Abstract
Introduction
The purpose of this study was to determine 1) if stable heart-failure patients with reduced ejection fraction (HFrEF) have elevated extravascular lung water (EVLW) versus healthy control subjects, and 2) the effect of acute β2AR agonist inhalation on lung fluid balance.
Methods
Twenty-two stable HFrEF patients and 18 age- and sex-matched healthy subjects were studied. Lung diffusing capacity for carbon monoxide (DLCO), alveolar-capillary conductance (DmCO), pulmonary capillary blood volume (Vc) (via rebreathe) and lung tissue volume (Vtis) (via computed tomography) were assessed before and within 30 min of administration of nebulized albuterol. EVLW was derived as Vtis – Vc.
Results
Pre-albuterol, Vtis and EVLW were greater in HFrEF vs. control (998 ± 200 vs. 884 ± 123 ml, P = 0.041; 943 ± 202 vs. 802 ± 133 ml, P = 0.015, respectively). Albuterol decreased Vtis and EVLW in HFrEF (−4.6 ± 7.8%, P = 0.010; −4.6 ± 8.8%, P = 0.018) and control (−2.8 ± 4.9%, P = 0.029; −3.0 ± 5.7%, P = 0.045). There was an inverse relationship between pre-albuterol values and the pre- to post-albuterol change for EVLW (r2 = −0.264, P = 0.015) and DmCO (r2 = −0.343, P = 0.004) in HFrEF only.
Conclusion
Lung fluid is elevated in stable HFrEF patients relative to healthy subjects. Stimulation of the β2ARs may cause fluid removal in HFrEF, especially in patients who exhibit greater evidence for increased lung water at baseline.
Keywords: pulmonary edema, albuterol, lung diffusing capacity, computed tomography
Summary statement
Stimulation of the β2-adrenergic receptors appears to reduce lung fluid in patients with stable heart-failure.
INTRODUCTION
Chronic heart failure (HF) is associated with an increase in pulmonary capillary pressure and wall tension secondary to the rise in left ventricle (LV) filling pressure consistent with a failing LV (1). Additionally, it has been shown that a chronic increase in adrenergic drive, as occurs with HF, elicits a down-regulation of the β receptors central to lung fluid removal mechanisms (2–4). In combination, it is possible that the aforementioned changes in the pulmonary system associated with HF may conspire to increase fluid flux across the pulmonary vasculature while impairing fluid clearance from the alveoli and interstitial space, thus making HF patients more susceptible lung fluid accumulation relative to their healthy counterparts. While pulmonary congestion, a key component of which is a significant increase in lung fluid, is a hallmark of acute decompensation in heart failure (5–7), whether stable HF patients exhibit increased extravascular lung fluid remains controversial with some (8) but not all (9) reporting an increase in lung water content in HF patients. Moreover, the role of alterations in lung β-adrenoreceptor system mediated fluid clearance mechanisms in the pulmonary edema associated with HF is unclear. Accordingly, in the present study we aimed to determine 1) whether stable HF patients with reduced ejection fraction (HFrEF) have elevated lung fluid compared to their healthy age and sex matched counterparts, and 2) the effect of an acute inhaled β2-adrenergic receptor agonist on lung fluid balance in stable HFrEF patients.
MATERIALS AND METHODS
Participants and ethical approval
Twenty-two adult patients with a history of heart failure with reduced ejection fraction (HFrEF) and 18 healthy age- and sex-matched controls volunteered to participate in the study (Table 1). The patients recruited for the study were required to meet the following criteria: 1) ≥1 y history of known HF, 2) ejection fraction of ≤40%, 3) New York Heart Association (NYHA) functional class I, II or III, 4) stable symptoms (i.e. receiving optimal medication and have had no change in disease status or medication) for >3 months, 5) free of uncontrolled systemic hypertension, anemia, or other comorbidities (e.g., COPD), 6) not pacemaker dependent, and 7) a BMI <36. The age-matched controls were recruited from the surrounding community and were current nonsmokers (past 15 years) with no history of cardiac or pulmonary disease. Each participant gave written informed consent after being provided a detailed description of the study requirements. The experimental procedures were approved by the Mayo Clinic Institutional Review Board and were performed in accordance with the Declaration of Helsinki.
Table 1.
Participant characteristics, medications and pulmonary function in heart-failure patients with reduced ejection fraction (HFrEF) and healthy control subjects.
| Control | HFrEF | P-value | |||||
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| n | 18 | 22 | |||||
| Female | 5 (28) | 6 (27) | |||||
| Age, y | 58 | ± | 9 | 63 | ± | 8 | 0.062 |
| Stature, cm | 174 | ± | 15 | 175 | ± | 10 | 0.754 |
| Body mass, kg | 78.7 | ± | 15.2 | 89.9 | ± | 16.7 | 0.034 |
| BMI, kg/m2 | 25.9 | ± | 4.4 | 29.2 | ± | 4.4 | 0.020 |
| BSA, m2 | 1.95 | ± | 0.21 | 2.08 | ± | 0.23 | 0.069 |
| Aetiology, n | 10 ISC/12IDC | ||||||
| HF duration, mo | 68 | ± | 75 | ||||
| LVEF, % | 28.5 | ± | 7.9 | ||||
| NYHA functional class | |||||||
| I | 6 (27) | ||||||
| II | 12 (55) | ||||||
| III | 4 (18) | ||||||
| Medications | |||||||
| ACE inhibitors | 22 (100) | ||||||
| Aspirin | 19 (86) | ||||||
| β-blockers | 21 (95) | ||||||
| Digitalis | 8 (36) | ||||||
| Diuretics | 16 (73) | ||||||
| Pulmonary function | |||||||
| FVC, % predicted | 103 | ± | 12 | 83 | ± | 15.8 | <0.001 |
| FEV1, % predicted | 106 | ± | 11 | 81 | ± | 18 | <0.001 |
| FEV1/FVC, % predicted | 97 | ± | 7 | 105 | ± | 19 | 0.145 |
| PEF, L/s | 98 | ± | 11 | 74 | ± | 22 | <0.001 |
| FEF25–75%, % predicted | 125 | ± | 32 | 84 | ± | 24 | 0.001 |
| IC, L | 109 | ± | 11 | 86 | ± | 21 | 0.002 |
| DLCO/VA | 10.9 | ± | 6.6 | 6.4 | ± | 4.6 | 0.015 |
Data are presented as group mean ± SD or n (%). BMI, body mass index; BSA, body surface area; ISC, ischemic; IDC, idiopathic dilated cardiomyopathy; HFrEF, heart-failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; ACE, angiotensin converting enzyme; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; PEF; peak expiratory flow rate; FEF25–75%, forced expiratory flow at 25–75% of FVC; IC, inspiratory capacity; DLCO, lung diffusing capacity for carbon monoxide; VA, alveolar volume. P-values, group mean control vs. group mean HF.
Experimental procedures
The experimental procedures were conducted during two separate laboratory visits separated by no longer than 1 week. At the first visit, complete blood count was assessed to rule out anemia before pulmonary function was assessed via body plethysmography (MedGraphics Elite Series Plethysmograph, Medical Graphics Corporation, St. Paul, MN, USA) according to standard procedures (10). During the second session, albuterol, a short acting β2-adrenergic receptor agonist, was administered at a dilution of 2.5 mg per 3 ml of saline using a nebulizer for 15 minutes in each participant. Pulmonary function, lung diffusing capacity for carbon monoxide (DLCO) and nitric oxide (DLNO), alveolar-capillary membrane conductance (DmCO) and pulmonary capillary blood volume (Vc) were assessed before and within 30 min after albuterol administration. Similarly, lung density and lung tissue volume (Vtis) were measured before and within 30 min post-albuterol via chest CT imaging.
Lung diffusing capacity
DLCO, DLNO, DmCO and Vc were assessed by simultaneously measuring the disappearance of CO and NO using a rebreathe technique, as we have described previously (11, 12). Participants sat upright and breathed through a two-way switching valve that was connected to a pneumotachometer, a mass spectrometer (Marquette 1100 Medical Gas Analyzer, Perkin-Elmer, St. Louis, MO) and a NO analyzer (Sievers 280i NOA, Sievers, Boulder, CO). The inspiratory port of the switching valve was set to either room air or a 5-L anesthesia bag filled with 0.3% CO (C18O), 40 parts per million (ppm) NO (diluted in the bag immediately before each rebreathe maneuver from an 800 ppm gas mixture), 35% O2 and N2 balance. The total volume of gas in the anesthesia bag was determined by the resting tidal volume of each participant. For each rebreathe maneuver, the participants breathed normally on room air for 4–5 breaths before, at the end of a normal expiration, they were switched to the rebreathe bag and told to “nearly empty the bag” with each inspiration for 10–12 consecutive breaths at a breathing frequency of 32 breaths per min. Each participant performed the rebreathe maneuver in triplicate before and within 30 min after administration of nebulized albuterol. DLCO, DLNO, DmCO and Vc were computed using custom analysis software.
Lung tissue volume & lung density via computed tomography
All CT scans were performed using the same scanner (GE LiteSpeed Spiral CT Scanner, GE Healthcare). Initial slices obtained for all scans were 2.5 mm thick with a 1.2 mm overlap and were then reconstructed to 1.25 mm thick with a 0.6 mm overlap. Prior to the baseline scan (i.e. pre-albuterol administration) a scout scan was performed to determine the location and size of the lungs. The anatomical location at the start of the scan was marked on each subject using indelible ink and the table height, field of view and the number of images obtained were recorded to ensure consistency between the CT scans taken pre- and post-albuterol administration. For each CT scan, the participants were instructed to breathe normally before inspiring fully and performing a breath-hold at total lung capacity (TLC) before the scan was initiated. During each scan, the participants breathed through a mouthpiece connected to a pneumotachometer that was integrated with a portable computer with custom analysis software so that accurate lung volumes could be measured; this protocol ensured that all pre- and post-albuterol CT measures of lung tissue volume and lung density were made at the same lung volume.
For analysis, the CT images were submitted to image analysis software (Pulmonary Analysis Software Suite, Physiological Imaging Laboratory, University of Iowa, Iowa City, IA) and all analyses were performed by a single member of the research team who was blinded to the condition (i.e. pre- or post-albuterol) of each CT scan. First, the Pulmonary Analysis Software was used to segment the images to separate lung tissue from surrounding structures and the mediastinum for analysis of parenchymal attenuation. In each picture element lung density was assumed to be a linear combination of air and lung tissue, which have an attenuation of −1,000 and 0 Hounsfield units (HU), respectively. A histogram analysis of picture elements within the lung tissue was performed to obtain mean lung density in HU and tissue volume by summation of each voxel among all elements in the lung fields.
Estimation of extravascular lung water
The parenchymal attenuation assessed by CT (i.e. tissue volume, Vtis) includes lung tissue, blood and water. By combining our CT derived measure of tissue volume and our measure of Vc from the lung diffusing capacity maneuvers (12), we were able to estimate the volume of extravascular lung water (EVLW) as:
where, EVLW is extravascular lung water in ml, Vtis is tissue volume in ml determined via CT, and Vc is pulmonary capillary blood volume in ml assessed via our CO and NO rebreathe technique.
Albuterol administration
Using a nebulizer, albuterol (a short acting β2-AR agonist) was administered at a dilution of 2.5 mg/3ml of saline for ~15 min during tidal breathing in each participant. Heart rate (HR) and cardiac rhythm were measured via a 12-lead ECG throughout albuterol administration. Similarly, arterial oxygen saturation (SaO2) was monitored continuously during albuterol administration using a pulse oximeter (Nellcor N-595, Tyco Healthcare Group, Nellcor Puritan Bennett Division, Pleasanton, CA, USA) and a forehead sensor. Manual blood pressure and the rating of perceived dyspnea were obtained before albuterol administration and at two minute intervals thereafter until 5 min post-albuterol.
Statistical analyses
Independent samples t-test was used to compare subject characteristics, measures of lung function and absolute measures of lung diffusion capacity and related variables, CT derived lung density and lung tissue volume, and extravascular lung water at equivalent time-points between the experimental groups (control vs. HFrEF). Paired samples t-test was used to compare absolute measures of lung diffusion capacity and related variables, CT derived lung density and lung tissue volume, and extravascular lung water across time (pre- vs. post-albuterol administration) within each experimental group (control and HFrEF). Pearson product-moment correlation coefficient (r) was computed to assess the relationships between baseline (i.e. pre-albuterol) measures of lung tissue volume, extravascular lung water and alveolar-capillary membrane conductance relative to pulmonary capillary blood volume and the change in these variables from before to after albuterol administration in control subjects and HFrEF patients. The acceptable type I error was set at P < 0.05. Results are expressed as means ± SD unless otherwise stated. Statistical analysis was performed using SPSS version 21.0 for Windows (SPSS, Chicago, IL).
RESULTS
Participant characteristics and pulmonary function measurements are shown in Table 1.
Lung fluid balance in HFrEF vs. healthy control subjects
Baseline pre-albuterol measures of DLCO, DmCO, Vc, lung density, Vtis, and EVLW are shown in Table 2. Pre-albuterol administration, group mean DLCO (P = 0.037) and Vc (P = 0.003) were greater in control subjects compared HFrEF patients; there was no such difference in DmCO between subject groups (Table 2). Baseline pre-albuterol group mean lung density (P < 0.001), Vtis (P = 0.041) and EVLW (P = 0.015) were greater in HFrEF patients compared to healthy control subjects (Table 2). These data suggest that lung fluid is elevated in stable HFrEF patients relative to healthy subjects.
Table 2.
Baseline (i.e. pre-albuterol) measures of cardiovascular function, lung diffusing capacity and indices of lung fluid balance in heart-failure patients with reduced ejection fraction (HFrEF) and healthy control subjects.
| Control | HFrEF | P-value | |||||
|---|---|---|---|---|---|---|---|
| Cardiovascular function | |||||||
| Q, L/min | 4.14 | ± | 0.82 | 3.41 | ± | 1.33 | 0.047 |
| CI, L/min/m2 | 2.15 | ± | 0.44 | 1.63 | ± | 0.58 | 0.003 |
| SV, ml | 69.7 | ± | 21.7 | 54.5 | ± | 19.5 | 0.016 |
| HR, bpm | 61 | ± | 10 | 65 | ± | 9 | 0.049 |
| SaO2, % | 98.9 | ± | 1.5 | 97.8 | ± | 1.7 | 0.032 |
| SBP. mmHg | 124 | ± | 17 | 120 | ± | 14 | 0.389 |
| DBP, mmHg | 78 | ± | 13 | 77 | ± | 11 | 0.740 |
| MAP, mmHg | 94 | ± | 11 | 92 | ± | 8 | 0.360 |
| Lung diffusing capacity | |||||||
| DLCO, ml/mmHg/min | 19.5 | ± | 3.5 | 16.0 | ± | 6.1 | 0.037 |
| DLNO, ml/mmHg/min | 68.5 | ± | 15.1 | 61.4 | ± | 24.8 | 0.294 |
| DmCO, ml/mmHg/min | 31.1 | ± | 6.9 | 29.7 | ± | 11.27 | 0.294 |
| Vc, ml | 81.5 | ± | 31.1 | 54.9 | ± | 22.1 | 0.003 |
| DmCO/Vc | 0.47 | ± | 0.26 | 0.57 | ± | 0.29 | 0.264 |
| Lung fluid balance | |||||||
| Lung density, HU | −867 | ± | 20 | −804 | ± | 35 | <0.001 |
| Vtis, ml | 884 | ± | 123 | 998 | ± | 200 | 0.041 |
| EVLW, ml | 802 | ± | 133 | 943 | ± | 202 | 0.015 |
Data are presented as group mean ± SD. Q, cardiac output; CI, cardiac index; SV, stroke volume; HR, heart rate; SaO2, arterial oxygen saturation; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP; mean arterial pressure; DLCO, lung diffusing capacity for carbon monoxide; DLNO; lung diffusing capacity for nitric oxide; DmCO, alveolar-capillary membrane conductance; Vc, pulmonary capillary blood volume; Vtis; lung tissue volume; EVLW, extravascular lung water. P-values, group mean control vs. group mean HFrEF.
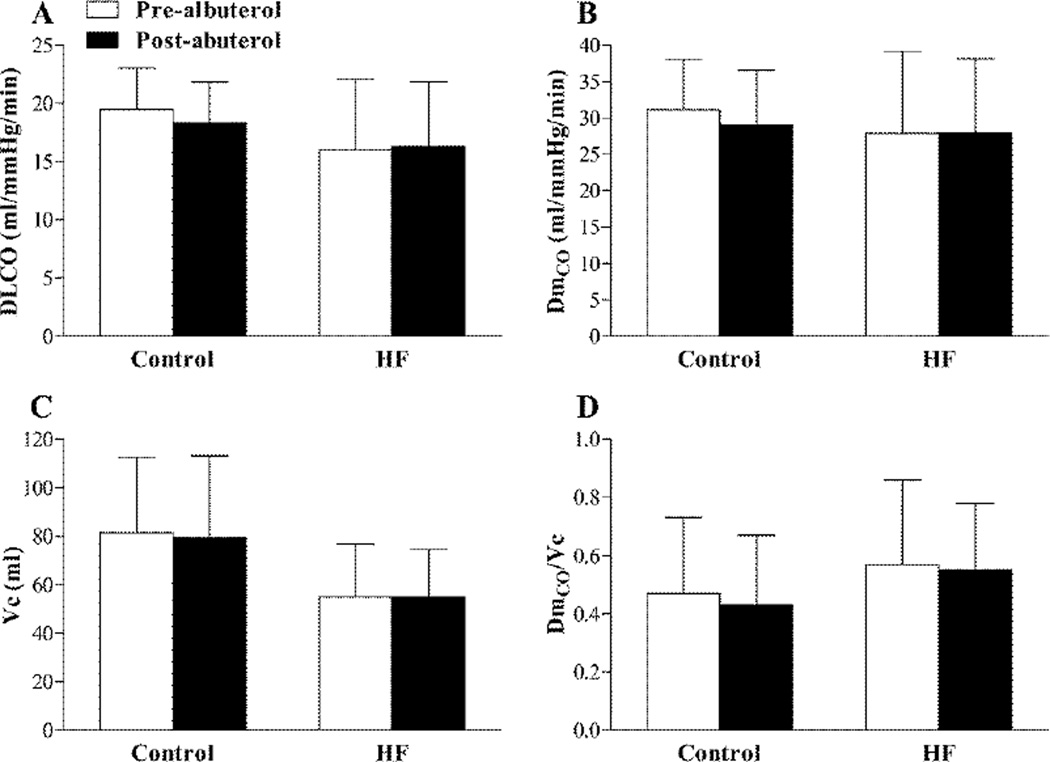
Effect of β2-AR stimulation in HFrEF and healthy controls
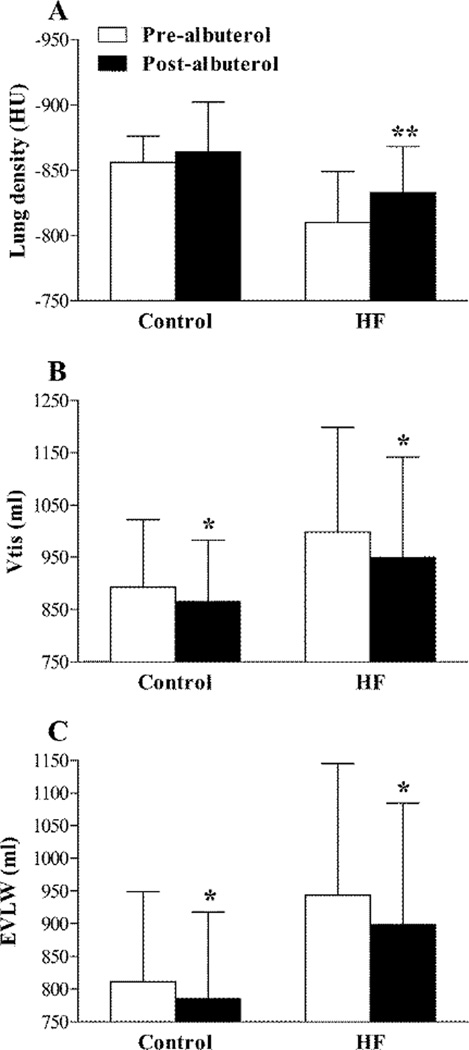
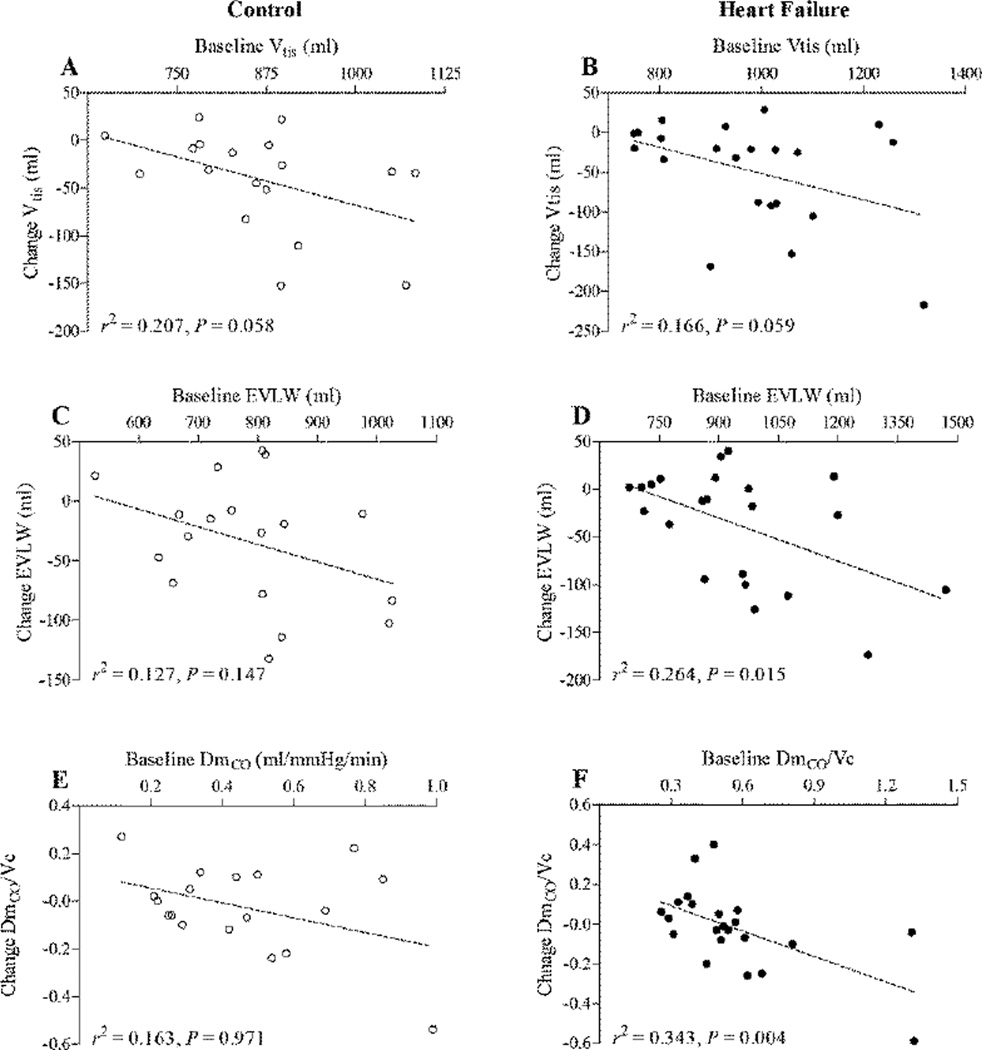
There was little/no change in either HR or blood pressure from before to immediately after albuterol administration in the HFrEF patients (HR 65 ± 9 vs. 69 ± 11 bpm, P = 0.129; MAP 92 ± 8 vs. 95 ± 7 mmHg, P = 0.098). Similarly, no evidence of cardiac arrhythmia was observed in any participant during albuterol administration. Albuterol administration had no effect on FVC, but caused an increase in FEV1, FEF25–75% and IC in both the control subjects and HFrEF patients (Table 3). DLCO, DmCO, Vc and DmCO/Vc were not different from before to after albuterol administration in neither the control subjects nor the HFrEF patients (Figure 1). Albuterol administration did, however, cause a significant reduction in Vtis in both the healthy control subjects and the HFrEF patients (control: −2.8 ± 4.9%, P = 0.029; HFrEF: −4.6±7.8%, P =0.010) (Figure 2). Similarly, there was a significant reduction in EVLW from before to after albuterol administration in both the control subjects and the HFrEF patients (control: −3.0 ± 5.7%, P = 0.045; HFrEF: −4.6±8.8%, P = 0.018) (Figure 2). There was a trend towards an inverse relationship between baseline values of Vtis, EVLW and DmCO/Vc ratio and the magnitude of the change in these variables from before to after albuterol in both the control subjects and the HFrEF patients; however, these relationships were statistically significant for EVLW and DmCO/Vc ratio in the HFrEF patients only (Figure 3). These data may suggest that stimulation of the β2-ARs via nebulized albuterol administration promoted a greater degree of lung fluid clearance in individuals with the greatest evidence lung fluid at baseline.
Table 3.
Measures of pulmonary function before and after albuterol administration in heart-failure patients with reduced ejection fraction (HFrEF) and healthy control subjects.
| Control | HFrEF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-albuterol | Post-albuterol | Pre- albuterol |
Post- albuterol |
|||||||||
| FVC, L | 4.5 3 |
± | 0.7 8 |
4.3 8 |
± | 0.81 | 3.5 0 |
± | 1.1 4 |
3.3 7 |
± | 1.11 |
| FEV1, L | 3.6 4 |
± | 0.6 3 |
3.7 0 |
± | 0.69 | 2.6 7 |
± | 1.0 3 |
2.7 7 |
± | 0.99 ** |
| FEV1/F VC, % |
81 | ± | 6 | 85 | ± | 6** | 75 | ± | 11 | 82 | ± | 7** |
| PEF, L/s | 9.1 2 |
± | 1.6 3 |
8.9 7 |
± | 1.59 | 6.8 4 |
± | 2.5 9 |
6.9 7 |
± | 2.87 |
| FEF25–75 %, L/s |
3.7 3 |
± | 0.9 3 |
4.3 1 |
± | 1.02 ** |
2.3 5 |
± | 1.2 8 |
2.9 2 |
± | 1.42 ** |
| IC, L | 3.3 1 |
± | 0.6 4 |
3.5 1 |
± | 0.74 ** |
2.6 1 |
± | 0.8 1 |
2.8 3 |
± | 0.90 * |
| DLCO/ VA |
10. 87 |
± | 6.6 0 |
10. 93 |
± | 6.83 | 6.3 7 |
± | 4.5 6 |
6.4 8 |
± | 3.08 |
Data are presented as group mean ± SD. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; PEF; peak expiratory flow rate; FEF25–75%, forced expiratory flow at 25–75% of FVC; IC, inspiratory capacity; DLCO, lung diffusing capacity for carbon monoxide; VA, alveolar volume.
P < 0.05 &
P < 0.01, value significantly different vs. pre-albuterol.
Figure 1.
Group mean ± SD lung density lung diffusing capacity for carbon monoxide (DLCO) (A), alveolar capillary membrane conductance (DmCO) (B), pulmonary capillary blood volume (Vc) (C) and the ratio of DmCO to Vc (DmCO/Vc) (D) before (pre-albuterol; white bars) and after (post-albuterol; black bars) nebulized albuterol administration in healthy control subjects (Control) and heart failure patients with reduced ejection fraction (HFrEF).
Figure 2.
Group mean ± SD lung density (A), lung tissue volume (Vtis) (B) and extravascular lung water (EVLW) (C) before (pre-albuterol; white bars) and after (post-albuterol; black bars) nebulized albuterol administration in healthy control subjects (Control) and heart failure patients with reduced ejection fraction (HFrEF). *P < 0.05, **P < 0.01; value significantly different vs. pre-albuterol.
Figure 3.
Scatter plots showing the relationships between the individual subject baseline values (i.e. before nebulized albuterol administration) and the before to after nebulized albuterol change in lung tissue volume (Vtis) (A & B), extravascular lung water (EVLW (C & D) and the ratio of alveolar capillary membrane conductance to pulmonary capillary blood volume (DmCO/Vc) (E & F) in in healthy control subjects (open circles) and heart failure patients with reduced ejection fraction (closed circles).
DISCUSSION
Main findings
The main findings of the present study were: 1) before albuterol administration, lung density, lung tissue volume (Vtis) and extravascular lung water (EVLW) were greater in the HFrEF patients compared to the healthy control subjects, 2) lung diffusing capacity for carbon monoxide (DLCO) and pulmonary capillary blood volume (Vc) were greater in control subjects compared to the HFrEF patients before albuterol administration, 3) albuterol administration caused a ~3 to 5% reduction in Vtis and EVLW in healthy control subjects and HFrEF patients, and 4) there was a trend towards an inverse relationship between baseline values of Vtis, EVLW and DmCO/Vc ratio and the magnitude of the change in these variables from before to after albuterol in both the control subjects and the HFrEF patients; however, this relationship was statistically significant for EVLW and DmCO/Vc ratio in the HFrEF patients only. In combination, the findings of the present study suggest that 1) lung fluid volume is elevated in stable, well compensated HFrEF patients relative to their healthy age and sex matched counterparts, and 2) acute stimulation of the β2-ARs appears to cause lung fluid removal in stable HFrEF patients, especially in patients with evidence of elevated lung fluid volume at rest. Our study demonstrates that pharmacological stimulation of the β2-ARs may help reduce lung fluid volume in stable HFrEF patients, and thus lends support to the hypothesis that β2-ARs play an important role in lung fluid balance in vivo in humans and that dysfunction of the β2-ARs may be a source of elevated lung fluid volume in stable heart failure patients.
The importance of the β2-AR system in the control of fluid balance
The β2-ARs are expressed throughout the pulmonary system, including in the airways, the alveolar spaces, the pulmonary vasculature and the pulmonary lymphatic tissue, where they appear to regulate lung fluid removal via two distinct mechanisms. First, it appears that stimulation of the β2-ARs facilitates fluid removal from the alveolar spaces through epithelial sodium channels (ENaC) located on both type I and type II alveolar cells (13, 14). Indeed, it has been shown previously that stimulation of the β2-ARs is associated with an increase in both the total number and open probability of ENaC on the apical portion of type I and type II alveolar cells secondary to an increase in the synthesis of cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) (13). Second, it has been shown that stimulation of the β2-ARs on lymphatic tissue causes dilation as well as active phasic contraction of the thoracic lymphatic ducts, which acts to clear lung fluid from the perivascular spaces to the hilar lymph nodes (15, 16).
The importance of the β2-AR system in the regulation of lung fluid in both animal models and in humans has been demonstrated through a number of key studies (17–26). For example, Tibayan et al (22) reported that administration of the nonselective β1 and β2 receptor agonist dobutamine, but not the selective β1 agonist dopamine, caused a substantial increase in alveolar liquid clearance (~50%) in anesthetized ventilated rats. In addition, it has been shown that alveolar β2-AR overexpression improves β2-AR function and maximally upregulates alveolar fluid clearance in a rat model (21, 23). In vivo in humans, it has been demonstrated that administration of the long acting β2-AR agonist salmeterol results in a ~50% decrease in the incidence of high-altitude pulmonary edema (HAPE) in subjects identified as HAPE susceptible (18). More recently, it has been shown that oral administration of the nonselective β1 and β2 receptor blocker Carvedilol, but not the selective β1blocker Bisoprolol, caused a significant decrease (~13%) in alveolar-capillary membrane conductance in healthy humans, which is indicative of an increase in extravascular lung water (17). In combination, the findings detailed above clearly identify the key role of the β2-AR system in the regulation lung fluid balance and in the maintenance of lung fluid homeostasis. In addition to the aforementioned findings, in the present study we found that acute administration of the selective β2-AR agonist albuterol caused a significant reduction in lung tissue volume and extravascular lung water (~3–5%) in both stable HFrEF patients and healthy control subjects (Figure 2). In addition, there was a trend towards an inverse relationship between baseline values of Vtis, EVLW and DmCO/Vc ratio and the magnitude of the change in these variables from before to after albuterol in both the control subjects and the HFrEF patients; however, this relationship was statistically significant for EVLW and DmCO/Vc ratio in the HFrEF patients only. These data suggest perhaps suggest that the greatest lung fluid clearance in response to acute albuterol administration occurs in the HFrEF patients who exhibit the greatest degree of lung fluid volume at rest. Accordingly, our study demonstrates that acute pharmacological stimulation of the β2-ARs reduces lung fluid volume in both stable HFrEF patients and healthy control subjects, and thus lends support to the hypothesis that 1) β2-ARs play a vital role in lung fluid balance in vivo in humans, and 2) that dysfunction of the β2-ARs may be a source of elevated lung fluid volume in stable HFrEF patients.
Why is lung fluid increased in stable HF?
It is well known that pulmonary congestion, a key component of which is a significant increase in lung fluid, is a common consequence of acute decompensation in HF (5–7). However, it has remained somewhat controversial whether stable HF patients exhibit elevated lung fluid volume (8, 9), with chronic HF patients often appearing to “resist” pulmonary edema (24). Indeed, in clinical practice it is often observed that patients with severe HF lack pulmonary rales on examination or alveolar edema on chest x-ray. Theoretically, the increase in pulmonary capillary hydrostatic pressure and wall tension due to the rise in LV filling pressure consistent with a failing LV (1) combined with down-regulation of the β receptors that are central to lung fluid removal mechanisms (2–4) secondary to a chronic increase in adrenergic drive should serve to make HF patients more susceptible to lung fluid accumulation relative to their healthy counterparts. However, it has also been shown that pulmonary microvascular permeability is decreased in severe HF patients, which would be expected to protect such patients from pulmonary edema (24). Presently, we found that lung fluid is elevated in stable HFrEF patients relative to healthy subjects (Table 2). These data perhaps suggest that, while somewhat preventative, the reduction in pulmonary microvascular permeability often observed in stable HFrEF patients does not fully protect against an increase in extravascular lung water in this population.
Clinical implications
Recently, we have reported that CT derived measures of large airway wall thickness and luminal area are not different in healthy control subjects relative to stable HFrEF patients (27). In combination with the findings of the present study, data from our laboratory suggest that stable, well compensated HFrEF patients have evidence of elevated lung fluid volume but not substantial large airway edema and/or engorgement relative to their healthy age and sex matched counterparts. Although the exact clinical ramifications of such an accumulation of lung fluid in these patients are unclear, it is possible that interstitial lung edema plays a role in abnormal pulmonary gas exchange and exaggerated ventilatory response to exercise associated with HF. HF is of course a complex disease and much of the impaired pulmonary gas exchange and hyperventilatory response to exercise in HF patients has been associated with pulmonary vascular dysfunction (28, 29), skeletal muscle dysfunction (30, 31), early onset of metabolic acidosis (32), heightened chemosensitivity, and exaggerated afferent signals from exercising muscles (30). However, in animal models, it has been demonstrated that artificial induction of pulmonary congestion causes a rapid shallow breathing pattern secondary to stimulation of pulmonary C-fibers (33). In addition, acute fluid loading in otherwise healthy humans has been shown to elicit a less efficient hyperventilatory response to incremental exercise (34). Indeed, Robertson et al. (34) reported that rapid intravenous saline infusion (30 mL/kg over 30 min) caused a ~12% and ~4% increase in extravascular fluid and intravascular fluid, respectively, with a concomitant ~12% increase in the ventilatory equivalent for carbon dioxide as well as a slight decrease in arterial PCO2 and a reduction aerobic exercise capacity. More recently, Paolillo et al (17) found that administration of the β2-AR antagonist Carvedilol caused a ~12% increase in the E2 slope in response to exercise that was always coincident with a ~13% decrease in DmCO (i.e. evidence of an increase in lung interstitial fluid volume). Based on the aforementioned considerations, it possible that lung interstitial edema provides an additional stimulus for the impaired pulmonary gas exchange and the inefficient hyperventilatory response to exercise commonly observed in HF patients. Moreover, given that a low DmCO and a high V̇E/V̇CO2 (slope or ratio) are key prognostic predictors in HFrEF patients (35, 36), it can be suggested that elevated extravascular lung water may represent an important target for therapeutic intervention in these patients. In the present study we found that acute low-dose albuterol administration appears to cause lung fluid clearance (~5%) in stable HFrEF patients. Whether such acute pharmacological stimulation of the β2-ARs may be indicated in the presence of clinical and/or radiological signs of pulmonary edema in stable HFrEF patients requires further investigation.
Conclusion
In conclusion, lung fluid volume is elevated in stable, well compensated heart failure patients with reduced ejection fraction relative to their healthy age and sex matched counterparts. Interestingly, stimulation of the β2-adrenergic receptors via acute low dose nebulized albuterol appears to promote lung fluid removal in both healthy control subjects and heart failure patients, but especially in HFrEF patients who exhibit evidence of elevated lung water at rest. Whether such acute β2-adrenergic receptor stimulation may provide a therapeutic aid to the excessive lung fluid commonly observed in HFrEF requires further investigation.
Acknowledgments
The authors thank Andrew Miller and Kathy O’Malley for assistance with data acquisition and management.
FUNDING SOURCES
This work was supported by National Institute of Health Grant Number HL71478, National Center for Research Resources (a component of NIH) Grant Number 1TL1RR024152 and NIH/NCRR CTSA Grant Number RR024150. BJT is supported by a Fulbright Commission UK Distinguished Scholar Award and American Heart Association Grant AHA12-POST12070084).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Borst MM, Beuthien W, Schwencke C, LaRosee P, Marquetant R, Haass M, et al. Desensitization of the pulmonary adenylyl cyclase system: a cause of airway hyperresponsiveness in congestive heart failure? J Am Coll Cardiol. 1999;34(3):848–856. doi: 10.1016/s0735-1097(99)00251-x. [DOI] [PubMed] [Google Scholar]
- 3.Nerme V, Abrahamsson T, Vauquelin G. Chronic isoproterenol administration causes altered beta adrenoceptor-Gs-coupling in guinea pig lung. J Pharmacol Exp Ther. 1990;252(3):1341–1346. [PubMed] [Google Scholar]
- 4.Nishikawa M, Mak JC, Shirasaki H, Harding SE, Barnes PJ. Long-term exposure to norepinephrine results in down-regulation and reduced mRNA expression of pulmonary beta-adrenergic receptors in guinea pigs. Am J Respir Cell Mol Biol. 1994;10(1):91–99. doi: 10.1165/ajrcmb.10.1.8292387. [DOI] [PubMed] [Google Scholar]
- 5.Clark AL, Cleland JG. Causes and treatment of oedema in patients with heart failure. Nat Rev Cardiol. 2013;10(3):156–170. doi: 10.1038/nrcardio.2012.191. [DOI] [PubMed] [Google Scholar]
- 6.Gustafsson M, Alehagen U, Johansson P. Imaging Congestion With a Pocket Ultrasound Device: Prognostic Implications in Patients With Chronic Heart Failure. Journal of cardiac failure. 2015;21(7):548–554. doi: 10.1016/j.cardfail.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Malfatto G, Caravita S, Giglio A, Rossi J, Perego GB, Facchini M, et al. Pulmonary congestion at rest and abnormal ventilation during exercise in chronic systolic heart failure. J Am Heart Assoc. 2015;4(5) doi: 10.1161/JAHA.114.001678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grover M, Slutsky RA, Higgins CB, Shabetai R. Extravascular lung water in patients with congestive heart failure. Difference between patients with acute and chronic myocardial disease. Radiology. 1983;147(3):659–662. doi: 10.1148/radiology.147.3.6342031. [DOI] [PubMed] [Google Scholar]
- 9.O'Dochartaigh CS, Kelly B, Riley MS, Nicholls DP. Lung water content is not increased in chronic cardiac failure. Heart. 2005;91(11):1473–1474. doi: 10.1136/hrt.2004.052829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 11.Ceridon ML, Beck KC, Olson TP, Bilezikian JA, Johnson BD. Calculating alveolar capillary conductance and pulmonary capillary blood volume: comparing the multiple- and single-inspired oxygen tension methods. J Appl Physiol (1985) 2010;109(3):643–653. doi: 10.1152/japplphysiol.01411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snyder EM, Beck KC, Hulsebus ML, Breen JF, Hoffman EA, Johnson BD. Short-term hypoxic exposure at rest and during exercise reduces lung water in healthy humans. J Appl Physiol (1985) 2006;101(6):1623–1632. doi: 10.1152/japplphysiol.00481.2006. [DOI] [PubMed] [Google Scholar]
- 13.Eaton DC, Chen J, Ramosevac S, Matalon S, Jain L. Regulation of Na+ channels in lung alveolar type II epithelial cells. Proc Am Thorac Soc. 2004;1(1):10–16. doi: 10.1513/pats.2306008. [DOI] [PubMed] [Google Scholar]
- 14.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, et al. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci U S A. 2006;103(13):4964–4969. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahe L, Chapelain B, Gargouil YM, Neliat G. Characterization of beta-adrenoceptor subtypes and indications for two cell populations in isolated bovine mesenteric lymphatic vessels. Eur J Pharmacol. 1991;199(1):19–25. doi: 10.1016/0014-2999(91)90632-z. [DOI] [PubMed] [Google Scholar]
- 16.Ikomi F, Kawai Y, Ohhashi T. Beta-1 and beta-2 adrenoceptors mediate smooth muscle relaxation in bovine isolated mesenteric lymphatics. J Pharmacol Exp Ther. 1991;259(1):365–370. [PubMed] [Google Scholar]
- 17.Paolillo S, Pellegrino R, Salvioni E, Contini M, Iorio A, Bovis F, et al. Role of alveolar beta2-adrenergic receptors on lung fluid clearance and exercise ventilation in healthy humans. PLoS One. 2013;8(4):e61877. doi: 10.1371/journal.pone.0061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sartori C, Allemann Y, Duplain H, Lepori M, Egli M, Lipp E, et al. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med. 2002;346(21):1631–1636. doi: 10.1056/NEJMoa013183. [DOI] [PubMed] [Google Scholar]
- 19.Sartori C, Fang X, McGraw DW, Koch P, Snider ME, Folkesson HG, et al. Selected contribution: long-term effects of beta(2)-adrenergic receptor stimulation on alveolar fluid clearance in mice. J Appl Physiol (1985) 2002;93(5):1875–1880. doi: 10.1152/japplphysiol.00275.2002. [DOI] [PubMed] [Google Scholar]
- 20.Vivona ML, Matthay M, Chabaud MB, Friedlander G, Clerici C. Hypoxia reduces alveolar epithelial sodium and fluid transport in rats: reversal by beta-adrenergic agonist treatment. Am J Respir Cell Mol Biol. 2001;25(5):554–561. doi: 10.1165/ajrcmb.25.5.4420. [DOI] [PubMed] [Google Scholar]
- 21.Dumasius V, Sznajder JI, Azzam ZS, Boja J, Mutlu GM, Maron MB, et al. beta(2)-adrenergic receptor overexpression increases alveolar fluid clearance and responsiveness to endogenous catecholamines in rats. Circ Res. 2001;89(10):907–914. doi: 10.1161/hh2201.100204. [DOI] [PubMed] [Google Scholar]
- 22.Tibayan FA, Chesnutt AN, Folkesson HG, Eandi J, Matthay MA. Dobutamine increases alveolar liquid clearance in ventilated rats by beta-2 receptor stimulation. Am J Respir Crit Care Med. 1997;156(2 Pt 1):438–444. doi: 10.1164/ajrccm.156.2.9609141. [DOI] [PubMed] [Google Scholar]
- 23.Factor P, Adir Y, Mutlu GM, Burhop J, Dumasius V. Effects of beta2-adrenergic receptor overexpression on alveolar epithelial active transport. J Allergy Clin Immunol. 2002;110(6 Suppl):S242–S246. doi: 10.1067/mai.2002.129706. [DOI] [PubMed] [Google Scholar]
- 24.Davies SW, Bailey J, Keegan J, Balcon R, Rudd RM, Lipkin DP. Reduced pulmonary microvascular permeability in severe chronic left heart failure. Am Heart J. 1992;124(1):137–142. doi: 10.1016/0002-8703(92)90931-k. [DOI] [PubMed] [Google Scholar]
- 25.Finley N, Norlin A, Baines DL, Folkesson HG. Alveolar epithelial fluid clearance is mediated by endogenous catecholamines at birth in guinea pigs. J Clin Invest. 1998;101(5):972–981. doi: 10.1172/JCI1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis B, Marin MG, Yee JW, Nadel JA. Effect of terbutaline on movement of Cl− and Na+ across the trachea of the dog in vitro. Am Rev Respir Dis. 1979;120(3):547–552. doi: 10.1164/arrd.1979.120.3.547. [DOI] [PubMed] [Google Scholar]
- 27.Chase SC, Wheatley CM, Olson LJ, Beck KC, Wentz RJ, Snyder EM, et al. Impact of chronic systolic heart failure on lung structure-function relationships in large airways. Physiol Rep. 2016;4(13) doi: 10.14814/phy2.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor BJ, Smetana MR, Frantz RP, Johnson BD. Submaximal Exercise Pulmonary Gas Exchange in Left Heart Disease Patients With Different Forms of Pulmonary Hypertension. J Card Fail. 2015;21(8):647–655. doi: 10.1016/j.cardfail.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guazzi M, Myers J, Peberdy MA, Bensimhon D, Chase P, Arena R. Ventilatory efficiency and dyspnea on exertion improvements are related to reduced pulmonary pressure in heart failure patients receiving Sildenafil. Int J Cardiol. 2010;144(3):410–412. doi: 10.1016/j.ijcard.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 30.Olson TP, Joyner MJ, Johnson BD. Influence of locomotor muscle metaboreceptor stimulation on the ventilatory response to exercise in heart failure. Circ Heart Fail. 2010;3(2):212–219. doi: 10.1161/CIRCHEARTFAILURE.109.879684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piepoli MF, Kaczmarek A, Francis DP, Davies LC, Rauchhaus M, Jankowska EA, et al. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation. 2006;114(2):126–134. doi: 10.1161/CIRCULATIONAHA.105.605980. [DOI] [PubMed] [Google Scholar]
- 32.Sun XG, Hansen JE, Oudiz RJ, Wasserman K. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation. 2001;104(4):429–435. doi: 10.1161/hc2901.093198. [DOI] [PubMed] [Google Scholar]
- 33.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- 34.Robertson HT, Pellegrino R, Pini D, Oreglia J, DeVita S, Brusasco V, et al. Exercise response after rapid intravenous infusion of saline in healthy humans. J Appl Physiol (1985) 2004;97(2):697–703. doi: 10.1152/japplphysiol.00108.2004. [DOI] [PubMed] [Google Scholar]
- 35.Guazzi M, Pontone G, Brambilla R, Agostoni P, Reina G. Alveolar--capillary membrane gas conductance: a novel prognostic indicator in chronic heart failure. Eur Heart J. 2002;23(6):467–476. doi: 10.1053/euhj.2001.2803. [DOI] [PubMed] [Google Scholar]
- 36.Woods PR, Bailey KR, Wood CM, Johnson BD. Submaximal exercise gas exchange is an important prognostic tool to predict adverse outcomes in heart failure. Eur J Heart Fail. 2011;13(3):303–310. doi: 10.1093/eurjhf/hfq187. [DOI] [PMC free article] [PubMed] [Google Scholar]