Abstract
The GAS3 family of tetraspan proteins has recently been implicated in the progression of cancer. Currently, six members of the GAS3 family have been identified in humans and mice, and while their expressions in disease vary, data suggest that they play a role in epithelial cell structure and function. In this review, we highlight the studies implicating four of the members in disease pathogenesis as well as probe the structural similarities between the family members. Finally, the impact of targeting select members of the family such as PMP22 and EMP2 is discussed.
Keywords: epithelial membrane proteins, peripheral membrane protein-22, tetraspan, GAS3, four-transmembrane, cancer
I. INTRODUCTION
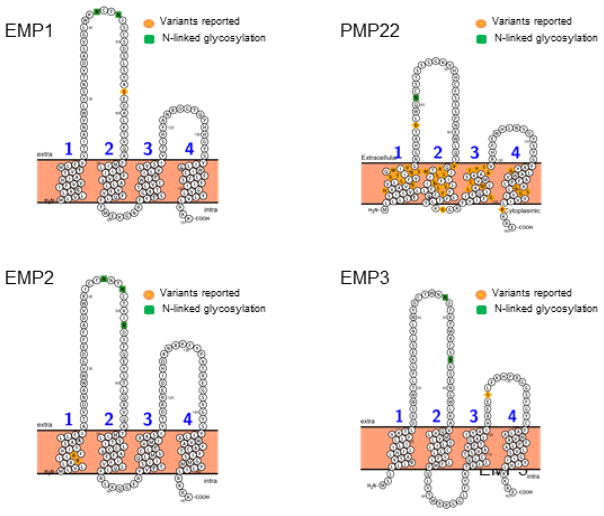
The tetraspan superfamily, also called transmembrane 4 superfamily (TM4SF) of membrane proteins, 1 is comprised of three subfamilies, namely, the connexins, tetraspanins, and growth arrest specific 3/PMP22 (GAS3) family. The current review focuses on the GAS3/PMP22 subfamily of TM4SF. This review will identify the known members of the GAS3/PMP22 family, describe their structure and function, and identify the different cancer types associated with each of the known members. Despite their association with many disease states, the GAS3 family of proteins largely remains enigmatic. Expressed in all metazoans, four members of the GAS3 family have been identified in humans and mice. This includes the prototype for this family peripheral myelin protein (PMP22), which has been linked to human demyelinating heredity neuropathies, 2,3 and all other members were identified based on their homology to PMP224,5 (Table 1). The GAS3 family can be distinguished from other four transmembrane proteins by their relatively small size, ranging from 157 (EMP1) to 160 amino acids (PMP22). They are characterized by two large extracellular domains of unequal sizes, which contain a number of N-linked glycosylation sites, and images are provided for each family member in Fig. 1.6 Members have been shown to be expressed on the plasma membrane or in intercellular vesicles, and unique tissue and/or cell specific functions have been implicated for each.7–10 In this review, we focus on identifying the structure, function, and the association of GAS3 family members PMP22, and the epithelial membrane proteins (EMPs) in cancer biology.
TABLE 1.
Amino acid homology between EMP2 family members
| EMP1 | EMP3 | PMP22 | EMP2 | |
|---|---|---|---|---|
| EMP1 | 100 | 31.41 | 40.65 | 41.56 |
| EMP3 | 31.41 | 100 | 43.12 | 39.75 |
| PMP22 | 40.65 | 43.12 | 100 | 45.28 |
| EMP2 | 41.56 | 39.75 | 45.28 | 100 |
FIG. 1.
Putative structure of the GAS3 family of proteins. The topographical structure was determined using Protter software (adapted from Ref. 6). Reported variants as well as the sites for posttranslational modifications have been indicated.
II. PMP22
A. Structure and Function
The PMP22 gene (previously designated PASII, SR13, and gas3 for growth arrest-specific gene-3) codes for a 22 kDA glycoprotein consisting of 160 amino acids.11–16 It has been proposed that PMP22 has four distinct functional roles. These include peripheral myelin formation, cell-cell interactions, cell proliferation, and peroxisomal biogenesis.14–16 PMP22 was originally identified in two different systems. First, as a peripheral nervous system (PNS) myelin protein that is downregulated after sciatic nerve injury in the distal nerve stump,12,13 and second as a mRNA that is strongly upregulated in growth-arrested NIH3T3 fibroblasts.3,8 Induction of PMP22 expression occurs during cell cycle arrest and apoptosis in fibroblasts,8,17 and hence, the family was named growth arrest specific or GAS3.
The highest levels of PMP22 expression are observed in myelinating Schwann cells in the PNS where it accounts for 2–5% of all myelin proteins.18 Altered gene expression of PMP22 has been documented in peripheral neuropathies where amplification, deletions, or mutations in PMP22 result in Charcot-Marie-Tooth disease type 1A (CMT1A), an autosomal dominant demyelinating peripheral neuropathy, and Dejerine-Sottas disease, an inherited neurological disorder that progressively affects mobility.19–22 Studies in mice have shown that PMP22 is required for the correct development of peripheral axons as mice devoid of PMP22 or carrying mutations in the gene display retarded myelination and develop hypermyelinated structures resulting in a Trembler phenotype.23,24
Outside of the peripheral nervous system, PMP22 transcripts have also been detected in the intestines, lungs, uterus, and heart, and it has been shown that PMP22 is regulated by two promoters that produce alternative gene transcripts in a tissue specific pattern.25 It is known that both progesterone and glucocorticosteroids can upregulate the expression of PMP22 in sciatic nerve, and anti-progesterone therapy has been shown to reduce PMP22 levels, reducing the CMT1A phenotype. 22,26,27 However, outside of the PNS, estrogen appears to regulate its expression as within the uterus, high PMP22 mRNA and protein levels have been reported in proliferative stroma and endometrium. 28,29 Additional studies will be required to determine if the differences in expression are the result of the alternate promoters as well as the function of PMP22 in cells outside the PNS.
B. PMP22 and Cancer
The history of PMP22 in cancer has been varied as a number of studies have reported either up- or downregulation of the protein in different models. For example, decreased expression of the PMP22 gene was observed during development of lung cancer in animal models.30 In contrast to lung cancer, several studies have shown amplification of the PMP22 transcript in cancer. Amplification of the chromosome 17p11.2 region and PMP22 expression have been associated with human osteosarcoma and glioblastoma in tissue and cell lines.31–36 Moreover, in vitro and in vivo data suggest PMP22 may play a role in the neoplastic transformation process of the normal pancreas to premalignant lesions to pancreatic cancer.37 This varied expression in different tumor types suggests that the role of PMP22 in growth arrest and differentiation may be cell and tissue specific.37
Similarly, the story in breast cancer is full of inconsistencies. Initial studies documented upregulation of PMP22 mRNA levels in a panel of invasive and noninvasive human mammary cancer cell lines,38 and these studies were expanded on to investigate its prognostic potential in 249 primary breast cancer patients.39 The authors concluded that patients who had higher than average median PMP22 gene expression were at higher risk of death from cancer when compared to patients with equal clinical covariables but lower PMP22 gene expression.39 However, complicating these conclusions, other studies in breast cancer showed reduced PMP22 mRNA levels. Mimori and colleagues compared differential gene expression of PMP22 between normal, primary carcinoma cells, and metastatic carcinoma cells, and identified PMP22 as typically having diminished expression when comparing both primary tumor cells to normal cells and metastatic cells with primary tumor cells.40
The contradiction in mRNA levels highlights the need for better protein analysis of PMP22 in cancer, and it is likely that posttranscriptional regulation for PMP22 exists. For example, Lauer et al. reported that PMP22 may exist within peroxisomes. His group showed that while PMP22 mRNA levels of PMP22 in colon cancer are not altered compared to normal tissue, its protein levels, as measured by immunohistochemistry and Western blot analysis, were significantly reduced in colon carcinoma samples compared to normal tissue.7 The authors conclude that the reduction of PMP22 along with other peroxisomal membrane proteins (for example, PMP70) in colon cancer may not be attributed to reduced transcription of their genes, as the mRNA levels were not altered in colon cancer, but possibly due to diminished rate of translation for these mRNAs in carcinoma cells.7
III. EMPS (EMP1, EMP2, AND EMP3)
A. EMP1: Structure and Function
The 25 kDa epithelial membrane protein 1 (EMP1), is a hydrophobic polypeptide protein composed of 160 amino acid residues that shares 40% of its sequence identity with PMP22.4 As the hydrophobicity profiles of PMP22 and EMP1 are comparable, particularly within the first two transmembrane domains, Taylor and colleagues proposed that EMP1 and PMP22 were members of the same family and may share similar molecular functions.41 Similarly, the N-linked glycosylation found in the extracellular domain of EMP1 is comparable to that of PMP22. The glycosylation domain in PMP22 carries an epitope known as L2/HNK-1, which is noted to mediate cell-cell recognition and adhesion.42 EMP1 carries the N-linked glycosylation in the identical position to PMP22, implicating EMP1 as having functional roles in cell proliferation and differentiation.
EMP1 has been isolated from human, rat, rabbit, and mouse tissues and has been labeled with several compellations: EMP1, Tmp, PAP, CL-20, and B4B.10,43–46 Despite being coexpressed on the transcriptional level in many tissues, especially PNS nerves, EMP1 and PMP22 show significant differences in their relative tissue-specific expression levels. For example, although PMP22 mRNA is present at low to absent levels in the gastrointestinal tract, lung, brain, and skin, EMP1 levels are more abundantly expressed in these tissues.10
Although EMP1 and PMP22 are coexpressed in the PNS, they appear to be differentially regulated. 10 In animal models of sciatic nerve injury, mRNA levels of EMP1 and PMP22 were inversely regulated with significantly lower EMP1 mRNA than that observed for PMP22. Instead, EMP1 mRNA was upregulated four days post rat sciatic nerve injury in proliferating cells, whereas PMP22 mRNA was significantly reduced.10 These results could be recapitulated in vitro using mitogen-expanded primary rat Schwann cells (pSc) and D6P2T Schwann cells grown in the presence or absence of forskolin, which induces PMP22 expression via axon-Schwann cell interactions during myelination. However, EMP1 mRNA levels decreased under similar experimental parameters. In other cell types such as NIH3T3 cells, similar results were observed. In a consecutive in vitro model, whereby NIH 3T3 fibroblasts were grown under serum deprivation, PMP22 mRNA expression was significantly increased but EMP1 mRNA decreased. This not only confirmed that PMP22 is regulated by growth arrest,47 but again illustrated the inverse regulation of EMP1 mRNA.4 The inverse regulation of EMP1 and PMP22 mRNAs during different stages of cell cycle provides indirect support for the role of these GAS3 family proteins in the regulation of cell proliferation and dormancy.4 Currently, EMP1 has been identified as having many biological functions in cancer, including cell proliferation, apoptosis, invasion, and metastasis.48–52
1. EMP1 and Cancer
Similar to PMP22, the expression profile of EMP1 in cancer is mixed, with conflicting reports between mRNA and protein studies. On the mRNA side, reports have suggested both an upregulation and downregulation of EMP1 in cancer. For example, gene expression profiling of 50 human gliomas was analyzed using a cDNA microarray platform. EMP1 mRNA was upregulated and correlated with an increase in the myc oncogene.53 Similar changes have been observed in other cancers. In breast cancer, immortalized human mammary luminal epithelial cells expressing moderate and high levels of the ERBB2 receptor, which is designated a proto-oncogene and belonging to the tyrosine kinase family (HER2/neu), were examined for changes in gene expression using cDNA microarrays corresponding to approximately 6000 genes.54 EMP1 gene expression was significantly elevated in cells overexpressing ERBB2.54 Finally, EMP1 overexpression was recently shown as a novel poor prognostic factor in pediatric leukemia that may regulate prednisolone resistance, cell proliferation, migration, and adhesion.55 BCP-ALL patients with high levels of EMP1 showed a significantly poorer five-year event-free survival compared to patients with low expression with pathway analysis suggesting that EMP1 signals through the Src family kinases.55
In contrast, in other cancer systems, EMP1 has been linked with tumor suppression. EMP1 mRNA is reduced in oral squamous cell carcinoma (OSCC), nasopharyngeal carcinoma, and prostate cancer.51,56,57 RT-PCR and immunohistochemistry were used to measure EMP1 mRNA levels and protein expression in OSCC and corresponding adjacent normal tissues. EMP1 mRNA levels and protein expression were significantly reduced in OSCC compared to control samples. In addition, decreased EMP1 expression was significantly correlated with clinical stage (p = 0.002) and lymph node metastasis (p = 0.044).52 The following findings suggest that EMP1 may function as a tumor suppressor. However, further studies are required to comprehend the functional mechanisms through which EMP1 may exert these effects in OSCC.
Similar to reports in OSCC, decreased EMP1 mRNA levels and protein expression have been reported in 75 cases of nasopharyngeal cancer relative to 31 cases of healthy controls. Decreased EMP1 expression significantly correlated with T stages (TNM staging system), lymph node metastasis, clinical stage, and histopathological grade. Furthermore, poor overall survival was significantly correlated with loss of EMP1 expression. In in vitro studies, transfection of the nasopharyngeal cancer cell line (CNE2) with overexpression of EMP1 resulted in significant decrease in cell proliferation but an increase in apoptosis. EMP1 overexpressing CNE2 cells exhibited increased caspase-9 (p < 0.05) but decreased VEGF-C protein levels.57 Similarly, Sun and colleagues56 again investigated the expression of EMP1 in colorectal carcinoma and prostate cancer. In both cases, decreased expression of EMP1 was significantly correlated with T stages, lymph node metastasis, clinical stage, and histopathological grade in patients with colorectal carcinoma as well as prostate cancer. In culture, transfection of human colorectal (SW-480) or human prostate PC-3 cancer cell lines with an EMP1 overexpression vector again resulted in increased apoptosis (p < 0.05) and decreased migration and invasion (p < 0.05). Both cell lines overexpressing EMP1 also showed significantly elevated caspase-9 protein levels but decreased VEGF-C levels. These findings suggest that EMP1 may induce apoptosis through the mitochondria-dependent pathway and suppress tumor metastasis through VEGF-C-mediated angiogenesis in OSCC, colorectal carcinoma, and prostate cancer.56,57
In cancers in women, a similar story exists in uterine leiomyomas and in cervical cancers. EMP1 gene expression was significantly downregulated (3.9-fold change) in leiomyoma samples compared to matched normal myometrium. These findings suggest that EMP1 may potentially alter smooth muscle cell differentiation in leiomyoma.58 In an in silico study, microarray samples of 24 normal and 102 cervical cancer biopsies from four independent, publicly accessible databases were analyzed for gene expression profiling. Once more, EMP1 gene expression was significantly downregulated in cervical cancer biopsies compared to matched healthy controls.59
Finally, studies investigating the role of EMP1 in breast cancer are limited but suggest similarity to observations in other cancer types. Sun and colleagues examined EMP1 protein levels in breast tissue. Immunohistochemistry and Western blot were used to analyze EMP1 protein levels in 67 cases of breast cancer and 35 normal tissues. EMP1 protein levels were significantly lower (p < 0.05) in breast cancer samples relative to normal controls.51 In addition, EMP1 protein levels were significantly correlated with T stages, lymph node metastasis, clinical stage, and histopathological grade. Poor overall survival was correlated (p < 0.05) with low EMP1 protein levels. Transfection of MCF-7 breast cancer cell lines in order to increase expression levels of EMP1 resulted in elevated apoptosis and a significant decline in migration and invasion. EMP1 significantly upregulated caspase-9 and decreased VEGF-C protein levels (p < 0.05). These findings suggest that EMP1 may negatively regulate breast cancer (in vitro) via modulation of caspase-9.51
B. EMP2
1. Structure and Function
Epithelial membrane protein-2 (EMP2) is believed to have resulted from a gene duplication of PMP22 as EMP2 and PMP22 are found on paralogous chromosomal regions.60 Analogous to EMP1, EMP2 shares ~40% of its amino acid sequence with PMP22. Structurally, EMP2 is 160 amino acids translating into an 18 kDa polypeptide core with three N-linked glycosylation sites on its first extracellular loop.5 Similar to the other tetraspan proteins, EMP2 mRNA is widely distributed with high expression in the lungs and moderate expression in the eyes, heart, thyroid, intestine, and uterus.4,5,61 Similar to PMP22, mutations in EMP2 expression have been associated with disease. Mutations in EMP2 have been linked to childhood onset nephrotic syndrome with patients carrying a 21C > G (p.Phe7Leu) mutation.62 Nonetheless, the protein expression of EMP2 is more discrete than its mRNA profile with limited expression observed in type 1 pneumocytes within the lung, endometrium, keratinocytes within the skin, and select sites including within retinal pigment epithelium and corneal epithelium of the eye.63–66
EMP2 expression is hormonally regulated, and in vivo studies have also delineated that upregulation of EMP2 expression within the endometrium occurs during the secretory phase.67 It has been proposed that its expression is critical for successful implantation as knockdown of EMP2 within the uterus resulted in a significant decrease in the number of implantation sites within the uterine horns.68
Functionally, under physiological conditions, EMP2 appears to regulate the expression of select integrins, GPI proteins, and class 1 major histocompatibility complex proteins where it helps to traffic these proteins into lipid raft domains.68–72 In this way, EMP2 appears to help stabilize select integrins, modulating adhesion onto various extracellular matrices.70,73
2. EMP2 and Cancer
Analogous to other tetraspan proteins, the history of EMP2 in cancer has been variable. Initially, EMP2 was also reported to have an apoptotic effect in a B-cell lymphoma cell line, and similar roles have been proposed in nasopharyngeal and urinary tract urothelial carcinoma.61,74,75 However, multiple other studies suggest that EMP2 functions as an oncoprotein. Using microarray technology, gene profiling suggests that EMP2 mRNA is upregulated in a number of cancers including breast, ovarian, endometrial, and primary CNS malignancies. 76–79 Moreover, in many of these models, EMP2 mRNA was further upregulated during disease progression and metastasis. In a study examining gene expression from circulating tumor cells, EMP2 was one of six genes identified to be significantly overexpressed in blood samples obtained from patients with advanced and patients with primary breast cancer.80 In addition, EMP2 was identified with twist expression in breast metastatic lesions in the bone, and its mRNA levels were upregulated in endocrine, dasatinib, and chemotherapy resistant tumors in multiple female tumors, suggesting that EMP2 promotes more aggressive disease.81–84 These findings suggest that EMP2 mRNA may serve to be a potential diagnostic marker for female cancer pathologies.
To understand how the differences in mRNA expression correlate with protein levels, several studies have assessed EMP2 protein expression. The first of these studies examined endometrial neoplasm samples for EMP2 expression and correlated these findings to patient clinical outcomes.85 Endometrial tumors with high EMP2 expression were more likely to be myometrium invasive, high stage, recurrent, or fatal. In addition, the medial survival for patients with high EMP2 tumors was 23 months, but patients with low-to-negative EMP2 expression in their tumors had significantly better prognosis. Wadehra and colleagues concluded that endometrial carcinomas (n = 99) with an assortment of histologic subtypes, grades, and stages rendered EMP2 an independent negative predictor of disease-free survival linked to high disease stage.85 These findings suggest that EMP2 may have significant prognostic implications in endometrial adenocarcinomas. To expand on the role of EMP2 in cancers in women, EMP2 protein expression was measured in patients with ovarian and breast cancers. EMP2 levels were upregulated in 63% of invasive breast cancers, and 73% in triple- negative breast tumors, and found to be normal in healthy controls. Similarly, EMP2 expression was observed in 68% of women with advanced ovarian cancers.86
Several studies have examined the mechanism through which EMP2 contributes to cancer progression. Using xenograft models in breast, endometrial, and primary CNS malignancies, alterations in EMP2 expression in turn modulated integrin-associated signaling cascades, specifically activating focal adhesion kinase (FAK)/Src.69,87 In fact, even in tumors where EMP2 did not promote tumor growth, alternations in integrin expression were observed.5 Subsequently, it has been shown that EMP2 also promotes induction of vascular endothelial growth factor (VEGF) and subsequently this leads to neoangiogenesis in endometrial tumors. This potentially occurs through a HIF-1a dependent mechanism mediated via hypoxia and FAK-Src activation.88 The high expression and function associated with EMP2 in cancers suggested that it may function as a novel diagnostic and therapeutic target.
Since previous studies have established that EMP2 protein is significantly upregulated in endometrial and ovarian cancers, the significance of the prognostic and foretelling diagnostic knowledge that can be gained by imaging EMP2 expression in malignant tumors may be of value. Therefore, Fu and colleagues generated anti-EMP2 antibody conjugated to 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and radiolabeled with Cu, which was then evaluated in mouse model of endometrial cancer as a positron emission tomography (PET) imaging molecule.65 Cu-DOTA anti-EMP2 minibody successfully established high uptake in xenograft models of endometrial cancer overexpressing EMP2. On the contrary, EMP2-negative tumors exhibited low uptake with anti-EMP2 minibodies.65 These findings suggest that patients with positive EMP2 malignancies may benefit from antibody specific imaging to localize EMP2-expressing tumors and eventually determining therapeutic efficacy.
To the best of our knowledge, EMP2 is the only protein in the GAS3 family that has been extensively investigated for its therapeutic potential in cancers in women.86–88 In vitro studies investigated the efficacy of anti-EMP2 diabodies for the treatment of human endometrial adenocarcinoma (HEC-1A). Significant cell death and elevated caspase- 3 levels were evident following treatment in HEC-1A cell line, suggesting that anti-EMP2 diabody may exert its effects via caspase-dependent apoptotic cell death. In vivo, subcutaneous human xenografts of HEC-1A cells were generated and subsequently treated with anti-EMP2 diabodies. Significant tumor growth suppression and cell death ensued following treatment with anti-EMP2 antibody.89 For the first time, these findings suggest that a GAS3 protein may have a pharmacological potential for the treatment of endometrial cancer. Given the successful treatment of endometrial tumors with the anti-EMP2 diabodies, fully human IgG1 antibodies were engineered. Anti-EMP2 IgG1 therapy significantly suppressed tumor growth devoid of any systemic toxicity.87 Collectively, these results suggest anti-EMP2 antibody may have a therapeutic potential for the treatment of aggressive endometrial cancers.65
Outside of cancers in women, the therapeutic potential of EMP2 was investigated in glioblastoma (GBM). A GBM array from 110 patients was examined for EMP2 protein by immunohotochemisty and scored in a masked fashion. Approximately 95% of GBM patients were positive for EMP2 expression but normal brain tissues had low-to-undetectable levels. In vivo, GBM cells expressing EMP2 significantly heightened tumor growth, partially through ανβ3 integrin upregulation, FAK/Src activation, and increased cell migration and invasion. Anti-EMP2 antibody therapy successfully decreased tumor load in subcutaneous mouse models and enhanced GBM cell death in vitro.90 These results suggest anti-EMP2 therapy may be of value in the pathogenesis of GBM.
C. EMP3
1. Structure and Function
Epithelial membrane protein-3 (EMP3) projects onto chromosome 19q13.3 band and consists of a165-amino acid sequence, four transmembrane domains, and two N-linked glycosylation sites within the first extracellular loop. The homology of EMP3 amino acid with PMP22, EMP1, and EMP2 is 41%, 33%, and 38%, respectively. The transmembrane domain is the site where homology is most significant among the GAS3 family proteins.5,41,91 EMP3 mRNA levels are heightened in peripheral leukocytes, ovary, intestine, and embryonic tissues. In contrast to PMP22 and EMP1, EMP3 (and EMP2) transcripts are present in the liver.5 Although the functional role of EMP3 has not been thoroughly investigated, speculations have been made that it may be involved in cell proliferation, cell-cell interactions, and apoptosis based on validated data from PMP22 and EMP1 studies.5 EMP3 gene overexpression has been reported in oligodendroglial tumors, breast cancer cell lines, glioblastomas, and prostate cancer.92–95 However, hypermethylation of EMP3 has extensively been studied in glioma and neuroblastoma, and has been reported to function as a tumor suppressor gene in these cancers.96,97 Subsequent studies have linked the tumor suppressor properties of EMP3 to solid tumors, such as esophageal squamous cell carcinoma and phenochromocytomas.94,98
2. EMP3 and Cancer
Using microarray analysis, 89 neuroblastoma tumors were analyzed for EMP3 gene expression. EMP3 expression was significantly downregulated in neuroblastoma tumors compared to benign ganglioneuroma samples. Hypermethylation-mediated silencing of EMP3 gene was reported to be one known mechanism by which EMP3 gene is silenced in neuroblastoma tumors, and decreased EMP3 expression correlated with poor survival after two years following the diagnosis (p = 0.03). In vitro, use of a demethylating agent (5-aza-2-deoxycytidine) restored EMP3 gene expression in neuroblastoma cells, and resulted in significant decline in colony formation. In vivo, re-methylation of EMP3 significantly reduced tumor volume in mouse xenograft models of neuroblastoma.96 These findings indicate that in neuroblastoma, EMP3 may function as a tumor suppressor gene, and hypermeythylation of EMP3 is an indication of poor prognosis in patients diagnosed with neuroblastoma. Similarly, transcript levels of EMP3 were evaluated in 57 samples of oliodendrioglial tumors (OTs) by quantitative real-time RT-PCR. Reduced EMP3 expression was reported in 18% of OTs compared to healthy brains. However, EMP3 was overexpressed in 19% of OTs as well, with eight samples having greater than tenfold increase in EMP3 gene compared to healthy controls. And almost all OTs were positive for EMP3 methylation, but there was no significant correlation between methylation status and EMP3 gene expression.99 These results suggest that in OTs, methylation alone does not regulate transcriptional silencing of EMP3, and the tumor suppressor properties of EMP3 that were reported in neuroblastoma do not apply to OTs.96 Further research is required to elucidate the function of EMP3 in neuroblastoma, OTs, and normal cells. EMP3 expression has been characterized in non-small cell lung cancer (NSCLC), during early and advanced stages and the prognostic validity of EMP3 in NSCLC patients has been evaluated. EMP3 levels in NSCLC were significantly lower than healthy control tissues and correlated with TMN stage (p < 0.05). No significant correlation was found between EMP3 expression and other clinical parameters.100 These findings provide further validation for the tumor suppressor properties of EMP3; however, further research is required to understand the molecular mechanisms of EMP3-mediated pathogenesis of NSCLC.
In upper urinary tract urothelial carcinoma (UUT-UC), EMP3 is associated with enhanced oncogenesis. In vitro and in vivo studies demonstrated that overexpression of EMP3 heightens cancer cell proliferation and migration, however suppresses cell adhesion (in vitro). In addition, the progression of urothelial carcinoma (UC) was positively correlated with EMP3 mRNA expression, providing evidence for the oncogenic property of EMP3 in UUT-UC. EMP3 mRNA and protein levels were evaluated in a cohort of UUT-UC patients (n = 77), and the most important critical indicator of poor prognosis was based on co-expression of EMP3 and ErbB2 integrin. In vitro studies validated the upregulation of ErbB2 in human UC cells and linked activation of ErbB2-PI3K-AKT pathway to increased expression of EMP3.101 These findings suggest EMP3 possess cell proliferative properties in UUT-UC. Further research is needed to validate the potential of EMP3-mediated ErbB2 targeted therapy in UUT-UC.
IV. CONCLUSIONS
The GAS3 family of tetraspan proteins was expanded 20 years ago in a search for genes homologous to PMP22. For the first time, the structure, function, and association of the four major members of the GAS3 family of proteins to cancer has been reviewed. Although there is no consensus on the level of expression, dysregulation of each epithelial membrane protein member has been implicated in multiple cancers, perhaps highlighting the differential tissue and cell type specific expression of each family member. In addition, there is no consensus as to the etiology in disease as in some cases select members function as tumor suppressors and in others as oncoproteins. Some of the discrepancies may simply be the result of posttranscriptional regulation. Many of the studies performed to date analyze mRNA expression, and given the robust mRNA but discrete protein expression for both PMP22 and EMP2, it is likely that extensive posttranscriptional regulation exists to determine the final protein levels of each of these family members. As such, targeting these proteins in specific cancers may yield new markers for diagnosis, prognosis, and therapy.
Acknowledgments
This work was generously supported by Grant No. NCI R01 CA163971 (M. W.). We acknowledge Dr. Lee Goodglick, our friend, mentor, and fellow scientist. We miss you.
ABBREVIATIONS
- 4-TM
four-transmembrane, tetraspan proteins
- EMP2
epithelial membrane protein-2
- GAS3
growth arrest stop 3
- PMP22
peripheral membrane protein-22
References
- 1.Wright MD, Tomlinson MG. The ins and outs of the transmembrane 4 superfamily. Immunol Today. 1994;15:588–94. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 2.Fabbretti E, Edomi P, Brancolini C, Schneider C. Apoptotic phenotype induced by overexpression of wild-type gas3/PMP22: its relation to the demyelinating peripheral neuropathy CMT1A. Genes Dev. 1995;9:1846–56. doi: 10.1101/gad.9.15.1846. [DOI] [PubMed] [Google Scholar]
- 3.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–93. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 4.Taylor V, Welcher AA, Program AE, Suter U. Epithelial membrane protein-1, peripheral myelin protein 22, and lens membrane protein 20 define a novel gene family. J Biol Chem. 1995;270:28824–33. doi: 10.1074/jbc.270.48.28824. [DOI] [PubMed] [Google Scholar]
- 5.Taylor V, Suter U. Epithelial membrane protein-2 and epithelial membrane protein-3: two novel members of the peripheral myelin protein 22 gene family. Gene. 1996;175:115–20. doi: 10.1016/0378-1119(96)00134-5. [DOI] [PubMed] [Google Scholar]
- 6.Omasits U, Ahrens CH, Müller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30:884–6. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- 7.Lauer C, Volkl A, Riedl S, Fahimi HD, Beier K. Impairment of peroxisomal biogenesis in human colon carcinoma. Carcinogenesis. 1999;20:985–9. doi: 10.1093/carcin/20.6.985. [DOI] [PubMed] [Google Scholar]
- 8.Manfioletti G, Ruaro ME, Del Sal G, Philipson L, Schneider C. A growth arrest-specific (gas) gene codes for a membrane protein. Mol Cell Biol. 1990;10:2924–30. doi: 10.1128/mcb.10.6.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor V, Suter U. Epithelial membrane protein-2 and epithelial membrane protein-3: two novel members of the peripheral myelin protein 22 gene family. Gene. 1996;175:115–20. doi: 10.1016/0378-1119(96)00134-5. [DOI] [PubMed] [Google Scholar]
- 10.Taylor V, Welcher AA, Program AE, Suter U. Epithelial membrane protein-1, peripheral myelin protein 22, and lens membrane protein 20 define a novel gene family. J Biol Chem. 1995;270:28824–33. doi: 10.1074/jbc.270.48.28824. [DOI] [PubMed] [Google Scholar]
- 11.Snipes GJ, Suter U, Welcher AA, Shooter EM. Characterization of a novel peripheral nervous system myelin protein (PMP-22/SR13) J Cell Biol. 1992;117:225–38. doi: 10.1083/jcb.117.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spreyer P, Kuhn G, Hanemann CO, Gillen C, Schaal H, Kuhn R, Lemke G, Muller HW. Axon-regulated expression of a Schwann cell transcript that is homologous to a ‘growth arrest-specific’ gene. Embo J. 1991;10:3661–8. doi: 10.1002/j.1460-2075.1991.tb04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welcher AA, Suter U, De Leon M, Snipes GJ, Shooter EM. A myelin protein is encoded by the homologue of a growth arrest-specific gene. Proc Natl Acad Sci USA. 1991;88:7195–9. doi: 10.1073/pnas.88.16.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant DD, Wilson GN. Differential evolution and expression of murine peroxisomal membrane protein genes. Biochem Mol Med. 1995;55:22–30. doi: 10.1006/bmme.1995.1027. [DOI] [PubMed] [Google Scholar]
- 15.Kaldi K, Diestelkotter P, Stenbeck G, Auerbach S, Jakle U, Magert HJ, Wieland FT, Just WW. Membrane topology of the 22 kDa integral peroxisomal membrane protein. FEBS Lett. 1993;315:217–22. doi: 10.1016/0014-5793(93)81167-x. [DOI] [PubMed] [Google Scholar]
- 16.Suter U, Snipes GJ. Peripheral myelin protein 22: facts and hypotheses. J Neurosci Res. 1995;40:145–51. doi: 10.1002/jnr.490400202. [DOI] [PubMed] [Google Scholar]
- 17.Zoidl G, D’Urso D, Blass-Kampmann S, Schmalenbach C, Kuhn R, Muller HW. Influence of elevated expression of rat wild-type PMP22 and its mutant PMP22Trembler on cell growth of NIH3T3 fibroblasts. Cell Tissue Res. 1997;287:459–70. doi: 10.1007/s004410050770. [DOI] [PubMed] [Google Scholar]
- 18.Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat Genet. 1995;11:274–80. doi: 10.1038/ng1195-274. [DOI] [PubMed] [Google Scholar]
- 19.Roa BB, Garcia CA, Suter U, Kulpa DA, Wise CA, Mueller J, Welcher AA, Snipes GJ, Shooter EM, Patel PI, Lupski JR. Charcot-Marie-Tooth disease type 1A. Association with a spontaneous point mutation in the PMP22 gene. N Engl J Med. 1993;329:96–101. doi: 10.1056/NEJM199307083290205. [DOI] [PubMed] [Google Scholar]
- 20.Roa BB, Dyck PJ, Marks HG, Chance PF, Lupski JR. Dejerine-Sottas syndrome associated with point mutation in the peripheral myelin protein 22 (PMP22) gene. Nat Genet. 1993;5:269–73. doi: 10.1038/ng1193-269. [DOI] [PubMed] [Google Scholar]
- 21.Patel PI, Roa BB, Welcher AA, Schoener-Scott R, Trask BJ, Pentao L, Snipes GJ, Garcia CA, Francke U, Shooter EM, Lupski JR, Suter U. The gene for the peripheral myelin protein PMP-22 is a candidate for Charcot-Marie-Tooth disease type 1A. Nat Genet. 1992;1:159–65. doi: 10.1038/ng0692-159. [DOI] [PubMed] [Google Scholar]
- 22.Sereda MW, zu Hörste GM, Suter U, Uzma N, Nave K-A. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A) Nat Med. 2003;9:1533–7. doi: 10.1038/nm957. [DOI] [PubMed] [Google Scholar]
- 23.Suter U, Welcher AA, Ozcelik T, Snipes GJ, Kosaras B, Francke U, Billings-Gagliardi S, Sidman RL, Shooter EM. Trembler mouse carries a point mutation in a myelin gene. Nature. 1992;356:241–4. doi: 10.1038/356241a0. [DOI] [PubMed] [Google Scholar]
- 24.Colby J, Nicholson R, Dickson KM, Orfali W, Naef R, Suter U, Snipes GJ. PMP22 carrying the trembler or trembler-J mutation is intracellularly retained in myelinating Schwann cells. Neurobiol Dis. 2000;7:561–73. doi: 10.1006/nbdi.2000.0323. [DOI] [PubMed] [Google Scholar]
- 25.Suter U, Snipes GJ, Schoener-Scott R, Welcher AA, Pareek S, Lupski JR, Murphy RA, Shooter EM, Patel PI. Regulation of tissue-specific expression of alternative peripheral myelin protein-22 (PMP22) gene transcripts by two promoters. J Biol Chem. 1994;269:25795–808. [PubMed] [Google Scholar]
- 26.Meyer zu Horste G, Prukop T, Liebetanz D, Mobius W, Nave KA, Sereda MW. Antiprogesterone therapy uncouples axonal loss from demyelination in a transgenic rat model of CMT1A neuropathy. Ann Neurol. 2007;61:61–72. doi: 10.1002/ana.21026. [DOI] [PubMed] [Google Scholar]
- 27.Desarnaud F, Bidichandani S, Patel PI, Baulieu EE, Schumacher M. Glucocorticosteroids stimulate the activity of the promoters of peripheral myelin protein-22 and protein zero genes in Schwann cells. Brain Res. 2000;865:12–6. doi: 10.1016/s0006-8993(00)02130-2. [DOI] [PubMed] [Google Scholar]
- 28.Yanaihara A, Otsuka Y, Iwasaki S, Aida T, Tachikawa T, Irie T, Okai T. Differences in gene expression in the proliferative human endometrium. Fertil Steril. 2005;83(Suppl 1):1206–15. doi: 10.1016/j.fertnstert.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 29.Rao RG, Sudhakar D, Hogue CP, Amici S, Gordon LK, Braun J, Notterpek L, Goodglick L, Wadehra M. Peripheral myelin protein-22 (PMP22) modulates alpha 6 integrin expression in the human endometrium. Reprod Biol Endocrinol. 2011;9:1–11. doi: 10.1186/1477-7827-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Re FC, Manenti G, Borrello MG, Colombo MP, Fisher JH, Pierotti MA, Porta GD, Dragani TA. Multiple molecular alterations in mouse lung tumors. Mol Carcinogen. 1992;5:155–60. doi: 10.1002/mc.2940050211. [DOI] [PubMed] [Google Scholar]
- 31.Both J, Wu T, Bras J, Schaap GR, Baas F, Hulsebos TJ. Identification of novel candidate oncogenes in chromosome region 17p11.2-p12 in human osteosarcoma. PloS One. 2012;7:e30907. doi: 10.1371/journal.pone.0030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huehne K, Rautenstrauss B. Transcriptional startpoints and methylation patterns in the PMP22 promoters of peripheral nerve, leukocytes and tumor cell lines. Int J Mol Med. 2001;7:669–75. doi: 10.3892/ijmm.7.6.669. [DOI] [PubMed] [Google Scholar]
- 33.Huhne K, Park O, Liehr T, Rautenstrauss B. Expression analysis of the PMP22 gene in glioma and osteogenic sarcoma cell lines. J Neurosci Res. 1999;58:624–31. [PubMed] [Google Scholar]
- 34.van Dartel M, Cornelissen PW, Redeker S, Tarkkanen M, Knuutila S, Hogendoorn PC, Westerveld A, Gomes I, Bras J, Hulsebos TJ. Amplification of 17p11.2 approximately p12, including PMP22, TOP3A, and MAPK7, in high-grade osteosarcoma. Cancer Genet Cytogenet. 2002;139:91–6. doi: 10.1016/s0165-4608(02)00627-1. [DOI] [PubMed] [Google Scholar]
- 35.van Dartel M, Hulsebos TJ. Characterization of PMP22 expression in osteosarcoma. Cancer Genet Cytogenet. 2004;152:113–8. doi: 10.1016/j.cancergencyto.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.van Dartel M, Leenstra S, Troost D, Hulsebos TJ. Infrequent but high-level amplification of 17p11.2 approximately p12 in human glioma. Cancer Genet Cytogenet. 2003;140:162–6. doi: 10.1016/s0165-4608(02)00683-0. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Kleeff J, Esposito I, Kayed H, Felix K, Giese T, Buchler MW, Friess H. Expression analysis of PMP22/Gas3 in premalignant and malignant pancreatic lesions. J Histochem Cytochem. 2005;53:885–93. doi: 10.1369/jhc.4A6546.2005. [DOI] [PubMed] [Google Scholar]
- 38.Evtimova V, Zeillinger R, Weidle UH. Identification of genes associated with the invasive status of human mammary carcinoma cell lines by transcriptional profiling. Tumour Biol. 2003;24:189–98. doi: 10.1159/000074429. [DOI] [PubMed] [Google Scholar]
- 39.Tong D, Heinze G, Pils D, Wolf A, Singer CF, Concin N, Hofstetter G, Schiebel I, Rudas M, Zeillinger R. Gene expression of PMP22 is an independent prognostic factor for disease-free and overall survival in breast cancer patients. BMC Cancer. 2010;10:682. doi: 10.1186/1471-2407-10-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mimori K, Kataoka A, Yoshinaga K, Ohta M, Sagara Y, Yoshikawa Y, Ohno S, Barnard GF, Mori M. Identification of molecular markers for metastasis-related genes in primary breast cancer cells. Clin Exp Metast. 2005;22:59–67. doi: 10.1007/s10585-005-4417-y. [DOI] [PubMed] [Google Scholar]
- 41.Liehr T, Kuhlenbaumer G, Wulf P, Taylor V, Suter U, Van Broeckhoven C, Lupski JR, Claussen U, Rautenstrauss B. Regional localization of the human epithelial membrane protein genes 1, 2, and 3 (EMP1, EMP2, EMP3) to 12p12.3, 16p13.2, and 19q13.3. Genomics. 1999;58:106–8. doi: 10.1006/geno.1999.5803. [DOI] [PubMed] [Google Scholar]
- 42.Snipes GJ, Suter U, Shooter EM. Human peripheral myelin protein-22 carries the L2/HNK-1 carbohydrate adhesion epitope. J Neurochem. 1993;61:1961–4. doi: 10.1111/j.1471-4159.1993.tb09840.x. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Porath I, Kozak CA, Benvenisty N. Chromosomal mapping of Tmp (Emp1), Xmp (Emp2), and Ymp (Emp3), genes encoding membrane proteins related to Pmp22. Genomics. 1998;49:443–7. doi: 10.1006/geno.1998.5238. [DOI] [PubMed] [Google Scholar]
- 44.Marvin KW, Fujimoto W, Jetten AM. Identification and characterization of a novel squamous cell-associated gene related to PMP22. J Biol Chem. 1995;270:28910–6. doi: 10.1074/jbc.270.48.28910. [DOI] [PubMed] [Google Scholar]
- 45.Ruegg CL, Wu HY, Fagnoni FF, Engleman EG, Laus R. B4B, a novel growth-arrest gene, is expressed by a subset of progenitor/pre-B lymphocytes negative for cytoplasmic mu-chain. J Immunol. 1996;157:72–80. [PubMed] [Google Scholar]
- 46.Schiemann S, Ruckels M, Engelholm LH, Schwirzke M, Brunner N, Weidle UH. Differential gene expression in human mammary carcinoma cells: identification of a new member of a receptor family. Anticancer Res. 1997;17:13–20. [PubMed] [Google Scholar]
- 47.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–93. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 48.Gnirke AU, Weidle UH. Investigation of prevalence and regulation of expression of progression associated protein (PAP) Anticancer Res. 1998;18:4363–9. [PubMed] [Google Scholar]
- 49.Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K, Kodama T, Aburatani H. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001;61:889–95. [PubMed] [Google Scholar]
- 50.Lai S, Wang G, Cao X, Li Z, Hu J, Wang J. EMP-1 promotes tumorigenesis of NSCLC through PI3K/AKT pathway. J Huazhong Univ Sci Technolog Med Sci. 2012;32:834–8. doi: 10.1007/s11596-012-1043-1. [DOI] [PubMed] [Google Scholar]
- 51.Sun GG, Wang YD, Lu YF, Hu WN. EMP1, a member of a new family of antiproliferative genes in breast carcinoma. Tumour Biol. 35:3347–54. doi: 10.1007/s13277-013-1441-4. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Cao W, Xu Q, Chen WT. The expression of EMP1 is downregulated in oral squamous cell carcinoma and possibly associated with tumour metastasis. J Clin Pathol. 2011;64:25–9. doi: 10.1136/jcp.2010.082404. [DOI] [PubMed] [Google Scholar]
- 53.Bredel M, Bredel C, Juric D, Harsh GR, Vogel H, Recht LD, Sikic BI. Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer Res. 2005;65:8679–89. doi: 10.1158/0008-5472.CAN-05-1204. [DOI] [PubMed] [Google Scholar]
- 54.Mackay A, Jones C, Dexter T, Silva RL, Bulmer K, Jones A, Simpson P, Harris RA, Jat PS, Neville AM, Reis LF, Lakhani SR, O’Hare MJ. cDNA microarray analysis of genes associated with ERBB2 (HER2/neu) overexpression in human mammary luminal epithelial cells. Oncogene. 2003;22:2680–8. doi: 10.1038/sj.onc.1206349. [DOI] [PubMed] [Google Scholar]
- 55.Aries IM, Jerchel IS, van den Dungen RESR, van den Berk LCJ, Boer JM, Horstmann MA, Escherich G, Pieters R, den Boer ML. EMP1, a novel poor prognostic factor in pediatric leukemia regulates prednisolone resistance, cell proliferation, migration and adhesion. Leukemia. 2014;28:1828–37. doi: 10.1038/leu.2014.80. [DOI] [PubMed] [Google Scholar]
- 56.Sun GG, Wang YD, Cui DW, Cheng YJ, Hu WN. Epithelial membrane protein 1 negatively regulates cell growth and metastasis in colorectal carcinoma. World J Gastroenterol. 2014;20:4001–10. doi: 10.3748/wjg.v20.i14.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun GG, Wang YD, Cui DW, Cheng YJ, Hu WN. EMP1 regulates caspase-9 and VEGFC expression and suppresses prostate cancer cell proliferation and invasion. Tumour Biol. 2014;35:3455–62. doi: 10.1007/s13277-013-1456-x. [DOI] [PubMed] [Google Scholar]
- 58.Arslan AA, Gold LI, Mittal K, Suen TC, Belitskaya-Levy I, Tang MS, Toniolo P. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod. 2005;20:852–63. doi: 10.1093/humrep/deh698. [DOI] [PubMed] [Google Scholar]
- 59.Koch M, Wiese M. Gene expression signatures of angiocidin and darapladib treatment connect to therapy options in cervical cancer. J Cancer Res Clin Oncol. 2013;139:259–67. doi: 10.1007/s00432-012-1317-9. [DOI] [PubMed] [Google Scholar]
- 60.Ben Porath I, Kozak CA, Benvenisty N. Chromosomal mapping of Tmp (Emp1), Xmp (Emp2), and Ymp (Emp3), genes encoding membrane proteins related to Pmp22. Genomics. 1998;49:443–7. doi: 10.1006/geno.1998.5238. [DOI] [PubMed] [Google Scholar]
- 61.Wang CX, Wadehra M, Fisk BC, Goodglick L, Braun J. Epithelial membrane protein 2, a 4-transmembrane protein that suppresses B-cell lymphoma tumorigenicity. Blood. 2001;97:3890–5. doi: 10.1182/blood.v97.12.3890. [DOI] [PubMed] [Google Scholar]
- 62.Gee HY, Ashraf S, Wan X, Vega-Warner V, Esteve-Rudd J, Lovric S, Fang H, Hurd TW, Sadowski CE, Allen SJ. Mutations in EMP2 cause childhood-onset nephrotic syndrome. Am J Hum Genet. 2014;94:884–90. doi: 10.1016/j.ajhg.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, Dobbs L. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol. 2004;31:309–16. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- 64.Wadehra M, Sulur GG, Braun J, Gordon LK, Goodglick L. Epithelial Membrane Protein-2 is expressed in discrete anatomical regions of the eye. Exp Mol Pathol. 2003;74:106–12. doi: 10.1016/s0014-4800(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 65.Fu M, Brewer S, Olafsen T, Wu A, Gordon L, Said J, Braun J, Wadehra M. Positron Emission Tomography Imaging of Endometrial Cancer Using Engineered Anti-EMP2 Antibody Fragments. Mol Imag Biol. 2013;15:68–78. doi: 10.1007/s11307-012-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wadehra M, Forbes A, Pushkarna N, Goodglick L, Gordon LK, Williams CJ, Braun J. Epithelial membrane protein-2 regulates surface expression of alphavbeta3 integrin in the endometrium. Dev Biol. 2005;287:336–45. doi: 10.1016/j.ydbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Wadehra M, Mainigi M, Morales SA, Rao RG, Gordon LK, Williams CJ, Braun J. Steroid hormone regulation of EMP2 expression and localization in the endometrium. Reprod Biol Endocrinol. 2008;6:1–11. doi: 10.1186/1477-7827-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wadehra M, Dayal M, Mainigi M, Ord T, Iyer R, Braun J, Williams CJ. Knockdown of the tetraspan protein epithelial membrane protein-2 inhibits implantation in the mouse. Dev Biol. 2006;292:430–41. doi: 10.1016/j.ydbio.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 69.Fu M, Rao R, Sudhakar D, Hogue CP, Rutta Z, Morales S, Gordon LK, Braun J, Goodglick L, Wadehra M. Epithelial membrane protein-2 promotes endometrial tumor formation through activation of FAK and Src. PLoS One. 2011;6:e19945. doi: 10.1371/journal.pone.0019945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Morales SA, Mareninov S, Wadehra M, Zhang L, Goodglick L, Braun J, Gordon LK. FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction. Invest Ophthalmol Vis Sci. 2009;50:462–9. doi: 10.1167/iovs.07-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wadehra M, Su H, Gordon LK, Goodglick L, Braun J. The tetraspan protein EMP2 increases surface expression of class I major histocompatibility complex proteins and susceptibility to CTL-mediated cell death. Clin Immunol. 2003;107:129–36. doi: 10.1016/s1521-6616(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 72.Wadehra M, Goodglick L, Braun J. The tetraspan protein EMP2 modulates the surface expression of caveolins and glycosylphosphatidyl inositol-linked proteins. Mol Biol Cell. 2004;15:2073–83. doi: 10.1091/mbc.E03-07-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wadehra M, Iyer R, Goodglick L, Braun J. The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J Biol Chem. 2002;277:41094–100. doi: 10.1074/jbc.M206868200. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y-W, Li W-M, Wu W-J, Chai C-Y, Chang T-Y, Sun Y, Cheng C-J, Shiue Y-L, Su S-J, Cheng H-L. Epithelial membrane protein 2 is a prognostic indictor for patients with urothelial carcinoma of the upper urinary tract. Am J Pathol. 2013;183:709–19. doi: 10.1016/j.ajpath.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y-H, Wu L-C, Wu W-R, Lin H-J, Lee S-W, Lin C-Y, Chang S-L, Chow N-H, Huang H-Y, Li C-F. Loss of epithelial membrane protein-2 expression confers an independent prognosticator in nasopharyngeal carcinoma: a cohort study. BMJ. 2012;2:e000900. doi: 10.1136/bmjopen-2012-000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Freije WA, Castro-Vargas FE, Fang Z, Horvath S, Cloughesy T, Liau LM, Mischel PS, Nelson SF. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004;64:6503–10. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- 77.Radvanyi L, Singh-Sandhu D, Gallichan S, Lovitt C, Pedyczak A, Mallo G, Gish K, Kwok K, Hanna W, Zubovits J, Armes J, Venter D, Hakimi J, Shortreed J, Donovan M, Parrington M, Dunn P, Oomen R, Tartaglia J, Berinstein NL. The gene associated with trichorhinophalangeal syndrome in humans is overexpressed in breast cancer. Proc Natl Acad Sci U S A. 2005;102:11005–10. doi: 10.1073/pnas.0500904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma X-J, Dahiya S, Richardson E, Erlander M, Sgroi D. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2004;11:2523–36. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao H, Langerød A, Ji Y, Nowels KW, Nesland JM, Tibshirani R, Bukholm IK, Kåresen R, Botstein D, Børresen-Dale A-L, Jeffrey SS. Different Gene Expression Patterns in Invasive Lobular and Ductal Carcinomas of the Breast. Mol Biol Cell. 2004;15:2523–36. doi: 10.1091/mbc.E03-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Obermayr E, Sanchez-Cabo F, Tea M-K, Singer C, Krainer M, Fischer M, Sehouli J, Reinthaller A, Horvat R, Heinze G, Tong D, Zeillinger R. Assessment of a six gene panel for the molecular detection of circulating tumor cells in the blood of female cancer patients. BMC Cancer. 2010;10:1–12. doi: 10.1186/1471-2407-10-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watson MA, Ylagan LR, Trinkaus KM, Gillanders WE, Naughton MJ, Weilbaecher KN, Fleming TP, Aft RL. Isolation and molecular profiling of bone marrow micrometastases identifies TWIST1 as a marker of early tumor relapse in breast cancer patients. Clin Cancer Res. 2007;13:5001–9. doi: 10.1158/1078-0432.CCR-07-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Creighton CJ, Massarweh S, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, Shou J, Malorni L, Schiff R. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. Cancer Res. 2008;68:7493–501. doi: 10.1158/0008-5472.CAN-08-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Györffy B, Surowiak P, Kiesslich O, Denkert C, Schäfer R, Dietel M, Lage H. Gene expression profiling of 30 cancer cell lines predicts resistance towards 11 anticancer drugs at clinically achieved concentrations. Int J Cancer. 2006;118:1699–712. doi: 10.1002/ijc.21570. [DOI] [PubMed] [Google Scholar]
- 84.Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, Lee F, Shaw P, Clark E. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–38. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 85.Wadehra M, Natarajan S, Seligson DB, Williams CJ, Hummer AJ, Hedvat C, Braun J, Soslow RA. Expression of epithelial membrane protein-2 is associated with endometrial adenocarcinoma of unfavorable outcome. Cancer. 2006;107:90–8. doi: 10.1002/cncr.21957. [DOI] [PubMed] [Google Scholar]
- 86.Fu M, Maresh EL, Soslow RA, Alavi M, Mah V, Zhou Q, Iasonos A, Goodglick L, Gordon LK, Braun J, Wadehra M. Epithelial membrane protein-2 is a novel therapeutic target in ovarian cancer. Clin Cancer Res. 2010;16:954–63. doi: 10.1158/1078-0432.CCR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu M, Maresh EL, Helguera GF, Kiyohara M, Qin Y, Ashki N, Daniels-Wells TR, Aziz N, Gordon LK, Braun J, Elshimali Y, Soslow RA, Penichet ML, Goodglick L, Wadehra M. Rationale and preclinical efficacy of a novel anti-EMP2 antibody for the treatment of invasive breast cancer. Mol Cancer Ther. 2014;13:902–15. doi: 10.1158/1535-7163.MCT-13-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gordon LK, Kiyohara M, Fu M, Braun J, Dhawan P, Chan A, Goodglick L, Wadehra M. EMP2 regulates angiogenesis in endometrial cancer cells through induction of VEGF. Oncogene. 2013;32:5369–76. doi: 10.1038/onc.2012.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shimazaki K, Lepin EJ, Wei B, Nagy AK, Coulam CP, Mareninov S, Fu M, Wu AM, Marks JD, Braun J, Gordon LK, Wadehra M. Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clin Cancer Res. 2008;14:7367–77. doi: 10.1158/1078-0432.CCR-08-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qin Y, Fu M, Takahashi M, Iwanami A, Kuga D, Rao RG, Sudhakar D, Huang T, Kiyohara M, Torres K. Epithelial membrane protein-2 (EMP2) activates Src protein and is a novel therapeutic target for glioblastoma. J Biol Chem. 2014;289:13974–85. doi: 10.1074/jbc.M113.543728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ben-Porath I, Benvenisty N. Characterization of a tumor-associated gene, a member of a novel family of genes encoding membrane glycoproteins. Gene. 1996;183:69–75. doi: 10.1016/s0378-1119(96)00475-1. [DOI] [PubMed] [Google Scholar]
- 92.Burmester JK, Suarez BK, Lin JH, Jin CH, Miller RD, Zhang KQ, Salzman SA, Reding DJ, Catalona WJ. Analysis of candidate genes for prostate cancer. Hum Hered. 2004;57:172–8. doi: 10.1159/000081443. [DOI] [PubMed] [Google Scholar]
- 93.Li KKW, Pang JCS, Chung NYF, Ng YL, Chan NHL, Zhou L, Poon WS, Ng HK. EMP3 overexpression is associated with oligodendroglial tumors retaining chromosome arms 1p and 19q. Int J Cancer. 2007;120:947–50. doi: 10.1002/ijc.22415. [DOI] [PubMed] [Google Scholar]
- 94.Zhou W, Jiang Z, Li X, Xu F, Liu Y, Wen P, Kong L, Hou M, Yu J. EMP3 overexpression in primary breast carcinomas is not associated with epigenetic aberrations. J Korean Med Sci. 2009;24:97–103. doi: 10.3346/jkms.2009.24.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ernst A, Hofmann S, Ahmadi R, Becker N, Korshunov A, Engel F, Hartmann C, Felsberg J, Sabel M, Peterziel H. Genomic and expression profiling of glioblastoma stem cell–like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin Cancer Res. 2009;15:6541–50. doi: 10.1158/1078-0432.CCR-09-0695. [DOI] [PubMed] [Google Scholar]
- 96.Alaminos M, Davalos V, Ropero S, Setien F, Paz MF, Herranz M, Fraga MF, Mora J, Cheung NK, Gerald WL, Esteller M. EMP3, a myelin-related gene located in the critical 19q13.3 region, is epigenetically silenced and exhibits features of a candidate tumor suppressor in glioma and neuroblastoma. Cancer Res. 2005;65:2565–71. doi: 10.1158/0008-5472.CAN-04-4283. [DOI] [PubMed] [Google Scholar]
- 97.Kunitz A, Wolter M, Van Den Boom J, Felsberg J, Tews B, Hahn M, Benner A, Sabel M, Lichter P, Reifenberger G. DNA hypermethylation and aberrant expression of the EMP3 gene at 19q13. 3 in human gliomas. Brain Pathol. 2007;17:363–70. doi: 10.1111/j.1750-3639.2007.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fumoto S, Hiyama K, Tanimoto K, Noguchi T, Hihara J, Hiyama E, Noguchi T, Nishiyama M. EMP3 as a tumor suppressor gene for esophageal squamous cell carcinoma. Cancer Lett. 2009;274:25–32. doi: 10.1016/j.canlet.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 99.Li KK, Pang JC, Chung NY, Ng YL, Chan NH, Zhou L, Poon WS, Ng HK. EMP3 overexpression is associated with oligodendroglial tumors retaining chromosome arms 1p and 19q. International journal of cancer J Int Cancer. 2007;120:947–50. doi: 10.1002/ijc.22415. [DOI] [PubMed] [Google Scholar]
- 100.Xue Q, Zhou Y, Wan C, Lv L, Chen B, Cao X, Ju G, Huang Y, Ni R, Mao G. Epithelial membrane protein 3 is frequently shown as promoter methylation and functions as a tumor suppressor gene in non-small cell lung cancer. Exp Mol Pathol. 2013;95:313–8. doi: 10.1016/j.yexmp.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y-W, Li W-M, Wu W-J, Chai C-Y, Liu H-S, Lai M-D, Chow N-H. Potential significance of EMP3 in patients with upper urinary tract urothelial carcinoma: crosstalk with ErbB2-PI3K-Akt pathway. J Urol. 2014;192:242–51. doi: 10.1016/j.juro.2013.12.001. [DOI] [PubMed] [Google Scholar]