Abstract
Visual search tasks support a special role for direct gaze in human cognition, while classic gaze judgment tasks suggest the congruency between head orientation and gaze direction plays a central role in gaze perception. Moreover, whether gaze direction can be accurately discriminated in the periphery using covert attention is unknown. In the present study, individual faces in frontal and in deviated head orientations with a direct or an averted gaze were flashed for 150 ms across the visual field; participants focused on a centred fixation while judging the gaze direction. Gaze discrimination speed and accuracy varied with head orientation and eccentricity. The limit of accurate gaze discrimination was less than ±6° eccentricity. Response times suggested a processing facilitation for direct gaze in fovea, irrespective of head orientation, however, by ±3° eccentricity, head orientation started biasing gaze judgments, and this bias increased with eccentricity. Results also suggested a special processing of frontal heads with direct gaze in central vision, rather than a general congruency effect between eye and head cues. Thus, while both head and eye cues contribute to gaze discrimination, their role differs with eccentricity.
Keywords: gaze discrimination, face perception, spatial attention, covert attention, peripheral vision
Introduction
Gaze direction is essential to nonverbal communication and is central to human social cognition (see George & Conty, 2008, and Itier & Batty, 2009 for reviews). Direct gaze or mutual gaze (eyes looking at you) conveys vital social information and may signal kindliness or affability (Kleinke, 1986), or potential threats such as anger or hostility (Ellsworth & Carlsmith, 1973). By contrast, averted gaze (eyes looking away from you) is mainly used as a cue to others’ focus of attention, and can also orient one’s attention to specific places or persons (Driver et al., 1999; Friesen & Kingstone, 1998; see Frischen et al., 2007 for a review), a phenomenon thought to play an important role in mental state attributions (Baron-Cohen et al., 1997). Accurate gaze discrimination is thus fundamental for proper communication and for navigating our social world.
Gaze discrimination is already seen in neonates (Batki et al., 2000; Farroni et al., 2002), and monkey cell recordings indicate that face selective neurons in the superior temporal sulcus (STS) are sensitive to gaze information (Perrett et al., 1985, 1990). An innate neurocognitive mechanism known as the Eye Direction Detector (EDD; Baron-Cohen, 1994) has been proposed, which would be specialized in detecting eyes and their gaze direction in the environment, and especially whether someone is looking at, or away from, the observer. However, neurons in the monkey STS are also sensitive to other cues such as head orientation and even body position (Perrett et al., 1982, 1985), which led some to propose a more general Direction of Attention Detector (DAD; Perrett & Emery, 1994) that would infer others’ attention based on the integration of directional information coming from the eyes (iris eccentricity), but also from the head and body positions.
Many behavioural studies in humans have shown that gaze judgments can be affected by the orientation of the head (Anstis et al., 1969; Kluttz et al., 2009; Langton, 2000; Langton et al., 2004; Ricciardelli & Driver, 2008; Seyama & Nagayama, 2005; Shirama, 2012; Todorović, 2006, 2009; Wollaston, 1824), thus supporting the DAD hypothesis. For example, reaction times to discriminate gaze direction are shorter when the eyes and head are oriented in the same direction than when they are oriented in different directions (Itier et al., 2007a, 2007b; Langton, 2000; Seyama & Nagayama, 2005; Todorović, 2009), reflecting a congruency effect between eye direction cues and head direction cues. Recent studies even suggest that although different, iris eccentricity and head direction cues jointly specify perceived gaze direction and that they are dependent on one another, each alone being insufficient for gaze perception (Todorović, 2006, 2009). This theoretical framework highlights the dependence of gaze perception on head direction, but makes no assumption as to whether one gaze direction is discriminated better and/or faster than the other and what role head orientation might play in this discrimination asymmetry.
In contrast, other lines of research support a special status for direct gaze discrimination. For instance, in visual search studies, direct gaze is discriminated faster and more accurately than averted gaze in a crowd of opposite-gaze distractors—a phenomenon known as the “stare-in-the-crowd effect.” This effect has been shown using pairs of schematic eyes (von Grünau & Anston, 1995), photographs of three-quarter view faces (Senju et al., 2005; see also Doi & Ueda, 2007; Doi et al., 2009), or photographs of eye regions from deviated heads (Conty et al., 2006). The fact that this effect has been shown with deviated head views suggests that attention capture by direct gaze is not just due to the visual symmetry between the dark iris and white sclera of the eyes in front-view faces, but rather to the perception that the gaze is directed at the observer (Conty et al., 2006; Doi & Ueda, 2007; Doi et al., 2009; Senju et al., 2005; but see Shirama, 2012 for the suggestion that what drives the stare-in-the-crowd effect is the frontal view of the face).
Importantly, the stare-in-the-crowd effect is seen in visual search paradigms which are very different from typical gaze discrimination paradigms in terms of stimuli presentation (arrays versus central presentation) and task (rapid discrimination of a given gaze direction among distractors versus looking-at-me/looking-away judgments or judgments of actual gaze direction angles). Furthermore, visual search paradigms involve the movement of the eyes to fixate on the target before responding, thus using overt attention. However, very little research has examined how gaze direction is discriminated when the stimuli are in the viewer’s peripheral field of view, using covert attention. If direct/mutual gaze is so important for social interactions, it would make sense that it also catches attention in the periphery. It has been proposed that faces could be detected by a subcortical face detection system (Johnson, 2005) that would also enable eye contact discrimination in the periphery, picking up contrast information between the circular dark iris and the white sclera of the eyes (Senju & Johnson, 2009). One visual search study reported better discrimination of direct than averted gaze looking characters situated in the visual periphery (Palanica & Itier, 2011). Other studies using change detection (e.g., Yokoyama et al., 2011) and interocular suppression (e.g., Stein et al., 2011; Yokoyama et al., 2013) also support the view of a preferential processing of direct gaze in faces presented covertly. However, these studies did not manipulate the eccentricity at which those faces were presented or the direction of the face. It is thus still unclear how gaze direction is discriminated covertly in the viewer’s peripheral field of view, and what role head direction plays in the periphery.
Loomis et al. (2008) presented face stimuli with various gaze directions at different visual eccentricities and found that participants’ judgments of gaze direction were only reliable in central vision, up to 4° of horizontal eccentricity1, while judgments of head orientation were reliable all the way to 90° of eccentricity. That study however, focused on gaze judgment accuracy (participants judged lookers’ facing direction using a haptic pointer) rather than on direct/averted gaze discrimination, and did not measure reaction times so the speed at which gaze was processed outside of foveal vision could not be determined. An important measure in many gaze discrimination paradigms (e.g., stare-in-the-crowd effect) is response time, since any hypothetical cognitive mechanism related to gaze discrimination (e.g., DAD) should function quickly to determine if one is the focus of attention (or possibly in danger). In a series of six experiments, Burton et al. (2009) showed that eye gaze could not be perceived from unattended faces presented in the vertical periphery even when their size was adjusted to match that of centrally presented items in terms of cortical representation, thus concluding that gaze processing requires focused attention. In contrast, the head direction (side profile views) of these distracter faces impacted responses to the centrally presented items, suggesting that head direction might be processed outside of focused attention (supporting Loomis et al., 2008). Recently, Yokoyama et al. (2014) refined this conclusion by conducting a dual-task paradigm in which participants discriminated a set of centrally-presented letters and discriminated the gaze direction of a parafoveally-presented face. Results showed that direct gaze faces could be perceived without focused attention while averted gaze perception required focused attention. However, similar to Loomis et al. (2008), reaction times were not measured, and Yokoyama et al. (2014) only used front-view faces presented within central vision (~5° eccentricity)2. Thus, it remains unknown whether gaze discrimination judgments could be made with faces presented in the periphery, beyond central vision, and whether head orientation affects these judgments.
The goal of the present experiment was three-fold. First, we examined whether and to what extent gaze direction could be covertly discriminated in the periphery above chance level. Second, we investigated whether direct gaze was faster and more accurately discriminated than averted gaze, and, if so, at what visual eccentricities. Third, we examined to what extent head orientation affected gaze discrimination in the periphery. We measured the speed and accuracy of gaze discrimination when faces were presented across the visual field (between 0° and ±12° of eccentricity) while fixation was centred and enforced by an eye-tracker. Based on previous research (Loomis et al., 2008; Burton et al., 2009; Yokoyama et al., 2014), we predicted that gaze discrimination would be above chance only in central vision (0° to ±5°) where acuity is high. As eccentricity increases and visual acuity decreases, perception of eye cues should become more difficult, and thus error rates and RTs should increase. Based on the visual search literature (e.g., Conty et al., 2006; Doi & Ueda, 2007; Doi et al., 2009; Senju et al., 2005; Shirama, 2012; von Grünau & Anston, 1995), we predicted that direct gaze faces would be discriminated faster and more accurately than averted gaze faces (across head orientations) in central vision, where gaze discrimination is accurate. Based on the gaze perception literature (e.g., Langton, 2000; Seyama & Nagayama, 2005; Itier et al., 2007a, 2007b; Todorović, 2009), we also predicted congruency effects between head and gaze directions, such that direct gaze discrimination should be fastest in frontal heads while that of averted gaze should be fastest in deviated heads. However, if gaze is accurately perceived in central vision only, while head orientation is accurately perceived in the periphery (Loomis et al., 2008; Burton et al., 2009), then a bias toward using head orientation cues to discriminate gaze should be seen in the periphery.
Methods
Participants
A total of 24 participants completed the experiment. Two participants were rejected due to eye calibration issues, one due to fatigue and one due to an insufficient amount of data, leaving a final sample of 20 participants included in the analyses (11 females, 9 males; 19 right-handed; age range 19–23 years, M = 20.7). Participants were undergraduate students from the University of Waterloo (UW), with normal or corrected-to-normal vision, who took part in the study for course credit. The study received full ethics clearance from the UW Research Ethics Board and all participants signed informed written consents.
Stimuli
Greyscale photographs of 8 individuals (four men, four women) with neutral expression were taken from George et al. (2001). Each face was photographed against a black background with the head pointed straight towards the camera (i.e., frontal heads) and with the head oriented 30° to the right side (i.e., deviated heads), with the eyes looking straight ahead at the camera (i.e., direct gaze) or 30° to the right side (i.e., averted gaze). These four pictures were then mirror-reversed using Adobe Photoshop to avoid any bias between the left and right sides. The face photographs subtended a visual angle of 4.4° horizontally by 6.6° vertically. The eye region subtended a visual angle of 2.5° horizontally by 0.5° vertically for frontal heads, and 2.2° horizontally by 0.5° vertically for deviated heads (slightly narrower as the faces were oriented to the side). The iris diameter subtended 20 minutes of arc for all face stimuli. Examples of the types of stimuli used in the study are shown in Figure 1; however, note that these are not the actual face stimuli used (see George et al., 2001 for accurate examples).
Figure 1.
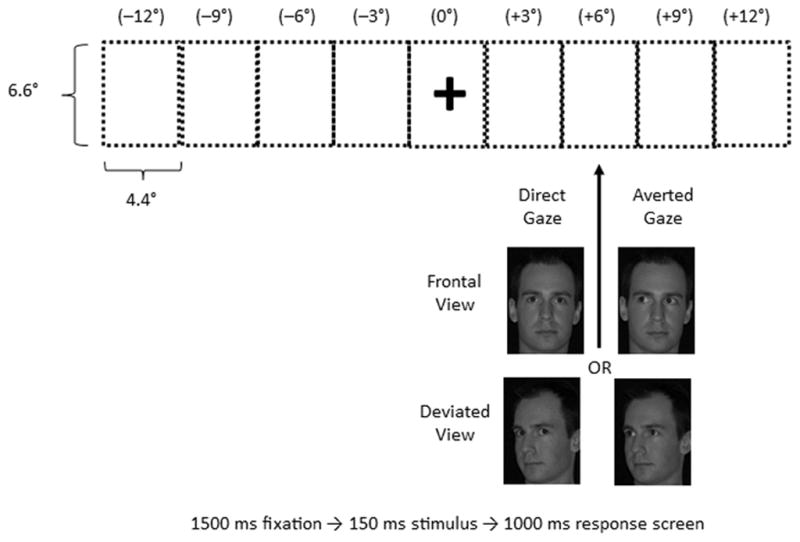
Schematic representation of stimulus presentation. The dotted rectangles are shown to represent all of the 9 possible locations of stimuli presentation, but were invisible during trials. Negative (−) eccentricities represent target positions to the left of fixation, while positive (+) eccentricities represent those to the right of fixation. The fixation cross was shown during the entire duration of each trial to keep participants’ fixation focused. Please also note that for averted gaze faces, both left- and right-looking faces were used, and for deviated head views, both left- and right-facing head orientations were used.
Apparatus
A Viewsonic PS790 CRT 19-inch colour monitor was used to present the stimuli (Intel Corel 2 Quad CPU Q6700; 1024 × 768 pixels; 60 Hz frame rate). We used a remote EyeLink 1000 eye-tracker from SR Research with a sampling rate of 1000 Hz to control for participants’ initial fixation and record any possible eye movements during stimulus presentation. Participants’ viewing position and distance were maintained by chin and forehead rests. At a viewing distance of 70 cm, the monitor subtended a visual angle of 29.2° × 22.2°.
Procedure
Participants were instructed to focus on a central fixation cross at all times (see Figure 1). Before each trial, the fixation cross (1° × 1°) was presented for 1200 ms, and then became a fixation trigger that participants must have focused on for 300 ms to activate the next trial. For each trial, an individual face was presented for 150 ms randomly in one of 9 possible locations aligned horizontally across the screen. Each face centre was positioned from −12° to +12° visual angle horizontally (the widest limit achieved with the computer screen). The centres of each adjacent face position were separated by 3° of visual angle horizontally. Following stimulus presentation, participants were required to discriminate whether the target face had a direct or an averted gaze as quickly and as accurately as possible, using a two-button press with their index and middle fingers of their dominant hand. Buttons were counterbalanced across participants. The next trial started 1000 ms after the end of stimulus presentation, regardless of whether a response was made or not. Before each study session, 9 practice trials (one for each possible stimulus location) were presented to familiarize participants with the stimuli and task.
Half of the stimulus trials consisted of frontal heads, while the other half consisted of deviated heads. For each head orientation, half of the trials consisted of direct gaze (DG) faces that looked straight ahead toward the observer while the other half of trials consisted of averted gaze (AG) faces (half looking to the left and half looking to the right). It may be argued that straight gaze in the periphery would be perceived as non-mutual gaze, and similarly, averted gaze in the periphery in the direction of the observer may be perceived as mutual gaze contact. However, at debriefing, all participants confirmed that they were aware of the difference between gaze directions across all eccentricities and did not perceive an averted gaze as a mutual gaze or a direct gaze as an averted gaze in the periphery.
For each eccentricity, there were 32 frontal DG trials, 32 frontal AG trials, 32 deviated DG trials, and 32 deviated AG trials, totalling 1152 trials which were divided into 8 blocks of 144 trials each. Head orientation, gaze direction, and eccentricity presentation were randomized within each block. A rest was given between blocks. The experiment lasted about 70 minutes.
Data Analysis
Left- and right-averted gaze directions were combined and averaged for each target position. Preliminary analyses revealed no significant differences between left- and right-averted gaze targets across eccentricities. Mean RT data were computed using only correct responses. For each subject, RTs that were below 150 ms (0.1% of the data) or exceeded 2.5 standard deviations (6.7% of the data) from the mean of each gaze condition per eccentricity were discarded. All trials where more than one fixation was made (i.e., when participants moved their eyes away from the centred fixation) were eliminated (2.8%). RTs exceeding 1000 ms were recorded as a miss (3.9%). Miss rates did not vary significantly across gaze conditions or eccentricities. Error rates were calculated as the number of false alarms (e.g., pressing the AG button when a DG face was presented, or vice versa) that were made out of the total amount of responses that could have been made for each individual eccentricity. As this was a two-button press, forced choice task, a 50% error rate to a particular condition is chance level performance. If a participant responded using only one button for each trial (e.g., DG button), then their error rate for DG would be 0% and their error rate for AG would be 100% (i.e., 100% false alarm rate and 0% hit rate for AG, assuming 0% misses). Preliminary analyses revealed that none of the participants followed this pattern of response. For clarity, we only reported error rates. We also reported the overall percentage of responses to each gaze condition regardless of whether participants were correct or not, indicating a possible response bias to press one button over the other. Preliminary analyses also revealed no significant effects of participant gender or stimulus gender on gaze discrimination.
RTs, percent error rates, and percent response rates were analyzed using a 2 (head orientations) by 2 (gaze directions) by 9 (eccentricities) repeated measures ANOVA. The Greenhouse-Geisser degrees of freedom correction was used when the sphericity assumption was violated (i.e., when the Mauchly’s test of sphericity was significant)3. Subsequently, for each dependent measure (RT, error rate, response rate), planned paired sample (two-tailed) t-tests were performed comparing the two gaze conditions within each head orientation at each target position, as well as comparing the two head orientations within each gaze direction at each target position. The Bonferroni correction was used for these planned t-tests comparing the two gaze conditions and two head orientations across the 9 eccentricities (i.e., 36 comparisons), making p value significance thresholds at .0014. To determine the peripheral eccentricities at which gaze was discriminated above chance level, error rates for each gaze condition were compared to chance level at each eccentricity using one-sample t-tests (two-tailed, with a test value of 50), with the same adjusted p values as above (i.e., .0014).
Results
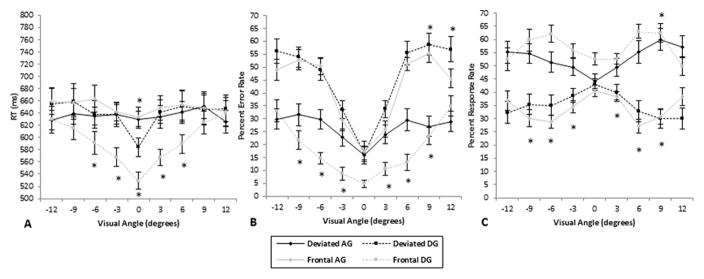
For RT results (Figure 2A), there were main effects of eccentricity (F(8, 152) = 9.20, MSE = 5316.78, p < .0001, ηp2 = .33), gaze direction (F(1, 19) = 9.86, MSE = 14278.18, p < .01, ηp2 = .34), and head orientation (F(1, 19) = 51.13, MSE = 907.50, p < .0001, ηp2 = .73), which were strongly modulated by interactions between eccentricity and gaze direction (F(8, 152) = 6.37, MSE = 3715.65, p < .001, ηp2 = .25), eccentricity and head orientation (F(8, 152) = 3.30, MSE = 1665.40, p < .01, ηp2 = .15), gaze direction and head orientation (F(1, 19) = 28.40, MSE = 5558.94, p < .0001, ηp2 = .60), and eccentricity by head orientation by gaze direction (F(8, 152) = 2.37, MSE = 1897.65, p < .05, ηp2 = .11). This complex pattern reflected that RTs to averted gaze faces were the longest and were not modulated by eccentricity (effect of eccentricity at p > .3 for AG faces tested separately). In contrast, RTs to DG faces increased with eccentricity. These RTs increased steadily up to the extreme eccentricities for frontal heads, while for deviated heads, the increase was sharp from 0 to ±3°, and levelled off by ±6°. RTs were also faster for DG than AG for frontal heads from −6° to +6° (planned t-tests, all p < .001), while for deviated heads, RTs were faster for DG than AG faces only at 0° (p < .001). RTs were also faster for DG frontal than DG deviated heads from −6° to +6° (planned comparison t-tests, all p < .001). By contrast, no differences in RTs were found between AG deviated and AG frontal heads. The overall pattern suggested a clear distinction between DG frontal heads and the other three conditions.
Figure 2.
Results, as a function of gaze direction and eccentricity, for (A) RT responses, (B) Error rates, and (C) Response presses (all shown with standard error bars). Please note that for (B) and (C), gaze direction data were computed within each head orientation (i.e., DG vs. AG for frontal heads, and DG vs. AG for deviated heads). DG vs. AG paired comparisons: stars above lines represent deviated head comparisons; stars below lines represent frontal head comparisons; *p < .001.
For error rates (Figure 2B), main effects of eccentricity (F(8, 152) = 117.70, MSE = 127.50, p < .0001, ηp2 = .86) and head orientation (F(1, 19) = 36.72, MSE = 200.63, p < .0001, ηp2 = .66) were modulated by interactions between eccentricity and head orientation (F(8, 152) = 3.84, MSE = 93.43, p < .005, ηp2 = .17), head orientation and gaze interaction (F(1, 19) = 80.83, MSE = 1122.97, p < .0001, ηp2 = .81), eccentricity and gaze direction (F(8, 152) = 2.87, MSE = 517.14, p < .05, ηp2 = .13), and eccentricity by head orientation by gaze direction (F(8, 152) = 14.88, MSE = 231.77, p < .0001, ηp2 = .44). The overall pattern clearly showed lower error rates for frontal heads with direct gaze followed by deviated heads with averted gaze (the two congruent conditions), and then by the two incongruent conditions, which did not differ. Error rates increased steadily with eccentricity for frontal view DG faces but performances well above chance level at all eccentricities (p < .001). In contrast, the increase in error rates levelled off for the other three conditions by about ±6°. For the deviated view AG, errors were also well above chance level at all eccentricities (p < .001). For the two incongruent conditions, performances reached chance level by ±6° (using a one-sample t-test, p > .1). For frontal heads, DG faces elicited lower error rates than AG faces from −9° to +9° (planned t-tests, all p < .001), except at 0°. For deviated heads, AG faces elicited lower error rates than DG faces from +9° to +12° (all p < .001). Additionally, lower error rates were found for DG frontal than DG deviated heads at all eccentricities (all p < .001), while lower error rates were found for AG deviated than AG frontal heads at all eccentricities (all p < .001), except between −3° and +3°.
For overall response rates (irrespective of hits or errors; Figure 2C), a main effect of head orientation (F(1, 19) = 8.97, MSE = 33.13, p < .01, ηp2 = .32) was found, which was strongly modulated by interactions between eccentricity and head orientation (F(8, 152) = 2.33, MSE = 9.50, p < .05, ηp2 = .11), eccentricity by gaze interaction (F(8, 152) = 3.14, MSE = 442.10, p < .05, ηp2 = .14), head orientation and gaze direction (F(1, 19) = 53.87, MSE = 1357.60, p < .0001, ηp2 = .74), and eccentricity, head orientation, and gaze direction (F(8, 152) = 14.14, MSE = 174.99, p < .0001, ηp2 = .43). For frontal heads, more DG than AG responses were made from −9° to +9° (planned t-tests, all p < .001), except at 0°. For deviated heads, more AG than DG responses were made only at +9° (all p < .001). Additionally, the DG responses were made more often for frontal heads than for deviated heads at all eccentricities (all p < .001, except at +12°), while the AG responses were made more often for deviated than frontal heads at all eccentricities (all p < .001), except between 0° and +3°.
Discussion
In this experiment, we investigated whether and to what extent gaze direction could be accurately discriminated in the periphery, and whether direct gaze was discriminated better than averted gaze when presented in both the central and peripheral visual fields. We also examined to what extent head orientation affected this gaze discrimination judgment. Both frontal and deviated faces were presented randomly across nine eccentricities while fixation on a centred fixation cross was enforced by an eye-tracker, thus ensuring the use of covert attention. Participants discriminated between direct gaze (DG) and averted gaze (AG) using two response buttons, while RTs, error rates, and response bias were recorded.
Error rates increased with eccentricity as expected, given the decrease in visual acuity from central to peripheral vision (e.g., Larson & Loschky, 2009) and the importance of iris position (a local detail) in gaze perception (e.g., Ricciardelli et al., 2000; Sinha, 2000). This increase was also steeper for the incongruent gaze-head conditions (i.e., AG frontal and DG deviated faces) than for the congruent conditions (i.e., DG frontal and AG deviated faces), with chance level reached at ±6° eccentricity for the incongruent conditions. This pattern seemed driven by the response bias, with participants making more “DG” than “AG” responses for frontal heads, and more “AG” than “DG” responses for deviated heads (although this bias was more pronounced for frontal heads). This response bias was also more pronounced in the periphery than in central vision, leading to more errors for the incongruent conditions with eccentricity (with a larger difference for frontal than deviated heads).
When faces were presented within foveal and parafoveal vision (i.e., from 0° to < ±6°), error rates were low for both gaze directions (across head orientations), suggesting true gaze discrimination. Thus, the limit of accurate gaze discrimination was between 3° and 6° of eccentricity, i.e., within central vision, in line with previous studies (Burton et al., 2009; Loomis et al., 2008; Yokoyama et al., 2014). Previous research using centrally presented faces has shown that gaze judgments can be strongly affected by head orientation (Anstis et al., 1969; Kluttz et al., 2009; Langton, 2000; Langton et al., 2004; Ricciardelli & Driver, 2008; Seyama & Nagayama, 2005; Shirama, 2012; Todorović, 2009; Wollaston, 1824), and thus that head and eye cues are both necessary for gaze judgments even in fovea. Using covert attention, the present study showed that head orientation starts biasing the gaze direction judgment as early as ±3° and that this bias increases with eccentricity. This effect is likely due to visual acuity diminishing very rapidly outside of foveal vision (Larson & Loschky, 2009), and thus eye cues receiving increasingly less importance for gaze judgments with eccentricity. That is, as the eye cues were more difficult to discriminate in the periphery, participants were more likely to press the direct gaze button when the face was in front view and the averted gaze button when the head was turned. The larger size of the head is easier to see in the periphery compared to the eyes (Loomis et al., 2008), and may lead to a global information bias (see Navon, 1977), where global information (i.e., head orientation) receives priority over local information (i.e., eye direction) for gaze judgments. The larger size of the head compared to the eyes could thus also account for the increased reliance on head orientation in covert gaze judgments.
Using head orientation as a primary indicator for gaze direction in the periphery makes sense since head direction can be seen from a much farther eccentricity than iris position (Loomis et al., 2008), and the two are normally congruent with each other. Additionally, if the eye region is occluded (e.g., by sunglasses; Nuku & Bekkering, 2008) or obscured by shadows, then the head can be used to infer attention direction, an idea already suggested by research in monkeys (Perrett et al., 1992). In instances of low eye region visibility, priority could be given to head direction as an indicator of social attention. Our results show that this is the case in the peripheral field when iris position is no longer clearly visible. Electrophysiological studies in monkeys provide evidence that neurons in the STS integrate information from iris position, head orientation and body position (Perrett et al., 1982, 1985). In humans, neuroimaging studies suggest that two distinct regions of the STS are involved in gaze processing. The anterior part (aSTS) has recently been shown to contain distinct neural populations sensitive to the different directions of gaze, as revealed by neural adaptation (Calder et al., 2007). The posterior part (pSTS) in contrast, has been involved in the processing of gaze (Allison et al., 2000; Hoffman & Haxby, 2000), but also biological movements (Puce & Perrett, 2003), and recent studies suggest that it is involved in the social relevance of gaze signals and the general analysis of social intentions of human actions (e.g., Pelphrey et al., 2003, 2004; Saxe et al., 2004) rather than in gaze direction discrimination per se. Although it is at present unclear how the coding of head orientation impacts the processing of gaze direction in the human brain, all of these findings support the general Direction of Attention Detector as a basis for social attention (Perrett & Emery, 1994). Future neuroimaging studies will have to investigate this coding issue more carefully, as the present behavioural findings can only speculate on possible cognitive mechanisms involved with these gaze discrimination processes.
For frontal heads, DG faces were discriminated faster than AG faces until ±6° eccentricity. Taking into account the fact that by ±6° performances were at chance level for incongruent conditions, these results reflect truly faster (and more accurate) discrimination of the DG frontal head condition below these eccentricity limits, i.e., within central vision. For deviated heads, faster RTs for DG than AG faces were also seen for the centred position, when participants were directly fixated on the target. This result is in line with the stare-in-the-crowd effect literature supporting a facilitation of direct gaze processing (Conty et al., 2006; Doi & Ueda, 2007; Doi et al., 2009; Senju et al., 2005; Shirama, 2012; von Grünau & Anston, 1995). Thus, even in this non-visual search paradigm, direct gaze processing was facilitated compared to averted gaze processing regardless of head orientation, within foveal vision. Faster responses to DG for centrally presented faces in front views were also reported in previous studies on gaze perception (e.g., Langton, 2000; Seyama & Nagayama, 2005; Itier et al., 2007a, 2007b; Pageler et al., 2003; Todorović, 2009). However, in contrast to the current study, for deviated head views, these previous studies usually reported either no gaze difference (e.g., Itier et al, 2007b; Pageler et al., 2003) or a congruency effect with faster responses for averted than direct gaze (e.g., Langton, 2000; Seyama & Nagayama, 2005; Itier et al., 2007a; Todorović, 2009). The specific design used might play an important role in these discrepant results for deviated heads, in particular, with the face always being centrally presented as in these previous studies, or presented randomly at different eccentricities as in the current study. Note that the lack of congruency effect in RTs for deviated heads was seen at all eccentricities in the present study. Thus, the present data support the idea of a real facilitation of processing for direct gaze in fovea that is not due to head orientation. Beyond fovea, the faster responses to DG might be driven by the combination of DG with a frontal head view.
Overall, our results suggest that the combination of a frontal head view with a direct gaze elicited the best and fastest response compared to the other three gaze-head combinations across most eccentricities (Figure 2). This specific combination of front view and direct gaze has been suggested to drive the stare-in-the-crowd effect (Shirama et al., 2012). Several factors might contribute to this front-view-direct-gaze effect. First, it corresponds to the innate face template for which human infants show a preference (Johnson et al., 1991; Morton & Johnson, 1991; Farroni et al., 2002). Second, parents usually direct both their head and eye gaze toward their offspring to capture their attention and also to perceive the infant’s wants and needs, making the front head with direct gaze the most prevalent visual stimulus infants are exposed to early in life. Third, in typical real-world situations, interpersonal communication usually occurs face to face. For instance, to grab another person’s attention, one will tend not only to shift their gaze, but also move their face toward the person of interest. Because it is physically uncomfortable to direct one’s eyes to the far side for a long period of time, people typically align their heads and eye direction to people/objects of interest. The intent in social interaction may also be perceived as stronger when both head and gaze directions are oriented toward us. Thus, the extra attention summoned by a frontal head with DG over and above the other conditions may be the result of both an innate preference for, and a learned response to that stimulus.
In conclusion, these findings support the notion that humans have a specialized cognitive mechanism capable of rapidly responding to important social signals in the environment. One such signal is gaze direction that gives cues to others’ attention and intentions. Our findings show that both head orientation and gaze direction contribute to gaze discrimination in the environment, supporting the Direction of Attention Detector that would integrate directional information from eyes, head, and body position (Perrett & Emery, 1994). While the integration of both eye and head orientation cues is necessary for accurate gaze perception even in fovea (Todorovic, 2009), the importance given to head orientation cues seems to increase beyond foveal view and especially in the periphery while eye cues might be given less importance due to decreased acuity. The data also highlight the special role played by frontal heads with a direct gaze, the most attention-capturing face stimulus. Different emphasis might be given to eye and head cues depending on whether overt or covert attention is applied, an idea that future studies will need to explore further.
Acknowledgments
This work was supported by CIHR (MOP-87393 and MOP-89822), CFI (#213322), and the Canada Research Chair (CRC) program (#959-213322) to RJ Itier.
Footnotes
Vision scientists discriminate between central vision, which encompasses foveal vision (~1° eccentricity on either side of fixation) and parafoveal vision (1–5° eccentricity), and peripheral vision, which encompasses everything beyond parafoveal vision (Calvo & Lang, 2005; Larson & Loschky, 2009). Foveal vision corresponds to the spatial focus of overt attention, whereas parafoveal and peripheral vision involve covert perception outside the focus of attention. This terminology will be used in the remainder of the paper.
Yokoyama et al. (2014) used facial images (3° × 3°) positioned along the edge of an imaginary rectangle subtending 8° × 10° of visual angle (i.e., 4° of visual angle from the vertical edge of the rectangle to centred fixation, and 5° of visual angle from the horizontal edge to centered fixation; see Figure 1 from their study). Thus, the distance from the centred fixation to the centre of the face (i.e., which is how we calculated the eccentricity measurements in the current study) would subtend less than 5° of visual angle.
For clarity, only the adjusted p-values were reported and the original degrees of freedom kept.
References
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Science. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Anstis SM, Mayhew JW, Morley T. The perception of where a face or television “portrait” is looking. American Journal of Psychology. 1969;82:474–489. [PubMed] [Google Scholar]
- Baron-Cohen S. How to build a baby that can read minds: Cognitive mechanisms in mind reading. Cahiers de Psychology Cognitive/Current Psychology of Cognition. 1994;13:513–552. [Google Scholar]
- Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: evidence from very high functioning adults with autism or Asperger Syndrome. Journal of Child Psychology and Psychiatry. 1997;38:813–822. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Batki A, Baron-Cohen S, Wheelwright S, Connellan J, Ahluwalia J. Is there an innate module? Evidence from human neonates. Infant Behavior and Development. 2000;23:223–229. [Google Scholar]
- Burton AM, Bindemann M, Langton SRH, Schweinberger SR, Jenkins R. Gaze perception requires focused attention: Evidence from an interference task. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:108–118. doi: 10.1037/0096-1523.35.1.108. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Winston JS, Dolan RJ, Jenkins R, Eger E, Henson RNA. Separate coding of different gaze directions in the superior temporal sulcus and inferior parietal lobule. Current Biology. 2007;17:20–25. doi: 10.1016/j.cub.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MG, Lang PJ. Parafoveal semantic processing of emotional visual scenes. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:502–519. doi: 10.1037/0096-1523.31.3.502. [DOI] [PubMed] [Google Scholar]
- Conty L, Tijus C, Hugueville L, Coelho E, George N. Searching for asymmetries in the detection of gaze contact versus averted gaze under different head views: a behavioural study. Spatial Vision. 2006;19:529–545. doi: 10.1163/156856806779194026. [DOI] [PubMed] [Google Scholar]
- Doi H, Ueda K. Searching for a perceived stare in the crowd. Perception. 2007;36:773–780. doi: 10.1068/p5614. [DOI] [PubMed] [Google Scholar]
- Doi H, Ueda K, Shinohara K. Neural correlates of the stare-in-the-crowd effect. Neuropsychologia. 2009;47:1053–1060. doi: 10.1016/j.neuropsychologia.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Visual Cognition. 1999;6:509–540. [Google Scholar]
- Ellsworth PC, Carlsmith JM. Eye contact and gaze aversion in aggressive encounter. Journal of Personality and Social Psychology. 1973;33:117–122. doi: 10.1037/h0035779. [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review. 1998;5:490–495. [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychological Bulletin. 2007;133:694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George N, Conty L. Facing the gaze of others. Clinical Neurophysiology. 2008;38:197–207. doi: 10.1016/j.neucli.2008.03.001. [DOI] [PubMed] [Google Scholar]
- George N, Driver J, Dolan RJ. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Batty M. Neural bases of eye and gaze processing: The core of social cognition. Neuroscience and Biobehavioural Reviews. 2009;33:843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier RJ, Alain C, Kovacevic N, McIntosh AR. Explicit versus implicit gaze processing assessed by ERPs. Brain Research. 2007a;1177:79–89. doi: 10.1016/j.brainres.2007.07.094. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Villate C, Ryan JD. Eyes always attract attention but gaze orienting is task-dependent: Evidence from eye movement monitoring. Neuropsychologia. 2007b;45:1019–1028. doi: 10.1016/j.neuropsychologia.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Kleinke CL. Gaze and eye contact: A research review. Psychological Bulletin. 1986;100:78–100. [PubMed] [Google Scholar]
- Kluttz NL, Mayes BR, West RW, Kerby DS. The effect of head turn on the perception of gaze. Vision Research. 2009;49:1979–1993. doi: 10.1016/j.visres.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Langton SRH. The mutual influence of gaze and head orientation in the analysis of social attention direction. Quarterly Journal of Experimental Psychology. 2000;53:825–845. doi: 10.1080/713755908. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Honeyman H, Tessler E. The influence of head contour and nose angle on the perception of eye-gaze direction. Perception & Psychophysics. 2004;66:752–771. doi: 10.3758/bf03194970. [DOI] [PubMed] [Google Scholar]
- Larson AM, Loschky LC. The contributions of central versus peripheral vision to scene gist recognition. Journal of Vision. 2009;9(10):6, 1–16. doi: 10.1167/9.10.6. [DOI] [PubMed] [Google Scholar]
- Loomis JM, Kelly JW, Pusch M, Bailenson JN, Beall AC. Psychophysics of perceiving eye-gaze and head direction with peripheral vision: Implications for the dynamics of eye-gaze behavior. Perception. 2008;37:1443–1457. doi: 10.1068/p5896. [DOI] [PubMed] [Google Scholar]
- Morton J, Johnson MH. CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychological Review. 1991;98:164–181. doi: 10.1037/0033-295x.98.2.164. [DOI] [PubMed] [Google Scholar]
- Navon D. Forest before trees: The precedence of global features in visual perception. Cognitive Psychology. 1977;9:353–383. [Google Scholar]
- Nuku P, Bekkering H. Joint attention: Inferring what others perceive (and don’t perceive) Consciousness and Cognition. 2008;17:339–349. doi: 10.1016/j.concog.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Pageler NM, Menon V, Merin NM, Eliez S, Brown WE, Reiss AL. Effect of head orientation on gaze processing in fusiform gyrus and superior temporal sulcus. Neuroimage. 2003;20:318–329. doi: 10.1016/s1053-8119(03)00229-5. [DOI] [PubMed] [Google Scholar]
- Palanica A, Itier RJ. Searching for a perceived gaze direction using eye tracking. Journal of Vision. 2011;11(2):19, 1–13. doi: 10.1167/11.2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown MJ, Goldstein J, Allison T, McCarthy G. Brain activity evoked by the perception of human walking: Controlling for meaningful coherent motion. Journal of Neuroscience. 2003;23:6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: The perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. Journal of Cognitive Neuroscience. 2004;16:1706–1716. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Emery NJ. Understanding the intentions of others from visual signals: Neurophysiological evidence. Current Psychology of Cognition. 1994;13:683–694. [Google Scholar]
- Perrett DI, Harries MH, Mistlin AJ, Hietanen JK, Bevan R, Thomas S, … Brierly K. Social signals analyzed at the single cell level: someone’s looking at me, something touched me, something moved! International Journal of Comparative Psychology. 1990;4:25–55. [Google Scholar]
- Perrett DI, Hietanen JK, Oram WM, Benson PJ. Organization and functions of cells responsive to faces in the temporal cortex. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 1992;335:23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Experimental Brain Research. 1982;47:329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PAJ, Potter DD, Mistlin AJ, Head AS, Milner AD, Jeeves MA. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proceedings of the Royal Society of London, Series B: Biological Sciences. 1985;223:293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Puce A, Perrett DI. Electrophysiology and brain imaging of biological motion. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2003;358:435–445. doi: 10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardelli P, Baylis G, Driver J. The positive and negative of human expertise in gaze perception. Cognition. 2000;77:B1–B14. doi: 10.1016/s0010-0277(00)00092-5. [DOI] [PubMed] [Google Scholar]
- Ricciardelli P, Driver J. Effects of head orientation on gaze perception: How positive congruency effects can be reversed. Quarterly Journal of Experimental Psychology. 2008;61:491–504. doi: 10.1080/17470210701255457. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42:1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Shirama A. Stare in the crowd: Frontal face guides overt attention independently of its gaze direction. Perception. 2012;41:447–459. doi: 10.1068/p7114. [DOI] [PubMed] [Google Scholar]
- Senju A, Hasegawa T, Tojo Y. Does perceived direct gaze boost detection in adults and children with and without autism? The stare-in-the-crowd effect revisited. Visual Cognition. 2005;12:1474–1496. [Google Scholar]
- Senju A, Johnson MH. The eye contact effect: Mechanisms and development. Trends in Cognitive Sciences. 2009;13:127–134. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Seyama J, Nagayama RS. The effect of torso direction on the judgment of eye direction. Visual Cognition. 2005;12:103–116. [Google Scholar]
- Sinha P. Here’s looking at you, kid. Perception. 2000;29:1005–1008. doi: 10.1068/p2908no. [DOI] [PubMed] [Google Scholar]
- Stein T, Senju A, Peelen MV, Sterzer P. Eye contact facilitates awareness of faces during interocular suppression. Cognition. 2011;119:307–311. doi: 10.1016/j.cognition.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorović D. Geometrical basis of perception of gaze direction. Vision Research. 2006;46:3549–3562. doi: 10.1016/j.visres.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Todorović D. The effect of face eccentricity on the perception of gaze direction. Perception. 2009;38:109–132. doi: 10.1068/p5930. [DOI] [PubMed] [Google Scholar]
- von Grünau M, Anston C. The detection of gaze direction: A stare-in-the-crowd effect. Perception. 1995;24:1297–1313. doi: 10.1068/p241297. [DOI] [PubMed] [Google Scholar]
- Wollaston WH. On the apparent direction of eyes in a portrait. Philosophical Transactions of the Royal Society of London B. 1824;114:247–256. [Google Scholar]
- Yokoyama T, Hishibashi K, Hongoh Y, Kita S. Attentional capture by change in direct gaze. Perception. 2011;40:785–797. doi: 10.1068/p7003. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Noguchi Y, Kita S. Unconscious processing of direct gaze: Evidence from an ERP study. Neuropsychologia. 2013;51:1161–1168. doi: 10.1016/j.neuropsychologia.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Sakai H, Noguchi Y, Kita S. Perception of direct gaze does not require focus of attention. Scientific Reports. 2014;4:3858. doi: 10.1038/srep03858. [DOI] [PMC free article] [PubMed] [Google Scholar]