Abstract
The bed nucleus of the stria terminalis (BNST) is known to play a critical role in mediating the behavioral and autonomic responses to stressors. The oval nucleus of the BNST (BNSTov) contains cell bodies that synthesize the stress hormone, corticotropin releasing factor (CRF). Although afferent fibers originating from the BNSTov have been shown to innervate several key structures of the neuroendocrine and central autonomic system, the question remains as to whether, some of these fibers are CRF-positive. To directly address this question, we injected a “floxed” anterograde tracer (rAAV5/EF1a-DIO-mCherry) into the BNSTov of CRFp3.0CreGFP transgenic mice, which express a green fluorescent protein (GFP) under the control of the CRF promoter. Serial sections were then analyzed for the presence of double-labeled fibers in potential projection sites. To determine whether CRF neurons in the rat BNSTov send comparable projections, we infused rat BNSTov with an AAV in which the human synapsin promoter drives enhanced GFP expression. We then used CRF immunoreactivity to examine double-labeled fluorescent fibers and axon terminals in projection sites from brain sections of the AAV-infused rats. We have observed several terminal fields in the mouse and rat brain with double-labeled fibers in the Dorsal raphe nucleus (DRD), the Paraventricular nucleus of the hypothalamus, and to a lesser extent in the Ventral tegmental area. We found double-labeled terminal boutons in the nucleus accumbens shell, prelimbic cortex, and posterior basolateral nucleus of the amygdala. The most intense double-labeling was found in midbrain, including substantia nigra pars compacta, red nucleus, periaqueductal gray, pontine nuclei, as well as DRD. The results of our study indicate that CRF neurons are the output neurons of the BNSTov and they send projections to the centers of neuroendocrine and autonomic regulation, but also regions modulating reward and motivation, vigilance, motor function, as well as affective behavior.
1. Introduction
Corticotropin-releasing factor (CRF) neurotransmission is essential for coordinating the adaptive response to stressful situations (1), but its prolonged activation as a result of chronic or traumatic stress is critically involved in the etiology of anxiety, depression, and post-traumatic stress disorder (PTSD) (2). The main clusters of CRF neurons outside the paraventricular nucleus of the hypothalamus (PVN) are in the extended amygdala, which includes the bed nucleus of the stria terminalis (BNST) and the central nucleus of the amygdala (CeA) (3). While CRF neurons in the PVN are associated with a fast endocrine stress response, the extra-hypothalamic CRF system is linked to the affective component of the stress response (4). The BNST is involved in stress adaptation and mediates autonomic and behavioral responses to stressors, including fear and anxiety (5). In humans, the BNST is active during anticipatory anxiety (6), in conditions of uncertainty and hyper-vigilant threat monitoring (7), and its activity is elevated further in patients with anxiety disorders (8). Although both the BNST and CeA are involved in mediating fear responses, the BNST is necessary for expression of long-duration fear responses that resemble anxiety (sustained fear), while the CeA mediates short-duration fear responses (phasic fear) (9). In addition, the BNST is required for anxiety to non-specific environmental cues, for example bright light exposure in rodents (unconditioned anxiety), while the CeA is required for fear responses to a specific cue (classic fear conditioning) (5). The anterolateral cell group of the BNST contains cell bodies that synthesize the stress hormone, CRF (10) in the oval (BNSTov) and fusiform nuclei, as well as CRF-positive fibers and terminals that originate mainly from the CeA (11). CRF, acting through its primary receptor, CRFR1, mediates anxiogenic responses in the BNST including potentiation of the acoustic startle reflex (12). Moreover, chronic overexpression of CRF in the BNST leads to anxiety- (13), and depressive-like behavior in experimental animal models (14). Hence, it is believed that CRF, primarily from the lateral division of the CeA, acts on CRF receptors type 1 (CRFR1) in the BNST to mediate anxiety and negative affect following a prolonged threat stimulus (9) or withdrawal from drugs of abuse (15).
However, although the BNSTov has one of the highest densities of neurons producing CRF in a rodent brain, the role and efferent projections of these neurons has not been fully elucidated. We have recently shown that repeated stress exposure leads to a long-term facilitation of synaptic plasticity selectively in putative CRF neurons of the BNSTov (measured as increased LTP), which contributes to the stress-induced persistent changes in behavior, including potentiated startle response and fear conditioning (16). This change in synaptic plasticity in CRF neurons could lead to the formation of a long-term memory in fear circuits; however the down-stream projection sites that potentially mediate these behaviors are largely unknown.
Neurons of the rat BNSTov are primarily GABA-ergic (17) and also express various neuropeptides besides CRF, including enkephalin, neuropeptide Y, dynorphin, calbindin and somatostatin (18). Therefore, not only is the BNST a heterogeneous structure containing several distinct nuclei exhibiting diverse neuropeptide expression profiles, but activation of the distinct nuclei can lead to contrasting physiological and behavioral effects, for review see (19). BNST projections arising from the oval and fusiform nuclei have been described in detail in a seminal study by Dong and colleagues (20). However, although afferent fibers originating from the BNSTov have been shown to innervate several key structures of the neuroendocrine and central autonomic system, the question remains as to whether some of these fibers are CRF-positive and, if so, what specific neuronal populations do they innervate. Therefore the aim of the current study was to understand specific projections of CRF neurons in the BNSTov and their relationship with downstream neuronal populations. Here, we have focused solely on the CRF neurons in the BNSTov, as there is growing evidence that their neurotransmitter phenotype and function might be different from the CRF neurons in the fusiform (subcomissural) nucleus of the BNST (19, 21), or from the CRF neurons in the PVN (17). To this end, we have identified downstream projections of BNSTov CRF neurons using a cell-type specific adeno-associated viral vector (AAV)-based approach in mouse and rat brain. Here, we propose that CRF neurons of the BNSTov are output neurons and send projections to hypothalamic, midbrain, and brainstem nuclei. Hence, these CRF neurons are uniquely positioned to mediate distinct features of fear and anxiety-like behavior.
2. Methods
All the procedures used were approved by the Institutional Animal Care and Use Committees (IACUC) of Emory University and Rosalind Franklin University of Medicine and Science, and were in compliance with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.
2.1. Cell-type specific targeting of the CRF neurons in the mouse BNSTov using a Cre-recombinase-dependent (floxed) AAV injection
To directly address the question of where BNSTov CRF cells send their projections in the mouse, we bilaterally injected a “floxed” anterograde tracer (rAAV5/EF1a-DIO-mCherry, Gene Therapy Center Virus Vector Core Facility, the University of North Carolina at Chapel Hill, Chapel Hill, NC, USA) into the BNSTov of five male (40–45 days old) CRFp3.0CreGFP transgenic mice, which express a green fluorescent protein (GFP) under the control of the CRF promoter (22). Mice were anaesthetized with an intraperitoneal injection of dexdormitor (Orion Pharma, Espoo, Finland) and ketamine hydrochloride (Bioniche Pharma, Bogart, GA, USA) mixture and infused with AAV (300 nl) during stereotaxic surgery using the following coordinates from Bregma: AP: +0.3 mm, ML: ±2.1 mm, DV: −4.2 mm with a 15° coronal angle to avoid the lateral ventricle. Two weeks after the AAV injection, following the standard 10% formalin fixation protocol (23), the brains were removed, post-fixed, and sliced. Serial sections (40 μm) were cut through the entire mouse brain and analyzed for the presence of double-labeled neurons in the BNSTov (GFP-mCherry) and dual-labeled fibers in potential projection sites using confocal spinning disk laser microscopy. We obtained high-resolution photomicrographs using an Orca R2 cooled CCD camera (Hamamatsu, Bridgewater, NJ) mounted on a Leica DM5500B microscope (Leica Microsystems, Bannockburn, IL) equipped with a CSU10B Spinning Disk (Yokagawa Electronic Corporation, Tokyo, Japan). Analysis of dual-labeled neurons in the BNST and fibers in the projection sites was performed with Simple PCI 6.6 software (Hamamatsu, Sewickley, PA).
2.2. Targeting of the CRF neurons in the rat BNSTov using an AAV injection combined with CRF immunoreactivity
To determine whether CRF neurons in the rat BNSTov have similar projections to those in the mouse brain, we injected six adult (60 days old) male Sprague-Dawley rats under ketamine/dexdormitor (as above) or isoflurane anesthesia with an AAV in which the human synapsin promoter-5 (hSyn) drives enhanced GFP (eGFP) expression (Gene Therapy Center Virus Vector Core Facility, the University of North Carolina at Chapel Hill, Chapel Hill, NC, USA). Here, 500 nl of the rAAV5-hSyn-eGFP was bilaterally injected into the BNSTov using the following stereotaxic coordinates from Bregma: AP: +0.1 mm; ML: ±3.4 mm, DV: −7.2 mm with a 15° coronal angle, modified from (24). Rats were perfused 4 weeks later following a standard 10% formalin fixation protocol, and the brains removed, post-fixed, and sliced for immunofluorescence staining with an anti-CRF antibody (rabbit polyclonal, 1:250, ab11133, Abcam, Cambridge, MA or guinea-pig polyclonal, 1:1000, T-5007, Peninsula, San Carlos, CA), as described previously (16, 17, 23). Specificity of the rabbit and guinea-pig CRF antibodies was assessed and described before (16, 17, 23, 25). Briefly, free-floating 50 μm sections were rinsed 3x (10 min each) in phosphate buffer saline (PBS) and permeabilized with 0.5 % Triton-X 100 in PBS. Normal goat serum (3%, Life Technologies, Thermofisher Scientific) was added to Triton-X/PBS to block non-specific binding and sections were then incubated for 48 hours at 4°C with the primary antibody diluted in 0.5% Triton-X/PBS solution. Sections were rinsed 3x (10 min each) in PBS and then incubated at room temperature for 2 hours with goat anti-rabbit or goat anti-guinea-pig AlexaFluor-568 secondary antibody (1:500, Life Technologies, Grand Island, NY, USA). Following incubation with the secondary antibody, sections were rinsed 3x (10 min each) in PBS and 1x in 0.05 M phosphate buffer (PB), mounted on gelatin-coated glass slides, cover-slipped using Mowiol 4–88 - Dabco Media (Polyvinyl alcohol, 81381; Sigma Aldrich, St. Louis, MO, USA), and dried overnight. Sections were then visualized using a Leica DM5500B microscope (as above), an Olympus Fluoview 500 Scanning Laser Confocal Microscope, or an Olympus FV10i Confocal Microscope (Olympus America, Inc., Center Valley, PA, USA). Sections were analyzed for the presence of double-labeled neurons and fibers with Simple PCI 6.6 software (Hamamatsu, Sewickley, PA) or FV10-ASW 3.0 software (Olympus).
2.3. Targeting neuronal populations innervated by CRF neurons from the BNSTov using dual-immunofluorescence protocol on brain sections from the AAV-hSyn-eGFP injected rats
Finally, to determine what subsets of neurons are innervated by the CRF projections originating in the BNSTov, we performed dual-immunofluorescence staining on brain sections from AAV-hSyn-eGFP infused rats (as above), in which the CRF antibody was used to label CRF-immunoreactivity (as above) in combination with one of the following antibodies: i) hypothalamic oxytocin (OT) neurons (mouse monoclonal anti-OT, 1:5000, MAB5296, Chemicon-Millipore, Billerica, MA); ii) midbrain dopamine (DA) neurons (mouse monoclonal anti-tyrosine hydroxylase, TH, 1:1000, MAB318, Chemicon-Millipore, Billerica, MA); iii) and dorsal raphe serotonin (5-HT) neurons (mouse anti-tryptophan hydroxylase, 5-TPH, 1:1000, ab82244, Abcam, Cambridge, MA). Following incubation with the primary antibodies, we then visualized local subsets of neurons with the following AlexaFluor secondary antibodies: Alexa633 goat anti-mouse IgG, Alexa568 goat anti-rabbit IgG or Alexa568 goat anti-guinea pig IgG (1:500, Life Technologies - Thermofisher Scientific). Confocal images were acquired using full signal distribution with sequential channel acquisition of each laser excitation on an Olympus Fluoview 500 Scanning Laser Confocal Microscope, or an Olympus FV10i Confocal Microscope and imaging was performed as above.
3. Results
The regional nomenclature used below is in accordance with the Rat Brain Atlas by (24).
3.1. Specific projections of CRF neurons originating from the mouse BNSTov
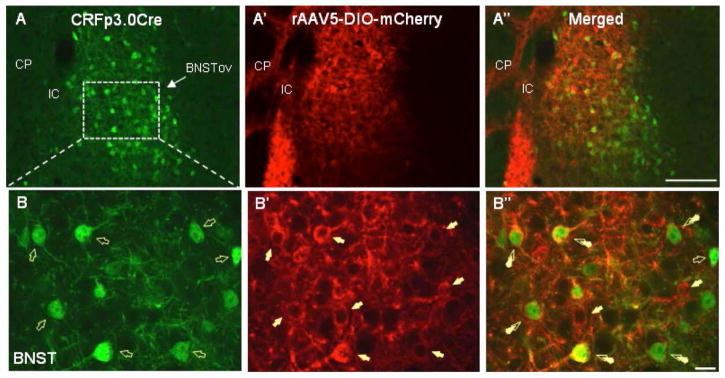
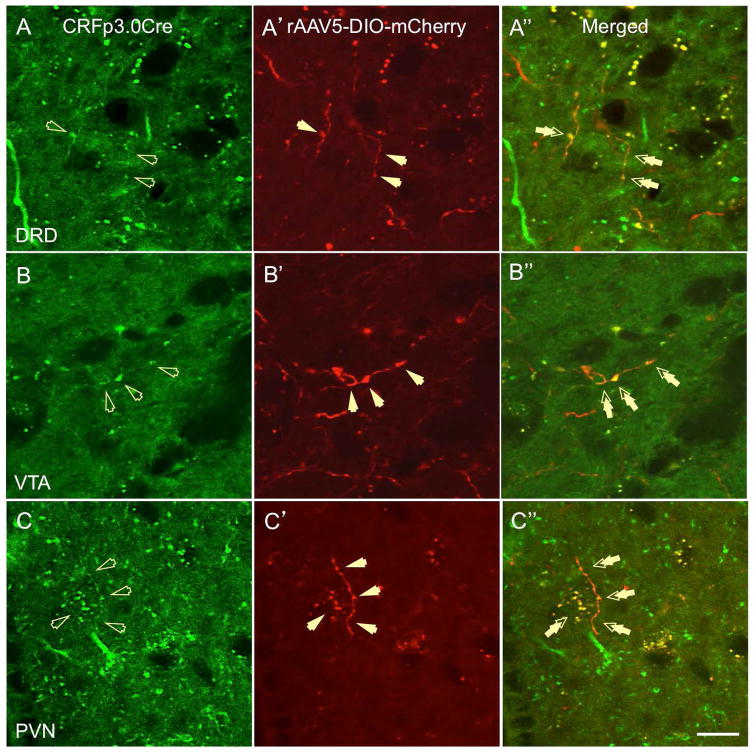
First, we analyzed serial brain sections from CRF-Cre-GFP transgenic mice injected with a floxed AAV5-mCherry into the BNSTov for the presence of double-labeled BNSTov neurons. We found a high level of co-expression between GFP- and m-Cherry-positive neurons in the BNSTov at the level of somata, dendrites, and axon fibers (Fig. 1A-B″), indicating successful AAV transfection of local CRF neurons in the BNSTov. We then investigated the presence of double-labeled (GFP-mCherry) fibers in potential projections sites using the study by Dong et al. (20) as a guideline for the general BNSTov projection patterns. Although we found single-labeled mCherry- and GFP-positive fibers in a variety of terminal sites, we have confirmed double-labeled fibers in the three major areas: the dorsal raphé nucleus, dorsal division (DRD, Fig. 2A-A''), the ventral tegmental area (VTA, Fig. 2B-B''), and the PVN (Fig. 2C-C″). These data strongly support the premise that CRF neurons of the mouse BNSTov project to downstream regions implicated in the regulation of stress, motivation, reward, and affective behavior.
Figure 1.
CRFp3.0CreGFP transgenic mice were injected with a floxed AAV5-DIO-mCherry viral vector into the BNSTov and analyzed for the presence of double-labeled BNSTov neurons. High level of co-expression between GFP- and m-Cherry-positive neurons was found in the BNSTov at the level of somata, dendrites, and axons (A–B″).
Figure 2.
Double-labeled (GFP-mCherry) fibers originating from the BNSTov were found in the following projections sites in CRFp3.0CreGFP transgenic mice injected with a floxed AAV5-DIO-mCherry: dorsal raphé nucleus, dorsal division (DRD, A-A″, double arrows), the ventral tegmental area (VTA, B-B″, double arrows), and the paraventricular nucleus of the hypothalamus (PVN, C-C″, double arrows, scale bar 10 μm).
3.2. Specific projections of CRF neurons originating from the rat BNSTov
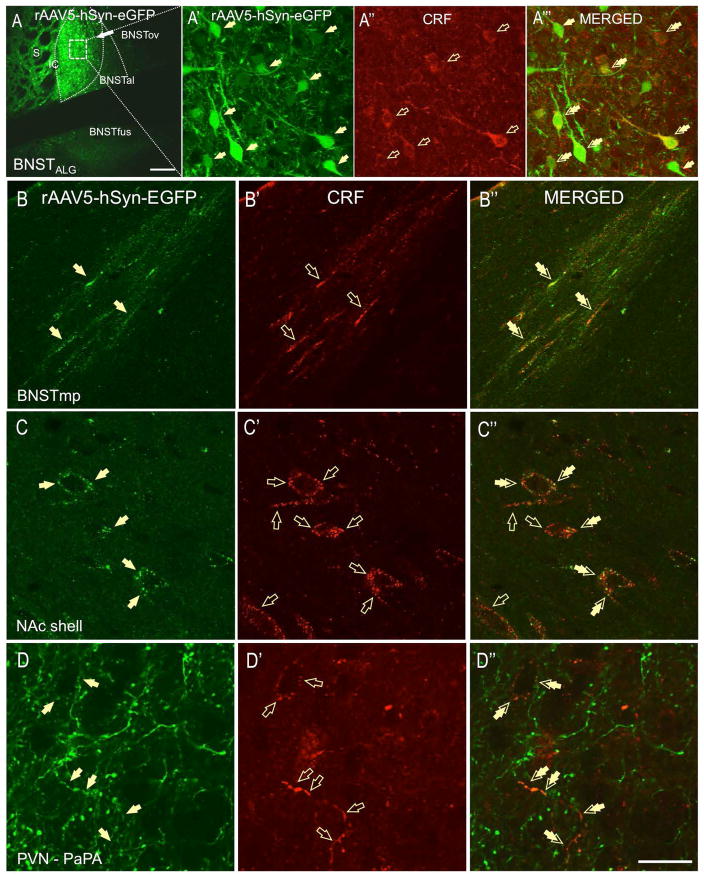
Next, we thoroughly analyzed serial brain sections from rats injected with the AAV-hSyn-eGFP (Fig. 3A), in which neuronal tract tracing of the BNSTov neurons was combined with CRF-fluorescent immunoreactivity (CRF-ir). First, we confirmed that a sub-population of BNSTov eGFP neurons co-expressed CRF at the level of somata, dendrites, and axons (Fig. 3A′-A‴), indicating that the AAV-hSyn-eGFP successfully transfected CRF neurons in the BNSTov. However, as expected, the AAV-hSyn-eGFP virus also transfected cells in the rat BNSTov that do not express CRF (eGFP-positive, CRF-ir negative, Fig. 3A′). Therefore we subsequently analyzed serial brain sections and noted only those fibers in which the presence of double-immunofluorescent labeling (eGFP and CRF) could be confirmed in potential projections sites. Once again, the study by (20) was used as a guideline for the general BNSTov projection patterns.
Figure 3.
Rats were injected with the AAV5-hSyn-eGFP viral vector into the BNSTov (A) and neuronal tract tracing of the BNSTov neurons was combined with CRF-fluorescent immunoreactivity (CRF-ir, A″). Sub-population of eGFP-transfected BNSTov neurons co-expresses CRF at the level of somata, dendrites, and axons (A′-A‴). BNSTov neurons send abundant projections to the ventral (subcomissural) division of the BNST, including the fusiform nucleus (BNSTfus, A) but these fibers are not CRF-ir. Double-labeled fibers (eGFP-CRF, double-arrows) from the BNSTov were found entering posterior-medial BNST (BNSTpm,) through the stria terminalis (B-B″). Double-labeled fibers and perisomatic boutons formed around putative neurons were found in the nucleus accumbens shell (NAc, C-C″). In the PVN, double-labeled fibers were found in several PVN divisions including PaAP-anterior parvicellular part (D-D″, double arrows, scale bar 10 μm).
3.2.1. Local projections of BNSTov CRF neurons within the rat BNST
As shown in Fig. 3A, eGFP-positive neurons from the rat BNSTov send abundant projections to the ventral (subcomissural) division of the BNST, including the fusiform nucleus (BNSTfus), which was previously identified as a major local projection site (20). Although we have confirmed rich eGFP-ir fibers and boutons in the rat BNSTfus, double-labeling with the CRF antibody revealed that the great majority of GFP-positive fibers in the ventral BNST were not CRF-positive, suggesting that non-CRF neurons of the BNSTov project to rat BNSTfus. However, intense somatodendritic CRF-ir in the BNSTfus might have potentially masked double-labeled boutons. In contrast, we have found double-labeled (CRF-eGFP) fibers entering rat posterior-medial BNST division (BNSTmp) through the stria terminalis (double arrows, Fig. 3B-B″).
3.2.2. CRF projections from the rat BNSTov extending rostrally
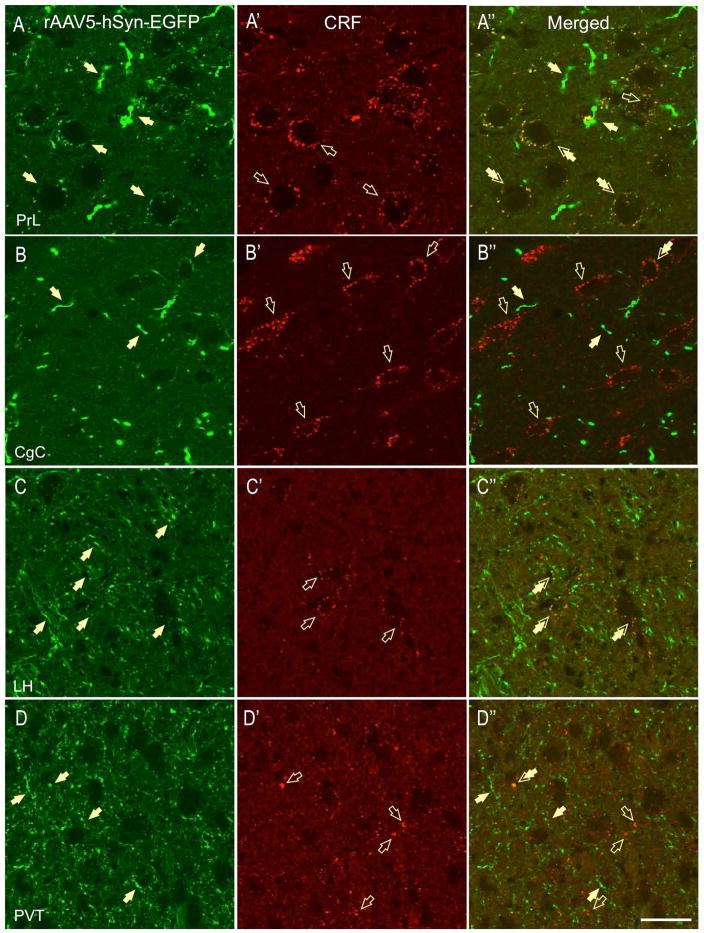
We found high expression of CRF-ir fibers and puncta in the rat nucleus accumbens shell (NAc, Fig. 3C′) and core and a moderate expression of eGFP-ir in fibers and terminals in both regions (Fig. 3C). Notably, a subset of CRF-ir fibers and puncta expressed eGFP in the NAc shell, suggesting that the rat NAc receives CRF innervation from the BNSTov. Here, the double-labeled puncta formed sparse perisomatic contacts around putative neurons and were also observed in the neuropil (Fig. 3C″). However, we also observed CRF-ir fibers and boutons that were not GFP-positive, suggesting an additional source of CRF innervation in the NAc (Fig. 3C′). In contrast, very sparse double-labeled puncta were observed in NAc core (not shown). Elsewhere, we found double-labeled (CRF-eGFP) fibers and perisomatic boutons formed around putative neurons in the prelimbic cortex (PrL, Fig. 4A-A″). In contrast, although intense CRF-ir (Fig. 4B′) and eGFP labeling (Fig. 4B) was observed in the cingulate cortex (CgC), very limited double-labeling was observed in this region (Fig. 4B-B″).
Figure 4.
Intense eGFP-CRF double-labeled perisomatic baskets were found in the prelimbic cortex (PrL, A-A″, double arrows), whereas very limited eGFP-CRF co-expression was found in the cingulate cortex (CgC, B-B″). High level of eGFP labeling (C) and moderate CRF-ir (C′) was observed in the lateral hypothalamus (LH), and moderate eGFP-CRF co-localization was found in puncta and terminal boutons (C″). In contrast, although moderate e-GFP and CRF-ir was also observed in the paraventricular thalamus (PVT), very limited co-labeling was observed in the region (D″, scale bar 10 μm).
3.2.3. CRF projections from the rat BNSTov extending caudally
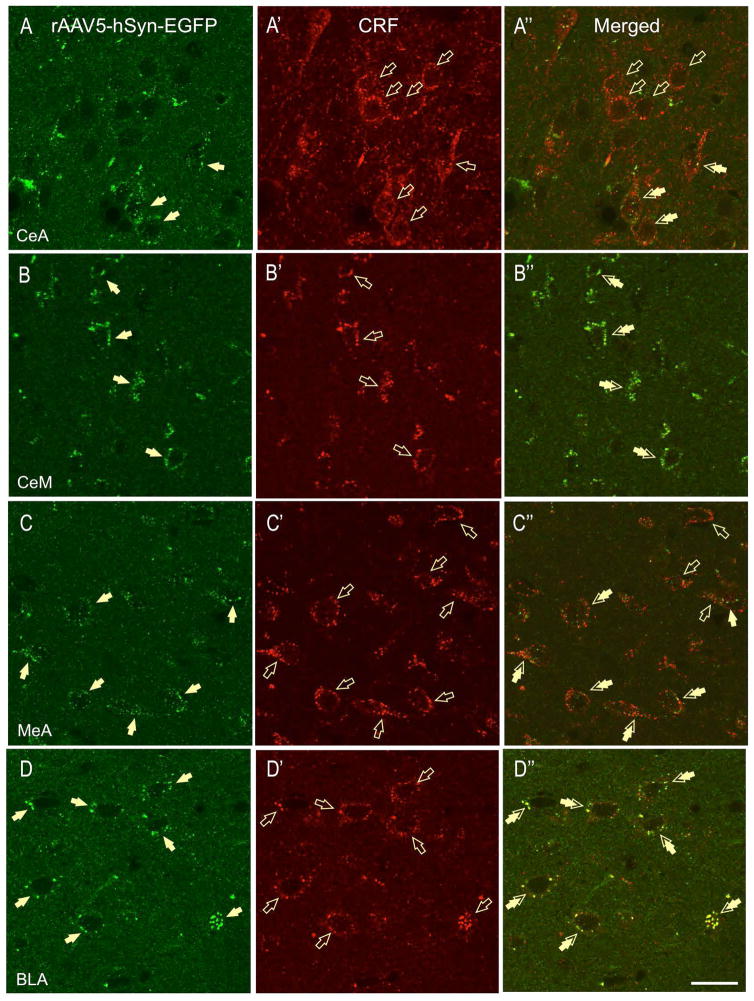
The great majority of the CRF-positive projections were found caudal to the rat BNSTov. In the PVN, we found double-labeled fibers in several PVN sub-divisions: namely the PaAP-anterior parvicellular part (Fig. 3D-D′), PaMP-medial parvicellular part, PaV-ventral part, PaPO-posterior part, and the PaLM-lateral magnocellular part. Most of the fibers did not form apparent perisomatic baskets around putative neurons, but contained multiple-beaded varicosities indicative of potential release-sites (Fig. 3D″). However, in the PaAP, and to a lesser extent in the PaLM, dual-labeled fibers were shown to make perisomatic baskets around subsets of putative neurons (Fig. 3D″). In the lateral hypothalamus (LH), we observed high level of eGFP labeling (Fig. 4C) and sparse CRF-ir (Fig. 4C′), as well as moderate eGFP-CRF co-localization on puncta and terminal boutons (Fig. 4C″). Although moderate e-GFP and CRF-ir was also observed in the paraventricular thalamus (PVT), very limited co-labeling was observed in the region (Fig. 4D-D″). Similarly, despite intense eGFP labeling, limited eGFP-CRF co-expression was observed in the lateral habenula (not shown). As expected, high CRF-ir was observed in the CeA at the level of somata, dendrites, and fibers (Fig. 5A′); but only limited CRF-ir puncta and contacts also co-expressed eGFP (Fig. 5A-A″). In contrast, we have found CRF-eGFP double-labeled puncta in medial division of CeA (CeM, Fig. 5B-B″). GFP-ir was also found in the rat medial (MeA, Fig. 5C-C″) and basolateral nucleus of the amygdala (BLA, Fig. 5D-D″). However, double-labeled perisomatic contacts were found primarily in the posterior BLA (Fig. 5D″), while the projections to the anterior BLA and the MeA were mostly non-overlapping with CRF-ir (Fig. 5C″).
Figure 5.
In the CeA (lateral part), high CRF-ir was found around putative neurons (A-A″, open arrows) and in the neuropil but limited CRF-ir boutons also co-expressed eGFP (A″, double arrows). In contrast, eGFP-CRF double-labeled puncta were found in the medial division of CeA (CeM, B-B″). High levels of CRF-ir were also found in the medial amygdala (MeA), but only sparse CRF-ir boutons also co-expressed eGFP (C-C″). In contrast, high level of CRF-eGFP co-expression was found in the posterior basolateral nucleus of the amygdala (BLA, D-D″, double arrows, scale bar 10 μm).
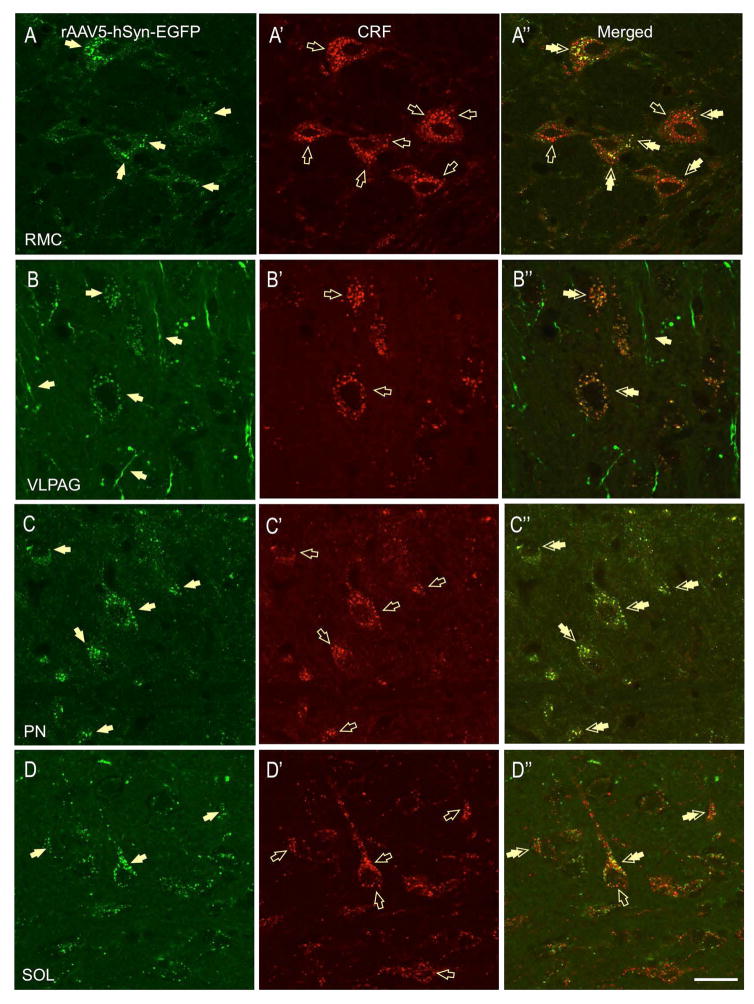
The most robust GFP-ir and CRF-ir co-expression in fibers and terminal boutons was found in midbrain sections. High level of eGFP-CRF-ir labeling was found in the red nucleus, magnocellular part (RMC, Fig. 6A-A″), a midbrain structure located dorsal to the VTA in which eGFP-CRF double-labeled puncta made baskets around putative magnocellular neurons. Interestingly, both co-labeled eGFP-CRF and single-labeled CRF-ir puncta were found on the RMC neurons (Fig. 6A″), suggesting that the same RMC neurons receive CRF input from two different sources. Similarly, in the ventrolateral periaqueductal gray (VLPAG), we found double-labeled perisomatic contacts (puncta) around subsets of putative neurons (Fig. 6B-B″), indicative of potential CRF-containing axon-terminals from the BNSTov. Limited dual-labeled fibers were also observed in the lateral PAG (not shown). Finally, high eGFP labeling was found in pontine nuclei (PN), lateral part, as well as in the nucleus of the solitary track (SOL). Although nearly complete eGFP-CRF co-expression was found in the PN (Fig. 6C-C″), only partial co-labeling was observed in the SOL (Fig. 6D-D″).
Figure 6.
In the red nucleus, magnocellular part (RMC), CRF-eGFP double-labeled puncta made baskets around putative magnocellular neurons (A-A″, double arrows). However, some putative neurons are innervated by CRF independent of GFP (A″, open arrow). In the ventrolateral periaqueductal gray (VLPAG), double-labeled (CRF-eGFP) perisomatic contacts (puncta) were found around subsets of putative neurons (B-B″), indicative of potential CRF-containing axon-terminals from the BNSTov. In the brainstem, high-intensity and nearly complete co-localization of CRF-GFP positive fibers and boutons was found around putative neurons in pontine nuclei (PN, C-C″, double-arrows). In contrast, limited double-labeled boutons were found on puncta and boutons in the nucleus of the solitary track (SOL, double arrows, D-D″, scale bar 10 μm).
In the VTA, CRF-ir was found primarily independent of GFP expression. In the rostral VTA (VTAR), despite the presence of CRF-ir, the majority of the CRF-ir fibers and puncta were not GFP-positive, suggesting that the BNSTov is not a primary source of CRF innervation in the VTAR (not shown). In contrast, in the parabrachial pigmented nucleus (PBP), a medial division of the VTA, we found limited co-labeled CRF-ir fibers and puncta (Fig. 7B″), as well intense CRF-ir puncta clearly independent of GFP-ir (Fig. 7B′). In the PBP, double-labeled puncta formed perisomatic contacts around a subset of putative neurons. In the interfascicular nucleus (Bregma −5.64), located dorsally to PBP, we found low levels of CRF-ir and very limited double-labeled fibers and puncta (not shown). Similarly, we also found sparse double-labeled puncta in the retrorubral field (RRF, DA cell A8, not shown). Notably, in the substantia nigra pars compacta (SNC), we found double-labeled perisomatic contacts (puncta) around subsets of SNC neurons (Fig. 7C-C″). Even more intense GFP-CRF double-labeling was found in the posterior substantia nigra reticulata (SNR, Fig. 7D-D″). In the DRD, we observed double-immunofluorescent puncta (perisomatic baskets) around a subset of putative neurons (Fig. 7E-E″). In the medial raphé nucleus (MRN), we also observed intense CRF-ir, but this was mostly independent of GFP expression (not shown).
Figure 7.
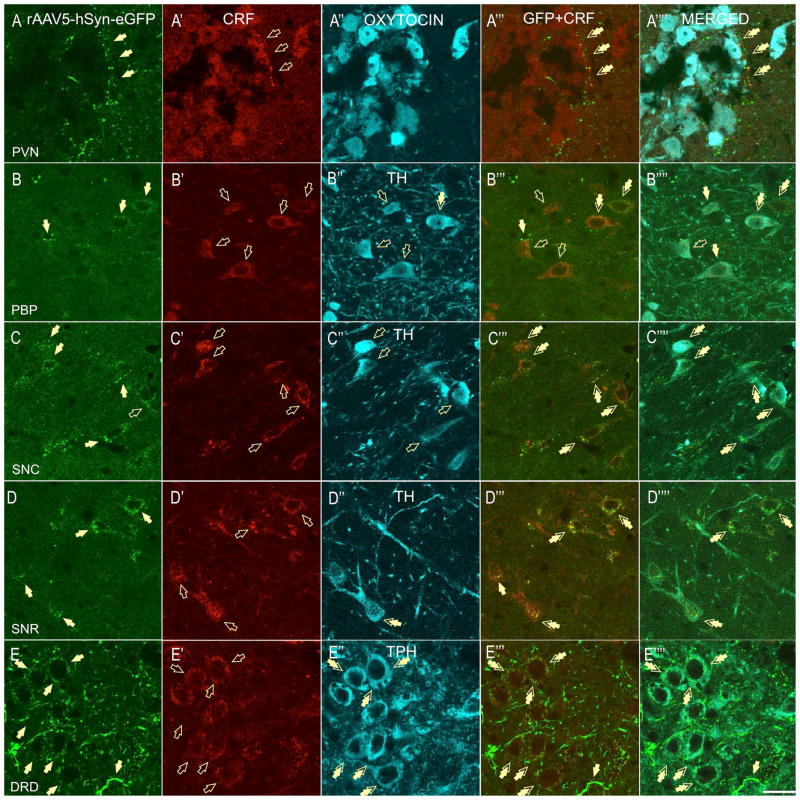
In the PVN (PaAP), eGFP-CRF-fibers (A-A″) were found in juxtaposition to local OT neurons (A-A‴prime;). Some of the OT neurons were also shown to co-express CRF (A′). In the VTA region, CRF-ir was found mostly independent of GFP in fibers and puncta. Sparse CRF-GFP terminals manifested as double-labeled puncta around cell bodies were found in the parabrachial pigmented nucleus (PBP) of the VTA, and the perisomatic contacts were found primarily with non-DA neurons, and only sporadic DA neurons (B-B‴′, TH-positive, double-arrows). In contrast, high level of CRF-GFP co-localization was found in the substantia nigra pars compacta (SNC) and double-labeled perisomatic contacts (puncta) were primarily formed around somata of DA-positive neurons (C-C‴, double-arrows). In the posterior substantia nigra reticulata (SNR), double-labeled contacts were found around both TH-negative and TH-positive, DA neurons (D-D‴, double arrows). In the dorsal raphé nucleus – dorsal division (DRD), double-immunofluorescent puncta (perisomatic baskets) were formed around a subset of 5-TPH-expressing, serotonergic neurons (E-E‴, double arrows, scale bar 10 μm).
3.3. Phenotype of neurons innervated by CRF-projections originating from BNSTov
In order to identify the neurotransmitter phenotype of neurons that receive CRF-projections from the BNSTov, we employed additional immunohistochemical staining against specific markers of local neurons in the different projection sites in which we identified double-labeled perisomatic baskets. Although previous studies have identified CRF innervation of OT neurons in the PVN (26, 27), the origin of these CRF inputs is not known. In the PVN, we found double-labeled fibers (eGFP-CRF) in juxtaposition to local OT neurons in the anterior parvicellular division (PaPA) (Fig. 7A-A‴). Interestingly, some of the parvicellular OT neurons in the PaPA that receive the BNSTov innervation were shown to also co-express CRF (Fig. 7A′).
Since we observed double-labeled puncta in a variety of midbrain sections, we used an additional marker (TH) to determine whether the CRF fibers originating from the BNSTov make appositions with populations of midbrain DA-ergic (TH-positive) neurons. In the VTA (DA cell group A10), sparse CRF-GFP terminals were observed in the PBP and were seen as double-labeled puncta around cell bodies; however these contacts were found primarily with non-DA neurons, and only occasionally with DA neurons (Fig. 7B-B‴). In contrast to the VTA, in the SNC (DA cell group A9) and SNR, double-labeled puncta were primarily observed around the somata of DA-positive neurons (Fig. 7C-C‴ and 7D-D‴). However, in the lateral division of the SNC, double-labeled puncta were primarily formed around non-DA neurons (not shown). Finally, we examined whether the intense double-labeled boutons observed in the DRD were in apposition to serotonin (5-HT) expressing neurons. Here we used an antibody against the enzyme necessary for 5-HT synthesis, namely 5-TPH. In the DRD, we observed CRF-GFP boutons primarily surrounding 5-TPH-expressing, serotonergic neurons (Fig. 7E-E‴).
4. Discussion
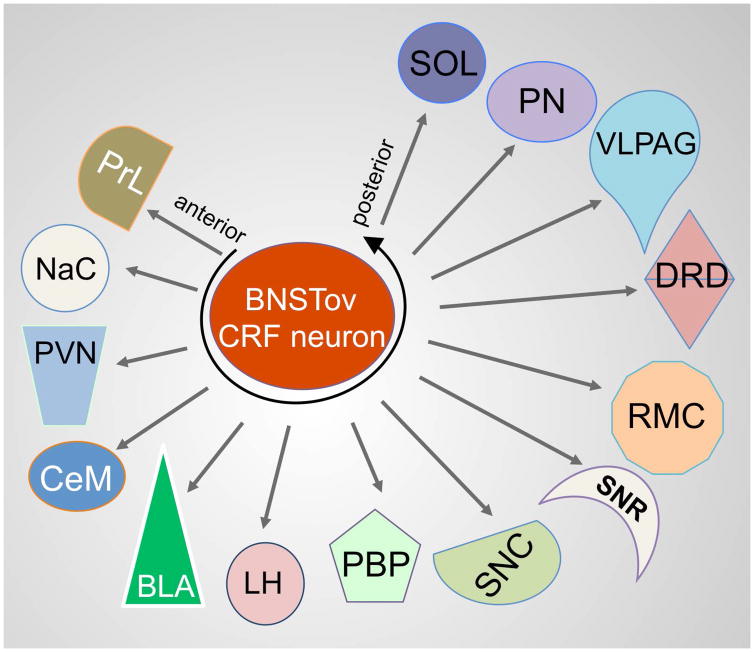
To the best of our knowledge, this is the first study describing in detail specific projections of BNSTov CRF neurons in the mouse and rat brain. Using a cell-type specific neuronal tracing approach, we have shown that CRF neurons of the BNSTov send projections to brain centers implicated in the regulation of the stress response (PVN), reward and motivation (VTA), as well as modulation of mood and affect (DRD). Furthermore, in the rat brain we found efferents of BNSTov CRF neurons in centers involved in pain processing and freezing behavior (PAG) and the regulation of motor function (RMC, SNC and PN, Fig. 8). Based on our results we propose that the CRF neurons should be considered one of the output neurons of the BNSTov.
Figure 8.
Schematic representation of the CRF projections from the BNSTov.
In the last decade, interest in the role of the BNST’s modulation of distinct features of anxiety (28) and divergent motivational states (29) has achieved circuit specificity with neuronal-specific promoters or promoters for GABA-ergic or glutamatergic markers to control BNST activity using opto- or chemogenetics. However, the BNST is a heterogeneous nucleus that contains a variety of neuropeptides (18), which are critical modulators of behavior, but often exert opposing behavioral effects (19, 28). Although these studies have undoubtedly advanced our knowledge of the BNST, to truly understand the role of its neurocircuitry in mediating anxiety-like behavior, we need to investigate peptide-specific inputs and outputs of the BNST. Specifically, although the BNSTov has one of the highest populations of CRF neurons in the rodent brain, their projection sites and the role of these neurons in regulating behavior remain largely unknown. Here, we have investigated specific projections of BNSTov CRF neurons using two independent neuronal track tracing approaches: i) a floxed-AAV-mCherry infused into the BNSTov of CRFp3.0CreGFP transgenic mice to achieve Cre-recombinase dependent expression of mCherry fluorescent protein selectively in the GFP-expressing CRF neurons of the BNSTov and ii) AAV5-hSyn-eGFP infused into the rat BNSTov combined with CRF-ir fluorescent labeling on fibers and axon terminals.
In the study by Dong et al., (20), the most abundant intrinsic projection of the BNSTov in the rat was to the ventral (subcomissural) BNST, including the BNSTfus. Although we also observed robust innervation of the BNSTfus after AAV-hSyn-eGFP injections into the rat BNSTov these projections did not co-express CRF, suggesting that other subsets of non-CRF BNSTov neurons innervate this region. However, high somatodendritic CRF-ir in the BNSTfus might have potentially masked double-labeled puncta and boutons. In agreement with (20), who demonstrated BNSTov projections to the medial CeA, we also observed CRF-positive BNSTov input to the rat CeM, and to a lesser extent to lateral CeA, suggesting that the CeA not only innervates the BNSTov (30) but also receives reciprocal CRF input from the BNSTov. In addition, we have observed double-labeled fibers entering rat posterior-medial BNST division (BNSTmp) through the stria terminalis. Interestingly, recent study on the role of the CRFR2 located in the posterior BNST has proven its critical contribution for the stress recovery (31). We also observed double-labeled perisomatic contacts around putative neurons in the posterior BLA, and to a lesser extent MeA. Previous studies have reported that the MeA expresses high levels of CRFR2, while the BLA expresses both CRFR1 and CRFR2 (10). During periods of stress, CRF is released into the BLA and local CRF receptor activation has been postulated as a substrate for stress-induced alterations in affective behavior (2).
Intriguingly, BNSTov CRF neuron innervation of the PVT was also very light considering the PVT has been reported to send dense projections to the BNSTov (32). However, as noted by (20) the densest innervation of the PVT arose from the BNSTfus. Thus, the BNST – PVT circuitry is not a simple reciprocal inhibitory feedback pathway. Glutamatergic efferents of PVT neurons are reported to make contact with CRF containing neurons in the BNST (33), and yet it is the non-CRF neurons of the BNSTov that send the densest input into the BNSTfus, which then projects back to the PVT. It is unknown at this juncture how this circuitry regulates the response to stress stimuli; however, the PVT has been shown to facilitate the hypothalamic-pituitary-adrenal (HPA) response to novel stimuli following chronic stress via the regulation of neural activity in the BNST and CeA (34).
Axonal projections originating from BNST divisions rich in CRF neurons, namely the BNSTov and BNSTfus, were previously reported to innervate neurons of the rat PVN (20). In agreement with this study, we have shown that CRF-ir fibers originating from the BNSTov innervate several subdivisions of the PVN in both the mouse and rat brain. Interestingly, CRF axon terminals were reported to make synaptic contacts with the soma and dendrites of OT-positive neurons of the rat PVN (26), but it was suggested they originate from local hypothalamic CRF sources (27). In the current study, we have shown that in the PaAP, CRF fibers originating from the rat BNSTov make perisomatic contacts with a subset of local OT neurons. Interestingly, some of the parvicellular OT neurons also co-expressed the CRF peptide, suggesting that CRF input from the BNSTov might modulate OT release from pre-autonomic neurons to the brainstem and/or CRF release to the anterior pituitary. Hence, CRF-containing fibers from the BNSTov innervate endocrine hypothalamic neurons and might, therefore, directly modulate the HPA axis activity and autonomic functions. In agreement with our results, previous studies have shown that distinct BNST nuclei differentially regulate the HPA axis activity. For example, the rat posterior BNST nuclei inhibit the HPA axis, whereas the antero-ventral BNST nuclei are involved in HPA axis activation (35). Stress-sensitive GABA-neurons projecting to the PVN have been also identified in the sub-commissural part of the anterior BNST (36). However, we believe that our report is the first to show direct CRF input from the BNSTov to the PaAP. Consistent with this observation, we have shown that OT neurons in the rat PVN express CRF receptor type 2 (CRFR2) (23), and our current results would suggest they might respond to CRF input from the BNSTov. Recently, we have demonstrated that activation of CRFR2 by its selective agonist, Urocortin 3 (stresscopin), decreased excitatory drive onto OT neurons in the PVN of male prairie voles (37). Together these data suggest that CRF innervation from the BNSTov might contribute to modulation of the OT neurons’ activity via a CRFR2-dependent mechanism. As we have shown before that CRF neurons in the BNSTov are GABA-ergic (17), terminals originating from the BNSTov might have fundamentally different function from local CRF sources in the PVN, which co-express glutamate (17). Dong and colleagues (20) also showed that the rat BNSTov sends projections to the LH through the medial forebrain bundle. Consistent with this observation, we observed eGFP-CRF-innervation of the LH. Previous studies have reported that neuronal activity of Orexin-A neurons in the LH is modulated by CRF receptor activation, and it was suggested that these neurons receive inputs from many CRF-rich neuronal fields including the PVN, CeA, and the BNST (38).
In the study of Dong and colleagues, descending BNSTov fibers were shown to course caudally toward the midbrain through the VTA and SNC (20). Here, we extend this observation to show the presence of double-labeled CRF-GFP BNSTov fibers in the mouse VTA. In rat brain, low levels of double-labeled puncta were observed in the posterior division of the VTA, including the PBP, and double-labeled terminal boutons were found primarily around non-DA neurons. These data are consistent with previous retrograde tracer studies reporting CRF-projections from the BNSTov to the VTA, where one-third of fluorogold-labeled neurons in the BNSTov were CRF-positive (39). However, the BNSTov is not the only BNST region exerting a direct influence over the VTA activity; projections have also been reported to originate from the anterodorsal and anterolateral BNST (40), but these two regions do not contain CRF neurons. Interestingly, the ventrolateral BNST, which does contains CRF neurons in the BNSTfus, has been reported to send an excitatory projection to the VTA and stimulation of this region increases the firing of VTA DA neurons (41). These data would suggest that whereas the ventral BNST directly excites DA neurons in the VTA, CRF neurons of the BNSTov primarily innervate local GABAergic interneurons in the VTA. Intriguingly, we also observed CRF-ir puncta and fibers in the rat VTA that did not originate from the BNSTov, indicating that DA neurons in the VTA receive direct innervation from other CRF sources, potentially the BNSTfus or the lateral CeA. We also observed high levels of GFP fibers in the VTA, which were not CRF-ir, suggesting that non-CRF BNSTov neurons also regulate the activity of VTA neurons. These data are consistent with the observation that the anterior BNST sends GABA/enkephalin projections to the VTA (42). In contrast to the VTA, we have shown that CRF projections from the BNSTov to the rat SNC primarily innervate DA neurons. Previous studies in the cat have also reported BNST projections to the SNC (43); here we extend this observation to show that some of these BNSTov projections contain CRF. Although the function of the projection is not well understood, significant changes in D2/D3 dopamine receptor binding have been reported in the SNC of nonhuman primates following deep brain stimulation of the BNST (44).
BNSTov CRF neurons also provide a dense input into the magnocellular part of the rat red nucleus (RMC), a caudal midbrain region implicated in a motor function. Here, rich double-labeled terminal boutons were formed around the magnocellular neurons. The RMC is primarily involved in motor control, in particular gait, and thus may be involved in the motoric component of the fight-or-flight response. In humans, however, the red nucleus has also been reported to participate in the regulation of cognitive circuits related to salience and executive control (45). Consistent with the observations of Dong and colleagues (20), we observed a subset of CRF-GFP fibers originating in BNSTov that formed rich terminal boutons in the rat VLPAG. These data are consistent with a previous retrograde tracer study that reported neuronal labeling in the BNSTov following tracer injections into the VLPAG, and that some of these neurons contained CRF (46). The VLPAG is reported to be a node in a “vigilance” circuit, and local administration of CRF into the VLPAG increases heart rate and contextual freezing (47). Hence, activation of the BNSTov – VLPAG circuit would be expected to facilitate contextual fear learning.
Double-labeled CRF-GFP boutons were also observed in the dorsal division of the DRN in both the mouse and rat brain. Moreover, these boutons appear to contact a subset of 5-HT positive neurons in rat DRN. In agreement with our observation, previous studies have reported fibers extending from the lateral BNST to the DRN (48), and overexpression of CRF in the dorsolateral BNST significantly down-regulates CRFR2 expression in the rat DRN (49). Together these data suggest that the CRF projections from the BNSTov might act via CRFR2 in the DRD to regulate affective behavior. 5-HT neurons of the DRN are activated in response to stress and high levels of CRF in the DRD mimic the effects of uncontrollable stress via activation of CRFR2 (50). Our results suggest that CRF originating from the BNSTov might mediate at least some of the behavioral effects mentioned above.
Here, we have used two technically different approaches to selectively track efferent projections of BNSTov CRF neurons in the mouse and rat brain. Although there has been some question about the selectivity of reporter expression patterns in transgenic mouse lines targeting CRF-neurons (51), the CRF projections identified in our CRFp3.0CreGFP mouse study were confirmed in the rat brain, suggesting that there is at least partial overlap in projection patterns across rodent species. From a methodological perspective, there is also the possibility that we have underestimated the density of the fibers in any given target site, and might have not captured all target sites of BNSTov CRF neurons in the rat brain, since not all CRF neurons were transfected by the AAV-hSyn-eGFP virus. However, we would emphasize that selective targeting was the main priority of our current study; therefore we have limited our analysis to fibers/terminal boutons that originated from BNSTov (expressed GFP) that also co-expressed CRF. As CRF peptide expression is largely stress-dependent and baseline CRF levels might be limited in non-stressed animals, we might have further underestimated CRF-ir in the relevant projections sites. Finally, sex differences have been reported in the responses of CRF neurons in the BNSTov to stress (52); therefore further studies are needed to establish whether comparable CRF projections from the BNSTov are observed in female rat brain.
In summary, the majority of the CRF projections from the BNSTov described herein parallel the general BNSTov projections described by Dong and colleagues (20), therefore we propose that BNSTov CRF neurons of the rat and mouse should be considered as output neurons. Consistent with this notion, we have shown that BNSTov CRF neurons may act as a key functional node to regulate distinct features of fear and anxiety-like behavior, affective behavior, autonomic function, motivational-behavior as well as executive function through their forebrain projections to the PVN, NAc, BLA, and PrL and midbrain connections to the DRD, SNC, RMC, VLPAG, PN, and to a lesser extent the VTA (Fig. 8).
Acknowledgments
This work was supported by Grant Number R00MH-096746 from the National Institute of Mental Health and start-up funds from the Chicago Medical School, Rosalind Franklin University of Medicine Science to JD, R01MH-072908 to DGR from the National Institute of Mental Health, and by the National Institutes of Health’s Office of the Director, Office of Research Infrastructure Programs, P51OD011132. The authors would also like to thank Dr. Kerry Ressler from the Department of Psychiatry, Division of Depression and Anxiety Disorders, McLean Hospital and Harvard Medical School for providing CRFp3.0CreGFP transgenic mice. We also thank Dr. Sarah Daniel for helpful comments and edits of the manuscript.
References
- 1.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 2.Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(14):3471–9. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain research. 1986;382(2):213–38. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- 4.Liang KC, Chen HC, Chen DY. Posttraining infusion of norepinephrine and corticotropin releasing factor into the bed nucleus of the stria terminalis enhanced retention in an inhibitory avoidance task. Chin J Physiol. 2001;44(1):33–43. [PubMed] [Google Scholar]
- 5.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17(23):9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. NeuroImage. 2007;37(4):1427–36. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological psychiatry. 2010;68(5):416–24. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yassa MA, Hazlett RL, Stark CE, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. Journal of psychiatric research. 2012;46(8):1045–52. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33(8):1291–308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004:44525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 11.Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1983;3(7):1355–68. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17(16):6434–46. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Molecular psychiatry. 2012 doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, Chen A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Molecular psychiatry. 2011;16(7):714–28. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- 15.Erb S, Shaham Y, Stewart J. Stress-induced relapse to drug seeking in the rat: role of the bed nucleus of the stria terminalis and amygdala. Stress. 2001;4(4):289–303. doi: 10.3109/10253890109014753. [DOI] [PubMed] [Google Scholar]
- 16.Dabrowska J, Hazra R, Guo JD, Li C, Dewitt S, Xu J, Lombroso PJ, Rainnie DG. Striatal-enriched protein tyrosine phosphatase-STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stria terminalis. Biological psychiatry. 2013;74(11):817–26. doi: 10.1016/j.biopsych.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG. Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Frontiers in neuroscience. 2013:7156. doi: 10.3389/fnins.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazra R, Guo JD, Dabrowska J, Rainnie DG. Differential distribution of neuropeptide mRNA in physiologically defined cell types in the oval subdivision of the anterolateral bed nucleus of stria terminalis. Program No 19101/UU14 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2011. 2011 Online. [Google Scholar]
- 19.Daniel SE, Rainnie DG. Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2016;41(1):103–25. doi: 10.1038/npp.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of comparative neurology. 2001;436(4):430–55. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 21.Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, Dadgar J, Kharazia V, De Guglielmo G, Crawford E, Janak PH, George O, Rice KC, Messing RO. A Transgenic Rat for Investigating the Anatomy and Function of Corticotrophin Releasing Factor Circuits. Frontiers in neuroscience. 2015:9487. doi: 10.3389/fnins.2015.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin EI, Ressler KJ, Jasnow AM, Dabrowska J, Hazra R, Rainnie DG, Nemeroff CB, Owens MJ. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biological psychiatry. 2010;67(12):1212–6. doi: 10.1016/j.biopsych.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36(9):1312–26. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Oxford, UK: Academic Press. Elsevier; 2007. [Google Scholar]
- 25.Das M, Vihlen CS, Legradi G. Hypothalamic and brainstem sources of pituitary adenylate cyclase-activating polypeptide nerve fibers innervating the hypothalamic paraventricular nucleus in the rat. The Journal of comparative neurology. 2007;500(4):761–76. doi: 10.1002/cne.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hisano S, Li S, Kagotani Y, Daikoku S. Synaptic associations between oxytocin-containing magnocellular neurons and neurons containing corticotropin-releasing factor in the rat magnocellular paraventricular nucleus. Brain research. 1992;576(2):311–8. doi: 10.1016/0006-8993(92)90695-6. [DOI] [PubMed] [Google Scholar]
- 27.Liposits Z, Paull WK, Setalo G, Vigh S. Evidence for local corticotropin releasing factor (CRF)-immunoreactive neuronal circuits in the paraventricular nucleus of the rat hypothalamus. An electron microscopic immunohistochemical analysis. Histochemistry. 1985;83(1):5–16. doi: 10.1007/BF00495294. [DOI] [PubMed] [Google Scholar]
- 28.Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219–23. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–8. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckerman MA, Van Kempen TA, Justice NJ, Milner TA, Glass MJ. Corticotropin-releasing factor in the mouse central nucleus of the amygdala: ultrastructural distribution in NMDA-NR1 receptor subunit expressing neurons as well as projection neurons to the bed nucleus of the stria terminalis. Experimental neurology. 2013:239120–32. doi: 10.1016/j.expneurol.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henckens MJ, Printz Y, Shamgar U, Dine J, Lebow M, Drori Y, Kuehne C, Kolarz A, Eder M, Deussing JM, Justice NJ, Yizhar O, Chen A. CRF receptor type 2 neurons in the posterior bed nucleus of the stria terminalis critically contribute to stress recovery. Molecular psychiatry. 2016 doi: 10.1038/mp.2016.133. [DOI] [PubMed] [Google Scholar]
- 32.Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neuroscience and biobehavioral reviews. 2015:5489–107. doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu DT, Kirouac GJ, Zubieta JK, Bhatnagar S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Frontiers in behavioral neuroscience. 2014:873. doi: 10.3389/fnbeh.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–39. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 35.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(8):2025–34. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radley JJ, Sawchenko PE. Evidence for involvement of a limbic paraventricular hypothalamic inhibitory network in hypothalamic-pituitary-adrenal axis adaptations to repeated stress. The Journal of comparative neurology. 2015;523(18):2769–87. doi: 10.1002/cne.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology. 2016 Feb;:6466–78. doi: 10.1016/j.psyneuen.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achua JK, Callahan JB, Brudvig J, Summers CH, Ronan PJ. Cross-talk between orexin/hypocretin and corticotropin releasing factor systems. Program No 7806/KK2 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2014. 2014 Online. [Google Scholar]
- 39.Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150(1):8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 40.Jalabert M, Aston-Jones G, Herzog E, Manzoni O, Georges F. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33(8):1336–46. doi: 10.1016/j.pnpbp.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(12):5173–87. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kudo T, Konno K, Uchigashima M, Yanagawa Y, Sora I, Minami M, Watanabe M. GABAergic neurons in the ventral tegmental area receive dual GABA/enkephalin-mediated inhibitory inputs from the bed nucleus of the stria terminalis. The European journal of neuroscience. 2014;39(11):1796–809. doi: 10.1111/ejn.12503. [DOI] [PubMed] [Google Scholar]
- 43.Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Experimental brain research. 1985;58(2):379–91. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- 44.Vandehey NT, Garell PC, Hampel JA, Murali D, Smith EM, Davidson R, Converse AK, Nickles RJ, Christian BT. PET measurement of changes in D2/D3 dopamine receptor binding in a nonhuman primate during chronic deep brain stimulation of the bed nucleus of the stria terminalis. Journal of neuroscience methods. 2009;176(2):129–35. doi: 10.1016/j.jneumeth.2008.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nioche C, Cabanis EA, Habas C. Functional connectivity of the human red nucleus in the brain resting state at 3T. AJNR American journal of neuroradiology. 2009;30(2):396–403. doi: 10.3174/ajnr.A1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13(3):451–60. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- 47.Carrive P, Leung P, Harris J, Paxinos G. Conditioned fear to context is associated with increased Fos expression in the caudal ventrolateral region of the midbrain periaqueductal gray. Neuroscience. 1997;78(1):165–77. doi: 10.1016/s0306-4522(97)83047-3. [DOI] [PubMed] [Google Scholar]
- 48.Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82(2):443–68. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 49.Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Molecular psychiatry. 2013;18(3):308–19. doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(3):1020–6. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y, Molet J, Gunn BG, Ressler K, Baram TZ. Diversity of Reporter Expression Patterns in Transgenic Mouse Lines Targeting Corticotropin-Releasing Hormone-Expressing Neurons. Endocrinology. 2015;156(12):4769–80. doi: 10.1210/en.2015-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Roubos EW, Peeters BW, Kozicz T. Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. Journal of neuroscience research. 2012;90(1):179–92. doi: 10.1002/jnr.22737. [DOI] [PubMed] [Google Scholar]