Abstract
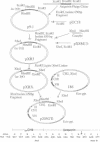
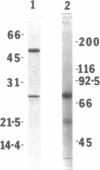
A fusion protein has been expressed from the relevant genes in mammalian cells consisting of the photoprotein aequorin and an anti-4-hydroxy-3-nitrophenacetyl antibody gene. This chimeric antibody has allowed the development of a sensitive luminescent immunoassay. Initially the cDNA of the photoprotein aequorin from Aequorea victoria was cloned and expressed in Escherichia coli. The gene was expressed as apoaequorin and, by using luciferin isolated from Renilla reniformis, its activity was found essentially identical to native aequorin. The aequorin gene was subcloned into a mammalian expression vector to produce a fusion protein directing secretion of apoaequorin; the aequorin gene was fused to the 3' terminus of an immunoglobulin heavy-chain gene that directed expression of an anti-4-hydroxy-3-nitrophenacetyl antibody. The gene fusion contained the variable region, the constant region domain 1, and part of domain 2 for the IgG2b mouse immunoglobulin, followed by the aequorin gene. Transfection of the chimeric gene into a cell line expressing the complementary lambda 1 light chain, J558L, allowed recovery of a chimeric antibody with binding specificity for the 4-hydroxy-3-nitrophenacetyl group and the related 4-hydroxy-3-iodo-5-nitrophenacetyl hapten. The Ca2(+)-dependent bioluminescent activity of aequorin was also recovered.
Full text
PDF

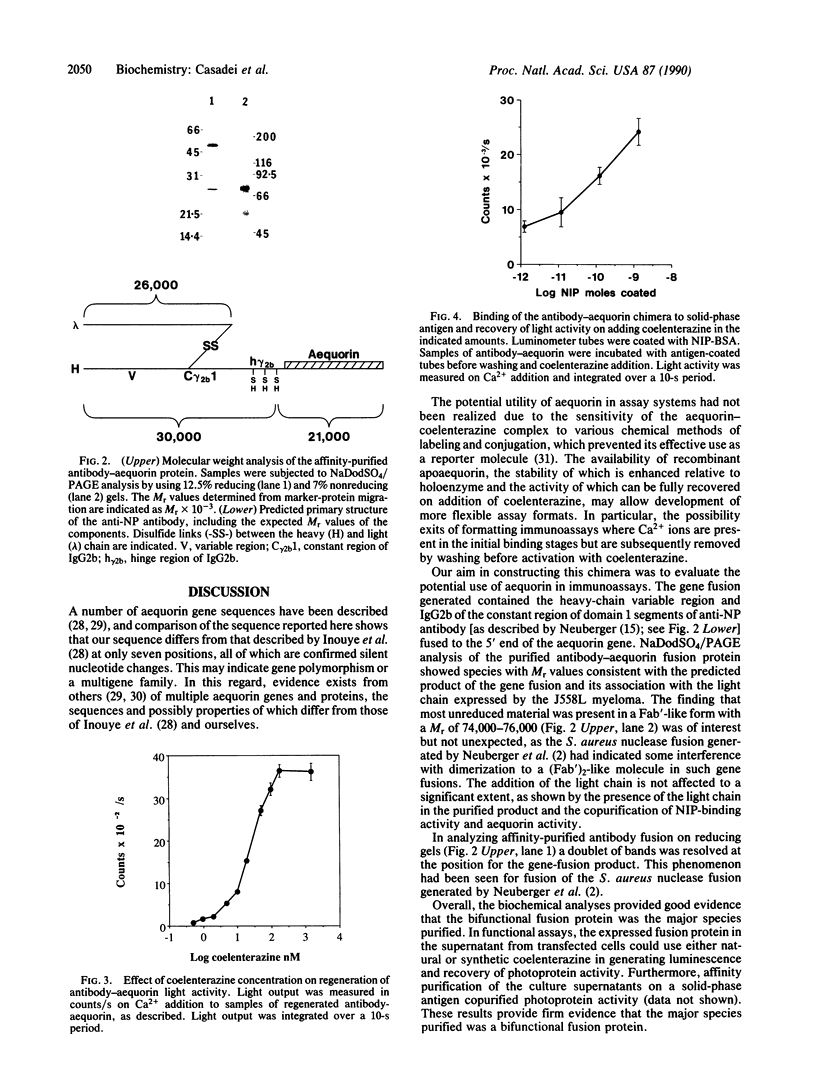

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Better M., Chang C. P., Robinson R. R., Horwitz A. H. Escherichia coli secretion of an active chimeric antibody fragment. Science. 1988 May 20;240(4855):1041–1043. doi: 10.1126/science.3285471. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Hallett M. B., Weeks I. Chemiluminescence as an analytical tool in cell biology and medicine. Methods Biochem Anal. 1985;31:317–416. doi: 10.1002/9780470110522.ch7. [DOI] [PubMed] [Google Scholar]
- Chaudhary V. K., Queen C., Junghans R. P., Waldmann T. A., FitzGerald D. J., Pastan I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989 Jun 1;339(6223):394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Faust C. H., Jr, Heim I., Moore J. Murine myeloma immunoglobulin heavy-chain mRNA. Isolation, partial purification, and characterization of gamma1, gamma2a, gamma2b, gamma3, micron and alpha heavy-chain mRNA'S. Biochemistry. 1979 Mar 20;18(6):1106–1119. doi: 10.1021/bi00573a027. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hori K., Charbonneau H., Hart R. C., Cormier M. J. Structure of native Renilla reinformis luciferin. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4285–4287. doi: 10.1073/pnas.74.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. H., Chang C. P., Better M., Hellstrom K. E., Robinson R. R. Secretion of functional antibody and Fab fragment from yeast cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8678–8682. doi: 10.1073/pnas.85.22.8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Noguchi M., Sakaki Y., Takagi Y., Miyata T., Iwanaga S., Miyata T., Tsuji F. I. Cloning and sequence analysis of cDNA for the luminescent protein aequorin. Proc Natl Acad Sci U S A. 1985 May;82(10):3154–3158. doi: 10.1073/pnas.82.10.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenten J. H., Molgaard H. V., Houghton M., Derbyshire R. B., Viney J., Bell L. O., Gould H. J. Cloning and sequence determination of the gene for the human immunoglobulin epsilon chain expressed in a myeloma cell line. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6661–6665. doi: 10.1073/pnas.79.21.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morin J. G., Hastings J. W. Biochemistry of the bioluminescence of colonial hydroids and other coelenterates. J Cell Physiol. 1971 Jun;77(3):305–312. doi: 10.1002/jcp.1040770304. [DOI] [PubMed] [Google Scholar]
- Moussebois C. H., Van Snick J., Masson P. L. A new method for coupling 4-hydroxy 3-iodo 5-nitrophenacetyl and 4-hydroxy 5-nitrophenacetyl to carrier proteins. J Immunol Methods. 1982 Oct 29;54(2):159–164. doi: 10.1016/0022-1759(82)90056-4. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Fox R. O. Recombinant antibodies possessing novel effector functions. Nature. 1984 Dec 13;312(5995):604–608. doi: 10.1038/312604a0. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher D. C., McCann R. O., Longiaru M., Cormier M. J. Sequence comparisons of complementary DNAs encoding aequorin isotypes. Biochemistry. 1987 Mar 10;26(5):1326–1332. doi: 10.1021/bi00379a019. [DOI] [PubMed] [Google Scholar]
- Prasher D., McCann R. O., Cormier M. J. Cloning and expression of the cDNA coding for aequorin, a bioluminescent calcium-binding protein. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1259–1268. doi: 10.1016/0006-291x(85)90321-3. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Regeneration of the photoprotein aequorin. Nature. 1975 Jul 17;256(5514):236–238. doi: 10.1038/256236a0. [DOI] [PubMed] [Google Scholar]
- Skerra A., Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988 May 20;240(4855):1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- Tsuji F. I., Inouye S., Goto T., Sakaki Y. Site-specific mutagenesis of the calcium-binding photoprotein aequorin. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8107–8111. doi: 10.1073/pnas.83.21.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]