Abstract
Specific membrane lipid composition is crucial for optimized structural and functional organization of biological membranes. Cardiolipin is a unique phospholipid and important component of the inner mitochondrial membrane. It is involved in energy metabolism, inner mitochondrial membrane transport, regulation of multiple metabolic reactions and apoptotic cell death. The physico-chemical properties of cardiolipin have been studied extensively but despite all these efforts there is still lingering controversy regarding the ionization of the two phosphate groups of cardiolipin. Results obtained in the 1990s and early 2000s suggested that cardiolipin has two disparate pKa values where one of the protons was proposed to be stabilized by an intramolecular hydrogen bond. This has led to extensive speculations on the roles of these two putative ionization states of cardiolipin in mitochondria. More recently the notion of two pKa values has been challenged and rejected by several groups. These studies relied on external measurements of proton adsorption or electrophoretic mobility of membranes but did not take into account the low pH phase behavior and chemical stability of cardiolipin. Here we used 31P NMR to show that in the physiologically relevant membrane phospholipid environment, cardiolipin carries two negative charges at physiological pH. We additionally demonstrate the pH dependent phase behavior and chemical stability of cardiolipin containing membranes.
Keywords: mitochondria, membrane electrostatics, cardiolipin-protein interactions, electron transport, lipid ionization, membrane structure
Graphical Abstract
Introduction
In spite of more than 50 years of studies of an unusual dimeric phospholipid of the inner mitochondrial membrane (IMM), cardiolipin (CL) [1], newly discovered features of its membrane behavior continue to perplex with the multiplicity and complexity of the possible mechanisms of CL’s involvement in many functions [2]. CLs (1,3-bis(sn-3′-phosphatidyl)-sn-glycerol) are structurally unique as they contain two phosphatidyl groups (linked to a glycerol backbone) and four fatty acyl chains (Fig. 1). This combination of high hydrophobicity and negative charges in one molecule define its binding and regulatory role towards many mitochondrial proteins from the major components of mitochondrial bioenergetics - mitochondrial electron carriers and the IMM proteins engaged in ATP synthesis [3, 4], to regulators of apoptosis and mitophagy [5, 6].
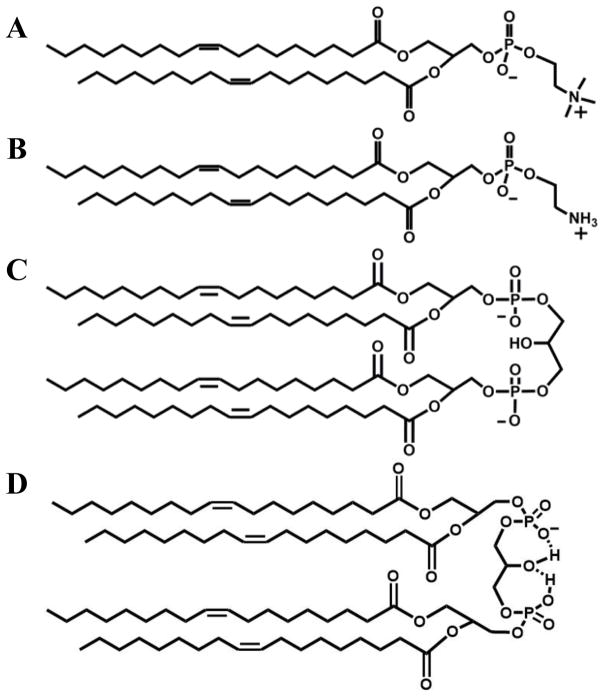
Figure 1.
Structures of the lipids used in our model mitochondrial membranes. A, DOPC; B. DOPE, and C & D, TOCL. D shows the cyclic-model proposed for CL around physiological pH. The structure shown in C should be considered the correct one based on our data, i.e. CL at neutral pH carries two negative charges.
Interestingly, some of the major physico-chemical propensities underlying CL’s engagement in the regulation of mitochondrial functions still remain enigmatic or debatable. One of them relates to the ionization of its two phosphate groups [3]. The predicted and initially documented low pK for both phosphate groups [7, 8] has been subsequently challenged based on the possible formation of a unique hydrogen bond in which the free hydroxyl on the central glycerol forms a cyclic intramolecular hydrogen-bonded structure with one protonated phosphate (P-OH group) resulting in the high (in the range of 7 or 8) pKa of the “2nd” phosphate [9]. This unusual electrochemical behavior of CL was associated with its participation in proton pumping across the membrane and important role in mitochondrial energy production [10]. Moreover, changing ionization of the CL molecule in the pH range where the physiological slightly basic (pH ~8) environment can undergo acidification (to pH ~7) immediately leads to many speculations on the significance of this transition in terms of possibly alternate interactions with a number of the IMM proteins, for example with mitochondrial uncoupling proteins [11]. Not surprisingly, significant efforts have been dedicated to refined measurements of the pK for CL’s phosphate groups. One of the most recent undertaking of this kind decisively rejected the presence of two pKa values and confirmed by the pH-titration and titration calorimetric experiments applied to two synthetic cardiolipins, 1,1′,2,2′-tetradecanoyl cardiolipin, CL (C14:0), and 1,1′,2,2′-tetraoctadecenoyl cardiolipin, CL (C18:1, CL (C18:1), that they behave as strong dibasic acids with pKa values about the same as the first pKa of phosphoric acid (2.15) [12]. The difference between the pKa values of the two phosphate groups in aqueous dispersions of CL was found to be small and of the order of one pH unit [12]. Another recent study used electrophoretic measurements to show that the charge on CL at neutral pH is most likely −2 [13]. These studies were performed on pure CL (Olofsson and Sparr [12]), and/or did not take into account the phase behavior of CL [13] which can change from bilayer to inverted hexagonal phase (HII) as a function of pH, where at low pH the HII phase dominates.
There is significant evidence that interactions of different phospholipids, including CLs, may affect their arrangements resulting in possible reciprocal effects on their membrane characteristics, including ionization state [14] and phase behavior [15]. It is therefore important to perform the assessment of CL’s pKa in the environment close to its natural “habitats” in mitochondrial membrane. One of the most powerful approaches may be magic angle spinning 31P NMR spectroscopy based titrations that can provide information on the state of the phosphate group ionization in the phospholipid membrane (bilayer) arrangement [16, 17]. With this in mind, we performed 31P-NMR titrations characterizing CLs behavior in two types of CL mixtures with most abundant mitochondrial phospholipids phosphatidylcholine (PC) and phosphatidylethanolamine (PE). We studied three systems, pure cardiolipin (to compare with previous work on CL), 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC)/CL at a 75:25 mol ratio, and DOPC/1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE)/CL at a 55:20:25 mol ratio. None of these systems showed much difference, and two ionization constants were not discerned as previously reported.
Materials and Methods
Materials
1′,3′-bis[1,2-dioleoyl-sn-glycero-3-phospho]-sn-glycerol (sodium salt) (TOCL), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), were purchased from Avanti Polar Lipids (Birmingham, AL). Lipid stocks (5–20 mM) were prepared gravimetrically in a 2:1 mixture of chloroform and methanol and the concentration was confirmed by phosphate assay according to Rouser [18]. Integrity of lipids was determined via thin layer chromatography (TLC), and only stocks that showed a single spot were used. HPLC grade water was purchased from Fisher Scientific. All other chemicals used were at least of HPLC grade and were purchased from VWR International, Radnor, PA.
Sample Preparation
NMR samples were prepared as described in Graber and Kooijman [16]. Briefly: dry lipid films of 10–15 μmol were prepared by mixing appropriate volumes of lipid stock solution in homemade borosilicate glass tubes (15 mm test tube size) and dried using a rotary evaporator. Resulting lipid films were further dried to remove trace amounts of organic solvents under high vacuum (~35mm Hg) in a vacuum oven at 35 °C. Lipid films were hydrated with 2 mL of buffer of appropriate pH and vortexed to form multilamellar vesicle (MLV) dispersions. The following buffers were used for the indicated pH ranges; 100 mM KCl-HCl for pH 1–2.5, 50 mM citric acid for pH 2.5–4, 20 mM citric acid, 30 mM MES for pH 4–6.5, 50 mM HEPES for pH 6.5–8.5, and 50 mM glycine for pH 8.5–10. Sample pH after hydration was measured with a Sentron pH meter fitted with a Sentron cupfet pH probe [16] and this bulk pH was used to prepare the 31P-NMR titration curves for CL. Buffers also contained 100 mM NaCl (except the pH 1–2.5 buffers) and 2 mM EDTA to complex residual amounts of divalent cations. Since divalent ions can dramatically influence the phase behavior of CL, and thus its ionization behavior this is an important precaution. MLV dispersions were subjected to two freeze-thaw cycles to homogenize the sample and to remove potential metastable lipid phases. Lipid dispersions were spun down at 14990 rpm in a Hettich 230R refrigerated centrifuge fitted with a 15000 rpm (24×3g) rotor at 4°C for a minimum of 30 min, except for the very low pH samples. Freeze-thaw cycles were omitted for the very low pH samples and these were spun down for ~15 min or less due to low stability and the formation of the hex (HII) phase. Lipid purity was checked after sample preparation by NMR and quantified after NMR spectra were obtained by Mass spectrometry.
NMR Spectroscopy
31P NMR spectra were recorded as previously described [16]. We used a 4.25 μs 90° pulse for 31P and in the case the static experiments also a 32 μs pulse of the 1H decoupling, on our 4 mm solid state probe. The sweep width was 98.9 ppm for the MAS spectra, and 398 ppm for the static spectra. Delay time between pulses was 1 s. An 85% H3PO4 standard solution was run before each sample as an external reference. Sample spectra were recorded under stable spinning conditions (5kHz) at 21 ± 1.0 °C. Generally, 200 to 2000 scans were collected. After the MAS spectra were recorded, the lipid phase of the sample was determined via static experiment. The static experiments were recorded on the same probe, and aided by low-power proton decoupling (the spinal64 pulse program was used for proton decoupling). For each static experiment, 300 to 7000 scans were collected unless noted otherwise. Free induction decays were processed using 5 Hz line broadening in the case of the MAS experiments and by 50 Hz in the case of the static (CSA) experiments.
Mass Spectrometry
Analysis of tetra-oleoyl-cardiolipin (TOCL) was performed using a Dionex Ultimate 3000 HPLC system coupled on-line to a Q-Exactive hybrid quadrupole-orbitrap mass spectrometer (ThermoFisher Scientific, San Jose, CA). TLCL, DOPC, MCL and LPC were separated on a normal phase column (Luna 3 μm Silica (2) 100 Å, 150×2 mm, (Phenomenex)) with flow rate 0.2 mL/min using gradient solvents A (propanol: hexane: ammonium acetate (10 mM): formic acid (0.01%): trimethylamine (0.5%), 57:43:1 (v/v)) and B (propanol:hexane:ammonium acetate (10mM): formic acid (0.01%): trimethylamine (0.5%), 57:43:8 (v/v)). The column was eluted during the first 15 min linear gradient from 10% solvent B to 37%, 15 – 23 min with a linear gradient from 37% solvent B to 50% solvent B, 23 – 25 min linear gradient to 100% solvent B, and then 25 – 47 min isocratic at 100% solvent B and flow 0.225 mL/min, 50–70 min isocratic at 10% solvent B and flow 0.2 mL/min for equilibrium column. Analysis was performed in negative ion mode at a resolution of 140,000 and an isolation window of 1.0 Da. Capillary spray voltage was set at 3.5 kV, and capillary temperature was 320 °C. The S-lens Rf level was set to 60. TMCL-(14:0)4 (Avanti Polar Lipids Inc., Alabaster, AL) and MCL-(14:0)3 were used as internal standards.
Results
Solid state 31P NMR of phospholipids is uniquely able to monitor the ionization state of the phosphate(s) present in the lipid headgroup in extended (i.e. flat) membrane systems [14]. An additional benefit of 31P NMR is that the lipid phase and stability of the lipid can be monitored for the same sample. We first determined the ionization and phase properties of pure CL since earlier work focused on this system.
Ionization and phase behavior of pure tetraoleoylcardiolipin
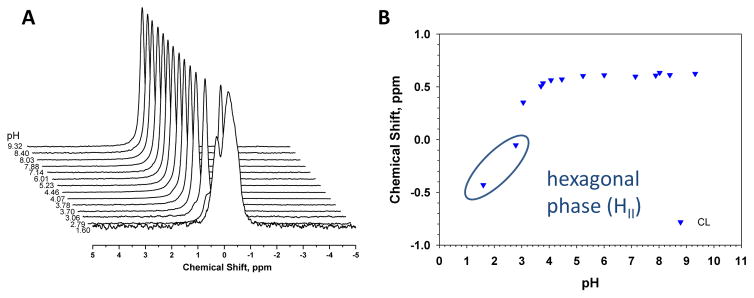
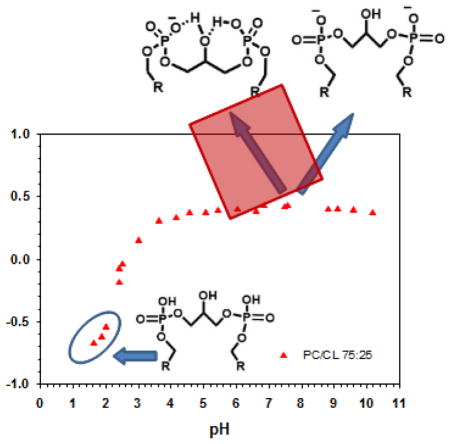
Figure 2A shows the chemical shift (CS) spectra as a function of pH for pure tetraoleoyl-cardiolipin. In figure 2B the chemical shift position of CL, taken from figure 2A is plotted as a function of pH. Between pH 4 and 10 only one peak for the two phosphates is observed and there is no significant change in the CS of this peak. Below pH 4 the CS of the CL peak moves upfield indicating a protonation of both phosphates. These data thus show that the two phosphodiesters of CL both become protonated below pH 4 and show no hint of a pKa at higher pH.
Figure 2.
Solid state 31P (magic angle spinning) NMR spectra for CL in multi-lamellar lipid dispersions as a function of pH. A. Waterfall plot showing all spectra as a function of pH. B. The peak position of CL plotted as a function of pH.
Several other important features are evident from these data. At very low pH pure CL (below pH 3) is highly unstable as evident from the complex peak (wide, with several overlapping peaks) at pH 2.8 and 1.6 (Supplementary Figure S1A). Even when the sample preparation time is reduced to less than 20 min (hydration, measurement of pH, and centrifugation) pure CL exhibited significant breakdown at pH < 3. The peak for CL included in Figure 2B for these low pH’s corresponds to the major peak found in these spectra and most likely corresponds to the unmodified lipid. We also observed that CL at pH below ~3 forms the hexagonal HII phase and that the CS of the CL peak shifts significantly upfield because of this (Supplementary Figure S1B). The HII phase has high negative curvature and the tight packing of the CL headgroup likely leads to a more upfield shift of the CS for the phosphate peaks and possibly a higher degree of protonation. This is consistent with previous observations for phosphatidic acid [14] which showed that shifts of the peak (CS) of PA occur upon the formation of the HII phase. The stability of CL in more relevant, mixed model membranes, via LC-MS analysis as discussed below.
Ionization and phase behavior of tetraoleoylcardiolipin in mixed lipid mixtures Model membranes of PC and CL
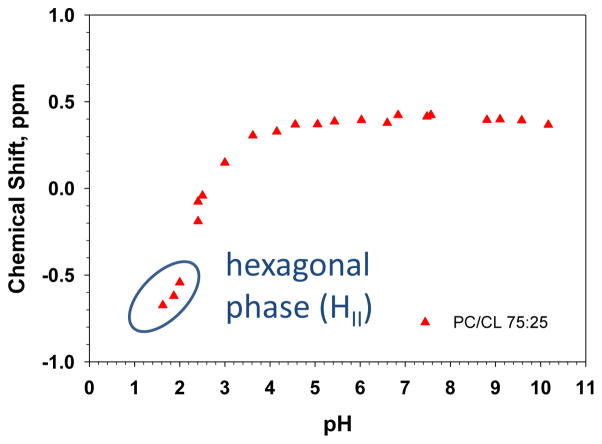
Next we determined the ionization behavior of 25 mol% CL in a matrix of DOPC. Figure 3 shows the titration behavior for the CL peak in the PC/CL mixed membranes (full CS spectra for the low pH samples are shown in the Supplementary Figure S2A). We observed only a single peak for the two phosphates of CL and no shift in this peak between pH 4 and 10. This indicates that there is no change in the ionization behavior of CL in a model membrane system that more closely mimics the native mitochondrial membrane compared to the pure system. In these mixed PC/CL systems the bilayer phase persisted to a slightly lower pH (to 2.2 < pH > 2.4). Interestingly we still observed the conversion of the bilayer phase to the HII phase for this lipid mixture. The samples that formed the HII phase are indicated in the titration curve in figure 3 and representative static spectra for these lipid mixtures as a function of pH are shown (between pH 1.64 and 6.84) in the Supplementary Figure S2B. These data show that CL is able to induce the HII phase in a DOPC bilayer solely as a function of pH. Similar behavior, i.e. not as a function of temperature and in pure CL systems, has been observed for CL as a function of divalent cations [19].
Figure 3.
Solid state 31P (magic angle spinning) NMR data for CL in PC:CL (at a 75:25 molar ratio) multi-lamellar lipid dispersions. The peak position of CL is plotted as a function of pH. At low pH the lipid samples undergo a pH dependent phase transition from the Lα to HII phase as indicated (data shown in Supplementary Figure S2B). Full 31P NMR spectra are shown in Supplementary Figure S2A.
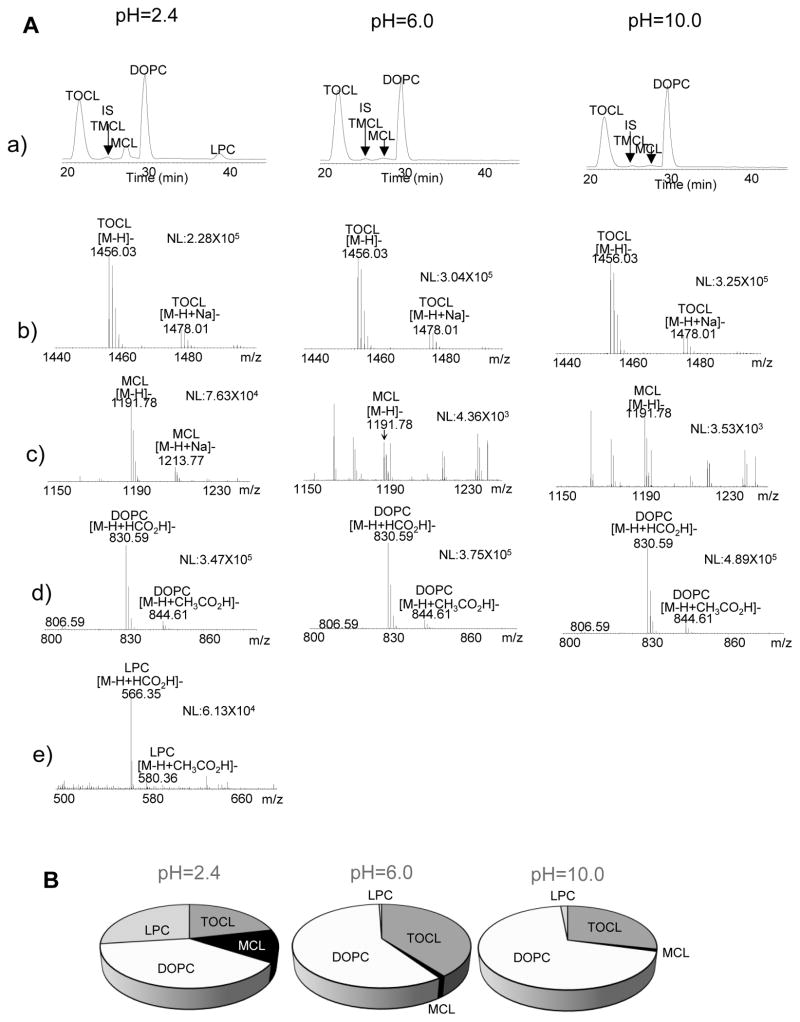
We also observed significantly less breakdown of CL in the mixed model membrane system at low pH. Provided that the samples were prepared quickly, spectra with no breakdown could be recorded down to a pH of 1.5. Breakdown of CL and PC did occur during long (overnight) static experiments for all pH values examined, unlike for systems without CL (Kooijman, personal observation). To determine the dominant breakdown products for these samples we employed LC-MS analysis to characterize the persistence of the phospholipids under different pH, see Figure 4. We found that, indeed, CL undergoes pH-dependent degradation that is particularly noticeable at very low pH (2.4, about 55–60mol%, Fig. 4). Notably, the major degradation product was monolyso-CL (MCL in the figure)– ie, still a derivative with both phosphate groups intact.
Figure 4.
LC/MS analysis of DOPC, TOCL, MCL and LPC in liposomes at different pH values. LC/MS analysis was performed after samples had been used for MAS 31P NMR analysis. A - LC/MS profiles (a) and mass spectra of TOCL (b), MCL (c), DOPC (d), and LPC (e); B - Quantitative assessment of TOCL, MCL, DOPC and LPC in liposomes.
Model membranes of PC, PE, and CL
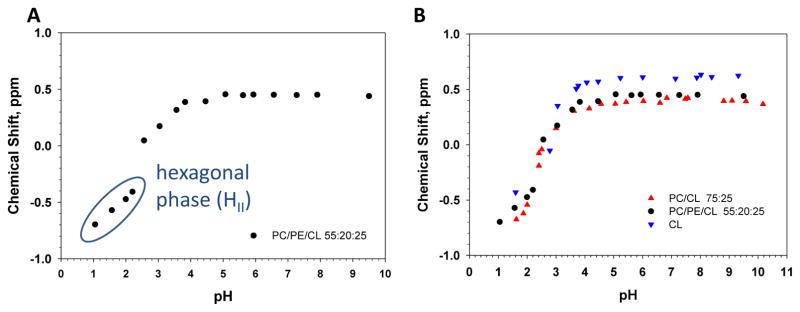
We have previously shown that the primary amine in the headgroup of PE is able to hydrogen bond with the phosphate headgroup in neighboring lipids. In the case of phosphatidic acid, lysophosphatidic acid, diacylglycerolpyrophosphate, ceramide-1-phopshate, and PI(4,5)P2 this leads to a deprotonation of the phosphomonoesters in the headgroup and thus a reduction of the second pKa [14, 17, 20, 21]. Additionally, PE is found in most eukaryotic cell membranes and is a significant lipid found in the mitochondrial inner membrane. We thus determined the ionization behavior of CL in a mixed lipid system of PC/PE/CL at a 55/20/25 molar ratio. Figure 5A shows the pH titration curve for CL in this lipid mixture. We again observed only one peak for CL in the pH range of 4 to 10, and a clear protonation event below this pH. The CS spectra from pH 6.5 till pH ~1.1 are shown in the Supplementary Figure S3. Since DOPE is a phospholipid with high negative curvature that above ~6C forms a HII phase on its own (physiological buffer at pH 7.4) the mixed system with PC and CL also converts to a hexagonal phase below pH 3 with a concomitant downfield shift of the CL peak as indicated in Figure 5A and Supplementary Figure S4. The large shift in the CS peak for CL upon formation of the HII phase is also clear in this lipid mixture. As in the case of PC/CL we observed a much improved stability for CL at low pH as can be seen in Supplementary Figure S3. A membrane environment containing PC and or PC:PE significantly improves the chemical stability of CL.
Figure 5.
A. Solid state 31P (magic angle spinning) NMR data for CL in PC:PE:CL (at a 55:20:25 molar ratio) multi-lamellar lipid dispersions. The peak position of CL is plotted as a function of pH. At low pH the lipid samples undergo a pH dependent phase transition from the Lα to HII phase as indicated (data shown in Supplementary Figure S4). Full 31P NMR spectra at low pH are shown in Supplementary Figure S3. B. Superposition of the three titration curves for CL when hydrated in buffer alone, for CL in DOPC, and CL in a DOPC/DOPE matrix.
Figure 5B shows all three titration curves for CL superimposed. The difference in CS for CL in the three different membrane systems (between pH 4 and 10) results from differences in electron density in the headgroup region. CL (blue data) is most deshielded compared to our reference followed by CL in the PC:PE membrane and finally CL in PC. More importantly these data indicate that the ionization behavior of CL is largely unaffected by membrane lipid composition and only depends on the pH.
Discussion
The full integration of mitochondria with the cell’s metabolic networks, requires coordinated transportation of numerous small compounds as well as macromolecules to and from the cytosolic sites [22]. To a large extent, the entire mitochondrial bioenergetics and regulatory metabolic functions are dependent, on the electrical and pH gradients across the mitochondrial inner membrane [23]. However specific mechanisms translating this general principle into molecular actions of the mitochondrial machinery remain enigmatic. It is believed that both protein and phospholipid components of the mitochondrial inner membrane (IMM) may contribute to the pH-driven regulation [24].
For example, the mitochondrial carriers (MCs) catalyze strict exchanges of substrates - nucleotides, metabolic intermediates, and cofactors that are required in cytoplasmic and matrix metabolism - across the mitochondrial inner membrane [25]. For different MCs, the transport characteristics vary depending on the electrical and pH gradients across the mitochondrial inner membrane [26]. For example, the ADP/ATP carrier is electrogenic (electrophoretic), the GTP/GDP carrier is dependent on the pH gradient, the aspartate/glutamate carrier is dependent on both, and the oxoglutarate/malate carrier is independent of them [25]. Many of these carrier oligomeric proteins, including uncoupling proteins, contain tightly bound CLs that is necessary for their optimized functions [27]. It is tempting to speculate that altered ionization state of the CL phosphate groups will change the binding profiles of the phospholipid with the carriers, hence will act as a pH-tunable sensor.
The remarkably diversified spectrum of CLs bound to numerous mitochondrial proteins is defined by electrostatic and hydrophobic interactions as well as by the combinations of them [28]. The electrostatic interactions are clearly dependent on the ionization state of the CL phosphate groups that may be affected by the specific characteristics of membrane lipid environment. Here, we employed MAS 31P NMR spectroscopy and characterized the ionization state of CL phosphate groups in phospholipid liposomes. Electrostatic interactions of the phosphate groups of CLs with many proteins define the activity, functions and trafficking of the latter. This emphasizes the essential role of CL ionization state. One of the good examples is an inter-membrane space hemoprotein cytochrome c (cyt c). Biosynthesis of cyt c involves translocation of apo-cyt c from the cytosol across the outer mitochondrial membrane to the sites where the heme group is attached [29]. The ability of apo-cyt c to induce fusion of both CL-containing vesicles reflects characteristics of protein/membrane interaction that pertain to its biological translocation. Binding of cyt c with CL triggers conformational re-arrangements in the protein that cause the “awakening” of its dormant peroxidase activity [30], the effect that is required for the execution of apoptotic program [31]. The equilibrium between the compact/extended conformers of the protein and the degree of the protein unfolding leading to the peroxidase activation are strongly dependent on the CL/cyt c ratio [32–34]. The general picture of cyt c/CL interactions - associated with the maximal peroxidase activation - shows that the electro-neutrality of the complex is achieved at the CL:cyt c ratio of 4 to 8 [30]. Given that cyt c has a surface charge of plus eight [35] this is compatible with the full ionization of CL’s two phosphate groups.
More detailed analysis of cyt c/CL interactions shows that the peroxidase function of cyt c may include several levels of “loosening” of the protein structure that are translated into different re-arrangements of the protein’s hexa-coordinated heme-iron environment [36, 37]. Detailed solid state NMR studies as well as crystallographic work suggest that small scale structural changes are sufficient for the initial induction of the peroxidase competence of cyt c/CL complexes [33, 38]. These results are in line with the kinetic studies [39] in which peroxidase activation occurred before any substantial changes in the coordination of heme Fe with one of the axial ligands, (Met80), were detectable. More profound structural changes take place when three binding sites are occupied by CL molecules on the cyt c surface as evidenced by solution NMR and molecular modeling data. The ability to bind CLs simultaneously at the proximal and distal sides of the heme and the consequently induced strain, opens up the heme crevice providing a path to the formation of the peroxidase “chanel”. Thus simultaneous binding of the distal and proximal sites can be considered as a “productive” binding mode leading to a robust peroxidase activation. Based on time-resolved FRET measurements of fluorescently-labeled cyt c the conclusion has been made about conformational diversity of the CL-bound protein with distinct populations of the polypeptide structures that varied in their degree of protein unfolding [40]. These heterogenous arrangements included the complexes with strongly unfolded protein structure almost equivalent to the fully denatured state of the hemoprotein (eg, in the presence of guanidine hydrochloride) with maximal peroxidase activity [41]. Finally, computational modeling revealed that increasing the CL concentration enhanced the affinity of cyt c for the membrane. This is due to the ability of cyt c to recruit CL molecules to form CL-containing membrane clusters, which demonstrates that multiple CL molecules (not individual molecules in isolation) stabilize the cyt c/CL complex. As a result, a model has been proposed [42] in which cyt c is partially and stably embedded in a locally curved CL-rich membrane patch. In the context of patho-physiologically relevant events, the structural data clearly indicate that the “deepness” of cyt c rearrangements are dictated by the amounts of available CL in the membrane microenvironment. Translocation of CLs from the IMM to the OMM may be the major factor controlling peroxidase activation of the hemoprotein enhancing CL oxidation.
Another important issue possibly related to the CL’s ionization state is the redistribution of CL between the IMM and the OMM [5, 43]. The physiologically significant remodeling of CLs from heteroacylated to homoacylated forms is catalyzed by three major protein mechanisms – TAZ1, MLCAT, and ALCAT [44]. The latter is localized to the mitochondria-associated membranes (MAMs) of ER [45]. This means that the remodeling mechanism should be dependent on the reverse re-distribution of the CLs and their hydrolysis products between the ER MAMs and IMM of mitochondria [44]. Clearly, an altered ionization state during these translocations occurring during the transfer from the neutral cytosolic pH to the slightly basic matrix pH of mitochondria may contribute to the translocation mechanisms [46].
Obviously, the existence of two largely different pKa for the two phosphate groups of CL – of which one may be relevant to the differences between the mitochondrial matrix and and the cytosol – is hugely attractive for explaining the CL-related translocations and other functions. This is the reason for extensive research that has revisited the initial, early, observations of the pKa(s) of CL over the last two decades. The latest published findings concluded that both phosphates of CL dispersions in water are strong dibasic acids similar to phosphoric acid (pKa ~2) [12], and recent zeta potential measurements also concluded that CL has only one pKa [13]. These results are all indirect measures of the charge on the entire membrane surface. Hence, there may still be an argument that in more physiologically relevant environments, interactions of the polar portions of the molecule with other phospholipids may be conducive of particular arrangements affecting the CL’s ionization states.
Our 31P NMR data clearly indicate that both in CL dispersions as well as in liposomes composed of PC/CL and PC/PE/CL taken at different ratios, the tetra-acylated phospholipid maintains its uniformity of the phosphate ionization at very low pH. The pKa of both phosphates of CL in all three systems investigated is ~2.5. Since low pH induces the HII phase and CL breakdown only an estimate of pKa is possible. These observations don’t change when we consider that our experiments were carried out at room T. At 37 °C the ionization of the phosphates will change slightly but won’t affect our observation of an essentially identical and very low pKa for both of the phosphates of CL. Similarly we don’t expect major changes to the observed ionization behavior in the presence of Mg2+ and Ca2+. The interaction of divalent cations with a bilayer interface will decrease the local membrane [H+] and thus increase the ionization of ionizable groups at the membrane interface. We have shown that the pKa of phosphatidic acid[14] and phosphoinositides[47] is decreased by Mg2+ and Ca2+, with Ca2+ having a significantly larger effect on PI4,5P2 ionization than Mg2+. However, because the pKa for both phosphates of CL is ~2.5 we don’t expect Mg2+ or Ca2+ to significantly alter this value. The observed pKa of ~2.5 is close to, but notably higher, than the 1st pKa of phosphoric acid due to the membrane environment. While the main conclusion of our work seems to exclude the possibility of the involvement of changed ionization state of CLs in regulatory processes, one cannot exclude that interactions with proteins – whereby strong anionic or cationic microenvironments might drastically change the local interactions leading to differences in pKa of one of its phosphate groups. Thus further work is necessary to assess CL ionization states in the presence of candidate binding proteins.
Supplementary Material
Highlights.
31P NMR reveals essentially one, very low, pKa value for both phosphates of Cardiolipin.
At low pH Cardiolipin has significantly reduced chemical stability as compared to other phospholipids.
At low pH Cardiolipin alone, and in mixtures with PC forms the hexagonal (HII) phase.
Acknowledgments
We thank Joseph Thomas and Mahinda Ganghoda for their assistance in managing the NMR spectrometer and organizing the 31P NMR data. This work was supported by the National Science Foundation under Grant No. CHE-1412920 and 1058719. This work was supported by NIH grants NS076511, NS061817, U19AI068021, P01 HL114453, ES020693, and CA165065 and the Human Frontier Science Program (HFSP-RGP0013/2014)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pangborn MC. Isolation and purification of a serologically active phospholipid from beef heart. J Biol Chem. 1942;143:247–256. [Google Scholar]
- 2.Kagan VE, Tyurina YY, Tyurin VA, Mohammadyani D, Angeli JP, Baranov SV, Klein-Seetharaman J, Friedlander RM, Mallampalli RK, Conrad M, Bayir H. Cardiolipin signaling mechanisms: collapse of asymmetry and oxidation. Antioxid Redox Signal. 2015;22:1667–1680. doi: 10.1089/ars.2014.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haines TH. A new look at Cardiolipin. Biochimica et biophysica acta. 2009;1788:1997–2002. doi: 10.1016/j.bbamem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Klingenberg M. Cardiolipin and mitochondrial carriers. Biochimica et biophysica acta. 2009;1788:2048–2058. doi: 10.1016/j.bbamem.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Qiang Wang KZ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nature cell biology. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalvez F, Pariselli F, Dupaigne P, Budihardjo I, Lutter M, Antonsson B, Diolez P, Manon S, Martinou JC, Goubern M, Wang X, Bernard S, Petit PX. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell death and differentiation. 2005;12:614–626. doi: 10.1038/sj.cdd.4401571. [DOI] [PubMed] [Google Scholar]
- 7.Coulon-Morelec MJ, Faure M, Marechal J. Controlled degradation of diphosphatidylglycerol (cardiolipid) in an acid medium. Study of the phosphatide derivatives obtained. Bull Soc Chim Biol (Paris) 1962;44:171–183. [PubMed] [Google Scholar]
- 8.Few AV, Gilby AR, Seaman GV. An electrophoretic study on structural components of Micrococcus lysodeikticus. Biochimica et biophysica acta. 1960;38:130–136. doi: 10.1016/0006-3002(60)91202-6. [DOI] [PubMed] [Google Scholar]
- 9.Kates M, Syz JY, Gosser D, Haines TH. pH-dissociation characteristics of cardiolipin and its 2′-deoxy analogue. Lipids. 1993;28:877–882. doi: 10.1007/BF02537494. [DOI] [PubMed] [Google Scholar]
- 10.Haines TH, Dencher NA. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS letters. 2002;528:35–39. doi: 10.1016/s0014-5793(02)03292-1. [DOI] [PubMed] [Google Scholar]
- 11.Hoang T, Smith MD, Jelokhani-Niaraki M. Expression, folding, and proton transport activity of human uncoupling protein-1 (UCP1) in lipid membranes: evidence for associated functional forms. The Journal of biological chemistry. 2013;288:36244–36258. doi: 10.1074/jbc.M113.509935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olofsson G, Sparr E. Ionization constants pKa of cardiolipin. PLoS One. 2013;8:e73040. doi: 10.1371/journal.pone.0073040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathappa M, Alder NN. The ionization properties of cardiolipin and its variants in model bilayers. Biochimica et biophysica acta. 2016;1858:1362–1372. doi: 10.1016/j.bbamem.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kooijman EE, Carter KM, van Laar EG, Chupin V, Burger KN, de Kruijff B. What makes the bioactive lipids phosphatidic acid and lysophosphatidic acid so special? Biochemistry. 2005;44:17007–17015. doi: 10.1021/bi0518794. [DOI] [PubMed] [Google Scholar]
- 15.Lopes S, Neves CS, Eaton P, Gameiro P. Cardiolipin, a key component to mimic the E. coli bacterial membrane in model systems revealed by dynamic light scattering and steady-state fluorescence anisotropy. Analytical and bioanalytical chemistry. 2010;398:1357–1366. doi: 10.1007/s00216-010-4028-6. [DOI] [PubMed] [Google Scholar]
- 16.Graber ZT, Kooijman EE. Ionization behavior of polyphosphoinositides determined via the preparation of pH titration curves using solid-state 31P NMR. Methods Mol Biol. 2013;1009:129–142. doi: 10.1007/978-1-62703-401-2_13. [DOI] [PubMed] [Google Scholar]
- 17.Kooijman EE, King KE, Gangoda M, Gericke A. Ionization properties of phosphatidylinositol polyphosphates in mixed model membranes. Biochemistry. 2009;48:9360–9371. doi: 10.1021/bi9008616. [DOI] [PubMed] [Google Scholar]
- 18.Rouser G, Fkeischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz A, Killian JA, Verkleij AJ, Wilschut J. Membrane fusion and the lamellar-to-inverted-hexagonal phase transition in cardiolipin vesicle systems induced by divalent cations. Biophys J. 1999;77:2003–2014. doi: 10.1016/S0006-3495(99)77041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kooijman EE, Sot J, Montes LR, Alonso A, Gericke A, de Kruijff B, Kumar S, Goni FM. Membrane organization and ionization behavior of the minor but crucial lipid ceramide-1-phosphate. Biophys J. 2008;94:4320–4330. doi: 10.1529/biophysj.107.121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strawn L, Babb A, Testerink C, Kooijman EE. The physical chemistry of the enigmatic phospholipid diacylglycerol pyrophosphate. Front Plant Sci. 2012;3:40. doi: 10.3389/fpls.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. The Journal of cell biology. 2016;212:379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skulachev VP. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis. 2006;11:473–485. doi: 10.1007/s10495-006-5881-9. [DOI] [PubMed] [Google Scholar]
- 24.Genova ML, Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochimica et biophysica acta. 2014;1837:427–443. doi: 10.1016/j.bbabio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Monne M, Palmieri F. Antiporters of the mitochondrial carrier family. Curr Top Membr. 2014;73:289–320. doi: 10.1016/B978-0-12-800223-0.00008-6. [DOI] [PubMed] [Google Scholar]
- 26.Dolce V, Cappello AR, Capobianco L. Mitochondrial tricarboxylate and dicarboxylate-tricarboxylate carriers: from animals to plants. IUBMB life. 2014;66:462–471. doi: 10.1002/iub.1290. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y, Willers C, Kunji ER, Crichton PG. Uncoupling protein 1 binds one nucleotide per monomer and is stabilized by tightly bound cardiolipin. P Natl Acad Sci USA. 2015;112:6973–6978. doi: 10.1073/pnas.1503833112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planas-Iglesias J, Dwarakanath H, Mohammadyani D, Yanamala N, Kagan VE, Klein-Seetharaman J. Cardiolipin Interactions with Proteins. Biophys J. 2015;109:1282–1294. doi: 10.1016/j.bpj.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter A, Margolis D, Mohan R, Blumenthal R. Apocytochrome c induces pH-dependent vesicle fusion. Membr Biochem. 1986;6:217–237. doi: 10.3109/09687688609065450. [DOI] [PubMed] [Google Scholar]
- 30.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 32.Gorbenko GP, Molotkovsky JG, Kinnunen PK. Cytochrome C interaction with cardiolipin/phosphatidylcholine model membranes: effect of cardiolipin protonation. Biophys J. 2006;90:4093–4103. doi: 10.1529/biophysj.105.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandal A, Hoop CL, DeLucia M, Kodali R, Kagan VE, Ahn J, van der Wel PC. Structural Changes and Proapoptotic Peroxidase Activity of Cardiolipin-Bound Mitochondrial Cytochrome c. Biophys J. 2015;109:1873–1884. doi: 10.1016/j.bpj.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul SS, Sil P, Haldar S, Mitra S, Chattopadhyay K. Subtle Change in the Charge Distribution of Surface Residues May Affect the Secondary Functions of Cytochrome c. The Journal of biological chemistry. 2015;290:14476–14490. doi: 10.1074/jbc.M114.607010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koppenol WH, Vroonland CA, Braams R. The electric potential field around cytochrome c and the effect of ionic strength on reaction rates of horse cytochrome c. Biochimica et biophysica acta. 1978;503:499–508. doi: 10.1016/0005-2728(78)90149-4. [DOI] [PubMed] [Google Scholar]
- 36.Atkinson J, Kapralov AA, Yanamala N, Tyurina YY, Amoscato AA, Pearce L, Peterson J, Huang Z, Jiang J, Samhan-Arias AK, Maeda A, Feng W, Wasserloos K, Belikova NA, Tyurin VA, Wang H, Fletcher J, Wang Y, Vlasova, Klein-Seetharaman J, Stoyanovsky DA, Bayir H, Pitt BR, Epperly MW, Greenberger JS, Kagan VE. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nat Commun. 2011;2:497. doi: 10.1038/ncomms1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maguire JJ, Tyurina YY, Mohammadyani D, Kapralov AA, Anthonymuthu TS, Qu F, Amoscato AA, Sparvero LJ, Tyurin VA, Planas-Iglesias J, He RR, Klein-Seetharaman J, Bayir H, Kagan VE. Known unknowns of cardiolipin signaling: The best is yet to come. Biochimica et biophysica acta. 2016 doi: 10.1016/j.bbalip.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClelland LJ, Mou TC, Jeakins-Cooley ME, Sprang SR, Bowler BE. Structure of a mitochondrial cytochrome c conformer competent for peroxidase activity. P Natl Acad Sci USA. 2014;111:6648–6653. doi: 10.1073/pnas.1323828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapralov AA, Kurnikov IV, Vlasova, Belikova NA, Tyurin VA, Basova LV, Zhao Q, Tyurina YY, Jiang J, Bayir H, Vladimirov YA, Kagan VE. The hierarchy of structural transitions induced in cytochrome c by anionic phospholipids determines its peroxidase activation and selective peroxidation during apoptosis in cells. Biochemistry. 2007;46:14232–14244. doi: 10.1021/bi701237b. [DOI] [PubMed] [Google Scholar]
- 40.Hanske J, Toffey JR, Morenz AM, Bonilla AJ, Schiavoni KH, Pletneva EV. Conformational properties of cardiolipin-bound cytochrome c. P Natl Acad Sci USA. 2012;109:125–130. doi: 10.1073/pnas.1112312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong Y, Muenzner J, Grimm SK, Pletneva EV. Origin of the conformational heterogeneity of cardiolipin-bound cytochrome C. Journal of the American Chemical Society. 2012;134:18713–18723. doi: 10.1021/ja307426k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammadyani D, Tyurin VA, O’Brien M, Sadovsky Y, Gabrilovich DI, Klein-Seetharaman J, Kagan VE. Molecular speciation and dynamics of oxidized triacylglycerols in lipid droplets: Mass spectrometry and coarse-grained simulations. Free Radic Biol Med. 2014;76:53–60. doi: 10.1016/j.freeradbiomed.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kagan VE, Jiang J, Huang Z, Tyurina YY, Desbourdes C, Cottet-Rousselle C, Dar HH, Verma M, Tyurin VA, Kapralov AA, Cheikhi A, Mao G, Stolz D, St Croix CM, Watkins S, Shen Z, Li Y, Greenberg ML, Tokarska-Schlattner M, Boissan M, Lacombe ML, Epand RM, Chu CT, Mallampalli RK, Bayir H, Schlattner U. NDPK-D (NM23-H4)-mediated externalization of cardiolipin enables elimination of depolarized mitochondria by mitophagy. Cell death and differentiation. 2016 doi: 10.1038/cdd.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu YW, Claypool SM. Disorders of phospholipid metabolism: an emerging class of mitochondrial disease due to defects in nuclear genes. Front Genet. 2015;6:3. doi: 10.3389/fgene.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao J, Liu Y, Lockwood J, Burn P, Shi Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. The Journal of biological chemistry. 2004;279:31727–31734. doi: 10.1074/jbc.M402930200. [DOI] [PubMed] [Google Scholar]
- 46.Tamura Y, Sesaki H, Endo T. Phospholipid transport via mitochondria. Traffic. 2014;15:933–945. doi: 10.1111/tra.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graber ZT, Gericke A, Kooijman EE. Phosphatidylinositol-4,5-bisphosphate ionization in the presence of cholesterol, calcium or magnesium ions. Chem Phys Lipids. 2014;182:62–72. doi: 10.1016/j.chemphyslip.2013.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.