Abstract
Background
The aim of the study was to investigate the expression of matrix metalloproteinase-9 (MMP-9) in rat abdominal aortic dissection (AD) induced by mechanical strain, so as to offer a better understanding of the possible mechanisms of AD.
Material/Methods
Experimental AD in rats was achieved by the injection of porcine pancreatic elastase. At days 0, 1, 3, 5, 7, 14, 21, and 30 after the establishment of AD model, serum MMP-9 levels were measured by enzyme-linked immunosorbent assay (ELISA). Four groups of vascular rings were stretched in vitro with a mechanical strength of 0 g, 1 g, 3 g, or 5 g for 30 min. Another four groups were pretreated with GdCl3, streptomycin, SN50, and SN50M, followed by stretching with 3 g for 30 min. The messenger RNA and the protein of MMP-9 were analyzed by real-time RT-PCR and Western blotting, and NF-κB p65 was detected by ELISA.
Results
After the establishment of rat abdominal AD model, the serum MMP-9 levels of AD groups increased significantly. The results showed increased expression of MMP-9 in rat AD vessels stretched with mechanical strength of 1 g, 3 g, and 5 g, but this effect was mostly blocked by Gd Cl3 and streptomycin. The NF-κB activity in aortic rings was activated by stretching with a mechanical strength of 3 g and was blocked by SN50, but not by SN50M.
Conclusions
The expression of MMP-9 in serum was increased significantly after rat abdominal AD formation. Mechanical strain induced MMP-9 expression in AD vessels, which was mediated through the activation of the stretch-activated channel-induced NF-κB pathway.
MeSH Keywords: Aortic Diseases, Matrix Metalloproteinase 9, Receptor Activator of Nuclear Factor-kappa B
Background
Aortic dissection (AD), which damages the integrity of the arterial wall, is a severe disease with high morbidity and mortality [1,2]. Reports indicate that hypertension is the major underlying disease factor for AD [3,4]. Blood pressure control is effective for prevention and treatment of AD. Blood pressure elevation increases the mechanical strain of the vessel wall and eventually leads to AD, although its detailed mechanism is not clear.
It has been confirmed that matrix metalloproteinase 9 (MMP-9) is very important in AD development, and mechanical strain induces expression of MMP-9 in vascular smooth muscle cells (VSMCs) [5,6]. However, it has not been clear how a mechanical signal (mechanical strain) is transferred into biological signals leading to the expression of MMP-9 in vessels. The aim of the study was to investigate serum MMP-9 levels after rat abdominal AD and the mechanism of mechanical strain-induced expression of MMP-9 in rat abdominal AD, so as to offer a better understanding of the possible mechanisms of aortic dissection.
Material and Methods
Animals
Male Sprague Dawley rats (7–8 weeks old, weighing 250–300 g) were provided by the Shanghai Sliaike Experimental Animal Co. Ltd., China. A total of 80 rats were divided into 2 groups (AD and sham control groups) for the measurement of serum MMP-9 levels, and another 40 rats were divided into 8 groups for the mechanical strain experiment. All experimental protocols complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and the Animal Care and Use committees of Fujian Medical University. The rats were housed at 22°C to 24°C in an alternating light-dark (12/12 h cycle) environment with access to normal rat chow and water.
Rat abdominal aortic dissection
The rat abdominal AD model was implemented according to a previously published study [7,8]. In brief, abdominal AD was induced by the injection of porcine pancreatic elastase into the tunicae media vasorum. In sham controls, the abdominal cavity was just opened and was not perfused with porcine pancreatic elastase. AD in this model was further confirmed by Doppler ultrasound.
To monitor serum MMP-9, blood samples were obtained from rats before the operation and at 1, 3, 5, 7, 14, 21, and 30 days after surgery.
Aortic ring collection
Rats were anesthetized with chloral hydrate (3.5 mL/kg i.p.) 24 hours after the induction of AD, and the dissected vessels were quickly removed. The isolated AD vessels were placed in 4°C Krebs-Henseleit (K-H) solution (118 mM NaCl, 4.76 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 1.25 mM CaCl2, 11.0 mM glucose). After rinsing the aortic vessels with 4°C saline, the vessels were cut into 4 mm ring segments according to the study protocol. The adventitia and intima of the aortic vessels were carefully removed.
Mechanical stress intervention
The AD ring segments were suspended vertically on stainless steel hooks placed in a tissue chamber containing 10 mL of K-H solution at 37°C in an atmosphere of 95% O2 and CO2. Mechanical strain generated by the aortic smooth muscle was measured by using a force transducer (Chengdu, TME Technology Co. Ltd., China) and recorded by using the BL-420S experimental system of biological function (Chengdu, TME Technology Co. Ltd., China) [9,10]. The resting tension was set to 0.2 g. At first, we equilibrated the aortic ring segments for 90 min, during which the K-H solution was changed every 15 min. The segments were then pre-contracted by prostaglandin F2α 1.5 μmol/L, and when the vascular contractions were stable, acetylcholine 0.5 μmol/L was added. If the relaxation amplitude of the aortic rings was not more than 5% of the contraction amplitude, the aortic rings were considered as completely devoid of endothelial cells, enabling us to start the experiment. The aortic rings were stretched by 0 g, 1 g, 3 g, or 5 g for 30 minutes, and there were four rings in each group.
The effect of GdCl3 and streptomycin (stretch-activated channel [SAC] inhibitors) and SN50 and its mutant, SN50M (cell-permeable peptide inhibitors), in the activation of the mechanical strength-induced SAC/NF-κB pathway on aortic vessel MMP-9 expression was evaluated. GdCl3, streptomycin, SN50, and SN50M were each dissolved in PBS as stock solution and then diluted in 10 mL of K-H solution to achieve working concentrations of 25 μM, 200 μM, 18 μM, and 18 μM, respectively. The ring segments were pretreated with these reagents for 1 h, prior to stretching with a mechanical strength of 3 g for 30 min. After that, the aortic rings were frozen in liquid nitrogen and stored at −80°C until analysis.
Serum MMP-9 measurement
Immediately after removal at various time points, venous blood samples were centrifuged for 20 min (1200 revolutions/min [rpm], 4°C), and serum was stored at −20°C until analysis. Serum MMP-9 levels were determined with a commercial immunoassay kit specific to rat MMP-9 (Immunology Consultants Laboratory, Inc., USA) as per the manufacturer’s instructions. This kit is a highly sensitive two-site enzyme-linked immunosorbent assay (ELISA) for measuring MMP-9. All experiments were performed in triplicate.
Immunohistochemistry assay
The AD tissues were dipped into 10% Formalin solution for 24 h and then cut into 4 μm sections. The monoclonal antibody against MMP-9 (Epitomics, Inc., California, USA) and the goat anti-mouse-IgG (Epitomics, Inc, California, USA) were used for immunostaining. Negative and positive controls were used for both staining procedures
Western blotting analysis of MMP-9
Aortic rings were lysed with protein buffer. Total proteins were transferred to polyvinyl difluoride membrane. The membrane was blocked with 5% milk solution and then incubated overnight at 4°C with monoclonal antibody against MMP-9. After being washed three times with TBS containing 0.2% Tween, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (goat anti-mouse IgG) for 1 h at 37°C. Protein bands were visualized with an enhanced chemiluminescence kit (Amersham Biosciences, Inc., USA).
Real-time RT-PCR analysis of MMP-9 mRNA
Total RNA was isolated from aortic rings with TRIzol according to the protocol of the manufacturer (Invitrogen, Carlsbad, California, USA). Oligonucleotide primers for β-actin and MMP-9 were as follows:
β-actin: sense: 5′-GAGGCCCAGAGCAAGAGAGGT-3′
Antisense: 5′-TTCACGGTTGGCCTTAGGGTT-3′
MMP-9: sense: 5′-GTAACCCTGGTCACCGGACTT-3′
Antisense: 5′-CAGATACGTTCCCGGCTGAT-3′
Reverse transcription reaction
These reagents were added in turn (on ice): 2×TS Reaction Mix 10 μL, HiScript® II Enzyme Mix 1 μL, gDNA wiper 1 μL, Oligo(dT) (0.5 μg/μL) 1 μL, total RNA 1 μL, and Rnase-free dH2O was made up to 20 μL. The reaction condition was 42°C for 30 min and 85°C for 5 min. The acquired cDNA was stored at −20°C until analysis.
Real-time PCR reaction
The modulation of reaction liquid was according to the protocol of the manufacturer (on ice): AceQ® qPCR SYBR® Green Master Mix 10 μL, PCR forward primer (10 μM) 0.5 μL, PCR reverse primer (10 μM) 0.5 μL, Rox Reference Dye II (50×) 0.4 μL, cDNA template 2 μL, and ddH2O was made up to 20 μL. The reaction condition was 94°C for 30 sec, 94°C for 5 sec, and 60°C for 34 sec, with 40 cycles. After running to the end, the data were saved for later analysis. The standard curve of the relative concentration of the sample was measured to calculate the slope, intercept, correlation coefficient, and amplification efficiency. The expression of β-actin, a constitutively expressed gene, was used as an internal control for the assay.
ELISA analysis of NF-κB p65 subunit
NF-κB p65 subunit was detected in the nuclear protein extraction of the aortic rings, using a commercially available ELISA kit (Millipore Corporation, Billerica, New York, USA), as described [9]. All experiments were performed in triplicate.
Statistical analysis
SPSS 16.0 software was used for statistical analysis. The data were expressed as mean ± standard deviation. For analysis of differences between the groups and time points, the two-way repeated-measures analysis of variance (ANOVA) was used, and in case of differences the least significant difference (LSD) test was applied. For analysis of differences between the groups, the one-way ANOVA was used, and in case of differences the LSD test was applied. P<0.05 was considered statistically significant.
Results
Induction of abdominal aortic dissection in rat model
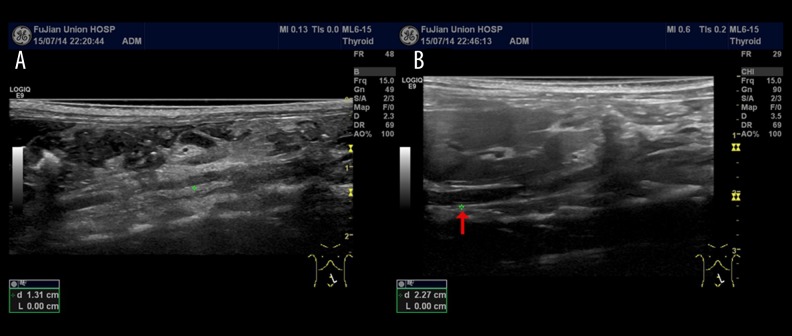
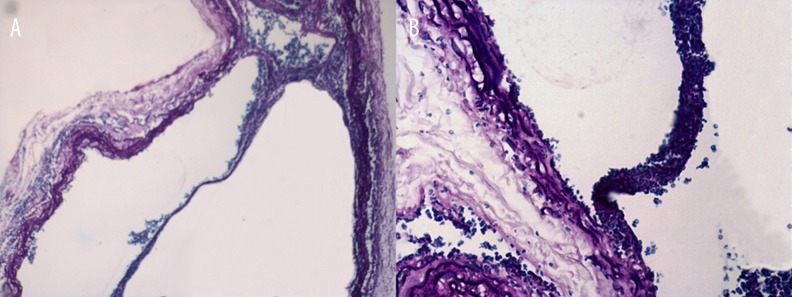
We used Doppler ultrasound to observe the formation of abdominal dissection in rats. The Doppler ultrasound longitudinal screenshots (Figure 1) confirmed the formation of abdominal AD. Further, Verhoeff-Van Gieson (VVG) staining of the abdominal aorta also confirmed the formation of abdominal AD (Figure 2).
Figure 1.
(A) Doppler ultrasound, longitudinal screenshot, showing the normal abdominal aorta. (B) Doppler ultrasound, longitudinal screenshot, showing the formation of abdominal aortic dissection (red arrows).
Figure 2.
Verhoeff-Van Gieson (VVG) staining to confirm the formation of abdominal aortic dissection (A ×40; B ×100).
Measurement of the aorta
The postoperative maximum, the thickness of the media, and the initial area from the AD group were significantly increased compared with those from the sham control group (P<0.05) (Table 1). There was no significance difference in weight and preoperative maximum diameter between the AD group and the sham group (P>0.05) (Table 1).
Table 1.
The differences in preoperative maximum diameter, postoperative maximum diameter, thickness media and initial area between the sham control group and aortic dissection group.
| Groups, P value | Weight (g) | Preoperative maximum diameter (mm) | Postoperative maximum diameter (mm) | Thickness of media (mm) | Initial area (mm2) |
|---|---|---|---|---|---|
| Sham control group (n=40) | 267±13 | 1.27±0.11 | 1.50±0.09 | 0.03±0.00 | 0.29±0.02 |
| Aortic dissection group (n=40) | 275±26 | 1.41±0.13 | 2.21±0.14* | 0.12±0.01* | 0.51±0.03* |
| P value | 0.57 | 0.79 | <0.01 | <0.01 | <0.01 |
P<0.05.
Serum MMP-9 after rat abdominal aortic dissection formation
Serum MMP-9 levels reached a peak at day 7 and maintained high expression at day 14 to day 30 after surgery in the AD group; however, in the sham group, serum MMP-9 levels reached a peak at day 3 and gradually decreased during the subsequent 2 weeks. Serum levels of MMP-9 in the AD group stayed significantly higher than the basal level at days 1 (5.21±0.32-fold), 3 (9.77±0.27-fold), 5 (15.28±0.41-fold), 7 (21.86±0.52-fold), 14 (18.35±0.76-fold), 21 (17.77±0.63-fold), and 30 (19.21±0.35-fold) (n=5, P<0.05). Serum MMP-9 levels in the sham group were higher than the basal level at days 1 (4.11±0.17-fold), 3 (6.23±0.11-fold), and 5 (5.63±0.23-fold) (n=5, P<0.05). They were not significantly different from the basal level at days 7, 14, 21, and 30 (n=5, P>0.05) (Figure 3, Table 2). Levels in the sham control group and the AD group were significantly different at days 5, 7, 14, 21, and 30 (n=5, P>0.05) (Figure 3, Table 2).
Figure 3.
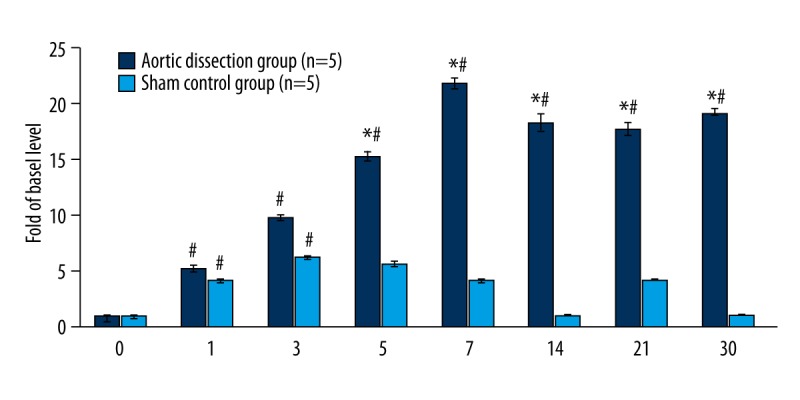
Dynamics of serum MMP-9 levels at different time points as compared to the basal serum MMP-9 level. # P<0.05 vs. basal level (0 d) (n=5). Aortic dissection group vs. sham control group, * P<0.05 (n=5).
Table 2.
Dynamics of serum MMP-9 levels at different time points in aortic dissection and sham control groups.
| Groups, P value | 0 d | 1 d | 3 d | 5 d | 7 d | 14 d | 21 d | 30 d |
|---|---|---|---|---|---|---|---|---|
| Sham control group (n=5) | 1.00±0.00 | 4.11±0.17# | 6.23±0.11# | 5.63±0.23# | 2.01±0.12 | 1.02±0.02 | 1.02±0.02 | 1.01±0.01 |
| Aortic dissection group (n=5) | 1.00±0.00 | 5.21±0.32# | 9.77±0.27# | 15.2±0.41*# | 21.86±0.52*# | 18.35±0.76*# | 17.77±0.63*# | 19.21±0.35*# |
| P value | >0.05 | 0.08 | 0.06 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
The sham control group vs. aortic dissection groups,
P<0.05, between different time points;
P<0.05 vs. basal level (0 d).
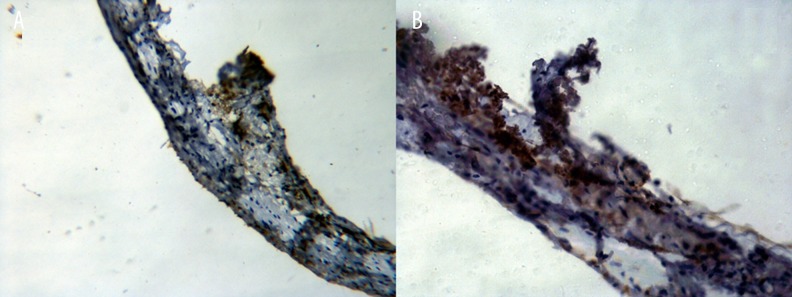
Immunohistochemical staining of aortic dissection
The representative micrographs of MMP-9 immunostained specimens are shown in Figure 4. The majority of MMP-9-positive cells were located in VSMCs in abdominal AD.
Figure 4.
The abdominal aortic dissection specimens were sectioned and labeled with MMP-9 antibodies as indicated (A ×200; B ×400).
Stretch-activated channel and MMP-9 in rat abdominal aortic dissection
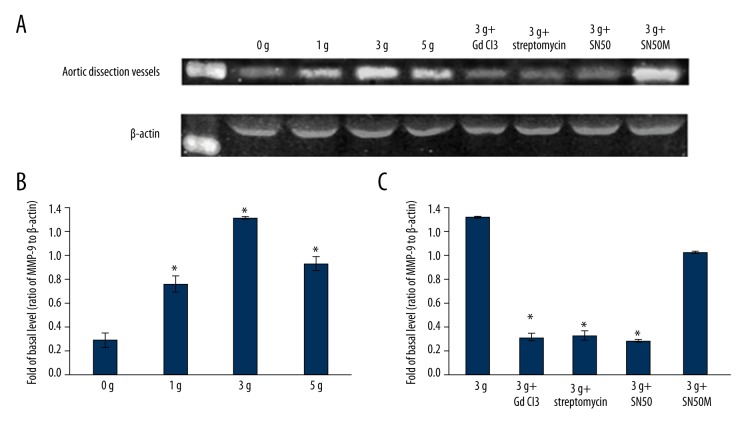
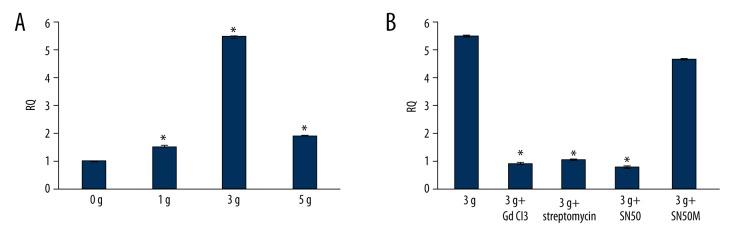
Increased expression of MMP-9 (92 KDa) in the aortic rings followed by mechanical stretching was verified by Western blotting. Applying mechanical strength of 1 g, 3 g, and 5 g resulted in the up-regulation of MMP-9 protein expression as compared to that observed with 0 g of strain (n=5, P<0.05 vs. the 0 g group). When the aortic rings were pretreated with SAC blockers, GdCl3 (25 μM) and streptomycin (200 μM), and the NF-κB peptide inhibitor SN50 (18 μM), mechanical strain of 3 g did not result in an increased expression of MMP-9 (n=5, P<0.05 vs. 3 g group). However, this effect was not seen when the aortic rings were pretreated with the inactive SN50 analogue SN50M (18 μM) (n=5, P>0.05 vs. 3 g group) (Figure 5, Tables 3, 4). Similar results were obtained for MMP-9 mRNA expression as measured by the real time PCR (Figure 6, Tables 5, 6).
Figure 5.
(A) Representative western blot analysis of MMP-9 expression in aortic dissection rings after being stretched by 0 g, 1 g, 3 g, and 5 g for 30 minutes (lanes 2–5, top panel); and after application of mechanical strain of 3 g in aortic rings pretreated with GdCl3, streptomycin, SN50, and SN50M (lanes 6–9, top panel). (B) Mean fold change from the ratio of MMP-9 to β-actin in the 0 g group, 1 g group, 3 g group, and 5 g group; * P<0.05 vs. 0 g group (n=5). (C) Mean fold change from the ratio of MMP-9 to β-actin in the 3 g, 3 g + GdCl3, 3 g + streptomycin, 3 g + SN50, and 3 g + SN50M groups; * P<0.05 vs. 3 g group (n=5).
Table 3.
The expression of MMP-9 induced with different mechanical strength.
| Group | MMP-9/β-actin | P value |
|---|---|---|
| 0 g (n=5) | 0.29±0.06 | |
| 1 g (n=5) | 0.76±0.07* | <0.01 |
| 3 g (n=5) | 1.31±0.01* | <0.01 |
| 5 g (n=5) | 0.93±0.06* | <0.01 |
P<0.05 vs. 0 g group.
Table 4.
The expression of MMP-9 in different groups.
| Group | MMP-9/β-actin | P value |
|---|---|---|
| 3 g (n=5) | 1.31±0.01 | |
| 3 g + GdCl3 (n=5) | 0.31±0.03* | <0.01 |
| 3 g + streptomycin (n=5) | 0.32±0.04* | <0.01 |
| 3 g + SN50 (n=5) | 0.28±0.01* | <0.01 |
| 3 g + SN50M (n=5) | 1.02±0.01 | 0.06 |
P<0.05 vs. 3 g group.
Figure 6.
(A) Representative real time PCR analysis of the MMP-9 expression in aortic dissection rings after being stretched by 0 g, 1 g, 3 g, or 5 g for 30 minutes; * P<0.05 vs. 0 g group (n=5). (B) Representative real time PCR analysis of the MMP-9 expression in aortic dissection rings after application of mechanical strain of 3 g in aortic rings pretreated with GdCl3, streptomycin, SN50, and SN50M; * P<0.05 vs. 3 g group (n=5).
Table 5.
The expression of MMP-9 mRNA induced with different mechanical strength.
| Group | RQ | P value |
|---|---|---|
| 0 g (n=5) | 1.00±0.00 | |
| 1 g (n=5) | 1.52±0.03* | 0.03 |
| 3 g (n=5) | 5.48±0.04* | <0.01 |
| 5 g (n=5) | 1.91±0.02* | 0.02 |
P<0.05 vs. 0 g group.
Table 6.
The expression of MMP-9 mRNA in different groups.
| Group | RQ | P value |
|---|---|---|
| 3 g (n=5) | 5.48±0.04 | |
| 3 g + GdCl3 (n=5) | 0.94±0.03* | <0.01 |
| 3 g + streptomycin (n=5) | 1.07±0.02* | <0.01 |
| 3 g + SN50 (n=5) | 0.81±0.03* | <0.01 |
| 3 g + SN50M (n=5) | 4.65±0.03 | 0.07 |
P<0.05 vs. 3 g group.
NF-κB activation in mechanically stretched AD rings
After mechanical stretching, NF-κB p65 activation was higher for the 3 g group (4.18±0.78-fold) and the 3 g + SN50M group (3.76±1.31-fold) as compared to the basal level (n=5, P<0.05). Similarly, NF-κB p65 activation levels for the 3 g + GdCl3, 3 g + streptomycin, and 3 g + SN50 groups were 1.39±0.34-fold, 1.42±0.45-fold, and 1.03±0.17-fold, respectively, and were not significantly different from the basal level (n=5, P>0.05) (Figure 7, Table 7).
Figure 7.
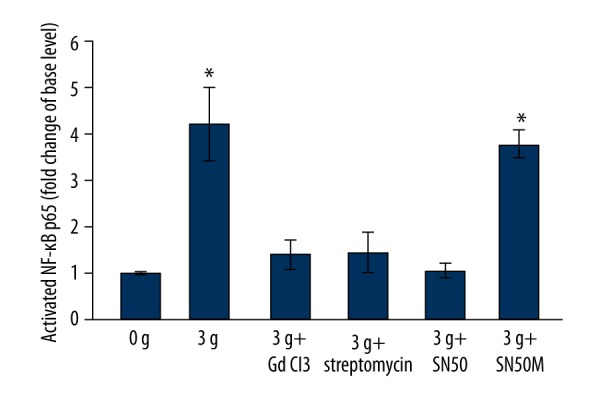
Representative ELISA analysis of activations of NF-κB p65 in aortic dissection rings after being stretched by 0 g and 3 g, and after application of mechanical strain of 3 g in aortic rings pretreated with GdCl3, streptomycin, SN50, and SN50M; * P<0.05 vs. 0 g group (n=5).
Table 7.
The activations of NF-kB P65 in different groups.
| Group | The activations of NF-κB | P value |
|---|---|---|
| 0 g (n=5) | 1.00±0.00 | |
| 3 g (n=5) | 4.18±0.78* | <0.01 |
| 3 g + GdCl3 (n=5) | 1.39±0.34 | 0.12 |
| 3 g + streptomycin (n=5) | 1.42±0.45 | 0.09 |
| 3 g + SN50 (n=5) | 1.03±0.17 | 0.21 |
| 3 g + SN50M (n=5) | 3.76±0.31* | <0.01 |
P<0.05 vs. 0 g group.
Discussion
In this study, we achieved a rat model for abdominal AD that persisted for 30 days. We applied the method of surgical puncture and injection of porcine pancreatic elastase to establish the model of rat abdominal AD. We punctured the aortic wall, which was divided into two layers to form the true lumen and false lumen, and then injected the elastase, which damaged the media, leading to arterial dilation. The advantage of this model is that the AD can be further confirmed by Doppler ultrasound.
The MMP-9 expressed by VSMCs plays an important role in the development of AD. Serum MMP-9 levels in patients with AD were higher than normal population levels [11–13]. In our AD model, an increased MMP-9 level was observed within 24 h after AD formation and reached a peak at day 7. The high serum MMP-9 level lasted for 30 days. The surgery-induced inflammation stimulated the release of cytokines, which mediated MMP-9 synthesis in inflammatory cells [14]. After AD formation, the VSMCs in the aortic wall expressed MMP-9 affected by abnormal hemodynamics, causing a significant increase in serum MMP-9 levels.
Abnormal hemodynamics may lead to hypertension, which increases the mechanical strain. Recent studies have recognized that mechanical strain is an important regulator of structure and function for cells and organs [15]. Mechanical strain sensed by cells is transmitted via intracellular signal transduction pathways to the nucleus, which causes the expression of different genes [9].
Mechanical strain activates SAC, which in turn transduces the external mechanical stimulation into an electrical or biochemical response. SAC was first found by Guharay and Sachs in skeletal muscle cells, and later studies showed that it widely exists in various kinds of cells [16]. Shaw et al. confirmed that mechanical strain could activate SAC and integrin receptors immediately in VSMCs and triggered a series of reactions, leading to migration of VSMCs and apoptosis [15]. Research has shown that the mechanism of mechanical stimuli transmuting into biological signals may be that SAC gating could be altered by mechanical strain, which led to the generation of ionic currents and subsequent transformation of a mechanical stimulus into an electrical or biochemical response [17].
We found that mechanical strain triggered MMP-9 expression in abdominal AD via the SAC-NF-κB pathway. In the present study, with a mechanical strength of 1 g, 3 g, or 5 g for 30 min, the AD rings could express large amounts of MMP-9. We obtained similar results when AD rings were stretched for 1 or 2 h (data not shown). With the use of GdCl3 and streptomycin, we demonstrated that mechanical strain-induced MMP-9 expression is mediated via activation of SACs.
The present research demonstrated that mechanical strain could activate several cell signaling pathways, such as MAPKs, ROS, AKT, and NO. In different cells and tissues, mechanical strain activated different signaling pathways [18–21], including interleukin-1β induced MMP-9 expression via p42/p44 MAPK, P38 MAPK, and JNK signaling pathways in human tracheal smooth muscle cells [22]. Amma et al. found that mechanical strain triggered COX-2 expression via the NF-κB pathway in human fibroblasts [23]. We demonstrated that mechanical strain induced MMP-9 expression via the NF-κB pathway in rat abdominal AD. As we all know, the p50/p65 heterodimer of NF-κB is the most widely distributed κB-binding factor [24]. Mechanical strain activated NF-κB through IκB kinase-dependent phosphorylation and subsequent degradation of IκB proteins. In this study, a mechanical strength of 3 g was able to activate NF-κB and induce MMP-9 expression. This effect was blocked by SN50 by inhibiting the translocation of NF-κB active complex into the nucleus [25].
Conclusions
Our study had shown that the expression of MMP-9 in serum was increased significantly after rat abdominal AD formation. Our data on mechanical strain-induced MMP-9 expression provide novel insights into the association between mechanical strain and AD. Mechanical strain triggered MMP-9 expression in rat abdominal AD, which was mediated through the SAC- NF-κ B signal pathway.
Footnotes
Source of support: This work was supported by funding provided by the National Natural Science Foundation of China (81370414)
References
- 1.Olsson C, Thelin S, Stahle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14000 cases from 1987to 2002. Circulation. 2006;114(24):2611–18. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 2.Mody PS, Wang Y, Geirsson A, et al. Trends in aortic dissection hospitalizations, interventions, and outcomes among medicare beneficiaries in the United States, 2000–2011. Cir Cardiovasc Qual Outcomes. 2014;7:920–28. doi: 10.1161/CIRCOUTCOMES.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fry JL, Shiraishi Y, Turcote R, et al. Vascular smooth muscle sirtuin-1 protects against aortic dissection during angiotensin II-induced hypertension. J Am Heart Assoc. 2015;4(9):e002384. doi: 10.1161/JAHA.115.002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hrar T, Yagi S, Akaike M, et al. Transdermal patch of bisoprolol for the treatment of hypertension complicated with aortic dissection. Int J Cardiol. 2015;198:220–21. doi: 10.1016/j.ijcard.2015.06.112. [DOI] [PubMed] [Google Scholar]
- 5.Kurihara T, Shimizu-Hirota R, Shimoda M, et al. Neutrophil-derived matrix metalloproteinase-9 triggers acute aortic dissection. Circulation. 2012;126(25):3070–80. doi: 10.1161/CIRCULATIONAHA.112.097097. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Wu D, Choi JC, et al. Matrix metalloproteinase levels in chronic thoracic aortic dissection. J Surq Res. 2014;189(2):348–58. doi: 10.1016/j.jss.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas G, Burquist J. Creation of dissecting thoracic aortic aneurysms in dogs. J Surg Res. 1970;10:333–36. doi: 10.1016/0022-4804(70)90052-1. [DOI] [PubMed] [Google Scholar]
- 8.Anidjar S, Salzmann JL, Gentric D, et al. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82(3):973–81. doi: 10.1161/01.cir.82.3.973. [DOI] [PubMed] [Google Scholar]
- 9.Huang G, Wang A, Li X, et al. Change in high-sensitive C-reactive protein during abdominal aortic aneurysm formation. J Hpertens. 2009;27:1829–37. doi: 10.1097/HJH.0b013e32832db36b. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Li Y, Huang G, et al. Interleukin 6 augments mechanical strain-induced C-reactive protein synthesis via the stretch-activated channel-nuclear factor κB signal pathway. Heart. 2013;99:570–76. doi: 10.1136/heartjnl-2012-303355. [DOI] [PubMed] [Google Scholar]
- 11.Wen D, Zhou XL, Li JJ, et al. Plasma concentrations of interleukin-6, C-reactive protein, tumor necrosis factor-α and matrix metalloproteinase-9 in aortic dissection. Clin Chim Acta. 2012;413(1–2):198–202. doi: 10.1016/j.cca.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Sangiorgi G, Trimarchi S, Mauriello A, et al. Plasma levels of metalloproteinases-9 and-2 in the acute and subacute phases of type A and type B aortic dissection. J Cardiovasc Med. 2006;7(5):307–15. doi: 10.2459/01.JCM.0000223251.26988.c5. [DOI] [PubMed] [Google Scholar]
- 13.Karapanagiotidis GT, Antonitsis P, Charokopos N, et al. Serum levels of matrix metalloproteinase-1,-2,-3,and-9 in thoracic aortic diseases and acute myocardial ischemia. J Cardiothorac Surg. 2009;4:59. doi: 10.1186/1749-8090-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakatos G, Hritzl, Varga MZ, et al. The impact of matrix metalloproteinases and their tissue inhibitors in inflammatory bowel diseases. Dig Dis. 2012;30(3):289–95. doi: 10.1159/000336995. [DOI] [PubMed] [Google Scholar]
- 15.Shaw A, Xu Q. Biomechanical stress-induced signaling in smooth muscle cells: An update. Curr Vasc Pharmacol. 2003;1:41–58. doi: 10.2174/1570161033386745. [DOI] [PubMed] [Google Scholar]
- 16.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 18.Ghantous CM, Kobeissy FH, Soudani N, et al. Mechanical stretch-induced vascular hypertrophy occurs through modulation of leptin synthesis-mediated ROS formation and GATA-4 nuclear translocation. Front Pharmacol. 2015;6:240. doi: 10.3389/fphar.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo KW, Lee SJ, Ye BH, et al. Mechanical stretch enhances the expression and activity of osteopontin and MMP-2 via the AKT1/AP-1 pathways in VSMC. J Mol Cell Cardiol. 2009;85:13–24. doi: 10.1016/j.yjmcc.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Du L, Empery P, Ji J, et al. Probenecid and N-acetylcysteine prevent loss of intracellular glutathione and inhibit neuronal death after mechanical stretch injury in vitro. J Neruotrauma. 2015;12(3):327–76. doi: 10.1089/neu.2015.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sears C, Kaunas R. The many ways adherent cell respond to applied stretch. J Biomech. 2016;49(8):1347–54. doi: 10.1016/j.jbiomech.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Lin WN, Luo SF, Lee CW, et al. Involvement of MAPKAs and NF-kappa B in LPS-induced VCAM-1 expression in human tracheal smooth muscle cells. Cell Signal. 2007;19(6):1258–67. doi: 10.1016/j.cellsig.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Amma H, Naruse K, Ishiguro N, et al. Involvement of reactive oxygen species in cyclic stretch-induced NF-κB activation in human fibroblast cells. Br J Pharmacol. 2005;145:364–73. doi: 10.1038/sj.bjp.0706182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan M, Ni J, Song D, et al. Activation of unfolded protein response protects osteosarcoma cells from cisplatin-induced apoptosis through NF-κB pathway. Int J Clin Exp Pathol. 2015;8(9):10204–15. [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LF, Greene WC. Regulation of distinct biological activities of the NF-kappa B transcription factor complex by acetylation. J Mol Med. 2003;81(9):549–57. doi: 10.1007/s00109-003-0469-0. [DOI] [PubMed] [Google Scholar]