Summary
Purpose
We describe two cases of intraoperative 4D microscope–integrated swept source optical coherence tomography (4D MIOCT) guided 27-gauge transvitreal retinochoroidal biopsy for choroidal melanoma.
Methods
Two 68-year-old females with choroidal melanomas underwent a transvitreal 27-gauge vitrectomy assisted chorioretinal biopsy for histologic and cytogenetic testing during I-125 radioactive episcleral plaque placement. A 4D (volumetric imaging through time) MIOCT device was used to simultaneously enable OCT image acquisition with surgical maneuvers during entry of and engagement of the vitreous cutter in the choroidal lesion.
Results
4D MIOCT permitted real-time visualization of the choroidal tumor for selection of biopsy site in an area of adequate thickness and free of subretinal fluid, relatively perpendicular entry of the vitrectomy probe into the choroidal lesion, confirmation of adequate penetration depth prior to and during engagement of the vitreous cutter, and absence of subretinal fluid surrounding the retinotomy following removal of the probe. In both cases an adequate sample for histological and cytogenetic testing was obtained and no tamponade was required.
Conclusion
Intraoperative 4D MIOCT imaging permits real-time visualization of vitreous cutter penetration and depth into the choroidal melanoma during a transvitreal choroidal biopsy.
Keywords: intraoperative optical coherence tomography, microscope-integrated optical coherence tomography, choroidal melanoma, transvitreal choroidal biopsy, 27-gauge vitrectomy, 27-gauge transvitreal choroidal biopsy
Introduction
Tissue sampling is desirable to understand prognostic genetic alterations in uveal melanoma. 1 A transvitreal vitrectomy-assisted retinochoroidal biopsy (TVB)) approach reduces the risk of insufficient tissue sampling compared with fine needle aspiration biopsy.
Although performed under direct visualization, it is challenging to assess the vitreous cutter probe depth in the choroidal tumor. TVB complications with a 25-gauge vitrector system include rhegmatogenous retinal detachment (RD), increased serous RD, vitreous hemorrhage, and seeding of tumor cells. 2
We demonstrate the use of a prototype swept-source 4D microscope-integrated optical coherence tomography (4D-MIOCT) device for live OCT volumetric acquisition during a 27-gauge TVB in choroidal melanoma.
Case Reports and Surgical Technique
A patient with a 3.2 mm choroidal lesion consistent with a choroidal melanoma underwent a TVB during placement of epislceral I-125 plaque brachytherapy, prior to application of the active plaque. Using a primed 27-gauge system (Constellation 27 Total Plus; Alcon Laboratories, Fort Worth, TX), three ports were placed for pars plana vitrectomy.
Once the vitreous cutter had penetrated into choroidal tumor, using a low cut-rate (300cpm), high-suction (600mmHg), and biased-open duty-cycle, it was engaged for 10–15 seconds or until tissue was observed in tubing. Contents of the probe and distal tubing were refluxed into a specimen tube and sent for cytopathology and gene expression profiling (GEP). The infusion was turned on and the intraocular pressure was raised to minimize perioperative bleeding and then slowly normalized. There was a vitreous hemorrhage overlying the macula, which was removed by performing a limited vitrectomy. The cannulas were removed, sclerotomies sutured and treated with double freeze-thaw cryotherapy.
The second patient with a 3.8mm peripapillary choroidal melanoma underwent a TVB in a similar fashion. The retinotomy was left untreated and no intravitreal tamponade was used in either case.
The 4D-MIOCT scanner (100,000 A-scans/second) integrated into the microscope allowed OCT acquisition (2–10 volumes/second) concurrent with live surgical maneuvers. Data was processed and displayed in three formats: en face maximum-intensity-projections (MIP), B-scans, and denoised-volumes rendered in real-time with lighting, edge, and depth-enhanced ray casting. All three formats were projected on a wall-mounted display and also using a binocular stereoscopic heads-up-display integrated into the microscope oculars. The 4D-MIOCT imaging protocol consisted of 7.5×7.5×6 mm (x,y,z) scans acquired during insertion of the vitrectomy probe into the choroidal lesion until it attained final depth, engagement of the cutter, and withdrawal from lesion.
Specific advantages of 4D-MIOCT guidance were:
Pre Choroidal Tumor Entry with Vitreous Cutter
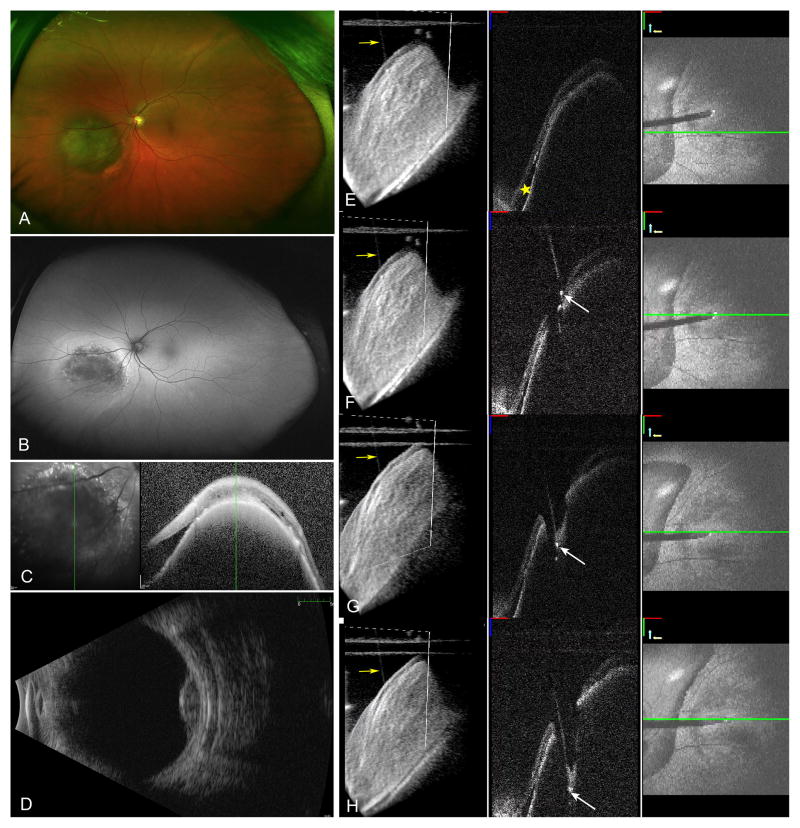
4D-MIOCT permitted volumetric visualization of the choroidal lesion and overlying subretinal fluid. This allowed selection of a biopsy site in an area without subretinal fluid (Figure-1E-yellow star) to minimize risk of RD following the retinotomy and at an area of maximal elevation to optimize the yield.
Figure 1.
Elevated pigmented choroidal lesion consistent with choroidal melanoma inferonasal to the nerve (A), with hyperautofluorescence surrounding the lesion consistent with subretinal fluid (B). Spectralis OCT (Heidelberg Engineering, Carlsbad, CA) obtained in clinic showing the choroidal tumor with overlying retinal pigment epithelium changes and surrounding subretinal fluid (C). Ultrasound B-scan showed low to medium internal reflectivity and a tumor height of 3.2 mm (D). Second column shows sequential 4D MIOCT images with the area of subretinal fluid (E, yellow star). The tip of the cutter is visualized as a hyperreflective area positioned against the surface of the retina in an area free of subretinal fluid (F, white arrow). Retinal indentation and the hyperreflective cutter tip are visualized as the vitrectomy probe enters the choroidal lesion (G). The 3D volumes show the cutter visualized in relation to the retinal surface (E–H, yellow arrows). Once the probe has penetrated to an adequate depth (H, white arrow), the cutter is engaged and the biopsy performed while visualizing the tip of the probe.
During Vitreous Cutter Engagement
4D MIOCT allowed real-time visualization of the probe entering the choroidal tissue (Online supplementary video). Retinal indentation (Figure-1G and Figure-2G) was visualized initially as the probe pushed into tumor surface in both B-scan and 3D volumes. Feedback from 3D volumes and B-scans allowed the probe to be positioned and maintained relatively perpendicular to surface.
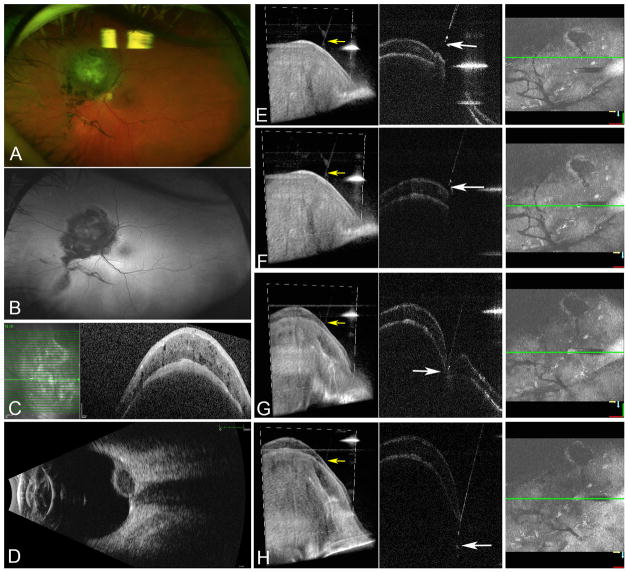
Figure 2.
Elevated pigmented peripapillary choroidal lesion consistent with choroidal melanoma superonasal to the nerve (A) with no surrounding hyperautofluorescence (B). Spectralis OCT (Heidelberg Engineering, Carlsbad, CA) obtained in clinic showing the choroidal tumor with no surrounding subretinal fluid (C). Ultrasound B-scan showed low to medium internal reflectivity and a tumor height of 3.8 mm (D). Second column shows sequential 4D MIOCT images with the cutter overlying the choroidal tumor (E). The tip of the cutter is visualized as a hyperreflective area positioned almost perpendicular to the surface of the retina (F). Retinal indentation and the hyperreflective cutter tip are visualized as the vitrectomy probe enters the choroidal lesion (G). The 3D volumes show the cutter visualized in relation to the retinal surface (E–H, yellow arrows). Once the probe has penetrated to an adequate depth (H), the cutter is engaged and the biopsy performed while visualizing the tip of the probe.
B-scan images allowed direct real-time visualization of cutter depth in the choroidal lesion (Figure-1 and 2H). The vitreous cutter tip was visualized as a hyperreflective edge. The cutter was engaged once appropriate penetration depth was confirmed on OCT images. 4D-MIOCT permitted direct visualization of maintenance of cutter depth and the small amount of excursion while the cutter was engaged to optimize the tissue yield. The heads-up-display was useful for accurate instrument-depth tracking.
Post Vitreous Cutter Removal
4D-MIOCT permitted visualization of the overlying hemorrhage, and confirmed absence of subretinal fluid surrounding the retinotomy site.
Discussion
We were able to harvest adequate sample for histopathologic diagnosis and GEP in both patients. Achieving an adequate depth using the cutter while balancing the risk of deep penetration triggering choroidal bleeding is important. Traditionally the cutter depth is assessed by visually burying the tip of the cutter through the retinal surface which, if not entered deep to the retina, may lead to inadvertent cutting of the neurosensory retina and consequently a larger retinotomy. Alternatively, the cutter may be pushed into the tumor until the cutter is felt against the sclera and then withdrawing slightly. 4D-MIOCT guided TVB allows directly visualizing the cutter depth, which may also help to reduce the chances of triggering more severe choroidal bleeding following the biopsy. Compared with the 25-gauge cutter, the 27-gauge vitrectomy port is larger (0.079 mm2 vs 0.066 mm2) and closer to the cutter tip (0.211 mm vs 0.330 mm). 3 This may allow more controlled sampling, especially of thinner tumors with MIOCT guided assessment of the tumor depth penetration. Even though the lesion heights in our report ranged from 3.2 to 3.8 mm, this system can be potentially used for smaller lesion, helping to more accurately assess the penetration depth
Complications during TVB include retinal breaks, RD, subretinal hemorrhage, vitreous hemorrhage, and cataracts. Earlier 20-gauge FNAB required laser barricade around the biopsy retinotomy site but 27-gauge retinotomies are smaller and do not require barricade or endotamponade. 4 4D-MIOCT allows assessment for the presence of subretinal fluid around the retinotomy site which can help with decisions regarding the need for placing a tamponade or retinopexy.
4D-MIOCT assisted chorioretinal or retinal biopsy can also be applied in lymphoma or infectious retinitis such as Candida, cytomegalovirus, acute retinal necrosis, tuberculosis, and toxoplasmosis.
Depth visualization during a choroidal biopsy with a 25-gauge vitrectomy cutter has been demonstrated using a commercially available spectral-domain-OCT (RESCAN-700; Carl Zeiss Meditec, Germany). 5 In contrast, we were not able to visualize the actual cutter port opening and closing. However the real-time volumetric data provided by our 4D-MIOCT allowed better tumor visualization and appropriate biopsy site selection. Other differences include our choroidal lesions being considerably thicker, use of smaller 27-gauge cutter, the increased depth of penetration allowing for visualization of the cutter tip at its final intended depth, which is more valuable information than visualization of port opening.
4D-MIOCT allows the ability to view B-scans in any region of interest, in contrast to current spectral-domain OCT systems, wherein an area of interest needs to be identified prior to image acquisition, and maintaining the area of interest in the same plane intraoperatively maybe challenging. Potential limitations of the system include inability to visualize deeper tumor areas and the increased costs.
Conclusion
27-gauge TVB under 4D-MIOCT guidance enabled intraoperative high-resolution cross-sectional visualization, provided near real-time volumetric data to track and confirm appropriate positioning of the cutter depth prior to engaging it. Such real-time visualization may optimize the yield of TVB by reducing the chances of an incomplete biopsy especially in smaller tumors and potentially reducing the risk of creating a larger retinotomy and known complications including choroidal bleeding. Further investigation into its utility is warranted.
Supplementary Material
Swept-source microscope-integrated optical coherence tomography (4D MIOCT) device for simultaneous OCT acquisition with choroioretinal biopsy for choroidal melanoma with a 27-gauge vitrectomy system. Surgical microscope view is shown in the inset with the 4D MIOCT B-scan and en face maximum intensity projection (MIP) video below. B-scan identifies the entry location of the vitreous cutter into the choroidal lesion. Intraoperative OCT allows for visualization of entry into the lesion, actual motion of the vitreous cutter, and final depth of the biopsy.
Summary Statement.
Intraoperative 4D Microscope Integrated Swept Source Intraoperative Optical Coherence Tomography imaging permits real-time visualization of vitreous cutter penetration and depth into the choroidal melanoma optimizing the yield for transvitreal choroidal biopsy.
Acknowledgments
This report adhered to the principles of the Declaration of Helsinki. Duke University institutional review board approval was obtained.
Funding/Support:
This project was funded by the NIH Bioengineering Research Partnership Grant: R01-EY-023039 “Intraoperative OCT Guidance of Intraocular Surgery” (Izatt/Toth) and NIH R01-EY024312 (Kuo)
Footnotes
Online supplementary material:
Video is provided as online supplementary material.
Presentation at a conference
This paper was presented in part at Vail Vitrectomy 2016
Conflict of interest:
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest: Dr. Izatt has patent licenses with Leica Microsystems. Dr. Toth receives financial support from Alcon and Genentech, has an intraoperative imaging patent with Duke University, and receives consulting fees from ThromboGenics. For the remaining authors no conflicts were declared.
The authors have full control of all primary data.
References
- 1.Bagger M, Andersen MT, Andersen KK, Heegaard S, Andersen MK, Kiilgaard JF. The prognostic effect of American Joint Committee on Cancer staging and genetic status in patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci. 2015;56(1):438–444. doi: 10.1167/iovs.14-15571. [DOI] [PubMed] [Google Scholar]
- 2.Glasgow BJ, Brown HH, Zargoza AM, Foos RY. Quantitation of tumor seeding from fine needle aspiration of ocular melanomas. Am J Ophthalmol. 1988;105(5):538–546. doi: 10.1016/0002-9394(88)90248-6. [DOI] [PubMed] [Google Scholar]
- 3.Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology. 2010;117(1):93–102. e102. doi: 10.1016/j.ophtha.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 4.Scherfig E, Prause JU, Jensen OA. Transvitreal retinochoroidal biopsy. Graefes Arch Clin Exp Ophthalmol. 1989;227(4):369–373. doi: 10.1007/BF02169415. [DOI] [PubMed] [Google Scholar]
- 5.Ehlers JP, Goshe J, Dupps WJ, et al. Determination of Feasibility and Utility of Microscope-Integrated Optical Coherence Tomography During Ophthalmic Surgery: The DISCOVER Study RESCAN Results. JAMA Ophthalmol. 2015;133(10):1124–1132. doi: 10.1001/jamaophthalmol.2015.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Swept-source microscope-integrated optical coherence tomography (4D MIOCT) device for simultaneous OCT acquisition with choroioretinal biopsy for choroidal melanoma with a 27-gauge vitrectomy system. Surgical microscope view is shown in the inset with the 4D MIOCT B-scan and en face maximum intensity projection (MIP) video below. B-scan identifies the entry location of the vitreous cutter into the choroidal lesion. Intraoperative OCT allows for visualization of entry into the lesion, actual motion of the vitreous cutter, and final depth of the biopsy.