Abstract
The engagement of sexual behaviors is regulated by a number of factors which include gene expression, hormone circulation, and multi-sensory information integration. In zebrafish, when a male and a female are placed in the same container, they show mating-like behaviors regardless of whether they are kept together or separated by a net. No mating-like behaviors are observed when same-sex animals are put together. Through the olfacto-visual centrifugal pathway, activation of the terminalis nerve in the olfactory bulb increases GnRH signaling in the brain and triggers mating-like behaviors between males. In zebrafish mutants or wild-type fish in which the olfacto-visual centrifugal pathway is impaired or chemically ablated, in response to odor stimulation the mating-like behaviors between males are no longer evident. Together, the data suggest that the combination of olfactory and visual signals alter male zebrafish's mating-like behaviors via GnRH signaling.
Introduction
The engagement of sexual behaviors is regulated by a number of factors, such as the expression of specific gene in sex-related cellular pathways, activation of hormone receptors, and stimulation of sensory cells [1–4]. Genetic loci that regulate the sexual behaviors have been identified. In flies (Drosophila melanogaster), for example, the expression of fruitless is required for initiating the courtship behaviors between the male and female animals; when the expression of fruitless is interrupted, the males alter their sexual behaviors [5,6]. Functional expression of fruitless in a variety of neural and non-neural tissues (e.g., olfactory bulb, visual cortex, auditory cells, and the gustatory system) is required for normal sexual behaviors [2, 7, 8].
Environmental cues such as pheromone modulation of sexual behaviors have been carefully examined [9, 10]. It is believed that in addition to pheromones, other sensory inputs, particularly those that trigger brain system GnRH signal transductions, may also have an impact on animal sexual behaviors [11, 12]. In vertebrates, different types of GnRH-containing cells have been identified [13–15]. Among them, the hypophyseotropic GnRH-containing neurons are found in all vertebrate species. These neurons are located in the pre-optic area and hypothalamus, and in some species, in the olfactory bulb [16, 17]. It has been speculated that the integration of cross-model sensory information may impact hypophyseotropic GnRH neural activity, which in turn, modulate animal’s sexual activity.
The zebrafish (Danio rerio) provide a model for studying the mechanisms underlying the integration of cross-model sensory information in sexual behaviors. In zebrafish, a paralogous form of mammalian hypophyseotropic GnRH-containing cells, known as the terminalis neurons (TNs) are found in the olfactory bulb [18–22]. Through the olfacto-visual centrifugal pathway, the TNs project axons to the neural retinas. In the retina, the TN axons synapse with dopaminergic interplexiform cells (DA-IPCs) and retinal ganglion cells (RGCs) [18, 23, 24]. While most of the RGC project axons to the visual cortex, some of the RGCs directly project axons to the hypothalamus [25]. This raises the possibility that through the olfacto-visual centrifugal pathway, the integration of multi-sensory information (i.e., between olfaction and vision) modulates neural functions in the hypothalamus, which in turn, alters animal’s sexual activity. In this research, using the zebrafish models we videotaped the sexual behaviors in adult zebrafish and recorded TN neural activities in transgenic animals under different experimental conditions. The data provide evidence that through the olfacto-visual centrifugal pathway, the integration of cross-model sensory information alters the dynamics of GnRH signaling in the brain and changes the sexual orientation in male animals.
Materials and methods
Animals
Zebrafish (Danio rerio) were maintained and handled in accordance with the NIH guidelines. All the experimental procedures were approved by the University of Notre Dame IACUC. Adult zebrafish were kept in 5-liter tanks (30 fish per tank, approximately half males and half females) or otherwise specified. The fish were maintained in the normal light-dark cycle (14:10; light, 07:00–21:00) and fed two times a day with freshly hatched brine shrimps.
Score of sexuality
Zebrafish were videotaped during their normal mating times (between 07:00 and 07:15). In the evening before the recording was taken, male and/or female zebrafish were transferred to the mating container (8 inches x 3 inches x 3 inches, long x wide x deep). The container was divided into two parts by an aluminum screen that was inserted in the middle of the container. The screen permitted water flow but separated the fish from physically contacting with each other. Each half of the container was further divided into 4 rectangle sections by lines marked on a paper that was placed underneath the container. The lines could be visualized through the bottom of the transparent container. Animal behaviors were videotaped and the traces of swimming behaviors were plotted in 15-sec intervals.
Several measures were put in place to indicate the animal’s sexuality levels. First, we recorded the speed of their swimming and scored each category of swimming behaviors: slow swimming or stationary was given a score of 0, normal swimming was given a score of 1, fast swimming was given a score of 3, and swimming against the net was given a score of 5. Data were collected in 3-min periods, and total 5 measurements were performed during the entire 15-min videotaping period. For each 3-min recording period, the percentage of time of each type of swimming was calculated, scaled down by a factor of 10, and then multiplied by the scores obtained from their swimming behaviors. Second, we examined the locations of the fish (at 15-second intervals) in the container in relation to the net. If the fish was recorded in the half of its side close to the net, it scored 5 points, and if the fish swam in the half of the side further from the net, it scored 0 points. Total scores from swimming speeds and locations were summated to indicate the sexuality levels. For the purpose of comparison, the scores from the most sexually active control male fish was normalized to 1, and the sexuality levels of other animals were determined by comparing to the base value of 1.
Electrophysiological recordings
Single-unit TN recordings were performed in developing transgenic zebrafish Tg(GnRH-3::GFP) between 4 and 5 days old. Prior to recordings, the fish were anesthetized with 0.5% 3-amino benzoic methylester and then embedded in 1% low-melting agrose. Patch pipettes (7–10 MΩ were fabricated from borosilicate glass (Sutter Instrument Co, CA) and filled with the pipette solutions (150 mM NaCl and 10 mM HEPES, pH 7.4). Whole-cell recording configurations were obtained by a brief pulse of suction after a gigaohm (GΩ) seal. For loose-patch recordings, once a spike was detected, light suction was applied to establish a low-resistance seal (50–100 MΩ). Voltages were recorded in the current-clamp mode using Clampex 8.0 software connected to an Axopatch 200B amplifier and a Digidata 1322A digitizer (Axon Instruments, CA). For each set of recording, data were collected in 60-second periods.
Drug solutions [glutamate, 6-cyano-7- nitroquinoxaline-2,3-dione disodium salt (CNQX; 10 μmol/L), D(–)-2-amino-7-phosphonoheptanoic acid (D-AP-7; 100 μmol/L); Sigma, MO] were delivered to the olfactory bulb (adjacent to the TNs, which were revealed by the expression of the GFP) at a rate of 10 μl/s.
Odor stimulation
Amino acids (methionine) were used for odor stimulation. Stock solutions (50 mM) were freshly prepared each day for individual experiment. Methionine (Sigma, MO) were dissolved in distilled water, and kept in room temperature. Methionine were delivered to swimming water (to the center of the container for adult zebrafish behavioral test) or perfused to the nostril using a glass pipette (for TN recordings) at the final concentration of 1.2 mM.
Chemical lesion
Olfactory neurons were ablated using 0.7% Triton X-100 [26]. Prior to drug treatment, the fish were anesthetized in a small container by 0.02% 3-aminobenzoic acid ethyl ester (applied to swimming water). Under a dissecting microscope, 0.5μl of 0.7% Triton X-100 (in PBS) were delivered to the nostril of the fish. Both sides of the nostril were treated. Control treatment was conducted using PBS. The fish were allowed to recover 2 days before used for mating tests.
DA-IPCs and RGCs were ablated by intraocular injections of 6-OHDA (5 μg/μl) and ouabian (3 μM), respectively. The fish were anesthetized by 0.02% 3-aminobenzoic acid ethyl ester before receiving injections. Under a dissecting microscope, 1 μl of drug solutions were injected into the vitreous. The fish were allowed to recover 4 days before used for mating tests.
Statistical analysis
Different numbers of zebrafish were used for different sets of the experiment. For the behavioral tests, under each condition (e.g., sham or methionine stimulation, control or lesion experiment) and for each mating-set (e.g., male-female, male-male, female-females), 6 pairs of zebrafish (between 4 and 6 months old) were tested. For electrophysiological studies, under each test condition (control, glutamate stimulation with or without receptor blockers, or in response to sham or methionine stimulation), 6 GFP-tagged TN cells from different transgenic animals (between 4 and 5 days old) were recorded. Data were analyzed using the double-blind methods. Statistical differences between the control and experimental or between sham and drug treatment groups were determined using the Student t-test.
Results
Activation of the olfacto-visual centrifugal pathway alters male zebrafish’s sexual orientation
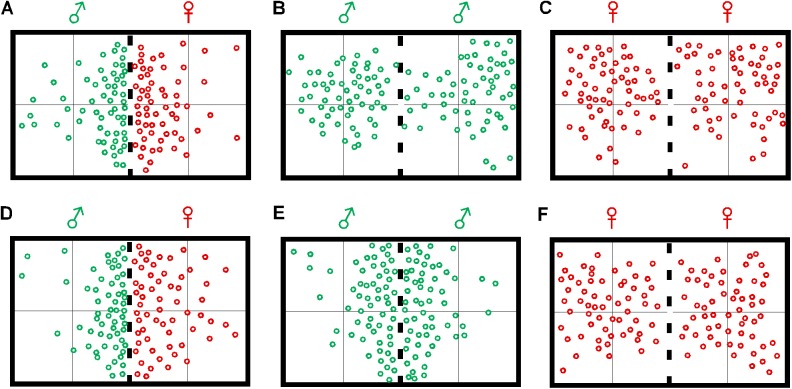
We videotaped the behaviors of adult male and female zebrafish during their normal mating times in the early morning. In the control setting (1 male and 1 female in the same container but separated by a net), both the male and female zebrafish displayed behavioral characteristic of mating. For example, for much of the time during the 15-min recording period, the male and female zebrafish swam close to the net, and often, bumped into the net in an attempt to interact with each other (Fig 1A). This was observed in 5 out 6 pairs tested on different days. In settings that two males (n = 6) or two females (n = 6) were kept in the same container and separated by a net, no obvious mating-like behaviors were observed. At most times during the 15-min recording period, the fish slowly swam around, and sometimes remained still. They did not spend much time swimming near the net (Fig 1B and 1C).
Fig 1.
Static positions of locomotor behaviors of male and female zebrafish recorded in control (A-C) and odor-stimulation conditions (D-F). The fish were separated by a net inserted in the middle of the container (indicated by bold dashed lines). Data were plotted in 15-sec intervals for a total of 15 min. Under control conditions (no odor stimulations), in pair-wise male-female setting, both the male and female fish show high sexuality levels, i.e., they displayed fast swimming behaviors and spent most of the time swimming close to the net in attempt to contact each other. In male-male or female-female settings, the fish randomly swam in the container, and they did not spend extra time swimming near the net. In response to odor stimulation, the behavior of the fish in the male-female setting remained unchanged. However, in the male-male setting, upon odor stimulation, the fish showed high sexuality levels. They increased the speed of swimming, and spent most of the time swimming close to the net in attempt to interact with each other. No obvious changes in swimming behaviors were seen in the female-female setting.
Animal sexual behaviors are regulated by internal circulation of GnRH [27]. In zebrafish, a group of GnRH-containing neurons (the TNs) are found in the olfactory bulb [18, 28]. The TNs express glutamate receptors and can be activated by glutamate or glutamate receptor agonists, such as NMDA or KA [23]. Because the TNs synapse with the RGCs [18], and because some of the RGCs directly project axons to the hypothalamus [25], it is possible that through the olfacto-visual centrifugal pathway, cross-model sensory information integration (i.e., between the olfactory bulb and retina) may alter the dynamics of GnRH signaling in the brain, which then impact the animal’s sexuality. We characterized the sexual behaviors of zebrafish in response to odor stimulation. Methionine was chosen as the odor stimulant [28, 29]. In the control setting (1 male and 1 female in the same container and they were separated by a net), activation of the olfacto-visual centrifugal pathway (by 1.2 mM methionine, applied to swimming water) produced no obvious effect on animals’ mating behaviors. For much of the time during the 15-min recording period, both the male and female fish swam close to the net in attempt to interact with each other (Fig 1D).
When two males were kept in the same container and separated by a net, stimulation by methionine led to increased mating-like behaviors. For example, both of the males showed increases in fast swimming behaviors, and they spent most of the time swimming close to the net. Often, they swam into the net in an attempt to make contact with each other (Fig 1E). However, when two females were kept in the same container and separated by a net, activation of the olfacto-visual centrifugal pathway by methionine produced no effect on the animal’s sexual behaviors, i.e., both fish swam slowly and did not appear to be interested in interacting with each other (Fig 1F).
Stimulation of olfactory neurons increases the activity of the TNs
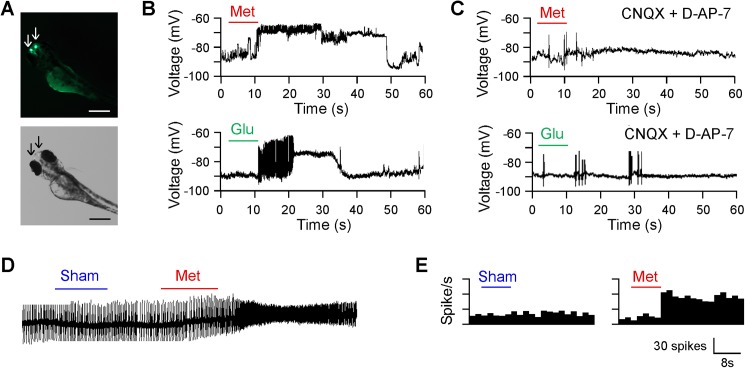
To test if the changes of the animal’s sexual behaviors after odor stimulation may relate to olfacto-visual centrifugal GnRH signaling, i.e., if the TN cells respond to increases in glutamate concentrations after odor stimulation, we recorded the TNs in live animals in response to odor stimulation using transgenic zebrafish Tg(GnRH3::GFP). In developing transgenic fish, the GFP-tagged TNs can be readily identified and manipulated (Fig 2A) [23, 30]. Stimulation of the olfactory neurons (by 1.2 mM methionine, applied to the nostril) produced long-lasting effects on the TNs, such as increases in membrane potentials (Fig 2B). Directly applying glutamate (0.5 mM, applied to the olfactory bulb) mimicked the effect of odor-mediated increase of membrane potential of the TNs (Fig 2B). In zebrafish that were treated with glutamate receptor antagonists CNQX or D-AP-7 (0.05 mM, applied to the olfactory bulb prior to odor stimulation to the nostril or glutamate stimulation to the TNs), the membrane potential of the TNs remained unchanged in response to olfactory odor or glutamate stimulations (Fig 2C).
Fig 2. Effects of odor or glutamate stimulation on TN activity.
(A) Fluorescent and bright-field images of transgenic zebrafish Tg(GnRH3::GFP). The TNs were identified by the expression of GFP (arrows) and were recorded by patch-clamp methods. Scale bar: 200 μm. (B) Whole-cell patch-clamp recordings of the TN in response to odor (methionine, 1.2 mM applied to the nostril) or glutamate (0.5 mM, applied to the olfactory bulb) stimulation. Note the increase in membrane potential of the TNs in response to odor or glutamate stimulation. In all cases (n = 6), the holding potential was set at -90 mV. (C) Whole-cell patch-clamp recordings of the TNs in response to odor (methionine, 1.2 mM applied to the nostril) or glutamate (0.5 mM, applied to the olfactory bulb) stimulation in zebrafish in which the glutamate receptors were blocked by CNQX or D-AP-7. In response to odor or glutamate stimulation, no obvious changes were detected. In all cases (n = 6), the holding potential was set at -90 mV. (D) Effects of odor stimulation (methionine, 1.2 mM applied to the nostril) on spontaneous firing of the TN neuron. Note the increase of the firing in response to odor stimulation. Sham stimulation (with distilled water) produced no effect on the firing rate of the TN neuron. The experiments were performed using different TN neurons (n = 6). (E) Histograms of the firing of the TNs (n = 6) in response to sham (distilled water) or odor stimulation (methionine, 1.2 mM; applied to the nostril). Note the increase of the firing in response to odor stimulation.
Loose-patch recordings of the TNs revealed that stimulation of olfactory neurons (with 1.2 mM methionine, applied to the nostril) increased the firing rate of the TNs (Fig 2D). As compared to the firing rete recorded prior to odor stimulation, on average the firing rate of the TNs increased 140.55 ±27.82% in response to odor stimulation (Fig 2E).
Interruption of the olfacto-visual pathway diminished mating-like behaviors between males
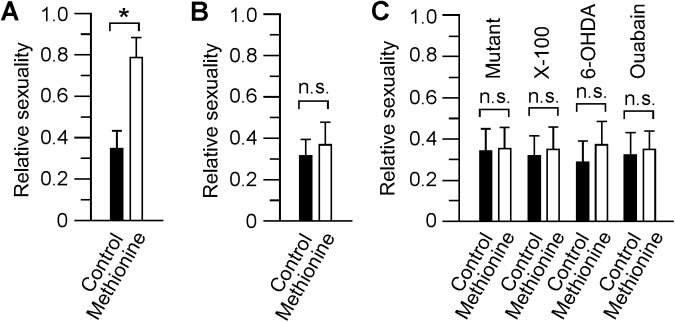
To further investigate the role of olfacto-visual sensory integration in male zebrafish’s sexual behaviors, we measured the sexuality levels of males that were placed in the same container without separation by the net. The experiments were conducted in wild-type fish and in fish with interruption of the olfacto-visual centrifugal pathway due to gene mutation or chemical lesion. In control wild-type fish, when two males were put together, for much of the time during the 15-min recording period both fish slowly swam around in the container and showed no interactions. Activation of the olfacto-visual centrifugal pathway (by 1.2 mM methionine, applied to swimming water) triggered mating-like behaviors. For example, upon the application of methionine, both males became occupied by chasing each other around the container, i.e., one male followed the other male by aligning its head next to the side of the other male slightly below the anal fin [31–33]. Based on their locomotory patterns (e.g., swimming speed) and mutual interactions (e.g., chasing behaviors), we scored the sexuality level of the fish. In males, activation of the olfacto-retinal centrifugal pathway increased the sexuality level by approximately 2-fold as compared to those measured under control conditions (Fig 3A). Interestingly, odor-triggered increases in sexuality were only observed in male animals. In wild-type females (the fish were kept in the same container without separation by the net), the fish showed no changes in sexual behaviors and the sexuality levels remained low with or without stimulation by odors (Fig 3B).
Fig 3. Relative sexuality levels of wild-type, mutant, and chemically lesioned animals tested under different conditions.
(A) Sexuality levels of male zebrafish recorded in the male-male setting before (black bar) and after odor stimulation (white bar). Note the increase of sexuality levels after odor stimulation. (B) Sexuality levels of female zebrafish recorded in the female-female setting before (black bar) and after odor stimulation (white bar). No obvious changes were observed before and after odor stimulation. (C) Sexuality levels of nbb and drug-treated males (Triton X-100, which destroyed the olfactory neurons; 6-OHDA, which ablated DA-IPCs; ouabain, which selectively lesioned RGCs) in the male-male setting recorded before (black bar) and after odor stimulation (white bar). In all cases, no obvious changes in sexuality levels were detected after odor stimulation. The data represent the Means ± SE. n = 8 in each group. * p < 0.05; n.s., not significant.
In zebrafish night blindness b (nbb) mutants, the olfacto-visual centrifugal pathway is interrupted, for example, the number of TN axons that project to the retina is reduced, the pattern of TN fiber distribution in the retina is disorganized, and the number of DA-IPCs is decreased [18]. In normal pair-wise setting, the sexual behaviors of nbb males were indistinguishable from wild-type animals, i.e., they mated with females and produced progenies. When two nbb males were placed together, they showed no mating-like behaviors either before or after the application of odors (1.2 mM methionine, applied to swimming water) (Fig 3C). In wild-type males in which the olfacto-visual centrifugal pathway is blocked (i.e., by treatment with Triton X-100, which ablate the olfactory neurons) [26], or the targets of the olfacto-retinal centrifugal pathway (i.e., DA-IPCs or RGCs in the retina) were excised by 6-OHDA or ocubain [34, 35], under normal pair-wise conditions, their mating behaviors were indistinguishable from wild-type males. However, when two chemically-treated males were kept together, in response to odor stimulation (1.2 mM methionine, applied to swimming water), during the full 15-min recording period, their sexuality levels remained low, similar to those measured before the application of methionine (Fig 3C).
Discussion
It is known that the circulation of GnRH is regulated by different genetic and cellular events [36]. However, the effect of environmental input on the regulation of synthesis and release of GnRH and how external modulation of GnRH signaling transduction impacts the sexual behaviors remain to be examined. Zebrafish provide a model system for addressing these issues. Zebrafish possess two distinctive forms of GnRH, which include cGnRH and sGnRH [37–41]. Previous studies have shown that the increase of cGnRH signaling promotes animals’ survival functions, such as food intake, whereas the modulation of sGnRH signaling transduction directly affects animals’ sexual activity and reproduction. In zebrafish, the olfactory TNs synthesize and release sGnRH [19]. Previously, we demonstrated that through the olfacto-visual centrifugal pathway, activation of the olfactory neurons decreases the release of dopamine from retinal DA-IPCs. This leads to two consequences: First, it increases the coupling between horizontal cells in the outer retina [42, 43]. Second, it lifts the inhibition of dopaminergic signaling on RGCs, thereby increasing inner retinal activity [44]. Together, the integration of olfacto-visual sensory information increases visual perception and retinal sensitivity. The increase of visual perception and retinal sensitivity directly impacts the animal’s social behaviors, which include sexual mating [45, 46].
Activation of the olfactory neurons increases the firing rate of the TNs, suggesting that through the olfacto-visual centrifugal pathway, sensory inputs alter the dynamics of GnRH signaling in the brain. The increase of TNs firing after odor stimulation is mediated by activating the glutamate receptors expressed on the TNs. When the glutamate receptors are blocked by receptor antagonists, stimulation of the olfactory neurons produces no effect on TN activity. The data suggest that through GnRH signaling, the olfacto-visual pathway may function as a bridge that incorporates external sensory signals to the sex-brain (see Fig 4). It works through the following mechanisms: Stimulation of olfactory neurons increases glutamate release in the olfactory bulb; since the TNs express glutamate receptors, thus odor stimulation will activate the TNs. Upon the activation of the TNs, internal GnRH-signaling will be increased. Through the olfacto-visual centrifugal pathway, the increase of GnRH signaling will be propagated to the retina. Because the RGCs express GnRH receptors [47], it is possible that the increase of GnRH signaling due to odor stimulation will alter the firing patterns of the RGCs. Because some of the RGCs project axons to the hypothalamus; the increase of GnRH signaling triggered by odor stimulation will then alter the activity of hypothalamus neurons. Alterations in GnRH signaling in the brain will directly impact the animal’s sexual activity.
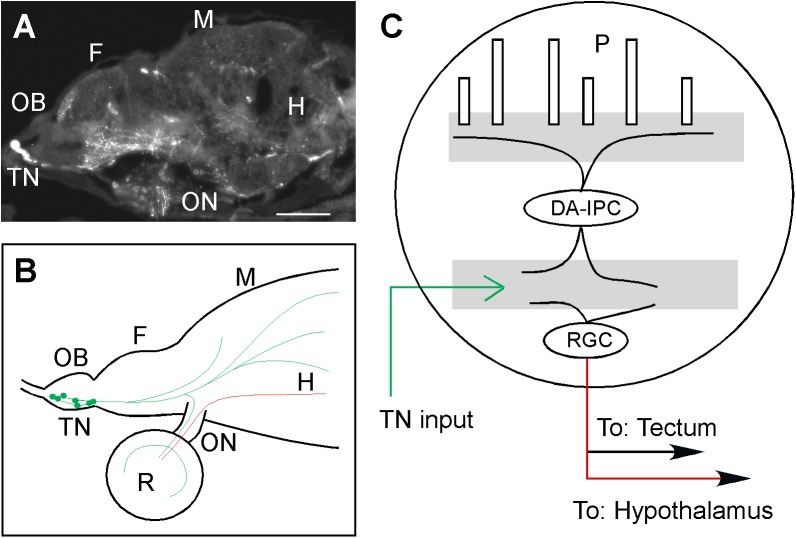
Fig 4. Effects of GnRH-mediated olfacto-visual sensory integration on animal sexuality.
(A) Lateral view of transgenic zebrafish (GnRH::3-GFP; anterior to the left) that shows the TNs and TN axons in the brain (revealed by the expression of the GFP). Scale bar, 50 μm. (B) A cartoon showing the projection of TN axons (red) and the projection of RGC axons (green) to the hypothalamus. (C) GnRH signaling in the eye. Upon entering the retina, the TN axons synapse with DA-IPCs and RGCs. Most of the RGCs project axons to the visual cortex, but some of the RGCs project axons to the hypothalamus. Abbreviations: F, forebrain; H, hypothalamus; M, midbrain; P, photo receptor cells; R, retina; DA-IPC, dopaminergic interplexiform cell; INL, inner nuclear layer; IPL, inner plexiform layer; OB, olfactory bulb; ON, optic nerve; OPL, outer plexiform layer; RGC, retinal ganglion cells; TN, terminalis neuron.
Our data provide evidence for the involvement and the physiological relevance of multi-sensory information integration in modulation of animal sexual activity. This is observed only in males. In pair-wise male-female or female-female settings, the activation of the olfacto-visual centrifugal pathway produced no obvious effect on animal sexual behaviors. It is possible that the GnRH signaling propagated by the olfacto-visual centrifugal pathway only targets male-specific dimorphic cells [6, 48, 49]. When the male fish encounter the other fish (regardless it is a male or a female), the male shows mating-like chasing behaviors.
Previous studies have demonstrated that in some of the mammalian species (e.g., rams), differences in brain structures due to the exposure to sex-related hormones, such as testosterone, may be involved in alterations in sex-orientations in male animals [50]. The increase of sexual activity induced by male-specific hormones, however, can be inhibited by chronic treatment with GnRH or its agonists [51]. In addition, sensory inputs, such as those mediated by the olfactory and/or vomeronasal systems, also play important roles in males’ sex behaviors [52]. The underlying mechanisms remain to be investigated.
Acknowledgments
This work was supported in part by grants from the Department of Defense (65632-LS-II; 68008-LS-II), the National Natural Science Foundation of China (81671242), and the National Basic Research Program of China (973-2012CB944700). The authors claim no potential conflicts of interest. All authors reviewed the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported in part by grants from the Department of Defense (65632-LS-II; 68008-LS-II), the National Natural Science Foundation of China (81671242), and the National Basic Research Program of China (973-2012CB944700). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gomez‐Diaz C, Benton R. The joy of sex pheromones. EMBO Rep. 2013; 14: 874–883. 10.1038/embor.2013.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto D, Koganezawa M. Genes and circuits of courtship behaviour in Drosophila males. Nature Rev Neurosci. 2013; 14: 681–692. [DOI] [PubMed] [Google Scholar]
- 3.Argiolas A, Melis MR. Neuropeptides and central control of sexual behaviour from the past to the present: a review. Prog Neurobiol. 2013; 108: 80–107. 10.1016/j.pneurobio.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 4.Cox KH, Bonthuis PJ, Rissman EF. Mouse model systems to study sex chromosome genes and behavior: relevance to humans. Front Neuroendocrinol. 2014; 35: 405–419. 10.1016/j.yfrne.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011; 69: 498–508. 10.1016/j.neuron.2010.12.017 [DOI] [PubMed] [Google Scholar]
- 6.Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V. Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron. 2015; 87: 1036–1049. 10.1016/j.neuron.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008; 452: 1002–1006. 10.1038/nature06850 [DOI] [PubMed] [Google Scholar]
- 8.Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GS, et al. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011; 478: 236–240. 10.1038/nature10428 [DOI] [PubMed] [Google Scholar]
- 9.Jallon JM. A few chemical words exchanged by Drosophila during courtship and mating. Behav Genet. 1984; 14: 441–478. [DOI] [PubMed] [Google Scholar]
- 10.Yew JY, Dreisewerd K, Luftmann H, Müthing J, Pohlentz G, Kravitz EA. A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr Biol. 2009; 19: 1245–1254. 10.1016/j.cub.2009.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster DL, Jackson LM, Padmanabhan V. Programming of GnRH feedback controls timing puberty and adult reproductive activity. Mol Cell Endocrinol. 2006; 254–255: 109–119. 10.1016/j.mce.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 12.Zohar Y, Muñoz-Cueto JA, Elizur A, Kah O. Neuroendocrinology of reproduction in teleost fish. Gen Comp Endocrinol. 2010; 165: 438–455. 10.1016/j.ygcen.2009.04.017 [DOI] [PubMed] [Google Scholar]
- 13.Fernald RD, White RB. Gonadotropin-releasing hormone genes: phylogeny, structure, and functions. Front Neuroendocrinol. 1999; 20: 224–240. 10.1006/frne.1999.0181 [DOI] [PubMed] [Google Scholar]
- 14.Dubois EA, Zandbergen MA, Peute J, Goos HJ. Evolutionary development of three gonadotropin-releasing hormone (GnRH) system in vertebrates. Brain Res Bull. 2002; 57: 413–418. [DOI] [PubMed] [Google Scholar]
- 15.Somoza GM, Miranda LA, Strobl-Mazzulla P, Guilgur LG. Gonadotropin-releasing hormone (GnRH): from fish to mammalian brains. Cell Mol Neurobiol. 2002; 22: 589–609. [DOI] [PubMed] [Google Scholar]
- 16.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci. 1989; 86: 8132–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacColl G, Quinton R, Rouloux P.M. GnRH neuronal development: insights into hypogonadotrophic hypogonadism. Trends Endocrinol. Metab. 2002; 13: 112–118. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Dowling JE. Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish J Neurosci. 2000; 20: 1883–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torgersen J, Nourizadeh-Lillabadi R, Husebye H, Aleström P. In silico and in situ characterization of the zebrafish (Danio rerio) gnrh3 (sGnRH) gene. BMC Genomics. 2002; 3: 25 10.1186/1471-2164-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steven C, Lehnen N, Kight K, Ijiri S, Klenke U, Harris WA, et al. Molecular characterization of the GnRH system in zebrafish (Danio rerio): cloning of chicken GnRH-II, adult brain expression patterns and pituitary content of salmon GnRH and chicken GnRH-II. Gen Comp Endocrinol. 2003; 133: 27–37. [DOI] [PubMed] [Google Scholar]
- 21.Whitlock KE. Development of the nervus terminalis: origin and migration. Microsc Res Tech. 2004; 65: 2–12. 10.1002/jemt.20094 [DOI] [PubMed] [Google Scholar]
- 22.Abraham E, Palevitch O., Ijiri S, Du SJ, Gothilf Y, Zohar Y. Early development of forebrain gonadotrophin-releasing hormone (GnRH) neurones and the role of GnRH as an autocrine migration factor. J Neuroendocrinol. 2008; 20: 394–405. 10.1111/j.1365-2826.2008.01654.x [DOI] [PubMed] [Google Scholar]
- 23.Wang XK, Huang L, Ray J, Li L. Characterization of GFP-tagged GnRH containing terminalis neurons in transgenic zebrafish. J Cell Physiol. 2011; 226: 608–615. 10.1002/jcp.22369 [DOI] [PubMed] [Google Scholar]
- 24.Esposti F, Johnston J, Rosa JM, Leung KM, Lagnado L. Olfactory stimulation selectively modulates the OFF pathway in the retina of zebrafish. Neuron. 2013; 79: 97–110. 10.1016/j.neuron.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore HA, Whitmore D. Circadian rhythmicity and light sensitivity of the zebrafish brain. PLoS ONE. 2014; 9: e86176 10.1371/journal.pone.0086176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iqbal T, Byrd-Jacobs C. Rapid degeneration and regeneration of the zebrafish olfactory epithelium after triton X-100 application. Chem Senses. 2010; 25: 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009; 30: 713–743. 10.1210/er.2009-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maaswinkel H, Li L. Olfactory input increases visual sensitivity in zebrafish: A possible function for the terminal nerve and dopaminergic interplexiform cells. J Exp Biol. 2003; 206: 2201–2209. [DOI] [PubMed] [Google Scholar]
- 29.Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997; 18: 737–752. [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Li L. Characterization of voltage-activated sodium and potassium currents in zebrafish terminalis neurons. Brain Res. 2011; 1367: 43–49. 10.1016/j.brainres.2010.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sessa AK, White R, Houvras Y, Burke C, Pugach E, Baker B, et al. The effect of a depth gradient on the mating behavior, oviposition site preference, and embryo production in the zebrafish, Danio rerio. Zebrafish. 2008; 5:335–339. 10.1089/zeb.2008.0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howard RD, Rohrer K, Liu Y, Muir WM. Mate competition and evolutionary outcomes in genetically modified zebrafish (Danio rerio). Evolution. 2015; 69: 1143–1157. 10.1111/evo.12662 [DOI] [PubMed] [Google Scholar]
- 33.Pradhan A, Olsson PE. Zebrafish sexual behavior: role of sex steroid hormones and prostaglandins. Behav Brain Funct. 2015; 11: 23 10.1186/s12993-015-0068-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Dowling JE. Effects of dopamine depletion on visual sensitivity of zebrafish. J Neurosci. 2000; 20: 1893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007; 27: 1712–1724. 10.1523/JNEUROSCI.5317-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarro VM, Tena-Sempere M. Neuroendocrine control by kisspeptins: role in metabolic regulation of fertility. Nat Rev Endocrinol. 2011; 8: 40–53. 10.1038/nrendo.2011.147 [DOI] [PubMed] [Google Scholar]
- 37.Gopinath A, Andrew-Tseng L, Whitlock KE. Temporal and spatial expression of gonadotropin releasing hormone (GnRH) in the brain of developing zebrafish (Danio rerio). Gene Exp Patterns. 2004; 4: 65–70. [DOI] [PubMed] [Google Scholar]
- 38.Abraham E, Palevitch O, Gothilf Y, Zohar Y. The zebrafish as a model system for forebrain GnRH neuronal development. Gen Comp Endocrinol. 2009; 164: 151–160. 10.1016/j.ygcen.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 39.Xia W, Smith O, Zmora N, Xu S, Zohar Y. Comprehensive analysis of GnRH2 neuronal projections in zebrafish. Sci Rep. 2014; 4: 3676 10.1038/srep03676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Lin MC, Mock A, Yang M, Wayne NL. Kisspeptins modulate the biology of multiple populations of gonadotropin-releasing hormoneneurons during embryogenesis and adulthood in zebrafish (Danio rerio). PLoS ONE. 2014; 9: e104330 10.1371/journal.pone.0104330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortés-Campos C, Letelier J, Ceriani R, Whitlock KE. Zebrafish adult-derived hypothalamic neurospheres generate gonadotropin-releasing hormone(GnRH) neurons. Biol Open. 2015; 4: 1077–1086. 10.1242/bio.010447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umino O, Dowling JE. Dopamine release from interplexiform cells in the retina: effects of GnRH, FMRFamide, bicuculline, and enkephalin on horizontal cell activity. J Neurosci. 1991; 11: 3034–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowling JE. Retinal neuromodulation: the role of dopamine. Vis Neurosci. 1991; 7: 87–97. [DOI] [PubMed] [Google Scholar]
- 44.Huang L, Maaswinkel H, Li L. Olfactoretinal centrifugal input increases zebrafish retinal ganglion cell sensitivity: A possible role for dopamine-mediated Ca2+ signaling pathways. J Physiol. 2005; 569: 939–948. 10.1113/jphysiol.2005.099531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CC, Fernald RG. Visual information alone changes behavior and physiology during social interactions in a cichlid fish (Astatotilapia burtoni). PLoS ONE. 2011; 6: e20313 10.1371/journal.pone.0020313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernald RD, Maruska KP. Social information changes the brain. Proc Natl Acad Sci. 2012; 109: 17194–17199. 10.1073/pnas.1202552109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grens KE, Greenwood AK, Fernald RD. Two visual processing pathways are targeted by gonadotropin-releasing hormone in the retina. Brain Behav Evol. 2005; 66: 1–9. 10.1159/000085043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246: 312–342. 10.1002/cne.902460304 [DOI] [PubMed] [Google Scholar]
- 49.Paredes RG, Baum MJ. Role of the medial preoptic area/anterior hypothalamus in the control of masculine sexual behavior. Ann Rev Sex Res. 1997; 8: 68–101. [PubMed] [Google Scholar]
- 50.Roselli CE, Larkin K, Schrunk JM, Stormshak F. Sexual partner preference, hypothalamic morphology and aromatase in rams. Physiol Behav. 2004; 83: 233–245. 10.1016/j.physbeh.2004.08.017 [DOI] [PubMed] [Google Scholar]
- 51.Tilbrook AJ, Galloway DB, Williams AH, Clarke IJ. Treatment of young rams with an agonist of GnRH delays reproductive development. Horm Behav. 1993; 27: 5–28 10.1006/hbeh.1993.1002 [DOI] [PubMed] [Google Scholar]
- 52.Keverne EB. Pheromones, vomeronasal function, and genderspecific behavior. Cell. 2002; 108: 735–738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.