Abstract
The production of Prostaglandin E2 (PGE2) is elevated in human breast cancer cells. The abnormal expression of COX-2, which is involved in the synthesis of PGE2, was recently reported as a critical determinant for invasiveness of human breast cancer cells. Autocrine and paracrine PGE2-mediated stimulation of the PGE2 receptor EP4 transduces multiple signaling pathways leading to diverse patho-physiological effects, including tumor cell invasion and metastasis. It is known that PGE2-induced EP4 activation can transactivate the intracellular signaling pathway of the epidermal growth factor receptor (EGFR). In malignant cancer cells, EGFR pathway activation promotes invadopodia protrusions, which further leads to degradation of the surrounding extracellular matrix (ECM). Despite the known influence of EP4 on the EGFR signaling pathway, the effect of EP4 stimulation on invadopodia formation in human breast cancer was never tested directly. Here we demonstrate the involvement of EP4 in invasion and its effect on invadopodia in breast cancer MDA-MB-231 cells using 2D invadopodia and 3D invasion in vitro assays as well as intravital microscopy. The results show that stimulation with the selective EP4 agonist CAY10598 or PGE2 promotes invadopodia-mediated degradation of the ECM, as well as the invasion of breast cancer cells in in vitro models. The effect on matrix degradation can be abrogated via direct inhibition of EP4 signaling as well as via inhibition of EGFR tyrosine kinase, indicating that EP4-mediated effects on invadopodia-driven degradation are EGFR dependent. Finally, using xenograft mouse models, we show that short-term systemic treatment with CAY10598 results in a >9-fold increase in the number of invadopodia. These findings highlight the importance of further investigation on the role of EP4-EGFR crosstalk in invadopodia formation.
Keywords: invadopodia, invasion, prostaglandin E2, EP4, spheroids, intravital microscopy
INTRODUCTION
During tumor progression, cancer cells develop the ability to degrade the extracellular matrix that allows them to move through the surrounding tissue and to breach blood vessel walls (Martin et al., 2000). This results in intravasation, the process of cancer cells entry into the blood vessels. Cancer cells further spread to secondary organs, where they can form metastases that are the main cause of patient mortality in most solid cancers.
Invadopodia, protrusions rich in F-actin and capable of degrading a number of proteins in extracellular matrix (ECM), have been hypothesized to facilitate the breach of the basement membrane necessary for several steps in metastasis (Beaty and Condeelis, 2014). Recent studies revealed that invadopodia assembly is directly molecularly linked with metastasis in mouse models and humans (Eckert et al., 2011; Gligorijevic et al., 2012). Further, real-time imaging in mice provided evidence that invadopodia are essential for both intravasation and extravasation of cancer cells into/from the blood vessels (Gligorijevic et al., 2014; Leong et al., 2014).
The assembly and maturation of invadopodia can be initiated by chemical stimuli such as the epidermal growth factor (EGF), as well as the mechanical signals from extracellular matrix (ECM). Upon binding, EGF activates the EGF receptor (EGFR), which further activates several intracellular signaling pathways (Cortesio et al., 2008), including the Src kinase which mediates the assembly of the invadopodium precursor and the phosphoinositide-3 kinase (PI-3K), necessary for anchoring the precursor to the cell membrane (Beaty and Condeelis, 2014). In addition to EGF, several other growth factors have been shown to initiate invadopodia formation. For example, transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF) and heparin binding EGF (HB-EGF) can induce invadopodia formation in several types of cancer cells (Díaz et al., 2013; Eckert et al., 2011; Pignatelli et al., 2012; Rajadurai et al., 2012; Yamaguchi et al., 2005). Moreover, autocrine signals can lead to the induction of invadopodium formation (Patsialou et al., 2009).
In addition to growth factors, a number of signals present in the tumor microenvironment, such as hypoxia, pH or direct interactions with stromal cells were shown to be associated with invasion and metastasis of cancer cells (Gould and Courtneidge, 2014). The production of the pro-inflammatory lipid Prostaglandin E2 (PGE2) is elevated in breast cancer due to high expression of the prostaglandin-endoperoxidase synthase 2 (COX2), which is a critical determinant for invasiveness of human breast cancer cells (Bocca et al., 2014; Miglietta et al., 2010; Park et al., 2012). However, the direct link between elevated PGE2 and increased invasiveness of human breast cancer cells remains elusive. PGE2 activity is mediated by four G-protein coupled receptor isotypes called EP1, -2, -3 and -4 (Narumiya et al., 1999; Pierce and Regan, 1998). The activation of the PGE2 receptor EP4 subtype (EP4) by its ligand PGE2 is known as a mechanism that increases the invasive capability of cancer cells (Konya et al., 2013). Hence, it is likely that EP4 is involved in the PGE2-mediated increase of cancer invasiveness. EP4 is capable of activating distinct intracellular signaling pathways. Its signaling is associated with the stimulating G alpha protein (Gαs), which leads to an increase of the intracellular secondary messenger cyclic adenosine monophosphate (cAMP) (Regan, 2003). Furthermore, EP4 is also capable of inhibiting the formation of cAMP by activating the inhibiting G alpha protein (Gαi) (Fujino and Regan, 2006).
The activated EP4 can be phosphorylated by a G protein-coupled receptor kinase (GRK), which enables the recruitment of β-arrestin to EP4 (Konya et al., 2013; Yokoyama et al., 2013). The EP4/β-arrestin complex then activates the membrane-bound Src, which is able to transactivate the EGFR receptor, thus showing that the EP4/β-arrestin/Src complex facilitates cell migration and metastasis (Buchanan et al., 2006; Kim et al., 2010). Moreover, it has been demonstrated that Src, activated by the EP4 signaling pathway, can also activate PI-3K (Yokoyama et al., 2013). This signaling cascade was demonstrated previously to be active in the migration and metastasis processes in colorectal carcinomas (Buchanan et al., 2006; Konya et al., 2013).
Taken collectively, these data demonstrate that stimulation of EP4 increases invasive behavior of cancer cells and that the EP4 and EGFR pathways cross-talk. However, the direct relationship between EP4 stimulation by PGE2 and invadopodia formation has not been studied yet. Here we focus on testing if EP4 stimulation influences invadopodia assembly and ECM degradation. Using in vitro invadopodia and spheroid assays as well as intravital imaging, our results provide novel insights into the effects of EP4 activation on breast cancer invasion. Understanding the interplay between different signaling receptors promoting cancer cell invasive behavior may provide novel targets to prevent or reduce cancer dissemination and metastasis.
RESULTS
Biphasic dose response of EGFR or EP4 on invasion of MDA-MB-231 cells
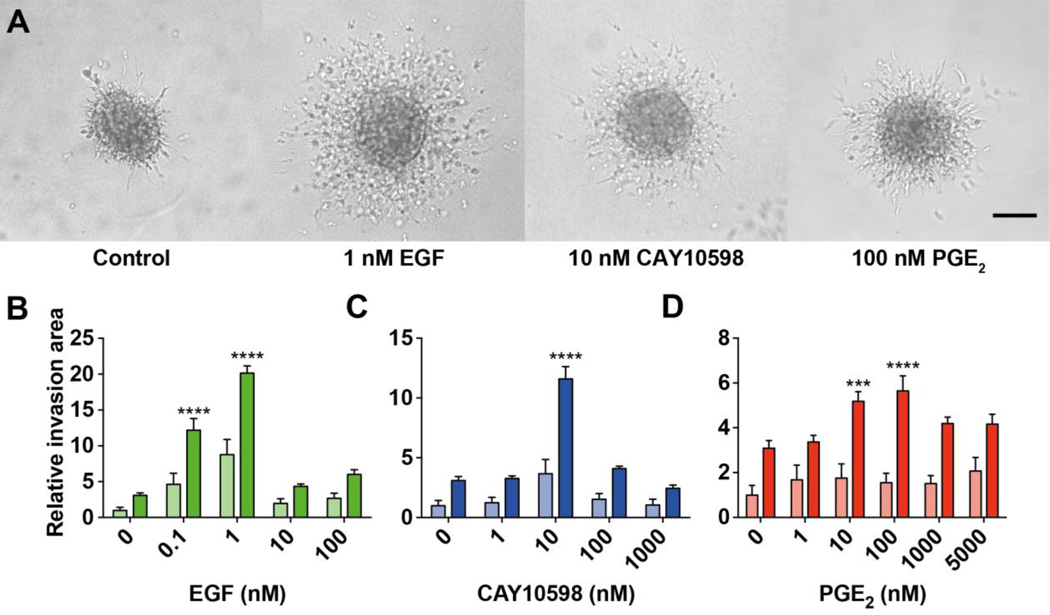
Cell cultures in 3D, including spheroids and related assays, have been exhaustively shown to better mimic physiological effects on cultured cells compared to 2D assays (Boudreau et al., 1996; Cukierman et al., 2002). Moreover, since the demonstration that MMP activity is necessary for the invasion of spheroids into the acid-soluble rat-tail collagen I (Sodek et al., 2008), spheroids are replacing the previous transwell assay, becoming a new standard for studying the invasiveness of cells (Friedl et al., 2012). Figure 1A shows representative spheroids of control (starved cells with no stimulation) as well as MDA-MB-231 cells stimulated by EGF-, CAY10598- (EP4 agonist) and PGE2. Dose-response curves of EGF, CAY10598 and PGE2 were obtained after 24 (light bars) and 48 h (dark bars, Figure 1B–D). To compare differences in the extent of cell invasion, the relative invasion area was calculated (see Materials and Methods; Figure S1). Figure 1B shows 1 nM is the peak of the biphasic response to EGF- stimulated invasion. EP4 agonist CAY10598 and PGE2 treatments showed peaks in their responses at a dose of 10 nM and 100 nM, respectively (Figure 1C, D). Following dose response determination, we proceeded with optimal doses determined, optimizing and automating the spheroid assay towards more precise high-throughput results.
Figure 1. Dosage optimization of EGF, CAY10598 and PGE2 in spheroid invasion assay.
MDA-MB-231 spheroids were stimulated with EGF, CAY10598 (CAY) or PGE2 to investigate involvement of EP4 and EGFR in invasive capability and to determine the optimal dosage. (A) Representative phase-contrast images of invasion of MDA-MB-231 spheroids (1,000 cells) embedded in rat tail collagen type I (4 mg/ml) control or stimulated with 1 nM EGF, 10 nM CAY10598 or 100 nM PGE2 for 48 h. Scale bar= 100 µm (B–D) Light bars, 24 h treatment; dark bars, 48 h treatment. Values of all graphs present the mean + SEM of ten individual spheroids from one experiment. Two-way ANOVA was used for the statistical analysis. The asterisks show the significance of the difference compared to the 0 nM condition. *** P<0.001; **** P<0.0001.
EGFR and EP4 stimulation significantly increase the invasive capability of MDA-MB-231 cells
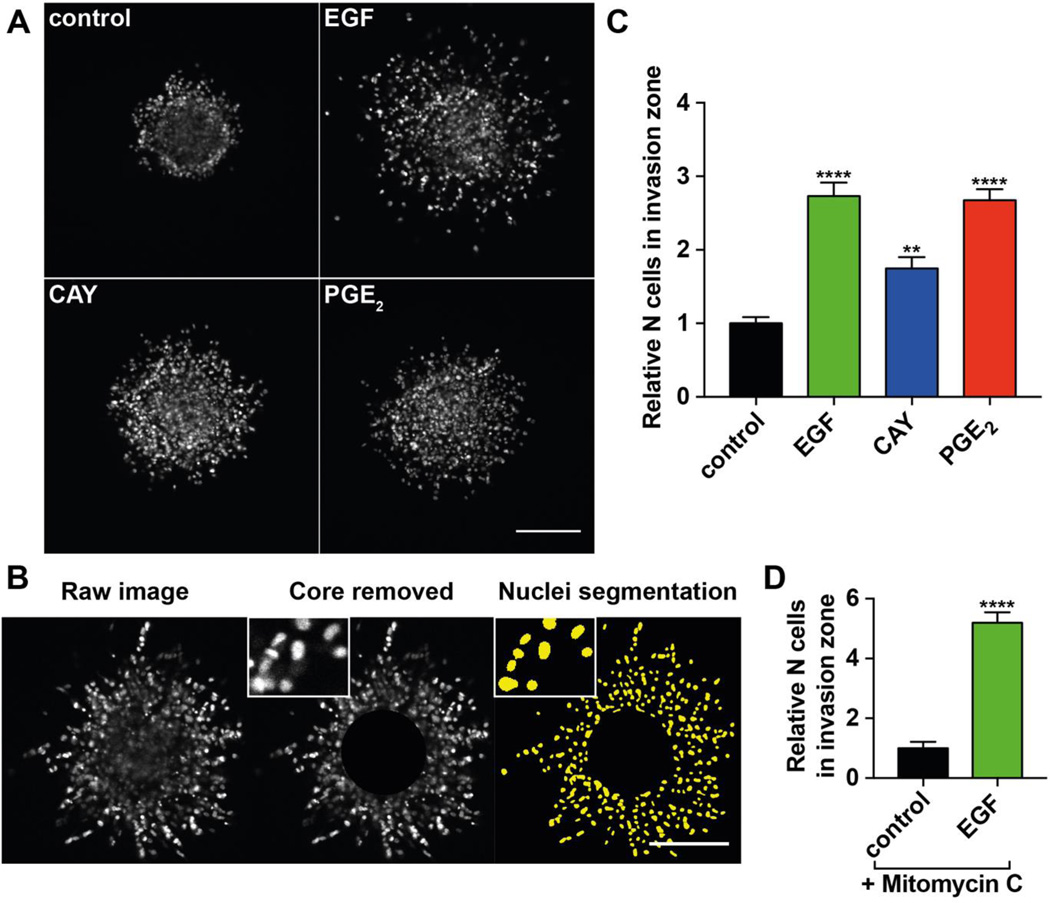
We next stained spheroids after 48 h with DAPI. This allowed a more accurate examination of invasion by quantification of nuclei in the invasion zone. Figure 2A shows representative images of control condition (starved cells with addition of DMSO), as well as EGF-, CAY10598- and PGE2-stimulated conditions. In Figure 2B, the automated analysis pipeline is demonstrated. First, the core is subtracted from the image followed by nuclei segmentation and quantification (see Materials and Methods). Under stimulation with EGF, a higher number of cells invade into the ECM and the invasion distance is higher compared to the control condition (Figure 2A, C). Similarly, the number of invading MDA-MB-231 cells was also significantly elevated by stimulation with CAY10598 or PGE2, suggesting that stimulation of EP4 induces invasion in 3D (Figure 2A, C). In the presence of the cell proliferation-inhibiting agent Mitomycin C, EGF stimulation still increases the number of cells in the invasion zone (Figure 2D) (An et al., 2015). This clearly indicates that the observed effect is due to invasion and not proliferation.
Figure 2. Spheroid invasion assay.
(A) Representative images of MDA-MB-231 spheroids (1,000 cells) embedded in collagen I (5 mg/ml). Control spheroid treated with 1 nM DMSO and spheroids stimulated with 1 nM EGF, 10 nM CAY10598 (CAY) or 1 µM PGE2 for 48 h were fixed and stained with DAPI. Scale bar= 200 µm. (B) Image processing workflow. The core of the spheroid was removed leaving only the cells in the invasion area for quantification. The nuclei were segmented and counted. (C) Quantification of cells in the invasion zone of spheroids shown in (A). Values present the mean + SEM of 15 – 25 spheroids per condition. One-way ANOVA was used for the statistical analysis. The asterisks show the significance of the difference compared to the DMSO control. ** P<0.01; **** P<0.0001. (D) Invasion assay in the presence of cell proliferation-inhibiting agent Mitomycin C (1 µg/µl). Values present the mean + SEM of 6 spheroids per condition. Students t-test was used for the statistical analysis. The asterisks show the significance of the difference compared to the DMSO control. **** P<0.0001.
EGFR and EP4 affect invadopodia maturation and degradative capability
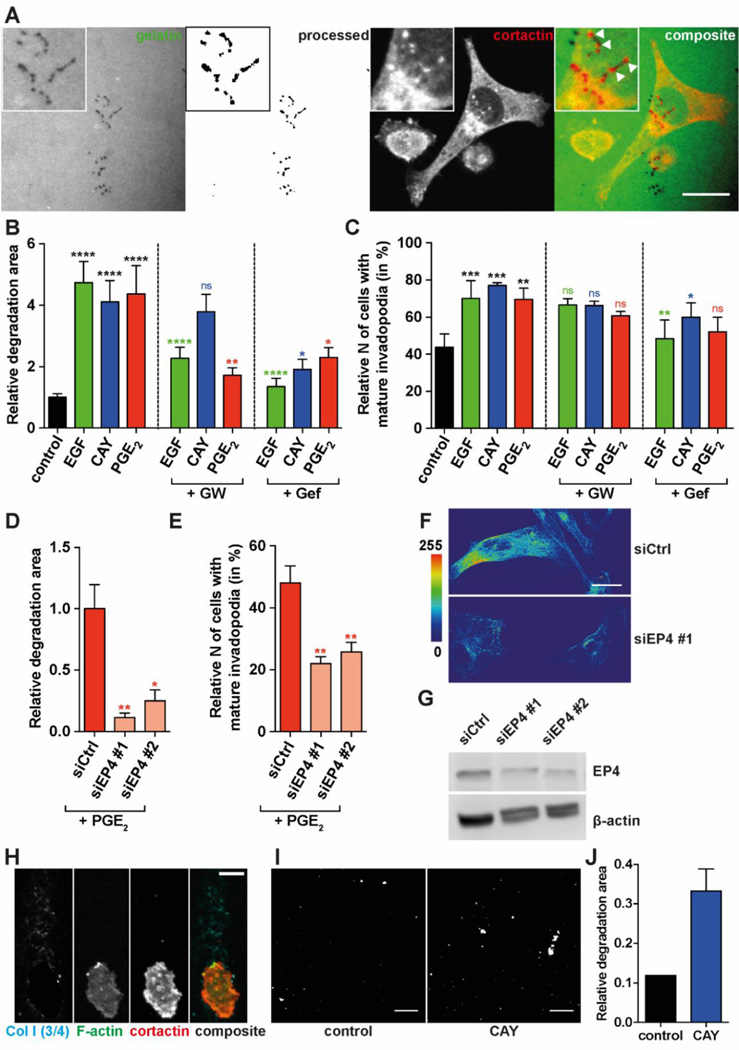
To investigate the role of EP4 in invadopodia stimulation, MDA-MB-231 cells plated on fluorescent gelatin were fixed after 16 hours (Figure 3A, left two panels) and the number of cells with mature invadopodia as well as the cumulative area of gelatin degradation were measured. Degradative activity of invadopodia was detected as non-fluorescent (dark) punctae inside fluorescent gelatin (Figure 3A, left). Mature invadopodia are represented as punctae with co-localized gelatin degradation and cortactin staining (Figure 3A, panels on the right). The measurements of the degradation area indicated that EGF, CAY10598 and PGE2 increase matrix degradation (Figure 3B). Similar measurements of invadopodia precursors did not show significant increase upon stimulation (data not shown). As expected, inhibition of the EGFR tyrosine kinase with gefitinib (Iressa) reversed the effect of EGF on degradation capability. Interestingly, the effect of CAY10598 and PGE2 on the degradation capability was also inhibited by gefitinib. This indicates that the EGFR tyrosine kinase is crucial for EP4-mediated stimulation of invadopodia. Inhibition of EP4 with GW627368X, a selective competitive antagonist, reverses the effect of PGE2 as well as EGF on degradation, indicating a crosstalk between EGFR and EP4. In contrast, GW627368X did not block CAY10598-induced stimulation significantly. This might be due to higher on/off ratio of EP4 and CAY10598 compared to EP4 and GW627368X, caused by differential binding affinities for EP4. In line with the gelatin degradation results, the number of cells with mature invadopodia was also significantly affected (Figure 3C). Again, GW627368X or gefitinib were used in order to test if the effect on invadopodia by the stimulants can be inhibited. Only inhibition of the EGFR was able to reverse the effect of EGF or CAY10598 significantly. To more directly test EP4 and PGE2 involvement in the invadopodia, we have further used siRNA-mediated knock-down of EP4. Both the degradative effect and the effect on the number of cells with mature invadopodia by PGE2 stimulation were abrogated by EP4 knock-down (Figure 3D, E). The knock-down of EP4 was confirmed by immunofluorescent staining and Western Blot of siCtrl- or siEP4-transfected MDA-MB-231 cells (Figure 3F, G). While the knock-down levels (51% for siEP4 #1 and 52% for siEP4 #2) seem to directly correlate to the number of mature invadopodia, both siEP4 knock-downs showed almost complete abrogation of the 16h-cumulative degradation.
Figure 3. Degradation and invadopodia formation of MDA-MB-231 cells in 2D and 3D.
Serum-starved MDA-MB-231 cells were plated on Oregon Green 488-labeled gelatin for 16 h; mature invadopodia were identified as co-localized punctae of gelatin degradation and cortactin. Examples are indicated by arrowheads. (A) Representative image of fluorescent gelatin, raw or processed, underneath the cells (1st and 2nd from left); MDA-MB-231 cell with invadopodia stained for cortactin on top of gelatin layer (3rd from left); composite of fluorescent gelatin and cortactin-stained cell (4th from left). Scale bar= 20 µm. Inserts zoom into mature invadopodia that degraded gelatin underneath; white arrows in composite insert point to individual invadopodia. The area of degradation (B) and the number of cells with mature invadopodia (C) were determined. Values present the mean + SEM of ≥ 9 images from 3 biological repeats. The area of degradation (D) and the number of cells with mature invadopodia (E) was also determined in cells transfected with siCtrl, siEP4 #1/2, and treated with PGE2. One-way ANOVA was used for the statistical analysis of area of degradation. Generalized linear regression model was used for the statistical analysis of number of cells with mature invadopodia. The color of the asterisks indicate the corresponding single treatment condition to which the statistical difference is displayed. * P<0.05; ** P<0.01; *** P<0.001; **** P<0.0001. (F) Representative pseudocolored images of MDA-MB-231 cells transfected with siCtrl or siEP4 #1 and stained for EP4 antibody. Pseudocolor look up table is shown on the left. (EP4 staining of siEP4 #1 and #2 showed similar results, not shown). (G) EP4 protein expression upon transfection with siCtrl, siEP4 #1/2 analyzed by Western Blot. Ratios of EP4/actin are 0.51 for siEP4 #1 and 0.52 siEP4 #2 compared to siCtrl. (H) Zoom into a cell in the invasion zone of MDA-MB-231 spheroids at 48 h and stained for collagen I (3/4), actin and cortactin. Scale bar= 10 µm. (I) Processed and thresholded images of collagen I (3/4) staining of spheroids- control and 10 nM CAY10598 (CAY) treatment. The degraded collagen is shown in white. Scale bar= 50 µm. (J) Quantification of degradation area of images from (I).
To assess the effect of EP4 stimulation on degradative capability in 3D spheroid assay, MDA-MB-231 spheroids were fixed and labeled at 48h. They were further stained for cortactin, F-actin and collagen I (3/4) antibody that binds to degraded collagen fibrils and demonstrated the trace of the invading cancer cell (Figure 3H) (Gligorijevic et al., 2012; Wolf et al., 2007). Similar cell paths were previously reported as single cell invasion tunnels and found to enable MMP-independent migration of following cancer cells (Fisher et al., 2009). Collagen I 3/4 staining of control and CAY10598-treated spheroids were compared (Figure 3I, J), indicating that stimulation with CAY10598 increases the degradative capability in 3D. Taken together, these results clearly suggest an association between stimulation of EP4 and an increased matrix degradation as well as an elevated level of invadopodia-driven ECM degradation.
Intravital Microscopy suggests role of EP4 in invadopodia stimulation in breast cancer cells
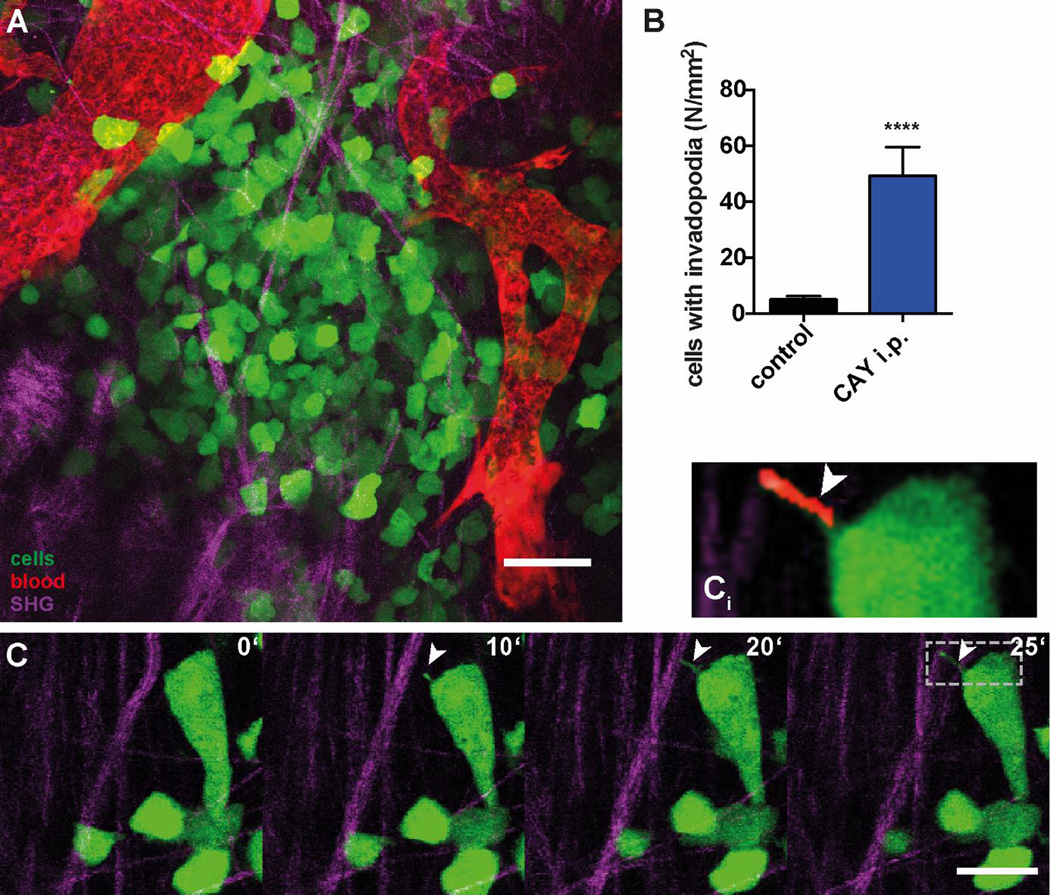
Multiphoton intravital imaging of Dendra2-MDA-MB-231 xenografts (Figure 4A) confirmed the results of in vitro assays (Figure 3). Time-lapse imaging of the xenografts was done exclusively in perivascular regions containing major blood vessels and high density of collagen fibers, where invadopodia can be detected by morphodynamic analysis (Figure 4A; Video S1). EP4 stimulation led to the 9-fold increase in the formation of invadopodia (Figure 4B) in perivascular niches (Gligorijevic et al., 2014), following a systemic delivery via intraperitoneal injection 4h earlier. The stills from invadopodia time-lapse show extension of invadopodia in vivo (Figure 4C; Video S2).
Figure 4. Systemic treatment with CAY10598 links EP4 stimulation with invadopodia formation in vivo.
(A) Representative image (still of Video S1) of Dendra2-MDA-MB-231 xenograft in mammary fat pad of mice (green: Dendra2-MDA-MB-231 cells, magenta: SHG signal from collagen I fibers, red: blood vessels). (B) Quantification of invadopodia in 4D stacks of Dendra2-MDA-MB-231 xenograft control animals and 4 h after i.p. injection of CAY10598 (CAY). Values present the mean + SEM of ≥ 5 stacks from ≥ 2 animals. Mann-Whitney test was used for the statistical analysis; **** P<0.0001. (C) Stills from time-lapse (Video S2) of a cell extending invadopodium. (Ci) Insert zoom in to invadopodium; red denotes invadopodium detected by morphodynamic analysis. Scale bar= 20 µm.
DISCUSSION
In this study, we investigated the role of EP4 on invasion and invadopodia in breast cancer cells. Our results demonstrate that EP4 activation promotes invadopodia-driven ECM degradation, in turn facilitating future intravasation and metastasis of breast cancer cells (Gligorijevic et al., 2012; Gligorijevic et al., 2014).
Our data demonstrates that EP4 stimulation elevates the invasive capability of breast cancer cells in ways similar to EGFR stimulation. Interestingly, both EGF and CAY10598-induced stimulation of EGFR and EP4 exhibit biphasic dose responses. It was recently suggested that two phosphorylation sites at Src are involved in this dose-dependent switch in EGF signaling, Src-Y416, which activates Src at low EGF concentrations, and the Src-527, which inactivates it at high EGF concentrations (X. Zhang et al., 2012). The fact that CAY10598 also exhibits the biphasic response may suggest that EP4 mediates the effect on the invasive capability via Src. The presence of EP4 and EGFR signaling cross-talk is supported by the fact that inhibition of EGFR reverses the positive effect of EP4 stimulation on matrix degradation, and the other way around. A number of papers proposed the EGFR pathway as effector for EP4-mediated signaling pathways in cancer (Du et al., 2015; Parida et al., 2016; Tveteraas et al., 2012; Yokoyama et al., 2013; M. Zhang et al., 2014). Recently, it has been shown that the selective EP4 inhibitor GW627368X restricts EGFR transactivation (Parida et al., 2016).
We here demonstrate that EP4 is capable of activating invadopodia-driven ECM degradation in vitro and might also be involved in ECM remodeling in vivo. This activation is dependent on the transactivation of the EGFR. The proposed signaling pathway, based on our findings and on existing literature (Buchanan et al., 2006; Kedziora et al., 2016), mediating the effect of EP4 on invadopodia maturation is depicted in Figure 5. Furthermore, we have shown that PGE2, or specific EP4 stimulation with CAY10598, can promote invadopodia in the absence of EGF. A substitutive effect of PGE2 on the EGFR signaling has been reported by Brocard et al., 2015 (Brocard et al., 2015).
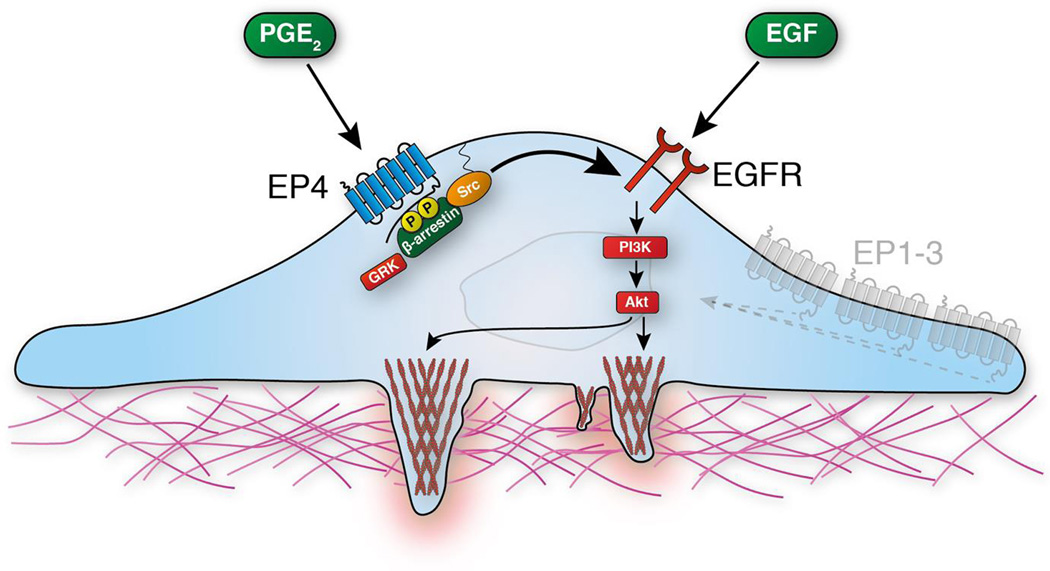
Figure 5. EP4 transactivates EGFR and thereby promotes the degradative capability of invadopodia.
EP4 is activated by the endogenous pro-inflammatory lipid PGE2. This leads to the phosphorylation of the activated EP4 by G protein-coupled receptor kinase (GRK) and allows recruitment and di-phosphorylation of β-arrestin and EGFR-dependent Src kinase phosphorylation. Further downstream phosphoinositide 3-kinase (PI3K) and the protein kinase B (Akt) stimulate invadopodia driven ECM degradation.
It is important to note that MDA-MB-231 cells are reported to express also EP1 and EP3 (Paquette et al., 2011). The activation of all present EP receptors might result in a complex interplay and intracellular processing of the signals. The dissection of the contribution of each receptor in transducing PGE2-dependent invasion and invadopodia signaling is a complex endeavor, but necessary to better understand the role of EP4 in particular. However, our preliminary results with EP2 specific agonist Butaprost showed it does not induce invasion and therefore EP2 is most likely not involved in promoting invasion (data not shown). Moreover, there was no significant difference in the stimulation of invadopodia matrix degradation as well as mature invadopodia between cells stimulated with the EP4-specific agonist CAY10598 and PGE2, which can activate all four EP receptors subtypes. This indicates that PGE2 mainly affects degradation and invadopodia through activation of EP4.
In summary, we have shown that EP4 stimulation increases the invasive capability of breast cancer cells as well as the degradative capability of invadopodia. Recently invadopodia became a very interesting therapeutic target for aggressive cancer because their inhibition is effective to limit invasion, extravasation and subsequent metastasis (Stoletov and Lewis, 2015). Since EP4 has been proposed as potential anti-cancer treatments (Kundu et al., 2014; Majumder et al., 2014) (Kundu et al., 2014; Majumder et al., 2014), further investigation of EP4 targeting to restrict invadopodia formation and cancer cell invasion is warranted.
MATERIALS AND METHODS
Cell culture and Animal Model
MDA-MB-231 cells (for high spheroid invasion assay, Invadopodium degradation assay) and Dendra2-MDA-MB-231 (for xenograft injection) were cultured in DMEM supplemented with 10% FBS, 50 units/mL penicillin and 50 µg/mL streptomycin. For serum starvation, cells at 70 % confluence were rinsed with PBS and starved in 0.5% FBS for 16–24 h.
Tumor formation in mice was carried out as described previously (Gligorijevic et al., 2014). Briefly, 106 Dendra2-MDA-MB-231 cells in 150 µl of 20% collagen I in PBS were injected into the mammary fat pads of 5–7 week old SCID mice. Intravital microscopy was done 12–18 weeks after injection, when tumors reached the size of 0.8–1 cm.
Spheroid Invasion Assay
The spheroids were formed by the hanging drop method with 1,000 cells/spheroid in DMEM containing 5.8% FBS, 50 units/mL penicillin and 50 µg/mL streptomycin, 4.8 mg/ml methyl cellulose and 20 µg/ml Nutragen (Advanced BioMatrix Inc., San Diego, CA) for 48 h. Then, the spheroids were embedded in 50 µl of 5 mg/ml rat-tail collagen I (Corning Inc., Corning, NY) and dispensed into each well of a 24-well plate. The collagen solution was prepared according to the manufacturer’s protocol (Alternate Gelation Procedure). Next, 500 µl of DMEM containing 0.5% FBS, 50 units/mL penicillin, 50 µg/mL streptomycin and the test substances were added into each well. The embedded spheroids were incubated for 48 h, fixed with 4% PFA and stained with DAPI. The images were captured using the Olympus laser-scanning confocal microscope (Fluoview 1200 IX 83).
For the image analysis of unstained spheroids (Figure 1), both the core and the distal margin of the invasion zone were outlined manually based on phase-contrast images. Then, the area of the core was subtracted to obtain the area of the invasion zone.
For the image analysis of DAPI-stained spheroids (Figure 2), a custom macro was used in Fiji. Briefly, the images were preprocessed by applying a Gaussian blur filter to reduce noise. Next, the core of the spheroid was selected using inverted threshold and subtracted from each slice semi-automatically. Further, we used a local adaptive threshold algorithm from Fiji (Auto Local Threshold, Bernsen method) for thresholding. Then a Median filter was applied to the thresholded image and the Watershed function was used to allow segmentation of nearby nuclei. The Particle Analyzer was then used for the segmentation and quantification of nuclei.
EP4 siRNA transfection
MDA-MB-231 cells were transfected using the Amaxa® Cell Line Nucleofector® Kit V, according to the manufacturer’s protocol. Briefly, 106 cells were resuspended in 100 µl Nucleofector® Solution and combined with 50 nM siRNA EP4 (Dharmacon, J-005714-06, siEP4 #1 and J-005714-07, siEP4 #2) or non-targeting control siRNA (Dharmacon, D-001810-01-05, siCtl). Samples were transferred into a 6-well plate and after 24 h, cells were serum-starved for 20 h before performing the invadopodium matrix degradation assay. Assessment of the knock-down efficiency was performed after 24 h by western-blot and immunofluorescence with EP4 antibody (Santa Cruz sc-55596; 1:100).
Invadopodium Matrix Degradation Assay
Lyophilized gelatin from pig skin, Oregon Green 488 conjugate (Life Technologies, Carlsbad, CA) was reconstituted in 2.5 ml H20 containing 2 mM NaN3 and stored at −20° C. For the preparation of a 0.2 % unlabeled gelatin solution, 50 mg gelatin from pig skin was dissolved in 25 mL PBS. The protocol for the preparation of fluorescent gelatin-coated dishes was adapted from Sharma et al. 2013 (Sharma et al., 2013). Briefly, MatTek dishes (MatTek corp., Ashland, MA) were treated with 300 µl 1N HCl for 10 min at room temperature. Between each of the subsequent steps the dishes were washed three times with PBS in 5 min intervals. Next, dishes were coated with 300 µl 50 µg/ml poly-L-lysine solution for 20 min at room temperature. Oregon Green 488 dye-labeled gelatin and unlabeled gelatin were brought to room temperature before mixing them to make solutions with a final ratio of 1:20. The mixture was incubated for 5–10 min at 37° C prior 200 µl of this solution was added to the dish for 10 min at room temperature. Dishes were then transferred on ice and gelatin was cross-linked with 2 mL of chilled 0.2% glutaraldehyde solution on ice for 15 min and then quenched with 2 mL of fresh 5 mg/ml sodium borohydride solution for 15 min at room temperature. For sterilization, 2 mL 70% ethanol was added and incubated for 15 min at room temperature. After addition of 2 mL 1,000 units/mL penicillin and 1,000 µg/mL streptomycin in PBS, the dishes were stored at 4° C for up to 10 days before using them.
Serum-starved cells (20,000) were plated on the gelatin-coated dishes at 0 hours in culturing media with addition of 1 µM DMSO, 1 nM EGF, 10 nM CAY10598 (CAY) or 1 µM PGE2. Treatments with inhibitors were done by adding 1µM GW627368X or 1 µM gefitinib to 1 nM EGF, 10 nM CAY10598 (CAY) or 1 µM PGE2, respectively. At 16 hours, dishes were fixed and staining with anti-cortactin antibody was used for the identification and quantification of invadopodia. The stacks were processed and analyzed in a custom plugin for ImageJ. Briefly, images of a stack were combined using average intensity projection. For the analysis of degradation, Subtract Background plugin was used to correct for unevenness of the coating, images were thresholded and degradation holes were measured with Particle Analyzer. Invadopodia, defined as punctae co-localizing in cortactin and gelatin degradation were counted manually with the Multi-point tool.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde in PBS for 15 min, permeabilized with 0.1% Triton-X100 in PBS for 5 min and blocked with 1% FBS/1% BSA in PBS for 2 h. Next, cells were incubated with the primary antibody in blocking buffer for 60–90 min, followed by the secondary antibody (1:250) in blocking buffer for 30 min. Primary antibodies against cortactin (Abcam ab33333; 1:100), EP4 (Santa Cruz sc-55596; 1:100) and degraded collagen I (Col I (3/4), ImmunoGlobe GmbH, Germany, 1:100) were used. For staining of spheroids embedded in rat-tail collagen, samples were shaken during all incubation steps, the primary antibody was incubated overnight at room temperature and the secondary antibody (1:400) was incubated for 4 h at room temperature, followed by four 15 min washes with PBS. Images of the samples were obtained with a Fluoview 1200 IX 83, hybrid confocal laser scanning / multiphoton microscope.
Intravital imaging
Skin flap surgery was performed on mice anaesthetized with Isoflurane before placing the tumor on a coverslip on top of an inverted hybrid confocal/multiphoton microscope, equipped with a multiphoton laser (MaiTai DeepSee) and a 30× immersion silicone objective (UPLSAPO30XSIR; NA: 1.05; Olympus). The selective EP4 agonist CAY10598 (Cayman; 100 µl of 1–10 µM) was injected intraperitoneally (250 µl of 10 µM), 3 h prior to imaging session. Presence of perfused blood vessels in chosen imaging area was confirmed prior to collecting each 4D stack. 4D stacks (14×5 µm) were collected at 3–5 min intervals, for up to 5 hours.
ImageJ was used for image processing and manual invadopodia counting. In our recently published work on breast carcinoma xenografts in vivo, we have demonstrated that invadopodia in vivo are highly dynamic protrusions whose assembly is limited to the perivascular niches (locations with major blood vessels, aligned collagen fibers and perivascular macrophages) (Gligorijevic et al., 2014). Invadopodia were the unique motility mode in such niches and can be identified by morphodynamic analysis. Briefly, morphodynamic analysis is done by first correcting timelapse recording for drift and breathing artefacts by StackReg (Thévenaz et al., 1998). Further, differential image is created by subtraction of frames taken at 0 and 3 (or 6) minutes, selecting for highly dynamic regions in the field of view. Finally, invadopodia can be localized and quantified using Particle Analysis by selecting for dynamic protrusions 2–20 µm2 in size.
Statistical analysis
The effects of different chemical treatments on invasion, measured on two timepoints or only after 48 h, were analyzed using two-way ANOVA or one-way ANOVA, respectively. The effect of Mitomycin C on the invasion was analyzed using a Student’s t-test. The effects of different chemical treatments and EP4 knock-down on the number of cells with mature invadopodia were analyzed using a generalized linear model. Invadopodia quantification from intravital imaging was analyzed with Mann-Whitney test.
Supplementary Material
Highlights.
Stimulation of EP4 promotes breast cancer cell invadopodia and invasion
EP4-stimulated ECM degradation is EGFR dependent
Acknowledgments
We are grateful to Antoine Khalil and the Microscopic Imaging Center of the Radboud Institute for Molecular Life Sciences, Nijmegen for instrumental help, sharing protocols and resources. We also thank Yujin Chung from the Center for Computational Genetics and Genomics at Temple University for her help in statistical analysis. This work was supported by the Radboud Honours Academy to FT, EU grant NANOVISTA [grant number 2882630] to AC and United States National Institute of Health [NIH 5K99CA172360] to BG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors contributions
FT, BG and AC conceived and designed the experiments. BB and FT performed the 3D invasion experiments; LP and FT performed the invadopodia experiments; FT performed the intravital microscopy experiments. FT, LP and BB collected and analyzed the data. FT, LP, LL, KD, BG and AC interpreted the data. FT, BG and AC wrote the manuscript.
REFERENCES
- An Q, Han C, Zhou Y, Li F, Li D, Zhang X, Yu Z, Duan Z, Kan Q. In vitro effects of mitomycin C on the proliferation of the non-small-cell lung cancer line A549. Int J Clin Exp Med. 2015;8:20516–20523. [PMC free article] [PubMed] [Google Scholar]
- Beaty BT, Condeelis J. Digging a little deeper: the stages of invadopodium formation and maturation. Eur. J. Cell Biol. 2014;93:438–444. doi: 10.1016/j.ejcb.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocca C, Ievolella M, Autelli R, Motta M, Mosso L, Torchio B, Bozzo F, Cannito S, Paternostro C, Colombatto S, Parola M, Miglietta A. Expression of Cox-2 in human breast cancer cells as a critical determinant of epithelial-to-mesenchymal transition and invasiveness. Expert Opin. Ther. Targets. 2014;18:121–135. doi: 10.1517/14728222.2014.860447. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proceedings of the National Academy of Sciences. 1996;93:3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard E, Oizel K, Lalier L, Pecqueur C, Paris F, Vallette FM, Oliver L. Radiation-induced PGE2 sustains human glioma cells growth and survival through EGF signaling. Oncotarget. 2015;6:6840–6849. doi: 10.18632/oncotarget.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN. Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc. Natl. Acad. SciU.SA. 2006;103:1492–1497. doi: 10.1073/pnas.0510562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesio CL, Chan KT, Perrin BJ, Burton NO, Zhang S, Zhang Z-Y, Huttenlocher A. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. The Journal of Cell Biology. 2008;180:957–971. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Current opinion in cell biology. 2002;14:633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- Díaz B, Yuen A, Iizuka S, Higashiyama S, Courtneidge SA. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. The Journal of Cell Biology. 2013;201:279–292. doi: 10.1083/jcb.201209151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Shi F, Zhang H, Xia S, Zhang M, Ma J, Bai X, Zhang L, Wang Y, Cheng S, Yang Q, Leng J. Prostaglandin E2 promotes human cholangiocarcinoma cell proliferation, migration and invasion through the upregulation of β-catenin expression via EP3-4 receptor. Oncol. Rep. 2015;34:715–726. doi: 10.3892/or.2015.4043. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KE, Sacharidou A, Stratman AN, Mayo AM, Fisher SB, Mahan RD, Davis MJ, Davis GE. MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. Journal of Cell Science. 2009;122:4558–4569. doi: 10.1242/jcs.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Sahai E, Weiss S, Yamada KM. New dimensions in cell migration. 2012;13:743–747. doi: 10.1038/nrm3459. [DOI] [PubMed] [Google Scholar]
- Fujino H, Regan JW. EP(4) prostanoid receptor coupling to a pertussis toxin-sensitive inhibitory G protein. Mol. Pharmacol. 2006;69:5–10. doi: 10.1124/mol.105.017749. [DOI] [PubMed] [Google Scholar]
- Gligorijevic B, Bergman A, Condeelis J. Multiparametric classification links tumor microenvironments with tumor cell phenotype. PLoS Biol. 2014;12:e1001995. doi: 10.1371/journal.pbio.1001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. Journal of Cell Science. 2012;125:724–734. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould CM, Courtneidge SA. Regulation of invadopodia by the tumor microenvironment. Cell Adhesion & Migration. 2014 doi: 10.4161/cam.28346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedziora KM, Leyton-Puig D, Argenzio E, Boumeester AJ, van Butselaar B, Yin T, Wu YI, van Leeuwen FN, Innocenti M, Jalink K, Moolenaar WH. Rapid remodeling of invadosomes by Gicoupled receptors: dissecting the role of Rho GTPases. J. Biol. Chem. 2016;291 doi: 10.1074/jbc.M115.695940. jbc.M115.695940–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Lakshmikanthan V, Frilot N, Daaka Y. Prostaglandin E2 promotes lung cancer cell migration via EP4-betaArrestin1-c-Src signalsome. Molecular cancer research : MCR. 2010;8:569–577. doi: 10.1158/1541-7786.MCR-09-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konya V, Marsche G, Schuligoi R, Heinemann A. E-type prostanoid receptor 4 (EP4) in disease and therapy. Pharmacology & therapeutics. 2013;138:485–502. doi: 10.1016/j.pharmthera.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu N, Ma X, Kochel T, Goloubeva O, Staats P, Thompson K, Martin S, Reader J, Take Y, Collin P, Fulton A. Prostaglandin E receptor EP4 is a therapeutic target in breast cancer cells with stem-like properties. Breast Cancer Res. Treat. 2014;143:19–31. doi: 10.1007/s10549-013-2779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, Hague MN, Ablack A, Carmine-Simmen K, McPherson VA, Postenka CO, Turley EA, Courtneidge SA, Chambers AF, Lewis JD. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014;8:1558–1570. doi: 10.1016/j.celrep.2014.07.050. [DOI] [PubMed] [Google Scholar]
- Majumder M, Xin X, Liu L, Girish GV, Lala PK. Prostaglandin E2 receptor EP4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci. 2014;105:1142–1151. doi: 10.1111/cas.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. Landes Bioscience. 2000 [Google Scholar]
- Miglietta A, Toselli M, Ravarino N, Vencia W, Chiecchio A, Bozzo F, Motta M, Torchio B, Bocca C. COX-2 expression in human breast carcinomas: correlation with clinicopathological features and prognostic molecular markers. Expert Opin. Ther. Targets. 2010;14:655–664. doi: 10.1517/14728222.2010.486792. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Paquette B, Therriault H, Desmarais G, Wagner R, Royer R, Bujold R. Radiation-enhancement of MDA-MB-231 breast cancer cell invasion prevented by a cyclooxygenase-2 inhibitor. Br. J. Cancer. 2011;105:534–541. doi: 10.1038/bjc.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida S, Pal I, Parekh A, Thakur B, Bharti R, Das S, Mandal M. GW627368X inhibits proliferation and induces apoptosis in cervical cancer by interfering with EP4/EGFR interactive signaling. Cell Death Dis. 2016;7:e2154. doi: 10.1038/cddis.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B-W, Park S, Park HS, Koo JS, Yang WI, Lee JS, Hwang H, Kim SI, Lee KS. Cyclooxygenase-2 expression in proliferative Ki-67-positive breast cancers is associated with poor outcomes. Breast Cancer Res. Treat. 2012;133:741–751. doi: 10.1007/s10549-012-1971-2. [DOI] [PubMed] [Google Scholar]
- Patsialou A, Wyckoff J, Wang Y, Goswami S, Stanley ER, Condeelis JS. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 2009;69:9498–9506. doi: 10.1158/0008-5472.CAN-09-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Regan JW. Prostanoid receptor heterogeneity through alternative mRNA splicing. Life Sci. 1998;62:1479–1483. doi: 10.1016/s0024-3205(98)00093-9. [DOI] [PubMed] [Google Scholar]
- Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE. Hic-5 promotes invadopodia formation and invasion during TGF-β-induced epithelial-mesenchymal transition. The Journal of Cell Biology. 2012;197:421–437. doi: 10.1083/jcb.201108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajadurai CV, Havrylov S, Zaoui K, Vaillancourt R, Stuible M, Naujokas M, Zuo D, Tremblay ML, Park M. Met receptor tyrosine kinase signals through a cortactin-Gab1 scaffold complex, to mediate invadopodia. Journal of Cell Science. 2012;125:2940–2953. doi: 10.1242/jcs.100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Sharma VP, Entenberg D, Condeelis J. High-resolution live-cell imaging and time-lapse microscopy of invadopodium dynamics and tracking analysis. Methods Mol. Biol. 2013;1046:343–357. doi: 10.1007/978-1-62703-538-5_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek KL, Brown TJ, Ringuette MJ. Collagen I but not Matrigel matrices provide an MMP-dependent barrier to ovarian cancer cell penetration. BMC Cancer. 2008;8:223. doi: 10.1186/1471-2407-8-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoletov K, Lewis JD. Invadopodia: a new therapeutic target to block cancer metastasis. Expert Rev Anticancer Ther. 2015;15:733–735. doi: 10.1586/14737140.2015.1058711. [DOI] [PubMed] [Google Scholar]
- Thévenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Tveteraas IH, Müller KM, Aasrum M, Ødegård J, Dajani O, Guren T, Sandnes D, Christoffersen T. Mechanisms involved in PGE2-induced transactivation of the epidermal growth factor receptor in MH1C1 hepatocarcinoma cells. J. Exp. Clin. Cancer Res. 2012;31:72. doi: 10.1186/1756-9966-31-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Current opinion in cell biology. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Yokoyama U, Iwatsubo K, Umemura M, Fujita T, Ishikawa Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacological reviews. 2013;65:1010–1052. doi: 10.1124/pr.112.007195. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhang H, Cheng S, Zhang D, Xu Y, Bai X, Xia S, Zhang L, Ma J, Du M, Wang Y, Wang J, Chen M, Leng J. Prostaglandin E2 accelerates invasion by upregulating Snail in hepatocellular carcinoma cells. Tumour Biol. 2014;35:7135–7145. doi: 10.1007/s13277-014-1963-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Meng J, Wang Z-Y. A switch role of Src in the biphasic EGF signaling of ER-negative breast cancer cells. PLoS ONE. 2012;7:e41613. doi: 10.1371/journal.pone.0041613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.