Abstract
The primary goal of translational research is to generate and apply knowledge that can improve human health. Although research conducted within the confines of a single discipline has helped us to achieve this goal in many settings, this uni-disciplinary approach may not be optimal when disease causation is complex and health decisions are pressing. To address these issues, we suggest that transdisciplinary approaches can facilitate the progress of translational research, and we review publications that demonstrate what these approaches can look like. These examples serve to 1) demonstrate why transdisciplinary research is useful, and 2) stimulate a conversation about how it can be further promoted. While we note that open-minded communication is a prerequisite for germinating any transdisciplinary work and that epidemiologists can play a key role in promoting it, we do not propose a rigid protocol for conducting transdisciplinary research, as one really does not exist. These achievements were developed in settings where typical disciplinary and institutional barriers were surmountable, but they were not accomplished with a single predetermined plan. The benefits of cross-disciplinary communication are hard to predict a priori and a detailed research protocol or process may impede the realization of novel and important insights. Overall, these examples demonstrate that enhanced cross-disciplinary information exchange can serve as a starting point that helps researchers frame better questions, integrate more relevant evidence, and advance translational knowledge more effectively. Specifically we discuss examples where transdisciplinary approaches are helping us to better explore, assess, and intervene to improve human health.
Keywords: Decision making, Epidemiology, Epidemiological methods, Methodology, Research methods
INTRODUCTION
The primary goal of translational biomedical research is to elucidate the determinants of disease and apply this knowledge to improve clinical or population health practices. Epidemiologists have been successful in advancing this goal, particularly in the context of conditions with causal factors that have consistently detectable marginal associations. However, in the context of etiologically heterogeneous complex disease, causal factors may not have reproducibly detectable marginal associations because these diseases have multiple interacting determinants. As a result progress in this area has been much slower. Here we take the perspective of epidemiologists and hope to generate further discussion by exploring a general approach for increasing our ability to address multifactorial health problems. Specifically, we advocate that epidemiologists take a transdisciplinary approach, and propose that enhancing the opportunity for cross-disciplinary information exchange can help by making relevant perspectives from multiple distinct fields available for scientific reasoning at each stage of the research process, but perhaps most importantly at the outset of defining a problem and designing a research strategy. This increases our chances of realizing information synergies, thereby allowing us to frame better questions, gather more comprehensive data, and better exploit existing information to guide health decisions.
This general approach addresses the key issues identified in two sets of recent commentaries concerning the future of epidemiologic research. The first group of commentaries proposes that innovative thinking will be central to progress in epidemiology and translational research (particularly in the current age of big data)1–3, and the second group posits that integrated approaches can streamline the development of effective interventions.4–7 Here we extend this discussion by exploring specific theoretical issues and examples that illustrate how cross-disciplinary information exchange has provided novel insights into disease processes, led to more complete knowledge of causation, and thereby spurred the development of more effective interventions. Thus, the overall purpose of this paper is to reveal the underappreciated utility of transdisciplinary research and fuel discussion about how it can be fostered.
The examples provided here clarify how enhanced communication between fields can cultivate creative approaches that make translational research both more effective and efficient. They also emphasize the often underappreciated advantages of teamwork.8 These examples do not argue for the development of a single pipeline for the conduct of transdisciplinary research or even that it is possible to know a priori how to design ensemble research strategies for all contexts. We also recognize that transdisciplinary research often involves difficult challenges9, and it cannot be forced. However, we propose that we can spur the development of productive transdisciplinary approaches if we create research and training environments that encourage cross-disciplinary information exchange. By extension, failing to take measures to increase our cross-disciplinary fluency will likely impede, or even prevent, the development of solutions to many human health problems. Stated differently, traditional silo-based research can only address a limited number of incomplete questions.
As the examples here demonstrate, open-minded cross-disciplinary communication can yield useful but unpredictable results. Before researchers begin talking to people from other fields (or at least start reading their papers), it will often not be clear how different disciplines may help each other address a given problem. Additionally, it will usually not be clear beforehand if the help will come in framing questions (the beginning), analytic methods (the middle), or information integration and intervention (the end). This communication could result in the cross-disciplinary transfer of a single critical piece of information or it could generate a long-term symbiotic relationship between researchers. The nature of this communication and character of its benefits are inherently unpredictable. Therefore, we will not unnecessarily constrain this process by proposing the use of rigid constructs or specific protocols.
Unfortunately, the utility of cross-disciplinary communication is frequently overlooked even though it has already produced significant advances. For example, how long would it have taken to reduce cholera transmission if John Snow had not: 1) thought beyond some existing concepts in his discipline (miasma), 2) looked for patterns in new ways (his maps) and 3) worked with relevant people from outside his immediate field (Rev. Henry Whitehead)?10 Essentially, the utilization of information from multiple disciplines throughout the research process creates a transdisciplinary approach11, 12 that can extend knowledge beyond the limitations of the contributing disciplines. Transdisciplinary research does not refer to the combination of fully formed ideas from distinct fields (multidisciplinary research), or the integration of ideas from distinct fields (interdisciplinary research), rather it refers to the generation and utilization of research frameworks and ideas that could not come from, or fit into, any one field.11, 13 This emergent property of transdisciplinary translational research can enable us to: 1) explore widely, 2) assess diversely, and 3) intervene effectively in our efforts to promote human health. These three areas provide the framework for our discussion below.
TRANSDISCIPLINARY PERSPECTIVES CAN HELP US GENERATE BETTER HYPOTHESES
A key to getting better answers is asking better questions, and transdisciplinary perspectives can generate hypotheses that uni-disciplinary perspectives might otherwise miss. If we utilize a fuller set of scientific perspectives, and tools from more than one discipline, we can frame critical questions that are not apparent from the data and tools of a single discipline. On the other hand, if we exclusively use canonical exploratory methods, we will define relatively simple questions that will likely fail to identify many of the complex phenomena that lead to health problems.
Example: Exploratory research in a single discipline often fails in the context of complex disease
“ . . . the problem of identifying and quantifying multiple component causes of disease is one of the most basic limitations in modern epidemiology”
-Paolo Vineis and David Kriebel14
In population health and medicine we often employ simple descriptive epidemiology techniques to generate hypotheses about the causes of illness and disease15 (e.g. univariate analyses from surveillance data, frequency tables, and histograms). Analytic epidemiology techniques can then be used to test these hypotheses, correcting for potential biases.15 This two-step process is foundational to epidemiology, but its effectiveness can be limited when the first step is insufficiently informative. Stated differently, if the relevant causal factors do not have detectable marginal associations then standard descriptive epidemiology techniques may not effectively direct our subsequent efforts.
Most epidemiology training focuses on analytic epidemiology and encourages the evaluation of effect modification (interaction) only if it is suspected a priori. This rule of thumb makes sense in the context of standard statistical models, because with these methods, screening for all possible interactions is at best problematic and at worst impossible. Therefore, if a critical cofactor is not suspected to be an effect modifier, it is not usually addressed in standard epidemiologic investigations. The ability of a cofactor to have important effects, however, is not contingent on our ability to suspect its role a priori. Therefore, we need to develop exploratory methods to identify putative component causes16 whose etiologic role is not evident from marginal associations. A variety of potentially useful new methods can be found in fields where techniques for analogous problems have already been developed (e.g. genetics, computer science, economics, and ecology). Such methods can advance complex disease research by expanding descriptive epidemiology beyond histograms and correlations, to include methods capable of generating novel multifactorial hypotheses.
An example of one such tool is Multifactor Dimensionality Reduction (MDR), a machine learning method that explores all possible combinations of categorical variables to identify combinations that best associate with the phenotype of interest.17, 18 This method was developed by geneticists to detect gene-gene and gene-environment interactions that are associated with a phenotype, and it has demonstrated great utility in this role.17–26 Thus, this method, perhaps in combination with other machine learning techniques27, 28, can be used as a tool to identify multifactor patterns associated with disease. For example, researchers have used MDR to identify putative gene-environment interactions in the development of lung cancer (predictive single-nucleotide-polymorphisms [SNPs] differ by smoking status)29, and childhood asthma (several SNPs interact with indoor dampness)30. Additional strategies for detecting multifactor patterns associated with disease continue to be developed in genetics31–33, and techniques such as these can extend our capacity to identify combinations of factors that are linked to disease risk.
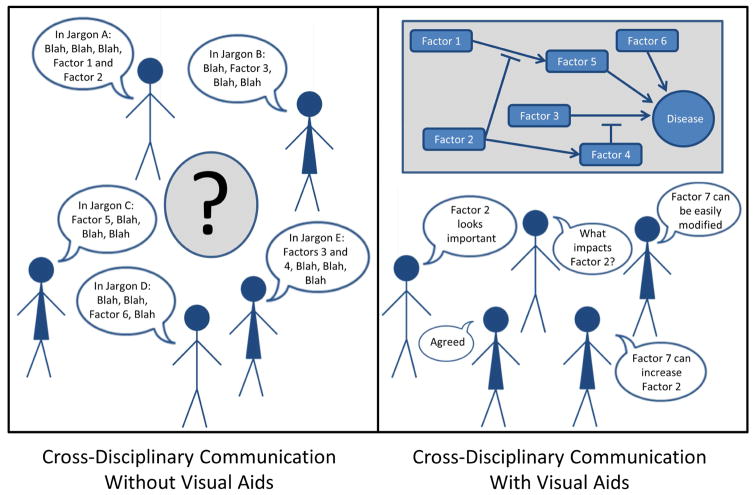
Another promising set of approaches from computational biology leverages visual data representations to help translate complex patterns into specific etiologic questions.34, 35 Visual methods may be of particular relevance in the development of transdisciplinary discovery epidemiology because they reduce jargon-based barriers to cross-disciplinary communication (Figure 1), by replacing field specific terminology with broadly-accessible visual aids. Additional non-standard computational tools, such as agent based models and other complex systems models, may be useful for learning about multifactor causes and system properties when disease risk is modulated by multiple non-linear interactions that vary temporally.36–38 Furthermore, establishing transdisciplinary teams can promote communication between diverse subject matter experts to advance the development of new complex systems models.39 These models can allow researchers to think about the relationships between putative causal factors from multiple fields in novel ways. Such methods create a unique opportunity to develop the multilevel hypotheses that are needed to address complex health problems.
Figure 1. Visualization techniques reduce jargon-based barriers to cross-disciplinary communication.
Visual information summaries can stimulate productive conversation in transdisciplinary teams by helping researchers to reason with relevant factors that are beyond their individual disciplinary expertise. In this example the putative causal factors in the etiology of a disease are summarized in a jargon-free visual schematic. Discussing this schematic allows the team to access additional relevant information from the collaborators (i.e. factor 7 may provide an intervention opportunity). In this way the team identified a previously unrecognized key modifiable factor even though no individual had enough information to think of it on their own.
Of course, thoughtful trials and discussion are required to better clarify the strengths and weaknesses of these non-traditional approaches. This process is already underway for MDR23–28 and agent based models.40–43 Because non-traditional discovery epidemiology methods (e.g. MDR) are prone to bias, as are all descriptive epidemiology methods (e.g. unadjusted associations), validation with traditional analytic epidemiology models is important. Furthermore, even analytic epidemiology approaches that properly account for known confounding and biases are limited in their ability to infer causality. Therefore, experimental, biological, and implementation research strategies will continue to be crucial for validating and characterizing the causal relationships suggested by any observed statistical associations.
We note that it is not surprising that transdisciplinary approaches can generate advances in descriptive epidemiology because in recent years techniques from other fields have enhanced the practice of analytic epidemiology. In particular, Directed Acyclic Graphs (DAGs) from computer science have advanced our ability to communicate causal structures and identify bias, thus allowing us to build better analytic epidemiology models (Table 1).44 Here we simply note that methods from other fields might help us advance descriptive epidemiology as well. Importantly, if we fail to utilize new pattern finding algorithms for discovery epidemiology, we will likely miss opportunities to identify modifiable component causes of disease.
Table 1.
A transdisciplinary advance in analytic epidemiology: Directed Acyclic Graphs
| Directed Acyclic Graphs (DAGs) adapted from computer science have: |
| 1. Helped us to better identify adjustments that introduce rather than reduce bias92 |
| 2. Provided a general analytic framework that can explain the “birthweight paradox”, and backs up our common sense notion that trials of prenatal smoking to reduce infant mortality are not a good idea93 |
TRANSDISCIPLINARY PERSPECTIVES CAN HELP US BETTER ASSESS AND INTERPRET EVIDENCE
Transdisciplinary perspectives can help us to gather and utilize more relevant and comprehensive evidence for vetting putative etiologic factors. This allows us to better address our concerns about potentially misleading findings.45 By including diverse types of data and encouraging multiple modes of assessment, transdisciplinary perspectives can rigorously evaluate the strength of evidence supporting a given hypothesis. The process of including more and diverse approaches that are encompassed by a transdisciplinary paradigm can result in a detailed understanding of the current uncertainties and thus clarify for decision makers which courses of action (or inaction) are most reasonable in light of the existing knowledge. It can also clarify for researchers what additional evidence is most needed to advance our understanding of disease etiology and potential interventions. In short, transdisciplinary approaches can improve our decision making by enhancing our ability to reason with imperfect and incomplete evidence from many sources.
Example: Transdisciplinary information allows for diverse convergent validation of findings
Genome Wide Association Studies (GWAS) are expensive endeavors and researchers would like to increase the usable knowledge gained from these studies to learn more about disease etiology and intervention options. It is becoming recognized that, when utilized in isolation, GWAS analyses have a variety of weaknesses that can hinder the discovery of genetic risk factors.46–52 Essentially GWAS, like all epidemiologic analyses, are prone to both type-1 and type-2 error, as well as the influence of unrecognized biases. However, if GWAS data is systematically evaluated in the context of relevant evidence from diverse areas, it can be part of a larger process that more effectively discovers and vets genetic risk factors for disease. For example, DiCE (Diverse Convergent Evidence) is an evidence integration process that combines information from observational association studies, bioinformatics, and laboratory experiments to yield a metric that reflects the likelihood that a given genetic factor is involved in the disease pathophysiology.52 As a proof of principle, this metric identified the role of Hemoglobin S in severe malaria resistance52–54 and the role of PPAR-gamma in type 2 diabetes52, 55–57 when standard GWAS validation criteria alone failed to detect these etiologically relevant factors. DiCE can also highlight potential false positive findings in GWAS analyses, including those that reach canonical thresholds for statistical significance, and suggest future research to address the ambiguous evidence. Overall, DiCE allows more diverse evidence to enter the process of determining what leads to follow and how to follow them. This promotes well informed decisions and faster knowledge acquisition.
Example: Transdisciplinary approaches allow us to work with disparate inconclusive evidence
How can we even begin to ameliorate a problem with as many potential causes as the obesity epidemic? Again, a broad perspective and systematic information integration can be useful. In 2010 the Institute of Medicine developed a framework to promote this type of translational approach for combatting obesity: the IOM L.E.A.D. framework.58 And recently Chatterji et al discussed the application of this type of approach in New York City’s policy decisions regarding fat and calorie information for restaurant food.59 In this framework researchers: A) Locate Evidence, B) Evaluate It, C) Assemble It, and D) Inform Decisions. The structure for this translation process reflects our need to make policy decisions when there are many disparate pieces of relevant but inconclusive evidence. Imperfect evidence may only provide decipherable guidance in our research and intervention decisions, if it is considered in its totality. Narrow assessments of the evidence from one field could prove misleading or just plain false. Thus, the structure and purpose of L.E.A.D. is analogous to DiCE, although it is more directly focused on implementation.
Example: Transdisciplinary teams can facilitate information synthesis and decision making
Physicians need to quickly learn what the research evidence suggests should be done for their patients. If that voluminous information is not comprehensively and cogently distilled in an ethical manner, then physicians cannot effectively use it to guide patient care decisions. For over 20 years the non-profit Cochrane Collaboration has been using a network of diverse working groups to synthesize medical research information and increase its utility in decision making.60 By promoting input from a broad array of sources, the available evidence and its quality have been considered and organized to address the types of questions that physicians and patients ask. For example this approach has translated the complex literature on vitamin C and the common cold into actionable information (Table 2).
Table 2.
Cochrane Reviews94 can convert literature that is unwieldy and inaccessible into evidence that is widely accessible and relevant to decision makersa
| Many doctors and patients may ask: Can vitamin C supplements prevent or treat the common cold (viral respiratory infections)? | |||
|---|---|---|---|
| Potential utility | Unclear utility | Feasibility | Safety |
| Strong evidence suggests that regular vitamin C supplementation can reduce the duration and severity of common colds that occur | The evidence is inconclusive as to whether vitamin C can prevent the common cold or reduce symptoms if it is started after cold onset | Inexpensive | Thought to be without adverse effects. |
Information extracted from a scientific abstract and plain language summary that are freely available (in several languages) at the Cochrane website.95
Example: Transdisciplinary approaches can clarify research and intervention priorities
How can we properly allocate limited research, remediation, and policy efforts to the environmental chemicals that pose the greatest risk to human health? This situation represents another instance where there is incomplete and non-definitive information and a need to advance knowledge quickly to minimize human health problems. One effective strategy can be found in the IARC monographs.61 In this approach multidisciplinary IARC working groups are assembled to discuss four aspects of a given exposure: 1) the potential for human exposure, 2) the evidence for association with cancer in humans, 3) the evidence for causation of cancer in animals, and 4) relevant mechanistic/toxicokinetic evidence. This broad scope of information is then converted by a cross-disciplinary consensus building process into a carcinogenicity assessment (e.g. probably not carcinogenic, not classifiable, possibly carcinogenic, probably carcinogenic, and carcinogenic). Recently, the National Toxicology Program (NTP) developed a similar general strategy for integrating human, animal, and in-vitro evidence in chemical assessments.62 The NTP also makes relevant evidence available for alternative integration analyses by compiling it into publically accessible databases (e.g. CEBS, DrugMatrix, and ToxFX).63 Overall, these transdisciplinary approaches can highlight the largest potential problems based on the available evidence and simply convey this information to both researchers and decision makers.
These examples indicate that more comprehensive and integrated information can allow for improved validation of potential risk factors and enhanced characterization of health problems. The long-standing transdisciplinary approaches (e.g. IARC monographs and Cochrane Collaboration) provide evidence that these strategies are very useful and the newer techniques (e.g. DiCE, LEAD, ToxFX) demonstrate that these approaches can be further optimized for efficiency.
TRANSDISCIPLINARY PERSPECTIVES CAN HELP US DEVELOP AND COORDINATE INTERVENTIONS
Discipline-specific strategies can limit our ability to develop policies and interventions that improve human health. For example, imagine our goal was to fill a barrel with water and keep it filled. If the barrel has a hole in it, how would we best coordinate the efforts of a cooper with that of a person getting water from a well? No matter how hard each individual works, their efforts will be inefficient or even ineffective unless they are applied in the right order. The development of effective interventions can suffer from similar issues.
Example: Interventions can be more effective when etiologic characterization is transdisciplinary
Chemical exposures, social exposures (neighborhood and family interaction styles), genetics, educational strategies, and nutrients can individually be evaluated for their association with neurodevelopmental outcomes. These variables may be studied separately by environmental epidemiologists, social epidemiologists, geneticists, developmental pediatricians, neuropsychologists, and nutritional epidemiologists, but if they work in isolation, information about how to most effectively intervene is likely to be obscured. How can you characterize the relationship between lead exposure and adverse neurodevelopmental outcomes without considering how psychosocial factors may generate potential confounding and other biases?64 Also, what good is an educational intervention if the child is still exposed to lead because the home is not properly remediated or the source of exposure remains unidentified? Educational, social, medical, and environmental interventions can fail or they can be synergistic. Understanding the relationships between component causes from a variety of traditionally separate fields can clarify the overall public health problem and intervention possibilities, and this principle has become a driving factor behind the emerging concept of “exposome” research.65, 66
Example: Moving from genes or environment, past genes and environment, to genes with environment
Studying genetic and environmental factors together can help us to avoid missing causal factors.67 The effect of each may depend on the context defined by the other, and thus some causal factors may not be detected by looking only at their marginal associations (note that here we are referring to environmental factors in the broadest sense: xenobiotic exposures, social/psychosocial factors, nutrition, etc.). Even when a marginal association is detectable, broader consideration of genetic and environmental variables taken together can illuminate the mechanisms that create this marginal association, and provide information about etiologic subtypes that may benefit from distinct interventions.68, 69 Furthermore, finding an isolated genetic cause of disease may not suggest obvious interventions but if a genetic factor is found to interact with a modifiable environmental factor, then knowledge of the environmental factor can create a prevention or treatment opportunity. For example: children with genetic disruptions of phenylalanine hydroxylase function (phenylketonuria) can avoid many adverse health consequences by eating phenylalanine-limited diets that would not be optimal for other children.70–72
Example: Diverse perspectives allow us to handle etiologies that change in response to intervention
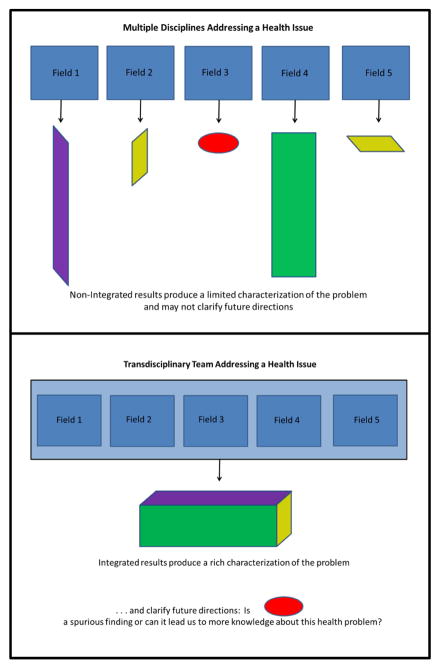
How can we design interventions that promote stable positive changes in complex dynamic systems when the effect of the same action may vary temporally? RCTs and experiments are ideal for learning about single factors in systems that you can randomize and control, but are less useful when multiple dynamic non-linear interactions are modulating disease risk. Some of the computational tools mentioned above (e.g. agent based modeling) may be useful for learning about how complex systems react to interventions, and thus they may also be useful for developing strategies that have consistently positive impacts.36, 37 These methods can allow us to ask important novel questions. Would a combination of interventions work well? Do certain policies only have a high probability of success in specific contexts? Can multifactorial interventions or contingency algorithms generate better outcomes in these settings? Along these lines the Cancer Intervention and Surveillance Modeling Network (CISNET)73 has developed a simulation process that leverages clear modeling assumptions and comparison of results from multiple simulations to acquire convergent evidence that highlights putative causal factors. Importantly, whatever is learned from complex intervention simulations and the careful observation of new interventions, can be fed back into an evolving knowledge base for guiding future research and interventions.74, 75 Overall, broad transdisciplinary approaches are essential to better coordinate both our knowledge and efforts (Figure 2).
Figure 2. Transdisciplinary approaches coordinate evidence to generate more useful knowledge.
Cross-disciplinary cooperation allows us to see how information from multiple sources can fit together to build our understanding of health issues. “Science is built up with facts, as a house is with stones. But a collection of facts is no more a science than a heap of stones is a house.” - Jules Henri Poincare 98, p. 127
EPIDEMIOLOGISTS CAN PLAY A KEY ROLE IN ADVANCING TRANSDISCIPLINARY APPROACHES
Epidemiologists are well positioned to facilitate transdisciplinary translational research74, 76 because good epidemiology training provides a familiarity with a broad range of causal factors, and makes practitioners aware of the “big picture”. In fact, many epidemiologists are already at the forefront when it comes to advancing transdisciplinary research, and a variety of transdisciplines that depend on study design and analytic principles from epidemiology have already emerged. For example, epidemiologists are working with social scientists to understand the social determinants of health (social epidemiology)77, and epidemiologists are cooperating with toxicologists to identify chemical etiologic factors (environmental epidemiology).78 These fields have even been further combined to allow transdisciplinary insights to flow from the consideration of social, ecological, and biological factors in infectious disease epidemiology.79, 80 These are just a few examples but we emphasize that epidemiology continues to spur synergy in new ways. Among the newest epidemiology-based transdisciplines are epigenetic epidemiology81–83 and molecular pathological epidemiology84, 85. These transdisciplines are demonstrating that when molecular biologists, pathologists, and epidemiologists collaborate, they can evaluate molecular factors in new ways that permit the identification of etiologic subgroups and the physiologic mechanisms of disease.
As a group, epidemiologists can further advance this approach by creating working environments that are more open to (and capable of) cross-disciplinary conversation at all stages of research. This allows for better integration and application of existing relevant information that can lead to more complete and useful knowledge86, and also promotes the more efficient acquisition of new relevant information. In Table 3 we list specific feasible strategies that can stimulate transdisciplinary thinking and create opportunities for intellectual crosspollination through better channels of communication.
Table 3.
Simple Ways that Epidemiologists Can Promote Transdisciplinary Translational Researcha
| A. Incorporate more non-epidemiology concepts and knowledge into epidemiology training |
|
| B. Diversify traditional epidemiology working environments |
|
It is possible that structural changes in research institutions and funding sources might further promote transdisciplinary thinking and the success of team science oriented researchers, but the small steps listed here are achievable in the near term and capable of informing potential next steps. Additionally, the epidemiologists that experiment with these small steps could serve as key resources in the development of large-scale transdisciplinary efforts such as the NIH’s Clinical and Translational Science Awards program.97 Beyond exploring the specific actions proposed here, the most important thing that we all can do to contribute to the conduct of effective transdisciplinary research is to “hold our knowledge lightly” 86 and promote a culture of open-mindedness. This receptive yet objective perspective is the oil for transdisciplinary engines. Essentially, it allows for discussions that illuminate crucial information from many disciplines to generate sound and comprehensive reasoning.
CONCLUSION
In this paper we cite examples which demonstrate that transdisciplinary approaches can cultivate and vet useful new strategies for dealing with complex health challenges. These ensemble science methods can develop whenever we make tangible efforts to improve cross-disciplinary information exchange, and they allow us to streamline the development of effective health interventions. Transdisciplinary approaches can be as sophisticated as an international team of specialists working together in a coordinated fashion, or they can be as simple as talking more often with people who have distinct training. The examples presented here are not intended to provide a blueprint for conducting transdisciplinary work. Instead they serve to 1) demonstrate what transdisciplinary insights can look like and 2) show that these insights can advance translational research. Overall, we have observed that being open to cross-disciplinary information exchange is the defining feature of transdisciplinary translational research, and it has tripartite utility. It helps us to explore widely, assess diversely, and intervene effectively in complex systems.
This review does not suggest that transdisciplinary collaboration is a panacea for health research challenges. However, it does suggest that transdisciplinary collaboration can help in some situations, and failing to enhance cross-disciplinary communication and subsequent research approaches may slow down our progress. That said, many barriers to the conduct of true transdisciplinary/team science are ingrained in our research infrastructure, including promotion criteria, funding, training strategies, and field specific jargon13, 87–91, and these barriers must be overcome if we are to realize the benefits of transdisciplinary research. Importantly, the limitations of uni-disciplinary research may not always be apparent, but if we utilize transdisciplinary approaches, those shortcomings are clarified and our ability to improve human health is enhanced. We cannot answer questions that we do not ask and we cannot guide our actions with evidence that we do not assess, but in these tasks transdisciplinary perspectives can expand the capabilities of epidemiologists and the translational research community.
Acknowledgments
Funding: This work was supported by the National institute of Mental Health at the National Institutes of Health (NIH-NIMH R01MH094609; UO1ES019457); the National Institute of Environmental Health Sciences at the National Institutes of Health (NIH-NIEHS R01ES022223); March of Dimes Ohio Collaborative for the Prevention of Preterm Birth, the National Institutes of Health (NIH P20 GM103534), and the National Cancer Institute at the National Institutes of Health (K07 CA172294) to [MCA]. The funders had no involvement in the writing of the manuscript or the decision to submit it for publication. All authors have read the journal’s policy on conflict of interest disclosure, and have read the journal’s authorship agreement.
Abbreviations
- SNP
Single Nucleotide Polymorphism
- MDR
Multifactor Dimensionality Reduction
- DAG
Directed Acyclic Graph
- GWAS
Genome Wide Association Study
- DiCE
Diverse Convergent Evidence
- L.E.A.D.
Locate Evidence, Evaluate It, Assemble It, and Inform Decisions
- IARC
International Agency for Research on Cancer
- NTP
National Toxicology Program
- CEBS
Chemical Effects in Biological Systems
- IOM
Institute of Medicine
- CISNET
Cancer Intervention and Surveillance Modeling Network
- CSTA
Clinical and Translational Science Awards program
Glossary
- Multidisciplinary
The aggregation of fully formed ideas that come from distinct fields
- Interdisciplinary
The integration, adaptation, and harmonization of ideas that come from distinct fields
- Transdisciplinary
The generation and utilization of research frameworks and admixed ideas that could not come from, or fit into, any one field
- Cross-Disciplinary
A general term referring to the unspecified involvement of more than one discipline
- Perspective
Intellectual orientation or viewpoint that can vary in its capacity to assess and adapt to external input
- Strategy, Approach, Process, or Method
A general code of conduct or way of proceeding that does not have a rigid, pre-specified, or detailed sequence or parameters
- Protocol or Procedure
A specific code of conduct or way of proceeding that has a rigid, pre-specified, and detailed sequence and parameters
- Communication
A general term referring to the exchange of information, strategies, protocols, hypotheses, or ideas (through talking, reading, graphical image presentation, etc.)
- Information
Data and facts
- Knowledge
Understanding of the relevant causal mechanisms that generated the data and facts (note that information and knowledge have similar meanings and are often used to define each other, however here we emphasize that knowledge implies an understanding of why the data or facts are as they are)
- Complex Systems
Systems with multiple interacting components and emergent properties that often cannot be accurately characterized with narrow or rigid research frameworks
- Marginal Association
The association between one exposure (factor) and one outcome (disease) independent of other variables. If potential biases and other observational data imperfections are properly accounted for, this association is thought to provide evidence for or against the involvement of the exposure with the disease.
Footnotes
Competing interests
None Declared
Contributors
TC proposed the idea of writing an article providing tangible examples of innovative methods in epidemiology. The general format of the piece was determined in a conference call with all authors. TC drafted the manuscript and all authors provided key feedback and input (RH, MA, CM, SW). SW is the senior author and provided substantial editorial input.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hiatt RA, Sulsky S, Aldrich MC, et al. Promoting innovation and creativity in epidemiology for the 21st century. Annals of epidemiology. 2013 Jul;23(7):452–4. doi: 10.1016/j.annepidem.2013.05.007. Epub 2013/06/26. eng. [DOI] [PubMed] [Google Scholar]
- 2.McKeown RE. Is epidemiology correcting its vision problem? A perspective on our perspective: 2012 presidential address for American College of Epidemiology. Annals of epidemiology. 2013 Oct;23(10):603–7. doi: 10.1016/j.annepidem.2013.07.016. Epub 2013/08/27. eng. [DOI] [PubMed] [Google Scholar]
- 3.Ness RB. Tools for innovative thinking in epidemiology. American journal of epidemiology. 2012 Apr 15;175(8):733–8. doi: 10.1093/aje/kwr412. Epub 2012/03/20. eng. [DOI] [PubMed] [Google Scholar]
- 4.Is Integrative Cancer Epidemiology the Next-Gen of Epidemiology? [6/27/2014];Epidemiology and Genomics Research Program of the National Cancer Institute's Division of Cancer Control and Population Sciences. 2014 Available from: http://blog-epi.grants.cancer.gov/2013/08/13/integrative-cancer524epidemiology/
- 5.Khoury MJ, Lam TK, Ioannidis JP, et al. Transforming epidemiology for 21st century medicine and public health. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013 Apr;22(4):508–16. doi: 10.1158/1055-9965.EPI-13-0146. Epub 2013/03/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spitz MR, Caporaso NE, Sellers TA. Integrative cancer epidemiology--the next generation. Cancer discovery. 2012 Dec;2(12):1087–90. doi: 10.1158/2159-8290.CD-12-0424. Epub 2012/12/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam TK, Spitz M, Schully SD, et al. "Drivers" of translational cancer epidemiology in the 21st century: needs and opportunities. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013 Feb;22(2):181–8. doi: 10.1158/1055-9965.EPI-12-1262. Epub 2013/01/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wuchty S, Jones BF, Uzzi B. The increasing dominance of teams in production of knowledge. Science (New York, NY) 2007 May 18;316(5827):1036–9. doi: 10.1126/science.1136099. Epub 2007/04/14. eng. [DOI] [PubMed] [Google Scholar]
- 9.Lynch J. It's not easy being interdisciplinary. International journal of epidemiology. 2006 Oct;35(5):1119–22. doi: 10.1093/ije/dyl200. Epub 2006/09/22. eng. [DOI] [PubMed] [Google Scholar]
- 10.Frerichs RR. [6/23/2014];John Snow. 2014 Available from: http://www.ph.ucla.edu/epi/snow.html#PUMP%20HANDLE.
- 11.Rosenfield PL. The potential of transdisciplinary research for sustaining and extending linkages between the health and social sciences. Social science & medicine (1982) 1992 Dec;35(11):1343–57. doi: 10.1016/0277-9536(92)90038-r. Epub 1992/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 12.Stokols D, Hall KL, Taylor BK, et al. The science of team science: overview of the field and introduction to the supplement. American journal of preventive medicine. 2008 Aug;35(2 Suppl):S77–89. doi: 10.1016/j.amepre.2008.05.002. Epub 2008/08/23. eng. [DOI] [PubMed] [Google Scholar]
- 13.Hall KL, Vogel AL, Stipelman B, et al. A Four-Phase Model of Transdisciplinary Team-Based Research: Goals, Team Processes, and Strategies. Translational behavioral medicine. 2012 Dec 1;2(4):415–30. doi: 10.1007/s13142-012-0167-y. Epub 2013/03/14. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vineis P, Kriebel D. Causal models in epidemiology: past inheritance and genetic future. Environmental health : a global access science source. 2006;5:21. doi: 10.1186/1476-069X-5-21. Epub 2006/07/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennekens CH, Buring JE. Chapter 2 Design Strategies in Epidemiologic Research. In: Mayrent SL, editor. Epidemiology in Medicine. Vol. 1. Philadelphia, PA: Lippincott Williams & Wilkins; 1987. pp. 16–29. [Google Scholar]
- 16.Rothman KJ. Causes. American journal of epidemiology. 1976 Dec;104(6):587–92. doi: 10.1093/oxfordjournals.aje.a112335. Epub 1976/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie MD, Hahn LW, Roodi N, et al. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. American journal of human genetics. 2001 Jul;69(1):138–47. doi: 10.1086/321276. Epub 2001/06/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore JH. [6/25/2014];2014 Available from: http://www.epistasis.org/index-3.html.
- 19.Moore JH. Computational analysis of gene-gene interactions using multifactor dimensionality reduction. Expert review of molecular diagnostics. 2004 Nov;4(6):795–803. doi: 10.1586/14737159.4.6.795. Epub 2004/11/05. eng. [DOI] [PubMed] [Google Scholar]
- 20.Park HW, Shin ES, Lee JE, et al. Multilocus analysis of atopy in Korean children using multifactor-dimensionality reduction. Thorax. 2007 Mar;62(3):265–9. doi: 10.1136/thx.2006.065482. Epub 2006/11/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins RL, Hu T, Wejse C, et al. Multifactor dimensionality reduction reveals a three-locus epistatic interaction associated with susceptibility to pulmonary tuberculosis. BioData mining. 2013;6(1):4. doi: 10.1186/1756-0381-6-4. Epub 2013/02/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afzal S, Gusella M, Jensen SA, et al. The association of polymorphisms in 5-fluorouracil metabolism genes with outcome in adjuvant treatment of colorectal cancer. Pharmacogenomics. 2011 Sep;12(9):1257–67. doi: 10.2217/pgs.11.83. Epub 2011/09/17. eng. [DOI] [PubMed] [Google Scholar]
- 23.Bastone L, Reilly M, Rader DJ, et al. MDR and PRP: a comparison of methods for high-order genotype-phenotype associations. Human heredity. 2004;58(2):82–92. doi: 10.1159/000083029. Epub 2005/02/16. eng. [DOI] [PubMed] [Google Scholar]
- 24.Ritchie MD, Hahn LW, Moore JH. Power of multifactor dimensionality reduction for detecting gene-gene interactions in the presence of genotyping error, missing data, phenocopy, and genetic heterogeneity. Genetic epidemiology. 2003 Feb;24(2):150–7. doi: 10.1002/gepi.10218. Epub 2003/01/28. eng. [DOI] [PubMed] [Google Scholar]
- 25.Gui J, Moore JH, Williams SM, et al. A Simple and Computationally Efficient Approach to Multifactor Dimensionality Reduction Analysis of Gene-Gene Interactions for Quantitative Traits. PloS one. 2013;8(6):e66545. doi: 10.1371/journal.pone.0066545. Epub 2013/06/28. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lou XY, Chen GB, Yan L, et al. A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. American journal of human genetics. 2007 Jun;80(6):1125–37. doi: 10.1086/518312. Epub 2007/05/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidema AG, Feskens EJ, Doevendans PA, et al. Analysis of multiple SNPs in genetic association studies: comparison of three multi-locus methods to prioritize and select SNPs. Genetic epidemiology. 2007 Dec;31(8):910–21. doi: 10.1002/gepi.20251. Epub 2007/07/07. eng. [DOI] [PubMed] [Google Scholar]
- 28.Andrew AS, Karagas MR, Nelson HH, et al. DNA repair polymorphisms modify bladder cancer risk: a multi-factor analytic strategy. Human heredity. 2008;65(2):105–18. doi: 10.1159/000108942. Epub 2007/09/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ihsan R, Chauhan PS, Mishra AK, et al. Multiple analytical approaches reveal distinct gene624 environment interactions in smokers and non smokers in lung cancer. PloS one. 2011;6(12):e29431. doi: 10.1371/journal.pone.0029431. Epub 2011/12/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su MW, Tung KY, Liang PH, et al. Gene-gene and gene-environmental interactions of childhood asthma: a multifactor dimension reduction approach. PloS one. 2012;7(2):e30694. doi: 10.1371/journal.pone.0030694. Epub 2012/02/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu T, Sinnott-Armstrong NA, Kiralis JW, et al. Characterizing genetic interactions in human disease association studies using statistical epistasis networks. BMC bioinformatics. 2011;12:364. doi: 10.1186/1471-2105-12-364. Epub 2011/09/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu T, Chen Y, Kiralis JW, et al. An information-gain approach to detecting three-way epistatic interactions in genetic association studies. Journal of the American Medical Informatics Association : JAMIA. 2013 Jul-Aug;20(4):630–6. doi: 10.1136/amiajnl-2012-001525. Epub 2013/02/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee J, Kwon MS, Park T, et al. A modified entropy-based approach for identifying gene-gene interactions in case-control study. PloS one. 2013;8(7):e69321. doi: 10.1371/journal.pone.0069321. Epub 2013/07/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore JH, Lari RC, Hill D, et al. Human microbiome visualization using 3D technology. Pacific Symposium on Biocomputing Pacific Symposium on Biocomputing. 2011:154–64. doi: 10.1142/9789814335058_0017. Epub 2010/12/02. eng. [DOI] [PubMed] [Google Scholar]
- 35.Hu T, Chen Y, Kiralis JW, et al. ViSEN: methodology and software for visualization of statistical epistasis networks. Genetic epidemiology. 2013 Apr;37(3):283–5. doi: 10.1002/gepi.21718. Epub 2013/03/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galea S, Riddle M, Kaplan GA. Causal thinking and complex system approaches in epidemiology. International journal of epidemiology. 2010 Feb;39(1):97–106. doi: 10.1093/ije/dyp296. Epub 2009/10/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auchincloss AH, Diez Roux AV. A new tool for epidemiology: the usefulness of dynamic-agent models in understanding place effects on health. American journal of epidemiology. 2008 Jul 1;168(1):1–8. doi: 10.1093/aje/kwn118. Epub 2008/05/16. eng. [DOI] [PubMed] [Google Scholar]
- 38.Diez Roux AV. Integrating social and biologic factors in health research: a systems view. Annals of epidemiology. 2007 Jul;17(7):569–74. doi: 10.1016/j.annepidem.2007.03.001. Epub 2007/06/08. eng. [DOI] [PubMed] [Google Scholar]
- 39.Hiatt RA, Porco TC, Liu F, et al. A multi-level model of postmenopausal breast cancer incidence. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014 Jul 13; doi: 10.1158/1055-9965.EPI-14-0403. Epub 2014/07/16. Eng. [DOI] [PubMed] [Google Scholar]
- 40.Marshall BD, Galea S. Formalizing the Role of Agent-Based Modeling in Causal Inference and Epidemiology. American journal of epidemiology. 2014 Dec 5; doi: 10.1093/aje/kwu274. Epub 2014/12/07. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernan MA. Invited Commentary: Agent-Based Models for Causal Inference-Reweighting Data and Theory in Epidemiology. American journal of epidemiology. 2014 Dec 5; doi: 10.1093/aje/kwu272. Epub 2014/12/07. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diez Roux AV. Invited Commentary: The Virtual Epidemiologist-Promise and Peril. American journal of epidemiology. 2014 Dec 5; doi: 10.1093/aje/kwu270. Epub 2014/12/07. Eng. [DOI] [PubMed] [Google Scholar]
- 43.Marshall BD, Galea S. Marshall and Galea Respond to "Data Theory in Epidemiology". American journal of epidemiology. 2014 Dec 5; doi: 10.1093/aje/kwu273. Epub 2014/12/07. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass) 1999 Jan;10(1):37–48. Epub 1999/01/15. eng. [PubMed] [Google Scholar]
- 45.Khoury MJ, Ioannidis JP. Medicine. Big data meets public health. Science (New York, NY) 2014 Nov 28;346(6213):1054–5. doi: 10.1126/science.aaa2709. Epub 2014/11/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraft P. Curses--winner's and otherwise--in genetic epidemiology. Epidemiology (Cambridge, Mass) 2008 Sep;19(5):649–51. doi: 10.1097/EDE.0b013e318181b865. discussion 57-8. Epub 2008/08/16. eng. [DOI] [PubMed] [Google Scholar]
- 47.Kraft P, Zeggini E, Ioannidis JP. Replication in genome-wide association studies. Statistical science : a review journal of the Institute of Mathematical Statistics. 2009 Nov 1;24(4):561–73. doi: 10.1214/09-STS290. Epub 2010/05/11. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vineis P, Brennan P, Canzian F, et al. Expectations and challenges stemming from genome-wide association studies. Mutagenesis. 2008 Nov;23(6):439–44. doi: 10.1093/mutage/gen042. Epub 2008/09/04. eng. [DOI] [PubMed] [Google Scholar]
- 49.Williams SM, Canter JA, Crawford DC, et al. Problems with genome-wide association studies. Science (New York, NY) 2007 Jun 29;316(5833):1840–2. Epub 2007/07/03. eng. [PubMed] [Google Scholar]
- 50.Greene CS, Penrod NM, Williams SM, et al. Failure to replicate a genetic association may provide important clues about genetic architecture. PloS one. 2009;4(6):e5639. doi: 10.1371/journal.pone.0005639. Epub 2009/06/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams SM, Haines JL. Correcting away the hidden heritability. Annals of human genetics. 2011 May;75(3):348–50. doi: 10.1111/j.1469-1809.2011.00640.x. Epub 2011/04/15. eng. [DOI] [PubMed] [Google Scholar]
- 52.Ciesielski TH, Pendergrass SA, White MJ, et al. Diverse convergent evidence in the genetic analysis of complex disease: coordinating omic, informatic, and experimental evidence to better identify and validate risk factors. BioData mining. 2014;7:10. doi: 10.1186/1756-0381-7-10. Epub 2014/07/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jallow M, Teo YY, Small KS, et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nature genetics. 2009 Jun;41(6):657–65. doi: 10.1038/ng.388. Epub 2009/05/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Timmann C, Thye T, Vens M, et al. Genome-wide association study indicates two novel resistance loci for severe malaria. Nature. 2012 Sep 20;489(7416):443–6. doi: 10.1038/nature11334. Epub 2012/08/17. eng. [DOI] [PubMed] [Google Scholar]
- 55.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science (New York, NY) 2007 Jun 1;316(5829):1331–6. doi: 10.1126/science.1142358. Epub 2007/04/28. eng. [DOI] [PubMed] [Google Scholar]
- 56.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science (New York, NY) 2007 Jun 1;316(5829):1341–5. doi: 10.1126/science.1142382. Epub 2007/04/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science (New York, NY) 2007 Jun 1;316(5829):1336–41. doi: 10.1126/science.1142364. Epub 2007/04/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.IOM. Bridging the Evidence Gap in Obesity Prevention: A Framework to Inform Decision Making. Washington DC: 2010 by the National Academy of Sciences; 2010. [PubMed] [Google Scholar]
- 59.Chatterji M, Green LW, Kumanyika S. L.E.A.D.: a framework for evidence gathering and use for the prevention of obesity and other complex public health problems. Health education & behavior : the official publication of the Society for Public Health Education. 2014 Feb;41(1):85–99. doi: 10.1177/1090198113490726. Epub 2013/06/21. eng. [DOI] [PubMed] [Google Scholar]
- 60.The Cochrane Collaboration. [9/8/2014]; Available from: http://www.cochrane.org/
- 61. [9/8/2014];IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Available from: http://monographs.iarc.fr/
- 62.Rooney AA, Boyles AL, Wolfe MS, et al. Systematic review and evidence integration for literature-based environmental health science assessments. Environmental health perspectives. 2014 Jul;122(7):711–8. doi: 10.1289/ehp.1307972. Epub 2014/04/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Databases, Searches & Other Resources. The National Toxicology Program, U.S. Department of Health and Human Services; [9/24/2014]. Available from: http://ntp.niehs.nih.gov/results/dbsearch/index.html. [Google Scholar]
- 64.Bellinger DC. Lead neurotoxicity and socioeconomic status: conceptual and analytical issues. Neurotoxicology. 2008 Sep;29(5):828–32. doi: 10.1016/j.neuro.2008.04.005. Epub 2008/05/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wild CP. Future research perspectives on environment and health: the requirement for a more expansive concept of translational cancer research. Environmental health : a global access science source. 2011;10( Suppl 1):S15. doi: 10.1186/1476-069X-10-S1-S15. Epub 2011/04/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rappaport SM. Implications of the exposome for exposure science. Journal of exposure science & environmental epidemiology. 2011 Jan-Feb;21(1):5–9. doi: 10.1038/jes.2010.50. Epub 2010/11/18. eng. [DOI] [PubMed] [Google Scholar]
- 67.Igl W, Johansson A, Wilson JF, et al. Modeling of environmental effects in genome-wide association studies identifies SLC2A2 and HP as novel loci influencing serum cholesterol levels. PLoS genetics. 2010 Jan;6(1):e1000798. doi: 10.1371/journal.pgen.1000798. Epub 2010/01/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho MM, Yoganathan P, Chu KY, et al. Diabetes genes identified by genome-wide association studies are regulated in mice by nutritional factors in metabolically relevant tissues and by glucose concentrations in islets. BMC genetics. 2013;14:10. doi: 10.1186/1471-2156-14-10. Epub 2013/02/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lusk CM, Dyson G, Clark AG, et al. Validated context-dependent associations of coronary heart disease risk with genotype variation in the chromosome 9p21 region: the Atherosclerosis Risk in Communities study. Human genetics. 2014 Jun 3; doi: 10.1007/s00439-014-1451-3. Epub 2014/06/04. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scriver CR. The PAH gene, phenylketonuria, and a paradigm shift. Human mutation. 2007 Sep;28(9):831–45. doi: 10.1002/humu.20526. Epub 2007/04/20. eng. [DOI] [PubMed] [Google Scholar]
- 71.Casey L. Caring for children with phenylketonuria. Canadian family physician Medecin de famille canadien. 2013 Aug;59(8):837–40. Epub 2013/08/16. eng. [PMC free article] [PubMed] [Google Scholar]
- 72.Robert M, Rocha JC, van Rijn M, et al. Micronutrient status in phenylketonuria. Molecular genetics and metabolism. 2013;110( Suppl):S6–17. doi: 10.1016/j.ymgme.2013.09.009. Epub 2013/10/12. eng. [DOI] [PubMed] [Google Scholar]
- 73.Cancer Intervention and Surveillance Modeling Network. [6/23/2014];2014 Available from: http://cisnet.cancer.gov/
- 74.Hiatt RA. Epidemiology: key to translational, team, and transdisciplinary science. Annals of epidemiology. 2008 Nov;18(11):859–61. doi: 10.1016/j.annepidem.2008.08.006. Epub 2008/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 75. [6/23/2014];Cancer Control Framework and Synthesis Rationale. 2014 Available from: http://cancercontrol.cancer.gov/od/about.html.
- 76.Hiatt R, Samet J, Ness RB. The role of the epidemiologist in clinical and translational science. Annals of epidemiology. 2006 May;16(5):409–10. doi: 10.1016/j.annepidem.2006.02.002. Epub 2006/05/02. eng. [DOI] [PubMed] [Google Scholar]
- 77.Berkman LF. Social epidemiology: social determinants of health in the United States: are we losing ground? Annual review of public health. 2009;30:27–41. doi: 10.1146/annurev.publhealth.031308.100310. Epub 2009/08/26. eng. [DOI] [PubMed] [Google Scholar]
- 78.Landrigan PJ. Children's Environmental Health: A Brief History. Academic pediatrics. 2016 Jan-Feb;16(1):1–9. doi: 10.1016/j.acap.2015.10.002. Epub 2015/10/27. eng. [DOI] [PubMed] [Google Scholar]
- 79.Gurtler RE, Yadon ZE. Eco-bio-social research on community-based approaches for Chagas disease vector control in Latin America. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2015 Feb;109(2):91–8. doi: 10.1093/trstmh/tru203. Epub 2015/01/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gonzalez JP, Lambert G, Legand A, et al. Toward a transdisciplinary understanding and a global control of emerging infectious diseases. Journal of infection in developing countries. 2011 Dec;5(12):903–5. doi: 10.3855/jidc.2425. Epub 2011/12/16. eng. [DOI] [PubMed] [Google Scholar]
- 81.Barrow TM, Michels KB. Epigenetic epidemiology of cancer. Biochemical and biophysical research communications. 2014 Dec 5;455(1–2):70–83. doi: 10.1016/j.bbrc.2014.08.002. Epub 2014/08/16. eng. [DOI] [PubMed] [Google Scholar]
- 82.Marsit CJ. Influence of environmental exposure on human epigenetic regulation. The Journal of experimental biology. 2015 Jan 1;218(Pt 1):71–9. doi: 10.1242/jeb.106971. Epub 2015/01/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ciesielski TH, Marsit CJ, Williams SM. Maternal psychiatric disease and epigenetic evidence suggest a common biology for poor fetal growth. BMC pregnancy and childbirth. 2015;15:192. doi: 10.1186/s12884-015-0627-8. Epub 2015/08/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogino S, Campbell PT, Nishihara R, et al. Proceedings of the second international molecular pathological epidemiology (MPE) meeting. Cancer causes & control : CCC. 2015 Jul;26(7):959–72. doi: 10.1007/s10552-015-0596-2. Epub 2015/05/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nishi A, Milner DA, Jr, Giovannucci EL, et al. Integration of molecular pathology, epidemiology and social science for global precision medicine. Expert review of molecular diagnostics. 2016;16(1):11–23. doi: 10.1586/14737159.2016.1115346. Epub 2015/12/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hiatt RA. Invited commentary: driving for further evolution. American journal of epidemiology. 2015 Apr 1;181(7):459–62. doi: 10.1093/aje/kwu476. Epub 2015/03/15. eng. [DOI] [PubMed] [Google Scholar]
- 87.Vogel AL, Stipelman BA, Hall KL, et al. Pioneering the Transdisciplinary Team Science Approach: Lessons Learned from National Cancer Institute Grantees. Journal of translational medicine & epidemiology. 2014;2(2) Epub 2015/01/03. Eng. [PMC free article] [PubMed] [Google Scholar]
- 88.Cooke NJ, Hilton ML, editors. Committee on the Science of Team Science. Board on Behavioral, Cognitive, and Sensory Sciences. Division of Behavioral and Social Sciences and Education. National Research Council. Enhancing the Effectiveness of Team Science. Washington DC: 2015 by the National Academy of Sciences; 2015. [PubMed] [Google Scholar]
- 89.Kemp SP, Nurius PS. Preparing Emerging Doctoral Scholars for Transdisciplinary Research: A Developmental Approach. Journal of teaching in social work. 2015;35(1–2):131–50. doi: 10.1080/08841233.2014.980929. Epub 2015/05/26. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawlor EF, Kreuter MW, Sebert-Kuhlmann AK, et al. Methodological innovations in public health education: transdisciplinary problem solving. American journal of public health. 2015 Mar;105( Suppl 1):S99–S103. doi: 10.2105/AJPH.2014.302462. Epub 2015/02/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Czajkowski SM, Lynch MR, Hall KL, et al. Transdisciplinary translational behavioral (TDTB) research: opportunities, barriers, and innovations. Translational behavioral medicine. 2016 Mar;6(1):32–43. doi: 10.1007/s13142-015-0367-3. Epub 2016/03/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Howards PP, Schisterman EF, Poole C, et al. "Toward a clearer definition of confounding" revisited with directed acyclic graphs. American journal of epidemiology. 2012 Sep 15;176(6):506–11. doi: 10.1093/aje/kws127. Epub 2012/08/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hernandez-Diaz S, Schisterman EF, Hernan MA. The birth weight "paradox" uncovered? American journal of epidemiology. 2006 Dec 1;164(11):1115–20. doi: 10.1093/aje/kwj275. Epub 2006/08/26. eng. [DOI] [PubMed] [Google Scholar]
- 94.Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. The Cochrane database of systematic reviews. 2013;1:CD000980. doi: 10.1002/14651858.CD000980.pub4. Epub 2013/02/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. John Wiley & Sons, Ltd; [3/17/2015]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000980.pub4/abstract. [Google Scholar]
- 96.Huang X, Bruce B, Buchan A, et al. No-boundary thinking in bioinformatics research. BioData mining. 2013;6(1):19. doi: 10.1186/1756-0381-6-19. Epub 2013/11/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clinical and Translational Science Awards. [6/23/2014];2014 Available from: https://www.ctsacentral.org/
- 98.Poincare H. In: The Foundations of Science. Cattell JM, editor. New York and Garrison N.Y: The Science Press; 1913. p. 127. [Google Scholar]