulcerans, ii) M. ulcerans: A. polyphaga (ratio = 2.96 x 104 CFU/ml: 1.6 x 106 cells/ml) and iii) 1.6 x 106 cells/ml A. polyphaga. Three other groups of mice were shaved on their rumps but not bruised. The skins of these groups were similarly treated as described above. Negative control groups (n = 12) were shaved on their rumps and the skin was either left intact or pinpricked. The intact and the pinpricked skins were treated with either distilled water or PYG medium or left untreated.
In the last treatment group (B), the right footpads of 12 mice, divided equally into four groups, were disinfected with 70% ethanol and injected with 50 μl suspensions of either; i) 2.25 x 106 CFU/ml M. ulcerans, or ii) 2.96 x 104 CFU/ml M. ulcerans, or iii) M. ulcerans: A. polyphaga (ratio = 2.96 x 104 CFU/ml: 1.6 x 106 cells/ml), or iv) 1.6 x 106 cells/ml A. polyphaga. In the negative control group of 6 mice, divided into two equal groups, the right footpads were injected with either 50 μl of distilled water or PYG medium. The mice were monitored daily for development of BU lesions. Mice from both M. ulcerans only and M. ulcerans: A. polyphaga treatment groups that had developed full-blown ulcers were euthanized by injecting intraperitoneally with an overdose of pentobarbital (200 mg/kg IP). The ulcerated skin tissues were surgically removed and immediately immersed in a 10x volume of neutral 10% formalin for subsequent histological processing.
Isolation of M. ulcerans from lesions and microbiological analysis
Samples of lesions from live and dead mice were taken and examined for the presence of M. ulcerans. In the case of live mice, fine needle aspirates (FNA) of lesions were taken aseptically. A portion of the sample was inoculated on LJ slants and incubated for 8 weeks at 32°C for M. ulcerans growth, while the remaining portion was smeared on glass slides and ZN-stained for detection of AFBs. Lesions from dead mice were excised, homogenized and decontaminated using the Petroff’s decontamination method [26]. Briefly, tissues were homogenized in 2ml sterile distilled water by grinding in a sterile mortar and mixed with 2ml of 4% NaOH to digest for 20 minutes with intermittent agitation. The suspension was then mixed with 2ml of 1% HCl containing Safranin red to neutralize the basic homogenate. The suspension was centrifuged at 3000 rpm for 20 minutes at 25°C. The pellet was washed twice with 10ml distilled water and centrifuged as described above and finally suspended in 1ml distilled water. One hundred microliters of the final suspension was inoculated on LJ slants and incubated at 32°C for M. ulcerans growth. The remaining suspension was also ZN-stained for AFB detection.
Histological examination of ulcerated skin tissues
The formalin preserved tissues obtained from the euthanized mice were embedded in wax and thereafter thin sections of 5 microns thickness were cut using a microtome (Bright 5040, England). The sections were then fixed on sterile slides and ZN-stained.
Results
Internalization and viability of M. ulcerans (acid-fast bacilli) within A. polyphaga trophozoites and cysts
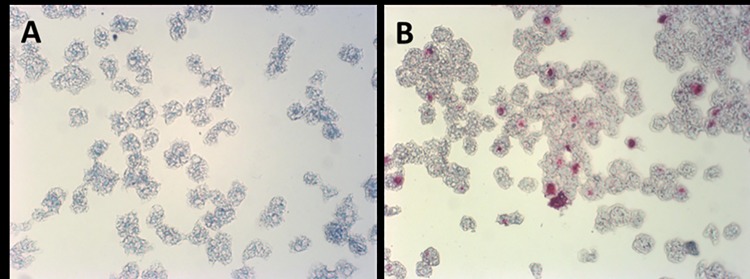
To determine whether A. polyphaga could successfully internalize and harbor M. ulcerans, axenic culture of A. polyphaga was cocultured with M. ulcerans for three days. No acid-fast particles were observed in axenic cultures of A. polyphaga when ZN stained [stained bluish] (Fig 1A). Acid-fast bacilli (stained reddish-pink) were consistently found internalized in trophozoites of M. ulcerans-infected A. polyphaga 3 days post-culture (Fig 1B). Profuse growth of M. ulcerans colonies were also observed after incubating M. ulcerans-infected A. polyphaga cell lysates on LJ slants for 8 weeks.
Fig 1. Visualization of M. ulcerans within A. polyphaga trophozoites.
Panel A shows ZN stain of A. polyphaga trophozoites. No acid-fast particle was seen in the axenic culture of A. polyphaga trophozoites. Panel B shows a ZN stain of A. polyphaga trophozoites infected with M. ulcerans. A. polyphaga trophozoites stained bluish and M. ulcerans stained reddish- pink (acid-fast particles). Acid fast particles denoting the presence of M. ulcerans can be seen inside trophozoites of A. polyphaga infected with M. ulcerans. The average rate of infection was 47% ± 8.6 (SD) (average counts in 5 fields randomly selected under 200x magnification).
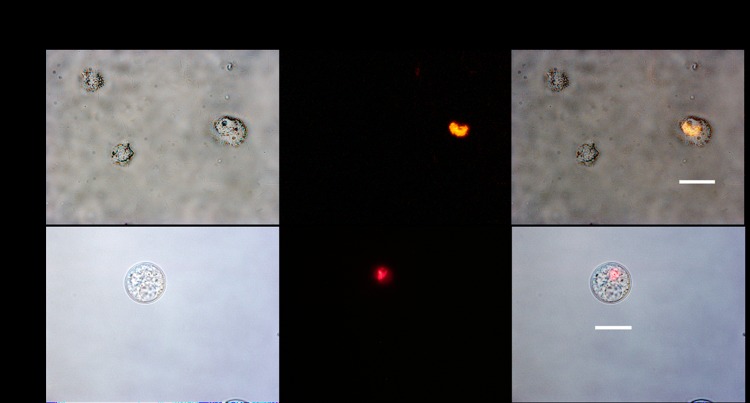
Strong red fluorescence from expressed red fluorescent proteins of M. ulcerans 1615::RFP were intracellularly localized in A. polyphaga trophozoites and cysts, three and 42 days respectively of coculturing (Fig 2).
Fig 2. Detection of strong RFP signals of M. ulcerans 1615::RFP inside trophozoites and cyst of A. polyphaga.
RFP signals were detected in trophozoites (3 days coculture) and also cyst (42 days coculture). Scale bar = 20μm.
Passive infection of mice with M. ulcerans only and M. ulcerans: A. polyphaga coculture
Previous studies have shown that M. ulcerans alone, without hypodermal injection, was incapable of spontaneously infecting lacerated skins of guinea pigs [25] by passive inoculation, therefore it was of interest to determine if A. polyphaga cocultured with M. ulcerans could confer passive infection of broken skin.
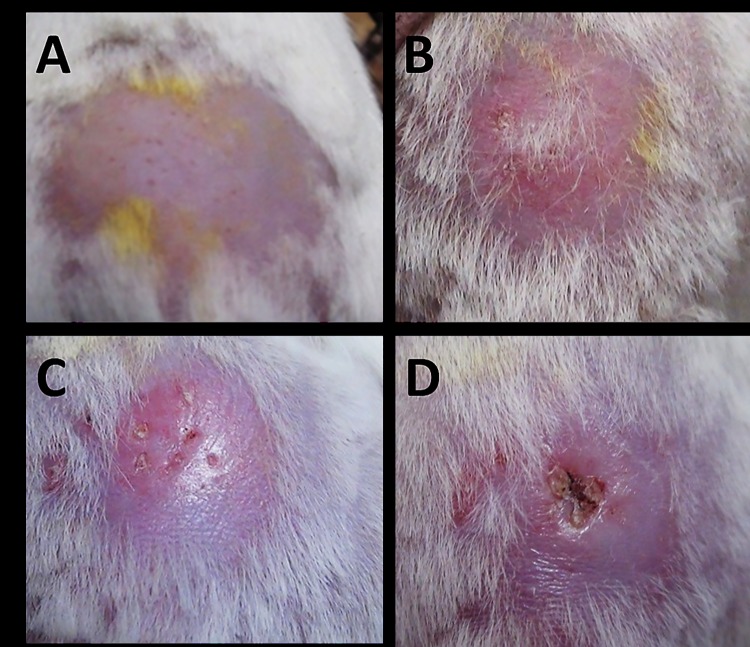
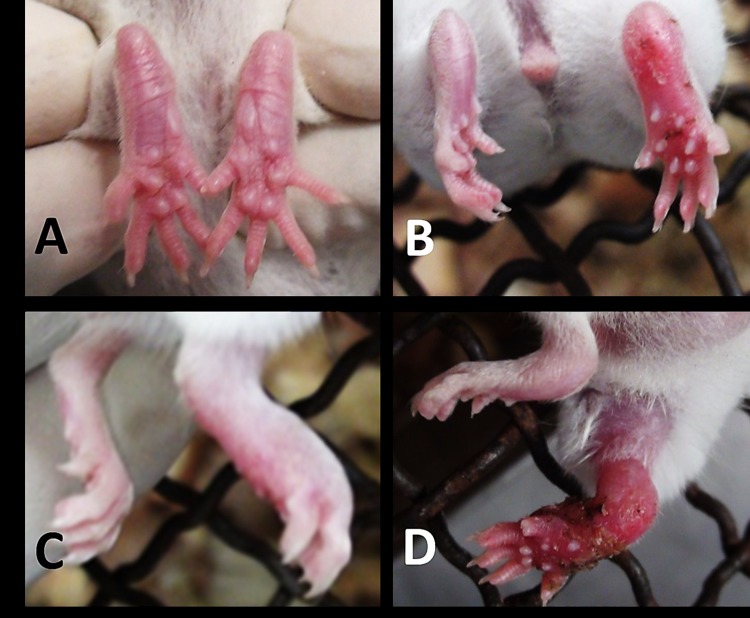
Progressive development of BU lesions were observed in mice when the punctured skins of the rump (Fig 3A) were topically treated with A. polyphaga-infected M. ulcerans. Inflammation (erythema) was observed at the punctured sites 31 days post inoculation (dpi) (Fig 3B), followed by edema on 44 dpi (Fig 3C) and ulcers on 49 dpi (Fig 3D). Fine needle aspirate (FNA) samples from the ulcers were all AFB positive.
Fig 3. The progressive development of BU in mouse topically treated with M. ulcerans-infected A. polyphaga at the punctured skin of the rump (lower back).
Panel A shows site of inoculation 1 day post-inoculation (dpi). Panel B shows inflammation (erythema) at site of inoculation 31 dpi. Panel C shows an edema at site of inoculation 44 dpi. Panel D shows ulcer at the site of inoculation 49 dpi. Photograph is representative of group (n = 3).
Similarly, topical application of 2.25 x 106 CFU/ml of M. ulcerans on punctured skin also elicited BU lesions (S1 Fig) with the same characteristics as that seen with M. ulcerans-infected A. polyphaga. This also elicited visible inflammation at the inoculation sites on 31 dpi (Table 1). However, in mice with intact shaved skins, none of the treatments elicited any noticeable pathological response even after five months of monitoring.
Table 1. The progression of BU signs in the various treatment groups post-inoculation.
| Experiments | Earliest time (days) of visible signs post-inoculation (respondents/number of mice) | AFB in fine needle aspirate (FNA) from ulcer | M. ulcerans culture from FNA and from ulcer | ||
|---|---|---|---|---|---|
| Infection by topical application of inoculum on punctured skin | Inflammation | Edema | Ulcer | ||
| 50 μl of M. ulcerans (2.25 x 106 CFU/ml), positive control (Group 1, n = 3) | 31 (3/3) | 45 (3/3) | 49 (3/3) | Yes (3/3) | Yes (3/3) |
| 50 μl of A. polyphaga (1.6 x 106 cells/ml) infected with M. ulcerans (2.96 x 104 CFU / ml) (Group 2, n = 3) | 31 (3/3) | 44 (3/3) | 49 (3/3) | Yes (3/3) | Yes (3/3) |
| 50 μl of A. polyphaga trophozoites (1.6 x 106 cells/ml) (Group 3, n = 3) | None (3/3) | None | None | Not applicable (N/A) | N/A |
| 50 μl of PYG (Group 4, n = 3) | None (3/3) | None | None | N/A | N/A |
| 50 μl of distilled water (Group 5, n = 3) | None (3/3) | None | None | N/A | N/A |
| Infection by injection into footpad | |||||
| 50 μl of M. ulcerans (2.25 x 106 CFU / ml,) positive control (Group 4, n = 3) | 1 (3/3) | 1 (3/3) | 21 (3/3) | Yes (3/3) | Yes (3/3) |
| 50 μl of A. polyphaga (1.6 x 106 cells/ml) infected with M. ulcerans (2.96 x 104 CFU / ml)) (Group 5, n = 3) | 3 (3/3)* | 7 (3/3) | 25 (3/3) | Yes (3/3) | Yes (3/3) |
| 50 μl of M. ulcerans (2.96 x 104 CFU / ml) (Group 6, n = 3) | 14 (3/3) | 31 (3/3) | 51 (3/3) | Yes (3/3) | Yes (3/3) |
| 50 μl of PYG (Group 4, n = 3) | None (3/3) | None | None | N/A | N/A |
| 50 μl of distilled water (Group 5, n = 3) | None (3/3) | None | None | N/A | N/A |
AFB = Acid fast bacilli, FNA = Fine needle aspirate
Effect of coculturing A. polyphaga with M. ulcerans on pathogenesis of BU
The virulence of some mycobacteria has been reported to be enhanced when cocultured with Acanthamoeba spp [16,19,20]. We therefore tested the hypotheses that coculturing M. ulcerans with A. polyphaga will enhance the pathogenesis of BU.
Footpads injected with M. ulcerans:A. polyphaga coculture (ratio = 2.96 x 104 CFU/ml: 1.6 x 106 cells/ml) showed inflammation on 3 dpi, whiles footpads injected with the same concentration of naked M. ulcerans showed inflammation 14 dpi (Table 1). Subsequent progressive signs appeared earlier for M. ulcerans:A. polyphaga than those of M. ulcerans alone of the same concentration (Table 1). However inoculation with a higher dose of naked M. ulcerans (2.25 x 106 CFU/ ml) showed inflammation earlier (1 dpi) than a lower dose (2.96 x 104 CFU/ ml) in coculture with A. polyphaga (3 dpi). Treatment with A. polyphaga alone at the same dose of 1.6 x 106 cells/ml did not show any lesion for the entire duration of study (5 months). M. ulcerans:A. polyphaga coculture and M. ulcerans alone showed similar signs (Fig 4), while no symptom was observed in the two controls; PYG and distilled water treatments.
Fig 4. The progressive stages of development of BU in mouse injected in the right footpad with M. ulcerans-infected A. polyphaga.
Panel A shows footpad 1 dpi. Panel B shows footpad with erythema 3 dpi. Panel C shows footpad with edema 7 dpi. Panel D shows swollen footpad and thigh 25 dpi. The photographs are typically representatives of each group (n = 3).
Microbiological and histopathological examination of lesions
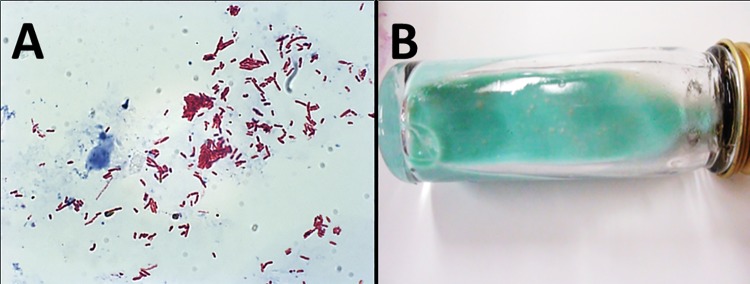
ZN staining of the homogenates of infected footpads of mice injected with M. ulcerans alone and M. ulcerans-A. polyphaga coculture confirmed the presence of acid-fast bacilli (Fig 5A). Also, viable M. ulcerans colonies were recovered from the culture of tissue homogenates (Fig 5B).
Fig 5. Demonstration of viable M. ulcerans in lesions.
Panel A shows a ZN stain of tissue homogenate from footpad of mouse injected with M. ulcerans-infected A. polyphaga. Profuse acid-fast particles (reddish pink) were seen in tissue homogenate. Panel B shows LJ slant (green) with colonies of M. ulcerans (yellowish) recovered from tissue homogenate of the footpad of mouse injected with M. ulcerans-infected A. polyphaga.
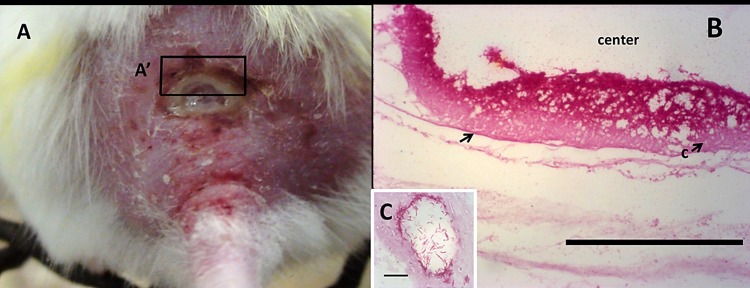
ZN stained thin sections of the ulcerated skin of mouse (Fig 6A) treated either with M. ulcerans-A. polyphaga coculture or M. ulcerans only showed presence of acid fast bacilli localized at the margins adjacent to the center of the ulcer (Fig 6B). Acid-fast bacilli appeared clustered in ring-like units (Fig 6C).
Fig 6. Gross morphology and ZN-stained section of the ulcer showing the distribution of acid-fast bacilli (i.e. M. ulcerans).
Panel A shows undermined ulcer at the back of a mouse. Panel B shows ZN stained thin section of A´ with an arrow showing bed of acid-fast bacilli localized at the edge of the ulcer. Panel C shows ring-like clustering unit of acid fast bacilli in panel B (the scale bar measures 10 μm).
Discussion
Several mycobacteria including M. ulcerans have been shown to survive in free living amoeba [27]. Studies by Gryseels et al. [22] and Amissah et al. [28] have also confirmed that M. ulcerans can survive in Acanthamoeba spps. Our study also confirms the survival of M. ulcerans 1615::RFP in trophozoites and cysts of A. polyphaga over 3 days and 42 days respectively. Cysts of Acanthamoeba spp have been reported to protect bacteria against extreme conditions of pH, humidity, nutrient availability, biocides and temperature [27]. The presence of viable M. ulcerans in cysts of A. polyphaga thus suggests the possibility of A. polyphaga cysts serving as protective reservoirs of M. ulcerans in unfavorable environmental conditions. Although Gryseels et al. [22] were not able to detect the presence of M. ulcerans-infected free living amoeba in the environment and further suggested their unlikely role in BU transmission, we are of the contrary view that this could not be ruled out and thus recommend further studies.
M. ulcerans-infected mosquitoes and aquatic bugs have been shown to cause BU lesions by biting intact tails of mice in controlled laboratory conditions [7,13]. Williamson et al. [25] also showed that hypodermal injection of M. ulcerans into the skin of guinea pigs was the only means by which infections could be established. BU lesions are mostly distributed specifically at the elbows and ankles, and generally at lower body extremities that are less protected by clothings [29,30]. In Cameron, women who reportedly covered their skin had less lesions on their trunks [29]. In Southeasten Australia, the odds of contracting BU was halved when insect repellents were applied and long trousers worn, both protecting the lower extremeties against insect bites [31]. Agreeably, the odds of having BU was found to be doubled for people who reported receiving mosquito bites at the lower extremeties [31]. These studies collectively support mechanical inoculation, amongst other possible means, as a possible route of BU transmission.
We have shown experimentally, and for the first time, that when both M. ulcerans alone and A. polyphaga-M. ulcerans coculture were applied externally on punctured skin, and not by mechanical inoculation, it resulted in infections and subsequent disease progression. Recently Jordan et al. [32] using molecular methods showed that M. ulcerans could be a contaminant on the skin. Skin abrasions happen in nature when passing through vegetation and also through normal human activities such as farming. We therefore propose that these abrasions and other sources of wounding may serve as portals of entry for M. ulcerans and M. ulcerans-infected Acanthamoeba spp that settle on the skin.
In this study, the skin infection caused by both naked M. ulcerans and M. ulcerans-A. polyphaga coculture progressed from an initial stage of inflammation (reddening, swelling), followed by eye-spot ulcers at the sites of infection, which progressed to become full-blown ulcers with undermined edges. The times of the appearance of signs were similar for both the higher dose of 2.25 x 106 CFU/ml M. ulcerans alone and the lower dose of 2.96 x 104 CFU/ml of M. ulcerans in the coculture.
The virulence of some mycobacteria has been shown to be enhanced by coculturing with Acanthameoba spps [16,19,20]. Based on this premise we hypothesized that A. polyphaga in coculture with M. ulcerans, will enhance the pathogenesis of BU. To test the A. polyphaga-enhanced BU pathogenicity hypothesis, we injected into mice footpads the same dose (2.96 x 104 CFU/ml) of M. ulcerans alone, and M. ulcerans:A. polyphaga coculture, and observed a marked difference in times of appearance of signs. M. ulcerans alone elicited inflammation (reddening) of the footpads 14 dpi whiles for M. ulcerans-A. polyphaga coculture, inflammation appeared 3 dpi. The corresponding times for edema were 31 and 7, and for the development of ulcers were 52 and 25 dpi respectively. Altogether these results suggest that A. polyphaga enhances the virulence of M. ulcerans when cocultured. Therefore, this study has for the first time demonstrated that the pathogenicity of BU is enhanced when M. ulcerans is cocultured with A. polyphaga. Our finding is consistent with reports that passage of some intracellular bacteria via free living amoeba selects for virulence traits and enhances pathogenicity such as the ability to evade destruction in mammalian macrophages [16,20,33,34].
We examined the sections of the ulcers and observed the classical localized bed of acid fast bacilli at the undermined sections of the ulcer similar to that found in BU patients. Regular release of exudates, also characteristic of BU in humans, were also observed. Cultures of the lesion aspirates and lesion homogenates revealed the presence of viable M. ulcerans suggesting that the lesions seen were due to M. ulcerans infection. This study has therefore discovered the ICR mouse as a potential animal model for BU studies.
To summarize, we have demonstrated clearly that passive inoculation via punctured skin could be another route of transmission of BU. We have also identified the ICR mouse as another animal model for BU studies. In addition to the footpad, we have also identified the rump as another site for M. ulcerans inoculations, and which in addition to the known BU presentations in mice, also elicits undermined ulcer—the hallmark of BU. Finally, our results demonstrate for the first time the possible role of A. polyphaga in the transmission of BU by enhancing the pathogenicity of BU when cocultured with M. ulcerans and possibly playing the role of a vector.
Supporting information
Panel A shows site of inoculation 1 dpi. Panel B shows inflammation (erythema) at site of inoculation 31 dpi. Panel C shows an edema at site of inoculation 45 dpi. Panel D shows ulcer at the site of inoculation 49 dpi. Photograph is representative of group (n = 3).
(TIF)
Acknowledgments
We are grateful to Professor Kwadwo Ansah Koram the Director of NMIMR for permission to publish this article. We thank the staff of the Department of Animal Experimentation especially Mona Abbas and Maxwell Quartey for their technical assistance. The study was funded with a grant award by the Office of Research Innovation and Development (ORID), University of Ghana to Phyllis Gertrude Addo (grant award #: URF/7/ILG-021/2013-2014).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded with a grant award by the Office of Research Innovation and Development (ORID), University of Ghana (www.orid.ug.edu.gh) to Phyllis Gertrude Addo (grant award #: URF/7/ILG-021/2013-2014). ORID had no role in the study design, data collection and analysis, and the decision to publish, or in the preparation of the manuscript.
References
- 1.Boleira M, Lupi O, Lehman L, Asiedu KB, Kiszewski AE (2010) Buruli ulcer. An Bras Dermatol 85: 281–298; quiz 299–301. [DOI] [PubMed] [Google Scholar]
- 2.Merritt RW, Walker ED, Small PL, Wallace JR, Johnson PD, Benbow ME, et al. (2010) Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl Trop Dis 4: e911 10.1371/journal.pntd.0000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobsen KH, Padgett JJ (2010) Risk factors for Mycobacterium ulcerans infection. Int J Infect Dis 14: e677–681. 10.1016/j.ijid.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 4.Huang GK, Johnson PD (2014) Epidemiology and management of Buruli ulcer. Expert Rev Anti Infect Ther 12: 855–865. 10.1586/14787210.2014.910113 [DOI] [PubMed] [Google Scholar]
- 5.Portaels F, Elsen P, Guimaraes-Peres A, Fonteyne PA, Meyers WM (1999) Insects in the transmission of Mycobacterium ulcerans infection. Lancet 353: 986 10.1016/S0140-6736(98)05177-0 [DOI] [PubMed] [Google Scholar]
- 6.Marsollier L, Aubry J, Milon G, Brodin P (2007) [Aquatic insects and transmission of Mycobacterium ulcerans]. Med Sci (Paris) 23: 572–575. [DOI] [PubMed] [Google Scholar]
- 7.Marsollier L, Robert R, Aubry J, Saint Andre JP, Kouakou H, Legras P, et al. (2002) Aquatic insects as a vector for Mycobacterium ulcerans. Appl Environ Microbiol 68: 4623–4628. 10.1128/AEM.68.9.4623-4628.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, et al. (2007) Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res 17: 192–200. 10.1101/gr.5942807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stinear TP, Jenkin GA, Johnson PD, Davies JK (2000) Comparative genetic analysis of Mycobacterium ulcerans and Mycobacterium marinum reveals evidence of recent divergence. J Bacteriol 182: 6322–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva MT, Portaels F, Pedrosa J (2009) Pathogenetic mechanisms of the intracellular parasite Mycobacterium ulcerans leading to Buruli ulcer. Lancet Infect Dis 9: 699–710. 10.1016/S1473-3099(09)70234-8 [DOI] [PubMed] [Google Scholar]
- 11.Lavender CJ, Fyfe JA, Azuolas J, Brown K, Evans RN, Ray LR, et al. (2011) Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in southeastern Australia. PLoS Negl Trop Dis 5: e1305 10.1371/journal.pntd.0001305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace JR, Gordon MC, Hartsell L, Mosi L, Benbow ME, Merritt RW, et al. (2010) Interaction of Mycobacterium ulcerans with mosquito species: implications for transmission and trophic relationships. Appl Environ Microbiol 76: 6215–6222. 10.1128/AEM.00340-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stinear PT, Wallace JR, Marcsisin R, Porter JL, Axford J, Omansen FT, et al. Mechanical transmission of Mycobacterium ulcerans may resolve the mystery of Buruli ulcer; 2015; Geneva, Switzerland: WHO; pp. 25. [Google Scholar]
- 14.Snelling WJ, Moore JE, McKenna JP, Lecky DM, Dooley JS (2006) Bacterial-protozoa interactions; an update on the role these phenomena play towards human illness. Microbes Infect 8: 578–587. 10.1016/j.micinf.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 15.Hilbi H, Weber SS, Ragaz C, Nyfeler Y, Urwyler S (2007) Environmental predators as models for bacterial pathogenesis. Environ Microbiol 9: 563–575. 10.1111/j.1462-2920.2007.01238.x [DOI] [PubMed] [Google Scholar]
- 16.Salah IB, Ghigo E, Drancourt M (2009) Free-living amoebae, a training field for macrophage resistance of mycobacteria. Clin Microbiol Infect 15: 894–905. 10.1111/j.1469-0691.2009.03011.x [DOI] [PubMed] [Google Scholar]
- 17.Lahiri R, Krahenbuhl JL (2008) The role of free-living pathogenic amoeba in the transmission of leprosy: a proof of principle. Lepr Rev 79: 401–409. [PubMed] [Google Scholar]
- 18.White CI, Birtles RJ, Wigley P, Jones PH (2010) Mycobacterium avium subspecies paratuberculosis in free-living amoebae isolated from fields not used for grazing. Vet Rec 166: 401–402. 10.1136/vr.b4797 [DOI] [PubMed] [Google Scholar]
- 19.Wheat WH, Casali AL, Thomas V, Spencer JS, Lahiri R, Williams DL, et al. (2014) Long-term survival and virulence of Mycobacterium leprae in amoebal cysts. PLoS Negl Trop Dis 8: e3405 10.1371/journal.pntd.0003405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cirillo JD, Falkow S, Tompkins LS, Bermudez LE (1997) Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun 65: 3759–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson MD, Boakye DA, Mosi L, Asiedu K (2011) In the case of transmission of Mycobacterium ulcerans in buruli ulcer disease Acanthamoeba species stand accused. Ghana Med J 45: 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gryseels S, Amissah D, Durnez L, Vandelannoote K, Leirs H, De Jonckheere J, et al. (2012) Amoebae as potential environmental hosts for Mycobacterium ulcerans and other mycobacteria, but doubtful actors in Buruli ulcer epidemiology. PLoS Negl Trop Dis 6: e1764 10.1371/journal.pntd.0001764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Addo P, Owusu E, Adu-Addai B, Quartey M, Abbas M, Dodoo A, et al. (2005) Findings from a Buruli ulcer mouse model study. Ghana Med J 39: 86–93. [PMC free article] [PubMed] [Google Scholar]
- 24.Addo P, Adu-Addai B, Quartey M, Abbas M, Okang I, Owusu E, et al. (2006) Clinical and histopathological presentation of Buruli ulcer in experimentally infected grasscutters (Thryonomys swinderianus). The Internet Journal of Tropical Medicine 3: 1–15. [Google Scholar]
- 25.Williamson HR, Mosi L, Donnell R, Aqqad M, Merritt RW, Small PL (2014) Mycobacterium ulcerans fails to infect through skin abrasions in a guinea pig infection model: implications for transmission. PLoS Negl Trop Dis 8: e2770 10.1371/journal.pntd.0002770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petroff SA (1915) A new and rapid method for the isolation and cultivation of tubercle bacilli directly from the sputum and feces. J Exp Med 21: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greub G, Raoult D (2004) Microorganisms resistant to free-living amoebae. Clin Microbiol Rev 17: 413–433. 10.1128/CMR.17.2.413-433.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amissah NA, Gryseels S, Tobias NJ, Ravadgar B, Suzuki M, Vandelannoote K, et al. (2014) Investigating the role of free-living amoebae as a reservoir for Mycobacterium ulcerans. PLoS Negl Trop Dis 8: e3148 10.1371/journal.pntd.0003148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bratschi MW, Bolz M, Minyem JC, Grize L, Wantong FG, Kerber S, et al. (2013) Geographic distribution, age pattern and sites of lesions in a cohort of Buruli ulcer patients from the Mapé basin of Cameroon. PLoS Negl Trop Dis 7: e2252 10.1371/journal.pntd.0002252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hospers IC, Wiersma IC, Dijkstra PU, Stienstra Y, Etuaful S, Ampadu EO, et al. (2005) Distribution of Buruli ulcer lesions over body surface area in a large case series in Ghana: Uncovering clues for mode of transmission. Trans R Soc Trop Med Hyg 99: 196–201. 10.1016/j.trstmh.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 31.Quek TYJ, Athan E, Henry MJ, Pasco JA, Redden-Hoare J, Hughes A, et al. (2007) Risk factors for Mycobacterium ulcerans infection, Southeastern Australia. Emerg Infect Dis 13: 1661–1666. 10.3201/eid1311.061206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan HR, Pechal JL, Small PLC, Tomberlin JK, Sanders M, Anagonou E, et al. Dectection of Mycobacterium ulcerans DNA on human skin; 2015; Geneva, Switzerland: WHO; pp. 23. [Google Scholar]
- 33.Danelishvili L, Wu M, Stang B, Harriff M, Cirillo SL, Cirillo JD, et al. (2007) Identification of Mycobacterium avium pathogenicity island important for macrophage and amoeba infection. Proc Natl Acad Sci U S A 104: 11038–11043. 10.1073/pnas.0610746104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau HY, Ashbolt NJ (2009) The role of biofilms and protozoa in Legionella pathogenesis: implications for drinking water. J Appl Microbiol 107: 368–378. 10.1111/j.1365-2672.2009.04208.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panel A shows site of inoculation 1 dpi. Panel B shows inflammation (erythema) at site of inoculation 31 dpi. Panel C shows an edema at site of inoculation 45 dpi. Panel D shows ulcer at the site of inoculation 49 dpi. Photograph is representative of group (n = 3).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.