Abstract
Emotion dysregulation is a core characteristic of patients with Borderline Personality Disorder (BPD), and is often attributed to an imbalance in fronto-limbic network function. Hyperarousal of amygdala, especially in response to negative affective stimuli, results in affective interference with cognitive processing of executive functions. Clinical consequences include the impulsive-aggression, suicidal and self-injurious behaviors which characterize BPD. Dysfunctional interactions between amygdala and its network targets have not been well characterized during cognitive task performance. Using psychophysiological interaction analysis (PPI), we mapped network profiles of amygdala interaction with key regulatory regions during a Go No-Go task, modified to use negative, positive and neutral Ekman faces as targets. Fifty-six female subjects, 31 BPD and 25 healthy controls (HC), completed the affectively valenced Go No-Go task during fMRI scanning. In the negative affective condition, the amygdala exerted greater modulation of its targets in BPD compared to HC subjects in Rt. OFC, Rt. dACC, Rt. Parietal cortex, Rt. Basal Ganglia, and Rt. dlPFC. Across the spectrum of affective contrasts, hypermodulation in BPD subjects observed the following ordering: Negative > Neutral > Positive contrast. The amygdala seed exerted modulatory effects on specific target regions important in processing response inhibition and motor impulsiveness. The vulnerability of BPD subjects to affective interference with impulse control may be due to specific network dysfunction related to amygdala hyper-arousal and its effects on prefrontal regulatory regions such as the OFC and dACC.
Keywords: Borderline Personality Disorder, fMRI, amygdala, psychophysiological interactions, cognition, impulsiveness
Introduction
Emotion dysregulation is a core diagnostic characteristic of Borderline Personality Disorder (BPD), and is considered by some to be the primary source of behavioral pathology in this illness (Linehan, 1993). In laboratory studies, patients with BPD respond more intensely and for longer durations to negative emotional stimuli, and are slower to return to baseline than healthy controls (Jacob et al., 2008, Levine et al., 1997). In studies using electronic monitoring in the natural environment, BPD subjects demonstrate more affective instability, hypersensitivity, extreme changes of mood, negative and conflicting emotions compared to controls (Ebner-Priemer et al., 2007a,b, 2008, Reisch et al., 2008, Trull et al., 2008). Current theories of emotion regulation postulate a balance between “top down” cortical modulation and “bottom up” limbic arousal. i.e. Dysregulation of emotion may result from either hyper-arousal of the limbic system, especially in response to aversive stimuli, or, conversely, diminished efficacy of tonic cortical inhibition (Ochsner & Gross, 2006, Gross & Thompson, 2006, Phillips et al., 2008, Davidson et al., 2000). The affective instability of the borderline patient is attributed to an imbalance in fronto-limbic network function involving the amygdala and associated regions of the limbic system, and regulatory regions in prefrontal cortex (PFC), including the orbital frontal cortex (OFC), anterior cingulate cortex (ACC), dorso-lateral PFC (dlPFC) and associated areas (Schulze et al., 2016). fMRI studies have repeatedly demonstrated increased arousal of the amygdala in BPD compared to control subjects in response to provocations using emotional stimuli such as the affectively valenced Ekman faces (Donegan et al., 2003, Minzenberg et al., 2007), aversive IAPS scenes (Herpertz et al., 2001, Schulze et al., 2010, Hazlett et al., 2012), unresolved life events (Beblo et al., 2006), emotional scripts (Schmahl et al,. 2003, Schmahl and Bremner, 2006), and negative social pictures (Koenigsberg et al., 2014). BPD subjects show prolonged BOLD responses in amygdala to emotional stimuli, indicating longer time to return to baseline, and a failure to down-regulate (habituate) amygdala responses with repeated presentations of emotional pictures, suggesting a deficit in regulating emotional arousal (Hazlett et al., 2012). Hyper-arousal of the amygdala is clinically important, given its role in appraising the affective salience of stimuli, especially facial expressions (Calder and Young, 2005), and in the appraisal of perceived threat and mediation of fear responses (LeDoux, 1993). In this investigation we extend previous work in BPD by assessing dysfunctional network profiles of the amygdala in the context of an affective impulsivity paradigm (Soloff et al., 2015).
Anatomical network connectivity, and functional interactions of the amygdala have been systematically studied using both animal and human models (Diwadkar et al., 2012; Phelps and LeDoux, 2005); however, dysfunctional interactions between the amygdala and its network targets have not been well-characterized in BPD. Thus, while hyper-activation of the amygdala is a characteristic of impaired functional brain responses in BPD, the directional network effects exerted by the amygdala in BPD are relatively under-studied using network analyses of fMRI signals. Understanding these network profiles will contribute greatly toward assessing the contribution of “bottom-up” signals in driving some of the core characteristics of emotional dysregulation and how they might affect cognitive processing in BPD.
While it is clear that the brain’s cognitive systems are not insulated from affective interference, the network profiles of the amygdala during cognitive processing have not been well characterized (Phelps, 2006). The OFC, ACC, dlPFC, and associated areas, are involved in executive cognitive functions such as focused attention, response inhibition, conflict resolution, encoding and recall of memory. Through extensive feedback loops to limbic structures, these prefrontal regions exercise a measure of “top-down” tonic control to maintain emotional homeostasis (Davidson and Irwin, 1999). In patients with BPD, affective interference, especially by negative stimuli, impairs functioning of brain networks that sub-serve cognitive processing of executive functions required for adaptive responding (Sebastian et al., 2013; Soloff et al., 2015; Winter et al., 2014).
We have been studying the effects of affect on brain responses during cognitive processing in BPD using paradigms specifically modified to utilize and permute affective context. In relying on affective appraisal to gate cognitive processing, we seek to force integration of affective and cognitive domains (Blair et al., 2007), and to assess if this integration differentially affects brain responses in BPD.
A particular focus of our work is the borderline patient’s trait impulsivity, a diagnostic characteristic of the disorder which is clinically associated with affective instability, aggression, suicidal and self-destructive behaviors (Brodsky et al., 2006). Impulsive-aggressive behavior in BPD is associated with structural, metabolic, and functional abnormalities in fronto-limbic networks (Berlin et al., 2005, Sebastian et al., 2013, Siever 2008). Impulsive, aggressive, and self-destructive behaviors in BPD occur most often in the context of negative affectivity, especially perceived rejection (Yen et al., 2004). fMRI studies of impulse control which incorporate negative emotional stimuli, demonstrate fronto-limbic dysfunction in BPD compared to control subjects (Jacob et al., 2013, Sebastian et al. 2014). Given the clinical relevance of impulsivity in BPD, we have focused attention on the role of emotional interference with this executive function using an affectively-modified Go-No-Go paradigm.
The classic version of this paradigm requires participants to gate their responses to rapidly presented stimuli based on perceptual identity. In our affective Go No-Go paradigm, positive, negative and neutral Ekman faces are used to mediate impulse control (i.e. response inhibition), depending on the affective (rather than perceptual) gating of the response (Soloff et al., 2015). As a result, it is possible to isolate the selective effects of the affective context of the response on fMRI responses, and particularly, on brain network interactions. Using the affective Go No-Go paradigm under negative affective conditions in subjects with BPD, we previously reported increased activation in amygdala, and increased and decreased activation in different regions of the middle-inferior OFC. Robust increases were also noted in areas reflecting task-relevant processing: the superior parietal/precuneus (for visuo-spatial processing and episodic visual memory), and the basal ganglia (for reward-based decision-making)(Soloff et al., 2015). The current extension of our work is specifically focused on the network profiles of the amygdala, relying on modeling psycho-physiological interactions (PPI) in the fMRI data (Friston et al., 1997; Horwitz et al., 2005; J. X. O’Reilly, M. W. Woolrich, T. E. Behrens, S. M. Smith, & H. Johansen-Berg, 2012).
The use of PPI was motivated by its analytic value, positioned as it is between techniques of functional and maximal effective connectivity analyses (Stephan et al, 2016, Silverstein et al., 2016), providing a robust model for investigation of seed-based network interactions (Friston, 2011; Friston et al., 1997; Kim & Horwitz, 2008; Woodcock, Wadehra, & Diwadkar, 2016). PPI estimates directional modulation by an a priori defined seed region (e.g., amygdala) on target regions (e.g., OFC) in the context of a psychological contrast of interest (e.g., negative > positive affective context) (Friston et al., 1997). The framework for network explorations using fMRI data is vast, with the availability of a rich set of well defined quantitative models. We chose PPI to explore differential interactions between the amygdala and its targets, in BPD relative to controls. PPIs afford rapid and efficient exploration of pairwise directional network effects between seed regions and targets (Silverstein et al., 2016) and are particularly useful when the choice of seed is well motivated (in our case, the amygdala). This model is more simplistic than more complex dynamic causal models of network interactions (or “effective connectivity” analyses) yet is a useful “first step” in divining dysfunctional network profiles in disorders ranging from schizophrenia (Wadehra et al., 2013) to obsessive compulsive disorder (Diwadkar et al., 2015; Friedman et al., In Press). In this analysis, we use PPI in elucidating “bottom-up” profiles of the amygdala during response inhibition in BPD, as well as mechanisms of affective interference at the level of brain network interactions.
Method
The study was approved by the University of Pittsburgh Institutional Review Board. Fifty-six (56) female subjects, 31 cases and 25 controls, 18 – 45 years of age, were recruited from the PI’s ongoing longitudinal studies of BPD, from psychiatric outpatient clinics, and by advertisement from the surrounding community. The study was restricted to females as they comprise 75% of BPD patients in clinical settings, avoiding any confounds due to gender (DSM V, 2013). All subjects gave written informed consent. To be included in the BPD sample, subjects were required to meet criteria for a probable or definite lifetime diagnosis of BPD on the International Personality Disorders Examination (IPDE) (Loranger, 1999), and a definite current diagnosis of BPD on the Revised Diagnostic Interview for Borderline Patients (DIB-R), using a two-year timeframe (Zanarini et al., 1989). Co-morbidity on Axis I was determined by the Structured Clinical Interview for DSM-IV (SCID), for current and lifetime diagnoses (First et al., 2005). Healthy control subjects (HC) did not meet criteria for any current or lifetime Axis I or II disorders and were free of psychoactive medication. BPD subjects on psychoactive medication were permitted to remain on their medication. Immediately preceding the scan, all subjects had negative urine toxicology for drugs of abuse (MedTox) and negative pregnancy tests. Activation profiles from a subset of this sample, including both BPD and HC subjects, were previously reported (Soloff et al., 2015).
Exclusion criteria
Exclusion criteria included: 1) a current or lifetime diagnosis of schizophrenia, delusional (paranoid) disorder, schizoaffective disorder, bipolar disorder, or psychotic depression; 2) a current diagnosis of Substance Dependence or any current drug and/or alcohol related CNS deficits. (A diagnosis of Substance Abuse was permitted so long as the subject had been abstinent for one week, showed no signs of withdrawal, and had a clean urine toxicology drug screen at the time of the scan.); 3) CNS pathology of any etiology, including acquired or developmental deficits or seizure disorder; 4) Physical disorders or treatments with known psychiatric consequence (e.g. hypothyroidism, steroid medications); 5) Mental Retardation (IQ <70 by WAIS); 6) standard exclusion criteria for MRI scans (i.e. ferromagnetic artifacts, inability to fit in the scanner, claustrophobia, inability to co-operate with instructions.)
Imaging Specifications
Anatomical images were acquired on the 3.0T Siemens Trio system in the axial plane parallel to the AC-PC line using a 3D MPRAGE sequence (TE/TI/TR=3.29ms/900ms/2200ms, flip angle=9, isotropic 1mm3 voxel, 192 axial slices, matrix size=256×192). fMRI data were acquired in the axial plane using gradient echo EPI (TR=2000 ms, TE=30 ms, flip angle=70 deg, 30 slices, slice thickness=3.1 mm, 3 mm x 3 mm in-plane, matrix size=64×64).
fMRI paradigm
The Go No-Go test is a neuropsychological measure of response inhibition and motor impulsiveness that requires subjects to chose to respond (“Go”) or not respond (“No-Go”) based on target class. The typical Go No-Go paradigm targets impulsivity (but independent of emotional context) but using affectively neutral stimuli. Given our motivation to assess affective interference in the context of cognitive processing, we modified the traditional version of the paradigm. Only Ekman faces depicting negative (angry, sad, fearful), positive (happy), or neutral affect were deployed as stimuli (Ekman and Friesen, 1976; Soloff et al., 2015). Before a block of trials, subjects were instructed on which affective context in the upcoming block of rapidly presented faces would require a “Go” response (e.g., “Negative”). Subjects were instructed to make a response only if a presented face was consistent with the instructed affective context (e.g., “Go” for a Negatively valenced face, but “No-Go” for a positively valenced face). Thus, in gating responses to the affective valence of the Ekman faces, we intended for the task to induce an interaction between affective context, and cognitive processing in the specific domain of impulsivity.
During each block of trials, Ekman faces were presented briefly (500 ms) in a mixed jittered event-related design (Inter-Stimulus interval range: 500–1500 ms in 250 ms increments) (Amaro and Barker, 2006; Donaldson et al., 2001). As noted the affective context for target stimuli was signaled at block onset and subjects responded if the affect in the face was consistent with the affective context. In any given block, 67% of faces were targets (i.e., consistent with the signaled context, and required a “Go” response). Four block types were employed (three repetitions, 30 s block length): negative, neutral and positive valence and distorted blocks (in which target images were scrambled faces), with three fixation blocks interspersed. All responses were recorded by button press.
fMRI processing
fMRI data were analyzed using SPM8 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). In all analyses, the first four images were discarded to account for EPI equilibration effects. The remaining images in the sequence were realigned to correct for head movements, corrected for slice timing, and subsequently spatially normalized according to the transformation matrix derived between the co-registered (to the mean EPI sequence image) T1-weighted image volume and the MNI template brain. The images were then smoothed spatially with a 3D Gaussian kernel of 6 mm FWHM and re-sampled (2 × 2 × 2 mm3). A high-pass filter (cutoff 1/128 s) was employed to remove low-frequency signal drifts. The data were modeled voxel-wise, applying a general linear model (GLM) based on boxcar waveforms to conform to each of the negative, positive and neutral affective contexts, and convolved with the canonical hemodynamic response function. All subjects’ head motion was within accepted limits (< 4 mm). Furthermore, in all first level models, the effects of motion were modeled by including the six motion parameters as covariates of no interest.
PPI analyses
To assess the network profiles of the amygdala, psychophysiological interaction (PPI) was employed. PPIs are constructed by extracting a time-series from a seed region of interest and multiplying the activity with a stimulus function or regressor encoding the psychological context. For PPI modeling, first time series were extracted from each participant based on their first-level activation maps. To achieve this, first an effects of interest contrast (p<.05FWE) was used to statistically distinguish amygdala voxels that could be reliably classified as “signal” rather than “noise” or false-positives (Woodcock et al., 2016). This allowed us to identify the specify loci of amygdala activations based on a typically employed statistical filtering. The time series itself was the average signal from a sphere (2 mm radius) centered at the statistical peak within the amygdala’s anatomical boundaries (Maldjian et al., 2003). This approach can be contrasted with approaches wherein the locus within a region is identified based on a second-level group map. We note that the current approach has a relative merit in that it respects individual differences in activation peaks, and enhances sensitivity for identifying peaks based on intra-subject maxima (Wadehra et al., 2013, Woodcock et al., 2016). Next, this time series was multiplied with three distinct contrasts each representing three separate affective contexts: These were, Negative > Positive, Neutral > Positive, Negative > Neutral. The positive weighting of the regressors modeled hypothesized “excitatory” modulation by the amygdala in the context of emotion processing (Phelps, 2006). The employed contrast structure allowed us to assess the relative hyper-modulatory effects of the amygdala across the spectrum of affective contexts (Positive to Negative)(Soloff et al., 2015). The intra-subject maps thus encode the strength of the interaction at the first level, and were submitted to separate (for each contrast) second level random effects analyses of co-variance, with group (HC vs. BPD) as the single factor, and age modeled as co-variate to accommodate age-differences between groups.
Significant differences (BPD ≠ HC) were assessed using directional contrasts. Cluster level correction was employed to identify significantly different modulation by the amygdala by estimating the minimum cluster extent in order for modulated clusters to be rejected as false positive (noise-only) clusters (Ward, 2000). This approach performs a Monte Carlo alpha probability simulation, computing the probability of a random field of noise (after taking into account the spatial correlations of voxels based on the image smoothness within each region of interest estimated directly from the data set), producing a cluster of a given size, after noise thresholding. The underlying principle is that true clusters will tend to occur over contiguous voxels within a region of relative functional homogeneity, whereas noise has much less of a tendency to form clusters of modulated voxels. A region-of-interest approach was used to focus analyses on anatomical structures of interest identified on the bases of previous studies. Thus, analyses were focused in a spatial mask that was derived from a combination of morphometric and fMRI analyses that has identified brain regions of clinical significance in BPD (Soloff et al., 2012; 2014, 2015). The network of anatomical regions is depicted in Supplementary Figure 1.
Results
Subject Characteristics
The mean (s.d.) age of the BPD sample was 30 (8.2) years, compared to 24.5 (5.5) years for healthy controls, (t 3.00, df 52.4, p =.004). At the time of the scan, current co-morbid Axis I diagnoses were noted in 27 BPD subjects (87.1%), the most frequent being MDD (in 19 subjects (61.3%)) and Generalized Anxiety Disorder (in 11 subjects (35.5%), with some overlap. A current Substance Use Disorder was noted in only 2 subjects (6.5%). Additional Axis II co-morbidity was diagnosed in 18 BPD subjects (58.1%), the most frequent being Paranoid PD (in 5 subjects (16.1%). Nineteen BPD subjects (61.3%) had histories of childhood abuse (14 sexually abused). Twenty-two (71%) BPD subjects had past histories of suicide attempts, 9 were non-attempters. Fifteen BPD subjects (48.8%) were taking one or more psychotropic medications: a.) antidepressants: venlafaxine (2), escitalopram (1), paroxitine (2), fluoxetine (2), sertraline (2), trazadone (1), citalopram (2), buproprion (2); b.) anxiolytics: clonazepam (1), alprazolam (1), lorazepam (2), hydroxyzine (2); c,) neuroleptics: aripiprazole (2), quetiapine XR (1); d.) mood stabilizers: topiramate (1), lamotrigine (2); e.) stimulants: methlyphenidate (1).
PPI Results (Table 1, Figures 1–3)
Table 1.
Amygdala modulation under three affective conditions during the Go No-Go task
| Anatomical ROI | Contrast | Ind. Cluster Ext. | p uncorrec. | Voxel Peak (MNI) |
|---|---|---|---|---|
| A. Neg > Neu | ||||
| Amygdala | BPD>HC | 160 | 0.002 | −28 0 −14 – L-Amygdala |
| Basal Ganglia | BPD>HC | 2681 | <0.001 | 34 10 −6 - R-BG |
| dACC | BPD>HC | 6005 | <0.001 | 9 12 27 – R-dACC |
| OFC | BPD>HC | 9600 | 0.001 | 38 20 −15 – R-OFC |
| Parietal | BPD>HC | 2939 | <0.001 | 46 −15 22 – R-Parietal |
| dPFC | BPD>HC | 651 | <0.001 | 46 28 21 – R_DLPFC |
| B. Neu > Pos | ||||
| OFC | BPD>HC | 677 | <0.001 | 36 15 −15 – R-OFC |
| OFC | BPD>HC | 1022 | <0.001 | −42 14 0 - L-OFC |
| Parietal
|
BPD>HC
|
170
|
0.005
|
−56 −48 39 – L-Parietal
|
| Parietal | HC>BPD | 1188 | 0.005 | 2 −57 33 – L-Precuneus |
| OFC | HC>BPD | 62 | 0.008 | 6 33 −15 R-Med Orb Frontal |
| C. Neg > Pos | ||||
| Amygdala | BPD>HC | 165 | 0.002 | −28 0 −14 – L-Amyg |
| Basal Ganglia | BPD>HC | 2681 | <0.001 | 34 10 −6 – R-BG |
| dACC | BPD>HC | 6621 | <0.001 | −6 34 18 – L-dACC |
| OFC | BPD>HC | 9640 | <0.001 | 38 20 −15 – R-OFC |
| Parietal | BPD>HC | 2939 | <0.001 | 46 −15 22 R-Parietal |
Figure 1.
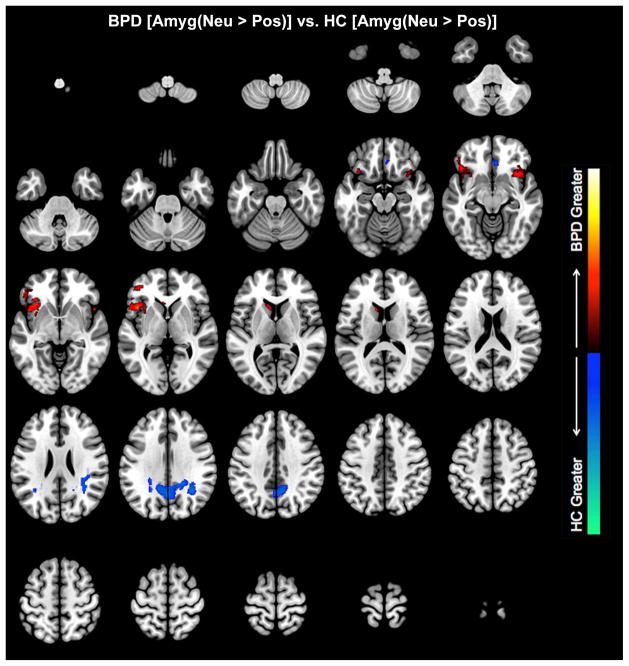
Neutral relative to positive conditions. Clusters depict where the amygdala differentially modulates brain regions in BPD and HC (see Table 1 for statistical and location information). BPD were characterized by increased amygdala modulation of the bilateral orbitofrontal cortex, whereas in HC, we observed increased modulation of the parietal cortex.
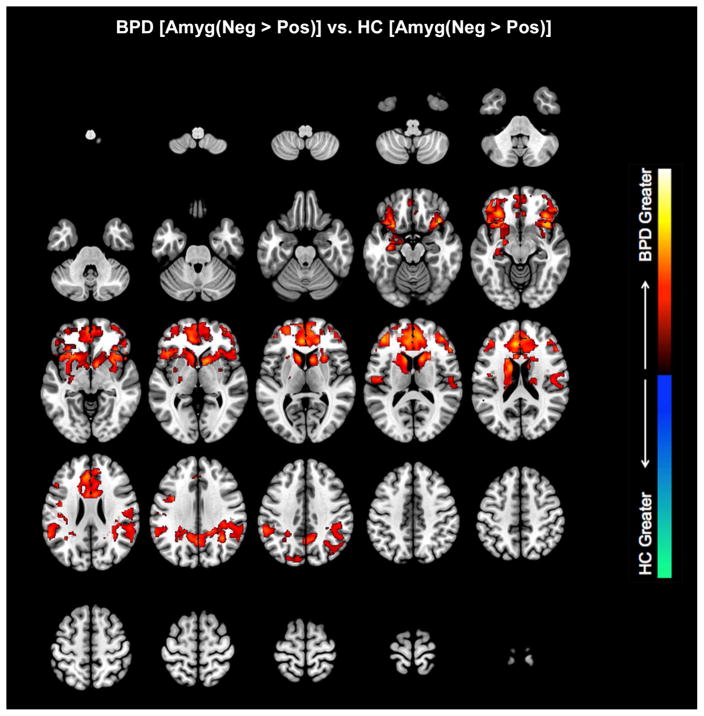
Figure 3.
Negative relative to Positive conditions. Clusters depict where the amygdala differentially modulates brain regions in BPD and HC (see Table 1 for statistical and location information). Again, BPD were characterized by increased amygdala modulation of a large network of frontal, cingulate and parietal regions.
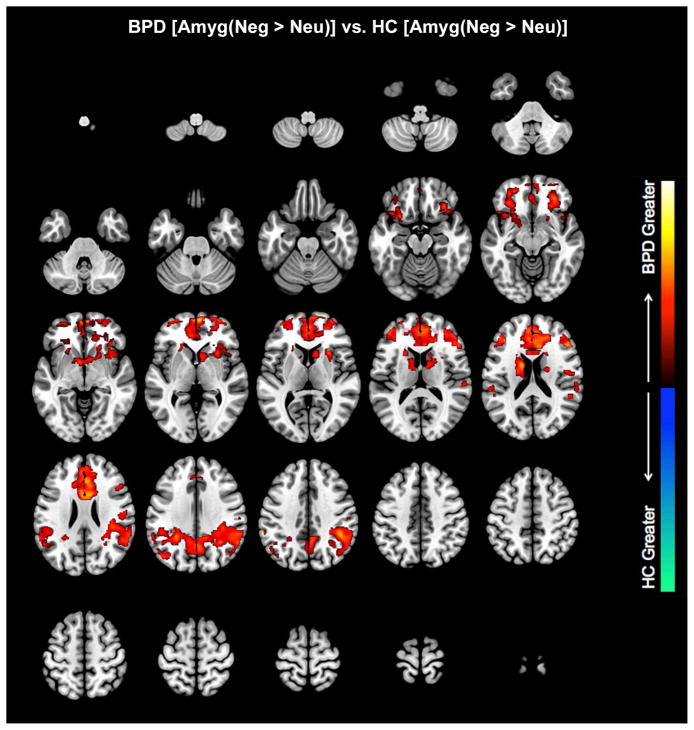
In the negative relative to the neutral affective context (Negative >Neutral), the amygdala exerted greater modulation of its targets in BPD compared to HC subjects in multiple areas. Voxel peaks were located in Rt. OFC, Rt. dACC, Rt. Parietal, Rt. Basal Ganglia, and Rt. dlPFC (in order of individual cluster extent) (Table 1). A small effect was also noted in Lt. amygdala. Results were remarkably similar in the Negative > Positive contrast, with greater modulation observed in Rt. OFC, Lt. dACC, Rt. Parietal, Rt. BG, and a small effect in Lt. amygdala. In the Neutral > Positive contrast, greater modulation in BPD than HC subjects was observed in two different OFC nodes (Lt. OFC, Rt. OFC), and a smaller area in L. Parietal cortex. HC subjects demonstrated greater amygdala modulation than BPD subjects only in the Neutral > Positive contrast, with activation in Lt. Parietal precuneus and a small area of Rt. OFC (medial orbital frontal cortex). The results suggest that across the spectrum of affective contexts, hyper-modulation in BPD observed the following ordering: Negative > Neutral > Positive.
Eleven (47.8%) of our BPD subjects were taking psychotropic medications, and medication use is a potentially confounding factor in fMRI studies of emotion processing (Schulze, et al., 2016). To test for medication effects on connectivity estimates, we compared connectivity parameters (that is, estimates of amygdala modulation) at the peak of each target. In investigating differences between medicated and non-medicated BPD participants, we found no differences in PPI parameter estimates, These null effects conform to our previously published results (Soloff et al., 2015), though we note that some meta-analyses report diminished activation of lt.amygdala and hippocampus among medicated subjects (Schulze, et al., 2016). While our BPD subjects were, on average, six years older than controls, age did not impact the results.
Discussion
Using an affectively valenced Go No-Go task, we evaluated network profiles of the amygdala that might underpin affective interference in BPD compared to control subjects. Consistent with a model of “bottom up” arousal to emotional stimuli, the amygdala exerted hyper-modulatory effects on specific target regions that are relevant in processing response inhibition and motor impulsiveness. The resulting pattern of network dysfunction suggests that BPD subjects may be more vulnerable to affective interference because of amygdala hyper-modulation that increases with negative context.
The amygdala is involved in perception and production of emotion, especially the processing of fear, in both conscious and non-conscious awareness (Davidson et al., 1999, Williams et al., 2006). It assigns salience to incoming emotional stimuli, and, through extensive reciprocal anatomical connections to prefrontal cortex, including OFC and dACC, modulates the expression of emotion, and behavior (Tekin and Cummings, 2002, Barbas, 2007, Bonelli and Cummings, 2007). The OFC acts in concert with the dACC to broadly regulate attention, expression of affect and impulse. Response inhibition, (as assessed by Go No-Go), is a function of the OFC, and selectively engages the OFC in fMRI studies (Casey et al., 1997, Horn et al., 2003). The dorsal and mid-ACC, in concert with the OFC, are engaged by tasks involving conflict resolution (competing choices), error detection, and decision-making (Carter et al. 2000). In concert with the ventro-medial PFC, they are also involved in emotion regulation (Hazlett et al., 2005; Phillips, Ladouceur, & Drevets, 2008). We predicted that hyper-arousal of amygdala during negative affective stimulation would modulate activity in specific cortical regulatory nodes, including OFC and dACC. In a negative affective context, hyper-activation of amygdala in BPD subjects is accompanied by diminished activation of OFC, and impaired behavioral performance, compared to healthy controls (Silbersweig et al., 2007). In a PET study, New et al. (2007) demonstrated diminished metabolic connectivity between amygdala and the OFC in patients with BPD, suggesting a functional vulnerability to disinhibited emotion and behavior. Greater modulation in BPD compared to control subjects between the amygdala, OFC and dACC during response inhibition under negative affective conditions may also reflect a relative decrease in strength of tonic cortical inhibition on limbic arousal. In the clinical context, diminished cortical inhibition during episodes of negative affective stress in BPD patients lowers the threshold for emotional and behavioral dyscontrol (Siever, 2008).
BPD subjects, compared to HC, also have increased amygdala modulation of the OFC in response to the neutral (> positive) affective condition. In fMRI studies, BPD subjects tend to project negative attributes onto neutral faces, and experience hyper-arousal of amygdala compared to healthy controls in response to neutral Ekman faces (Donegan et al., 2003).
Increased modulation by the amygdala of the parietal cortex/precuneus in BPD subjects may reflect a heightened response to specific task demands of the affective Go No-Go paradigm, especially the processing of visuo-spatial inputs and spatial attention. Increased modulation by the amygdala of the parietal cortex in HC subjects compared to BPD in the neutral condition, suggests a basic role for parietal cortex in task performance. The posterior parietal cortex is part of the central executive network. In concert with the dPFC, posterior parietal cortex is involved in rule based problem solving and decision making in the context of goal directed behavior (Menon, 2011). In the presence of negative affect, increased modulation by the amygdala of the parietal cortex in BPD may contribute to affective interference with task performance (Cavanna and Trimble, 2006).
Similarly, increased modulation of the basal ganglia (BG) in BPD compared to HC under negative affective conditions may reflect specific task demands requiring attention and reward-based decision-making, which are functions of the BG (Herrero et al., 2002, Voytek and Knight, 2010). Impulsive decision-making, and even suicidal behavior, have been associated with structural deficits in the BG (Vang et al., 2010, Dombrovski et al., 2012). Both parietal cortex and BG were activated in fMRI studies comparing BPD to HC subjects on the affective Go No-Go task (Soloff et al., 2015).
Cognitive defenses against affective interference
The “top down/bottom up” hypothesis of emotion regulation is supported by studies of cognitive defenses against affective interference in healthy subjects and patients with BPD. Cognitive defenses against affective interference include voluntary reappraisal, suppression and distancing techniques, and involuntary habituation. In fMRI studies involving healthy subjects, cognitive reappraisal and distancing in response to aversive stimuli are associated with increased cortical and decreased limbic activation (Koenigsberg et al., 2010, Schulze et al., 2011, Banks et al., 2007). In contrast, among BPD subjects, voluntary efforts at distancing emotional response to negative social cues are associated with failure to down-regulate amygdala or to activate cortical regulatory centers compared to controls (Koenigsberg et al., 2009). In a study of voluntary, effortful down-regulation of negative emotional responses to aversive IAPS scenes, BPD subjects showed decreased activity in the lt. OFC and increased activation in the insula, bilaterally (Schulze et al., 2011). Difficulties in emotion regulation during cognitive reappraisal were positively associated with insular activation and negatively associated with activity in the OFC (Schulze et al., 2011). PPI studies of voluntary cognitive reappraisal in healthy subjects report increased amygdala-frontal coupling (Banks et al. 2007).
In implicit habituation paradigms, healthy subjects demonstrate diminished emotional responses with repeated exposure to negative pictures, PPI analyses reveal increased insula-amygdala coupling associated with greater success in habituation (Denny et al., 2014). Among subjects with BPD, fMRI studies using the habituation paradigm demonstrate diminished activation in dACC and temporal gyri during repeated negative pictures, and increased activation in amygdala and insula (Koenigsberg et al., 2014). PPI analyses of habituation trials among subjects with BPD demonstrate diminished coupling of Lt. mid-posterior insula with amygdala bilaterally compared to healthy controls. Increases in coupling were associated with greater behavioral habituation (Koenigsberg et al., 2014).
Successful treatment in BPD depends, in part, on diminishing affective instability. Dialectical Behavior Therapy (DBT) is a cognitive behavioral treatment directed at enhancing emotion regulation in patients with BPD (Linehan, 1993). A one year treatment study of DBT in non-medicated patients with BPD produced normalization of amygdala hyper-reactivity to provocative IAPS pictures relative to healthy control subjects. Decreased reactivity of amygdala following DBT was associated with improved emotion regulation (Goodman et al., 2014).
Using the affectively valenced Go No-Go paradigm, these network-based analyses by PPI show greater differences between BPD and HC subjects than studies using conventional fMRI methods (Soloff et al., 2015). This increased sensitivity suggests that PPI network profiles more closely approximate brain network interactions, and provide a more meaningful assessment of dysfunctional neurobiology in psychiatric conditions (Friston et al., 1997; J. X. O’Reilly, M. W. Woolrich, T. E. J. Behrens, S. M. Smith, & H. Johansen-Berg, 2012). This increase in sensitivity appears even though PPI analyses constitute relatively limited models of network interactions. i.e. PPI models capture statistical dependencies between signals in the seed and its targets depending on the psychological context (Silverstein et al., In Press, Stephan, 2004). The choice of seed and the psychological context are free parameters of the model, and are chosen based on prior knowledge regarding task characteristics and the putative network relationships of the seed (Friston et al., 1997; Horwitz et al., 2005; Wadehra, Pruitt, Murphy, & Diwadkar, 2013; Woodcock et al., 2016). PPIs constitute a model of directed functional connectivity and are distinguishable from more sophisticated models of brain network function (e.g., dynamic causal models) that provide estimates of effective connectivity, or causal interactions exerted between neuronal populations (Friston et al., 2012; Diwadkar et al., 2014; Jagtap & Diwadkar, 2016). Given our focus on assessing “bottom-up” network profiles in BPD, the amygdala was a logical choice of seed. Additional seeds or psychological contexts would add complexity to our network model.
The BPD patient is vulnerable to emotion dysregulation, and to affective interference with cognitive functioning. These aberrant behaviors arise from discoverable interactions in the neural substrate and are related to dysfunctional brain networks. The vulnerability of BPD subjects to affective interference with impulse control may be due to specific network dysfunction related to amygdala hyper-arousal and its effects on prefrontal regulatory regions such as the OFC and dACC.
Limitations
A limitation of our approach was that the choice of seed was agnostic with respect to the sub-divisions of the amygdala. The amygdala is composed of functionally variegated clusters of nuclei (Pitkanen & Amaral, 1998) but their identification using MRI depends on high-resolution techniques (Hrybouski et al., 2016) or as we have previously shown (Barbour et al., 2010), approximations based on maximum probability maps of the structure’s sub-nuclei. In our analyses, the choice of seed was statistically motivated, and it is expected that the locations of statistically significance peaks (see Methods) are subject to inter-participant variability. Thus we are unable to (and do not) make claims regarding the specific anatomical pathways that might underpin the patterns of amygdala modulation that we reveal. Rather, our results speak to the functional transactions from the amygdala to its targets in the context of our task (Silverstein et al., 2016; Friston, 2011), and how these functional transactions are distorted in BPD.
We chose to study women with BPD because of the preponderance of women subjects with BPD compared to men in a clinic setting. However, gender differences in the borderline patient’s response to emotional stimuli, especially aggressive responses to negative stimuli, limit generalization of our results. e.g. Women with BPD tend to internalize reactive aggressive feelings (as in self-injury), while men with BPD tend to externalize aggression (as in antisocial behaviors) (Johnson et al., 2003). Such gender differences may result in differing fMRI activation patterns.
We also used healthy control subjects to compare to our BPD sample, introducing uncontrolled variables associated with BPD such as diagnostic co-morbidities, adverse life experiences (e.g. childhood abuse), and medication use. These uncontrolled variables could potentially confound results. The use of a clinical control group could reduce, though never fully eliminate, this limitation to interpretation.
The use of medication in nearly half of our BPD subjects poses an additional limitation to interpretation. All of our BPD subjects were currently symptomatic, and were seen in an ambulatory setting. We assessed the effects of medication use on activation metrics among our BPD subjects by comparing medicated to non-medicated subjects; however, this comparison is relatively underpowered and might not identify such effects if they existed.
Supplementary Material
Figure 2.
Negative relative to Neutral conditions. Clusters depict where the amygdala differentially modulates brain regions in BPD and HC (see Table 1 for statistical and location information). No effects were observed for HC, However, BPD were characterized by increased amygdala modulation of a large network of frontal, cingulate and parietal regions, with the effects more pronounced than those observed comparing neutral to positive contexts.
Highlights.
Emotion dysregulation in BPD may result from an imbalance in fronto-limbic network function.
PPI analysis demonstrated network dysfunction in BPD during an affective Go No-Go task.
In the negative condition, amygdala exerted greater modulation of its targets in BPD compared to HC subjects.
Amygdala modulation was greatest in regions relevant for processing response inhibition.
BPD vulnerability to affective interference with cognition is related to underlying dysfunctional brain networks.
Acknowledgments
This work was funded by the National Institute of Mental Health, grant # MH 048463 to Dr. Soloff.
Role of Funding Source
This work was supported by the National Institute of Mental Health (grant number MH 048063 to Dr. Soloff). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Contributors.
Paul Soloff was involved in the study design, analysis, interpretation and manuscript writing. Kristy Abraham, Karthik Ramaseshan, and Ashley Burgess were involved in data analysis. Vaibhav A. Diwadkar was involved in the study design, analysis, interpretation, and manuscript writing.
Conflicts of Interest:
There are no conflicts of interest with any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaro E, Barker GJ. Study design in fMRI: Basic principles. Brain Cogn. 2006;60(3):220–232. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. J Anat. 2007;211(2):237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour T, Murphy E, Pruitt P, Eickhoff SB, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA. Reduced intra-amygdala activity to positively valenced faces in adolescent schizophrenia offspring. Schizophr Res. 2010;123:126–136. doi: 10.1016/j.schres.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beblo T, Driessen M, Mertens M, Wingenfeld K, Piefke M, Rullkoetter N, Silva-Saavedra A, Mensebach C, Reddemann L, Rau H, Markowitsch HJ, Wulff H, Lange W, Berea C, Ollech I, Woermann FG. Functional MRI correlates of the recall of unresolved life events in borderline personality disorder. Psychol Med. 2006;36(6):845–856. doi: 10.1017/S0033291706007227. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Iverson SD. Borderline personality disorder, impulsivity and the orbitofrontal cortex. Am J Psychiatry. 2005;162:2360–2373. doi: 10.1176/appi.ajp.162.12.2360. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9(2):141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky BS, Groves SA, Oquendo MA, Mann JJ, Stanley B. Interpersonal precipitants and suicide attempts in borderline personality disorder. Suicide Life Threat Behav. 2006;36:313–322. doi: 10.1521/suli.2006.36.3.313. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW. Understanding the recognition of facial identity and facial expression. Nat Rev Neurosci. 2005;6(8):641–651. doi: 10.1038/nrn1724. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97(4):1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J Cogn Neurosci. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289(5479):591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Denny BT, Fan J, Liu X, Guerreri S, Mayson SJ, Rimsky L, New AS, Siever LJ, Koenigsberg HW. Insula-amygdala functional connectivity is correlated with habituation to repeated negative images. Soc Cogn Affect Neurosci. 2014 Nov;9(11):1660–1667. doi: 10.1093/scan/nst160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA. Critical perspectives on causality and inference in brain networks: Allusions, illusions, solutions?: Comment on: “Foundational perspectives on causality in large-scale brain networks” by M. Mannino and S.L. Bressler. Phys Life Rev. 2015 Dec;15:141–4. doi: 10.1016/j.plrev.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Bakshi N, Gupta G, Pruitt P, White R, Eickhoff SB. Dysfunction and dysconnection in cortical-striatal networks during sustained attention: Genetic risk for schizophrenia or bipolar disorder and its impact on brain network function. Front Psychiatry. 2014;5:50. doi: 10.3389/fpsyt.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Wadehra S, Pruitt P, Keshavan MS, Rajan U, Zajac-Benitez C, Eickhoff SB. Disordered cortico-limbic interactions during affective processing in children and adolescents at risk for schizophrenia revealed by fMRI and Dynamic Causal Modeling. Arch Gen Psychiatry. 2012;69(3):231–242. doi: 10.1001/archgenpsychiatry.2011.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychological Medicine. 2012;42(6):1203–1215. doi: 10.1017/S0033291711002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13(1):129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashen TH, Wexler BE. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiat. 2003;54(11):1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Kuo J, Kleindienst N, Welch SS, Reisch T, Reinhard I, Lieb K, Linehan MM, Bohus M. State affective instability in borderline personality disorder assessed by ambulatory monitoring. Psychological Medicine. 2007a;37(7):961–970. doi: 10.1017/S0033291706009706. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Kuo J, Schlotz W, Kleindienst N, Rosenthal MZ, Detterer L, Linehan MM, Bohus M. Distress and affective dysregulation in patients with borderline personality disorder: A psychophysiological ambulatory monitoring study. J Nerv Ment Dis. 2008;196(4):314–320. doi: 10.1097/NMD.0b013e31816a493f. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Welch SS, Grossman P, Reisch T, Linehan MM, Bohus M. Psychophysiological ambulatory assessment of affective dysregulation in borderline personality disorder. Psychiatry Res. 2007b;150(3):265–275. doi: 10.1016/j.psychres.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P, 4/2005 revision) Biometrics Research Department, New York State Psychiatric Institute; New York: 2005. [Google Scholar]
- Friedman A, Burgess A, Ramaseshan K, Easter P, Khatib D, Arnold PD, Hanna GL, Rosenberg DR, Diwadkar VA. Brain network dysfunction in obsessive-compulsive disorder induced by simple uni-manual behavior: The role of the dorsal anterior cingulate cortex. Psychiatry Res Neuroimaging. doi: 10.1016/j.pscychresns.2016.12.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Li B, Daunizeau J, Stephan KE. Network discovery with DCM. Neuroimage. 2012;56:1202–1221. doi: 10.1016/j.neuroimage.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Carpenter D, Tang CY, Goldstein KE, Avedon J, Fernandez N, Mascitelli KA, Blair NJ, New AS, Triebwasser J, Siever LJ, Hazlett EA. Dialectical behavior therapy alters emotion regulation and amygdala activity in patients with borderline personality disorder. J Psychiatr Res. 2014;57:108–16. doi: 10.1016/j.jpsychires.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Conceptual foundations. In: JGJ, editor. Handbook of Emotion Regulation. Guilford Press; New York: 2006. pp. 5–18. [Google Scholar]
- Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, Chen AD, Mitropoulou V, Minzenberg M, Siever LJ, Buchsbaum MS. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biol Psychiat. 2005;58(8):614–623. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Zhang J, New AS, Zelmanova Y, Goldstein KE, Haznedar MM, Meyerson D, Goodman M, Siever LJ, Chu KW. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol Psychiatry. 2012;72(6):448–56. doi: 10.1016/j.biopsych.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SC, Willmes K, Thron A, Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: A functional MRI study. Biol Psychiat. 2001;50(4):292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Child’s Nervous System. 2002;18(8):386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41(14):1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Warner B, Fitzer J, Tagamets MA, Husain FT, Long TW. Investigating the neural basis for functional and effective connectivity. Application to fMRI. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1093–1108. doi: 10.1098/rstb.2005.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrybouski S, Aghamohammadi-Sereshki A, Madan CR, Shafer AT, Baron CA, Seres P, Beaulieu C, Olsen F, Malykhin NV. Amygdala subnuclei response and connectivity during emotional processing. Neuroimage. 2016;133:98–110. doi: 10.1016/j.neuroimage.2016.02.056. [DOI] [PubMed] [Google Scholar]
- Jacob GA, Guenzler C, Zimmermann S, Scheel CN, Rusch N, Leonhart R, Nerb J, Lieb K. Time course of anger and other emotions in women with borderline personality disorder: A preliminary study. J Behav Ther Exp Psy. 2008;39(3):391–402. doi: 10.1016/j.jbtep.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Jagtap P, Diwadkar VA. Effective connectivity of ascending and descending frontalthalamic pathways during sustained attention: complex brain network interactions in adolescence. Hum Brain Mapp. 2016;37:2557–2570. doi: 10.1002/hbm.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Shea MT, Yen S, Battle CL, Zlotnick C, Sanislow CA, Grilo CM, Skodol AE, Bender DS, McGlashan TH, Gunderson JG, Zanarini MC. Gender differences in borderline personality disorder: Findings from the Collaborative Longitudinal Personality Disorders Study. Compr Psychiatry. 2003;44(4):284–292. doi: 10.1016/S0010-440X(03)00090-7. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Denny BT, Fan J, Liu X, Guerreri S, Mayson SJ, Rimsky L, New AS, Goodman M, Siever LJ. The neural correlates of anomalous habituation to negative emotional pictures in borderline and avoidant personality disorder patients. Am J Psychiatry. 2014;171(1):82–90. doi: 10.1176/appi.ajp.2013.13070852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Horwitz B. Investigating the neural basis for fMRI-based functional connectivity in a blocked design: application to interregional correlations and psycho-physiological interactions. Magn Reson Imaging. 2008;26(5):583–593. doi: 10.1016/j.mri.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise K, Pizzarello S, Dorantes C, Tecuta L, Guerreri S, Goodman M, New A, Flory J, Siever LJ. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48(6):1813–1822. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993;58(1–2):69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- Levine D, Marziali E, Hood J. Emotion processing in borderline personality disorders. J Nerv Ment Dis. 1997;185(4):240–246. doi: 10.1097/00005053-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Linehan M. Cognitive-behavioral treatment of borderline personality disorder. Guilford Press; New York: 1993. [Google Scholar]
- Loranger AW. DSM-IV and ICD-10 Interviews. Psychological Assessment Resources, Inc; Lutz, FL: 1999. International Personality Disorder Examination. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: An event-related fMRI study. Psychiatry Res. 2007;155(3):231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. Guilford Press; New York: 2006. pp. 87–89. [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, Trisdorfer R, Haznedar MM, Koenigsberg HW, Flory J, Siever LJ. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32(7):1629–1640. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7(5):604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13 (9) 2008;829:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Reisch T, Ebner-Priemer U, Tschacher W, Bohus M, Linehan M. Sequences of emotions in patients with borderline personality disorder. Acta Psychiatr Scand. 2008;118(1):42–48. doi: 10.1111/j.1600-0447.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Bremner JD. Neuroimaging in borderline personality disorder. J Psychiatr Res. 2006;40(5):419–427. doi: 10.1016/j.jpsychires.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl CG, Elzinga BM, Vermetten E, Sanislow C, McGlashan TH, Bremner JD. Neural correlates of memories of abandonment in women with and without borderline personality disorder. Biol Psychiat. 2003;54(2):142–151. doi: 10.1016/s0006-3223(02)01720-1. [DOI] [PubMed] [Google Scholar]
- Schulze L, Domes G, Kruger A, Berger C, Fleischer M, Prehn K, Schmahl C, Grossmann A, Hauenstein K, Herpertz SC. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol Psychiat. 2011;69(6):564–573. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Schulze L, Schmahl C, Niedtfeld I. Neural Correlates of Disturbed Emotion Processing in Borderline Personality Disorder: A Multimodal Meta-Analysis. Biol Psychiatry. 2016;79(2):97–106. doi: 10.1016/j.biopsych.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Jacob G, Lieb K, Tuescher O. Impulsivity in borderline personality disorder: a matter of disturbed impulse control or a facet of emotional dysregulation? Curr Psychiatry Rep. 2013;15(2):339. doi: 10.1007/s11920-012-0339-y. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Jung P, Krause-Utz A, Lieb K, Schmahl C, Tuescher O. Frontal dysfunctions of impulse control - a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Front Hum Neurosci. 2014;8:698. doi: 10.3389/fnhum.2014.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165(4):429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, Brendel G, Pan H, Beutel M, Pavony MT, Epstein J, Lenzenweger MF, Thomas KM, Posner MI, Stern E. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry. 2007;164(12):1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Silverstein B, Bressler S, Diwadkar VA. Inferring the dysconnection syndrome in schizophrenia: Interpretational considerations on methods for the network analyses of fMRI data. Frontiers in Psychiatry. 2016;7:132. doi: 10.3389/fpsyt.2016.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res. 2012;46(4):516–525. doi: 10.1016/j.jpsychires.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, White R, Diwadkar VA. Impulsivity, aggression and brain structure in high and low lethality suicide attempters with borderline personality disorder. Psychiatry Research: Neuroimaging. 2014;222(3):131–139. doi: 10.1016/j.pscychresns.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, White R, Omari A, Ramaseshan K, Diwadkar VA. Affective context interferes with brain responses during cognitive processing in borderline personality disorder: fMRI evidence. Psychiatry Res. 2015;233(1):23–35. doi: 10.1016/j.pscychresns.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE. On the role of general system theory for functional neuroimaging. J Anat. 2004;205:443–470. doi: 10.1111/j.0021-8782.2004.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Schlagenhauf F, Huys QJ, Raman S, Aponte EA, Brodersen KH, Rigoux L, Moran RJ, Daunizeau J, Dolan RJ, Friston KJ, Heinz A. Computational neuroimaging strategies for single patient predictions. Neuroimage. 2016 Jun 21; doi: 10.1016/j.neuroimage.2016.06.038. S1053-8119(16)30287-7. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. J Psychosom Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Solhan MB, Tragesser SL, Jahng S, Wood PK, Piasecki TM, Watson D. Affective instability: measuring a core feature of borderline personality disorder with ecological momentary assessment. J Abnorm Psychol. 2008;117(3):647–661. doi: 10.1037/a0012532. [DOI] [PubMed] [Google Scholar]
- Vang FJ, Ryding E, Traskman-Bendz L, van Westen D, Lindstrom MB. Size of basal ganglia in suicide attempters, and its association with temperament and serotonin transporter density. Psychiatry Research: Neuroimaging. 2010;183(2):177–179. doi: 10.1016/j.pscychresns.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Voytek B, Knight RT. Prefrontal cortex and basal ganglia contributions to visual working memory. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(42):18167–18172. doi: 10.1073/pnas.1007277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadehra S, Pruitt P, Murphy ER, Diwadkar VA. Network dysfunction during associative learning in schizophrenia: Increased activation, but decreased connectivity: an fMRI study. Schizophr Res. 2013;148(1–3):38–49. doi: 10.1016/j.schres.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Ward BD. AFNI 3d Deconvolve Documentation. Medical College of Wisconsin; 2000. Simultaneous inference for fMRI data. [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. 2006;26(36):9264–9271. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Elzinga B, Schmahl C. Emotions and memory in borderline personality disorder. Psychopathology. 2014;47(2):71–85. doi: 10.1159/000356360. [DOI] [PubMed] [Google Scholar]
- Woodcock EA, Wadehra S, Diwadkar VA. Network profiles of the dorsal anterior cingulate and dorsal prefrontal cortex in schizophrenia during hippocampal-based associative memory. Front Systems Neurosci. 2016;10:32. doi: 10.3389/fnsys.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S, Shea MT, Sanislow CA, Grilo CM, Skodol AE, Gunderson JG, McGlashan TH, Zanarini MC, Morey LC. Borderline personality disorder criteria associated with prospectively observed suicidal behavior. Am J Psychiatry. 2004;161:1296–1298. doi: 10.1176/appi.ajp.161.7.1296. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Gunderson JG, Frankenburg FR, Chauncey DL. The Revised Diagnostic Interview for Borderlines: Discriminating BPD from other Axis II disorders. J Pers Disord. 1989;3(1):10–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.