Abstract
Introduction
Lifestyle change programs implemented within healthcare systems could reach many Americans, but their impact on cardiovascular disease (CVD) remains unclear. The MOVE! program is the largest lifestyle change program implemented in a healthcare setting in the U.S. This study aimed to determine whether MOVE! participation was associated with reduced CVD incidence.
Methods
This retrospective cohort study, analyzed in 2013–2015, used national Veterans Health Administration databases to identify MOVE! participants and eligible non-participants for comparison (2005–2012). Patients eligible for MOVE!—obese or overweight with a weight-related health condition, and no baseline CVD—were examined (N=1,463,003). Of these, 169,248 (12%) were MOVE! participants. Patients were 92% male, 76% white, with mean age 52 years and BMI of 32. The main outcome was incidence of CVD (ICD-9 and procedure codes for coronary artery disease, cerebrovascular disease, peripheral vascular disease, and heart failure).
Results
Adjusting for age, race, sex, BMI, statin use, and baseline comorbidities, over a mean 4.9 years of follow-up, MOVE! participation was associated with lower incidence of total CVD (hazard ratio [HR]=0.83, 95% CI=0.80, 0.86), coronary artery disease (HR=0.81, 95% CI=0.77, 0.86), cerebrovascular disease (HR=0.87, 95% CI=0.82, 0.92), peripheral vascular disease (HR=0.89, 95% CI=0.83, 0.94), and heart failure (HR=0.78, 95% CI=0.74, 0.83). The association between MOVE! participation and CVD incidence remained significant when examined across categories of race/ethnicity, BMI, diabetes, hypertension, smoking status, and statin use.
Conclusions
Although participation was limited, MOVE! was associated with reduced CVD incidence in a nationwide healthcare setting.
INTRODUCTION
Obesity and cardiovascular disease (CVD) are major causes of morbidity, mortality, and healthcare cost in the U.S.1, 2 Much of this burden is avoidable through management of CVD risk factors, and lifestyle change programs that offer nutrition and physical activity counseling are a recommended prevention strategy.3, 4 Several large randomized trials have demonstrated that lifestyle change programs can achieve weight loss and reductions in diabetes incidence,5-7 and implementation in healthcare settings has been recommended. However, evidence of impact on CVD risk factors is mixed: Few studies have had sufficient sample size to study CVD incidence, and programs translated into healthcare settings are rare.8-13
The Veterans Health Administration (VA) is the largest integrated healthcare system in the U.S., serving more than 8 million patients.14 Eligibility for VA care is based on poverty or disability related to military service. Nearly three quarters of VA patients are overweight or obese15 and 58% have dyslipidemia, hypertension, or both.16 The VA’s lifestyle change program, MOVE!® Weight Management Program for Veterans, is the largest such program in the country, with more than 500,000 participants since 2005.17 MOVE! participation is associated with modest but sustained weight loss18 and lower diabetes incidence.19 This study examined the association between MOVE! participation and CVD incidence, including coronary artery disease (CAD), cerebrovascular disease (CBD), peripheral vascular disease (PVD), and heart failure (HF).
METHODS
Data Sample
The VA’s Corporate Data Warehouse (CDW) contains data on all veterans receiving care in VA facilities, including demographics, vital signs, diagnoses, procedures, and prescriptions. Data were accessed through the Veterans Informatics, Information, and Computing Infrastructure data processing environment.20 This secondary data analysis was approved by the Emory University IRB and the Atlanta VA Medical Center’s Research and Development Committee.
The MOVE! program involves group-based educational sessions on nutrition, physical activity, and goal setting.21 Eligible patients who are either obese (BMI ≥30 kg/m2) or overweight (BMI ≥25 kg/m2) with a weight-related health condition (diabetes, hypertension, dyslipidemia, sleep apnea, or osteoarthritis) are referred to the program by their physicians. The standard ten-session core curriculum includes topics such as evaluating portion sizes, walking with a pedometer or modifying exercise for wheelchair users, and overcoming barriers to change (www.move.va.gov/grpSessions.asp). The program was rolled out nationwide with no designated additional funds or staff, and organization varies across VA facilities.22 Most sessions are in person and group based, although some are offered remotely or one on one. Participants were defined as those who attended at least one session, in any format, as indicated by the use of MOVE!-specific clinic codes in the VA CDW.
From nearly 10 million veterans receiving care between 2005 and 2012 (Appendix Figure 1), 4.5 million had at least one outpatient visit per year for at least 3 consecutive years, and were eligible to participate in MOVE! based on weight and health criteria. From these, patients aged >70 years were excluded because MOVE! is not targeted at individuals above this age given uncertainty about adverse effects of overweight.23, 24 Consistent with a previous study of MOVE!,18 veterans were excluded if they would be unlikely to be able to participate, such as those with malignant cancer, anorexia, or receipt of hospice care. After excluding those with missing data for key demographic and clinical indicators, and restricting the study population to veterans without CVD at baseline, there were 1,463,003 patients eligible for analysis.
Measures
Incident CVD was identified with ICD-9 and procedure codes for CAD, CBD, PVD, and HF. Total CVD was defined as incidence of any of these four conditions. Additional laboratory and clinical values (systolic blood pressure [SBP], random plasma glucose [RPG], high-density lipoprotein cholesterol [HDL] level, non-HDL cholesterol level) were available for a subset of patients (n=701,930), using the most recent value within 12 months prior to baseline visits. Follow-up measures were recorded as mean values captured within subsequent time windows (6 months, 3–9 months; 12 months, 9–15 months; 24 months, 21–27 months; 36 months, 33–39 months).
Patient’s BMI was assessed using clinically measured weight and height, after excluding implausible values (approximately 0.1%).25 Mean height was used if multiple measures were available. Weight was recorded as the patient’s baseline weight. Medical record text was used to classify patients as “current smoker,” “former smoker,” or “never/lifetime non-smoker,” by updating a previously validated approach.26 Baseline statin use was determined by any prescription of a statin medication prior to the baseline visit (list available upon request). Illnesses and comorbidities were assessed individually using ICD-9 codes and procedure codes, and also summed using the Charlson Comorbidity Index.27
Available demographic data included age, sex, race/ethnicity (white, African American, and other, the latter combining Hispanics, Asian/Pacific Islanders, and American Indians/Alaska Natives—each <2% of the population), marital status, and VA facility. An administrative indicator of SES and disability status was used28: Disability is assessed by the VA in percentages, with higher percentages indicating more severe disability, and “no disability” indicating that a veteran qualified for VA care based on low SES. Distance to the nearest VA facility offering MOVE! was calculated for each patient, based on distance between the midpoint of the patient’s home ZIP code and the coordinates of the nearest facility offering MOVE!.
Statistical Analysis
Descriptive characteristics were calculated for MOVE! participants and non-participants, and bivariate associations were analyzed using ANOVA (continuous variables) and chi-squared tests (categorical variables). Least square means were used to obtain mean SBP, HDL cholesterol, non-HDL cholesterol, and RPG over time, among participants compared with non-participants, controlling for baseline value, BMI, age, sex, race/ethnicity, and diabetes status.
After evaluating model assumptions including proportionality, Cox proportional hazards models were constructed to estimate hazard ratios (HRs) for CVD incidence. Robust sandwich covariance matrix estimates were used to adjust for clustering at the clinic level, and models were stratified by baseline diabetes status.29 In addition to demographic and clinical characteristics, multivariable models were further adjusted for a propensity score that reflected each patient’s likelihood of participating in MOVE!. Propensity scores, which help to adjust for differing distribution of baseline covariates between participants and non-participants, were calculated on all available demographic and clinical indicators.30 Baseline was assigned as a veteran’s first MOVE! visit for participants and as the first visit at which weight was recorded after January 1, 2005 (the initial year of MOVE! rollout) for non-participants. Person time was censored at either the date of CVD incidence or the date of the last recorded VA visit within the study period.
Additional analyses were performed to examine the association between participation and CVD incidence among subgroups likely to have differing CVD risk (sex, race, age, BMI, diabetes status, hypertension status, smoking status, and statin use). Three sensitivity analyses were conducted:
restricted to veterans aged <65 years, in order to exclude predominantly Medicare-eligible patients;
adjusted for baseline year as a categorical variable, to allow for potential differences in management across years; and
separately examining those who met the VA-defined criteria for “intense and sustained” participation in MOVE! (attending eight or more sessions within 6 months, with a span of at least 129 days between first and last attended session), which is a level of participation that has been previously associated with greater weight loss.
Analyses, conducted in 2013–2015, used SAS version 9.2.
RESULTS
Compared with eligible non-participants, participants were more likely to be women, African American, and obese (Table 1). At baseline, participants had more diagnosed illnesses and risk factors than non-participants, including diabetes, hypertension, and dyslipidemia, and were more likely to have used a statin medication (each p<0.001). Participants were also more likely to have mental health disorders, including depression, post-traumatic stress disorder, and psychoses (each p<0.001). However, participants were less likely to be current smokers than non-participants (p<0.001).
Table 1.
Characteristics of Participants and Eligible Non-Participants, VA 2005-2012
| Characteristic | All | Non-participants | Participants |
|---|---|---|---|
| N=1,463,003 | N=1,293,755 | N=169,248 | |
| Age at baseline, years ±SD | 51.97 ± 11.71 | 51.91 ± 11.77 | 52.39 ± 11.18 |
| Sex | |||
| Male | 92.14% | 93.16% | 84.42% |
| Female | 7.86% | 6.84% | 15.58% |
| Race | |||
| White | 76.24% | 77.03% | 70.13% |
| African American | 19.40% | 18.62% | 25.40% |
| Other | 4.36% | 4.35% | 4.47% |
| BMI at baseline, mean (kg/m2) ±SD | 31.89 ± 5.15 | 31.38 ± 4.76 | 35.80 ± 6.27 |
| 25-29.9 | 41.22% | 44.66% | 14.97% |
| 30-34.9 | 36.91% | 36.86% | 37.25% |
| 35.0-39.9 | 14.67% | 13.09% | 26.79% |
| ≥40 | 7.19% | 5.39% | 20.98% |
| Diabetes | 18.77% | 17.21% | 30.72% |
| Hypertension | 49.00% | 47.05% | 63.91% |
| Dyslipidemia | 40.76% | 38.33% | 59.33% |
| Statin use at baseline | 12.43% | 9.71% | 33.29% |
| Mental health conditions | |||
| Depression | 20.94% | 18.32% | 40.99% |
| Psychoses | 18.49% | 16.09% | 36.80% |
| PTSD | 10.69% | 9.26% | 21.64% |
| Smoking | |||
| Current smoker | 35.84% | 36.70% | 29.25% |
| Former smoker | 28.71% | 28.46% | 30.63% |
| Lifetime non-smoker | 35.45% | 34.84% | 40.12% |
| Prescription for weight loss medication | 4.30% | 3.79% | 8.14% |
| Prescription with weight gain risk | 69.93% | 68.55% | 80.44% |
| Married | 56.79% | 57.40% | 52.17% |
| No disability | 50.51% | 51.42% | 43.52% |
| No. primary care visits/year ±SD | 3.34 ± 2.08 | 3.26 ± 2.02 | 3.97 ± 2.45 |
| No. years of VA care ±SD | 8.40 ± 3.41 | 8.35 ± 3.41 | 8.81 ± 3.35 |
| Distance to MOVE! Clinic >30 mi*±SD | 43.50 ± 33.52 | 43.91 ± 33.61 | 40.38 ± 32.60 |
Note: Boldface indicates statistical significance (p<0.05).
VA, Veterans Health Administration; PTSD, post traumatic stress disorder; No., number
The median number of attended MOVE! sessions was two, with 54% of participants engaging in only one or two sessions. Twenty-six percent of participants attended three to seven sessions, and 20% engaged in at least eight sessions. Among those with recorded weights available, participants decreased weight by −0.9% and −0.6% at 12 months and 3 years, respectively, whereas non-participants increased weight by 0.2% and 0.6%. In sensitivity analyses, “intense and sustained” participants lost more weight (−3.0% at 12 months, −2.1% at 3 years) compared with less active participants (−0.7% at 12 months, −0.5% at 3 years). Detailed weight loss outcomes associated with MOVE! participation have been described.18, 19
In this population without baseline CVD, the observed incidence rate of total CVD was 35 per 1,000 person years. Individually, incidence rates of CAD, CBD, PVD, and HF were 21, 8, 7, and 5, respectively, per 1,000 person years. Mean per-patient observation time was 59 months (range, 1–95). In multivariable Cox proportional hazards models adjusting for the aforementioned demographic and clinical characteristics (Table 2), MOVE! participation was associated with 17% lower incidence of total CVD (HR=0.83, 95% CI=0.80, 0.86), as well as CAD (HR=0.81, 95% CI=0.77, 0.86), CBD (HR=0.87, 95% CI=0.82, 0.92), PVD (HR=0.89, 95% CI=0.83, 0.94), and HF (HR=0.78, 95% CI=0.74, 0.83). In sensitivity analyses, results remained consistent when restricted to people aged <65 years (for total CVD, HR=0.84, 95% CI=0.80, 0.87; Appendix Table 1), indicating that observed effects were not primarily driven by Medicare-eligible patients. A slight dose–response effect was observed (total CVD: HR 0.79, 95% CI=0.73, 0.85 for “intense and sustained” participants versus non-participants; HR=0.83, 95% CI=0.80, 0.87 for less active participants versus non-participants).
Table 2.
Association Between CVD Incidence and MOVE! Participation, along with Select Demographic and Clinical Characteristics; VA 2005-2012a
| Characteristic | Total CVD | Coronary artery disease | Cerebrovascular disease | Peripheral vascular disease | Heart failure | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| MOVE! Participation | 0.83 | 0.80-0.86 | 0.81 | 0.77-0.86 | 0.87 | 0.82-0.92 | 0.89 | 0.83-0.94 | 0.78 | 0.74-0.83 |
| Age at baseline, 5-year increments | 1.30 | 1.29-1.31 | 1.28 | 1.27-1.29 | 1.32 | 1.31-1.33 | 1.38 | 1.36-1.39 | 1.33 | 1.32-1.35 |
| Female | 0.76 | 0.73-0.79 | 0.69 | 0.65-0.73 | 1.01 | 0.96-1.06 | 0.74 | 0.70-0.79 | 0.64 | 0.59-0.68 |
| Race (ref=White) | ||||||||||
| African American | 0.97 | 0.92-1.03 | 0.89 | 0.82-0.97 | 0.97 | 0.92-1.01 | 0.99 | 0.92-1.07 | 1.38 | 1.30-1.46 |
| Other | 0.83 | 0.79-0.86 | 0.82 | 0.78-0.87 | 0.80 | 0.75-0.85 | 0.83 | 0.76-0.91 | 0.81 | 0.75-0.87 |
| BMI at baseline, 5-unit increments | 1.06 | 1.05-1.07 | 1.06 | 1.05-1.07 | 0.92 | 0.90-0.93 | 0.99 | 0.97-1.00 | 1.35 | 1.33-1.38 |
| Charlson Comorbidity Index (ref=none) | ||||||||||
| One | 1.07 | 1.05-1.09 | 1.03 | 1.01-1.05 | 1.08 | 1.05-1.11 | 1.06 | 1.02-1.09 | 1.28 | 1.22-1.34 |
| Two+ | 1.12 | 1.07-1.17 | 1.03 | 0.97-1.08 | 1.27 | 1.18-1.36 | 1.13 | 1.03-1.23 | 1.22 | 1.12-1.32 |
| Hypertension (ICD-9) | 1.16 | 1.14-1.17 | 1.10 | 1.09-1.12 | 1.29 | 1.26-1.32 | 1.17 | 1.14-1.19 | 1.28 | 1.25-1.32 |
| Dyslipidemia (ICD-9) | 0.97 | 0.96-0.99 | 1.01 | 0.99-1.04 | 0.99 | 0.97-1.02 | 0.93 | 0.90-0.96 | 0.80 | 0.78-0.83 |
| Statin use at baseline | 0.96 | 0.94-0.96 | 0.99 | 0.97-1.01 | 0.97 | 0.94-1.01 | 0.90 | 0.87-0.94 | 0.96 | 0.91-1.01 |
| COPD | 1.07 | 1.04-1.10 | 1.11 | 1.07-1.14 | 0.98 | 0.94-1.03 | 0.99 | 0.94-1.05 | 1.29 | 1.23-1.35 |
| Smoking status | ||||||||||
| Current smoker (ref=Never) | 1.42 | 1.38-1.46 | 1.28 | 1.23-1.33 | 1.46 | 1.39-1.53 | 2.34 | 2.18-2.52 | 1.42 | 1.35-1.49 |
| Former smoker (ref=Never) | 1.08 | 1.04-1.13 | 1.07 | 1.02-1.14 | 1.07 | 1.00-1.14 | 1.21 | 1.12-1.30 | 1.08 | 1.01-1.14 |
| Prescriptions for weight loss | 1.14 | 1.11-1.17 | 1.14 | 1.10-1.17 | 1.11 | 1.06-1.16 | 1.29 | 1.19-1.29 | 1.18 | 1.13-1.24 |
| Prescriptions with weight gain risk | 1.44 | 1.42-1.47 | 1.38 | 1.33-1.40 | 1.50 | 1.45-1.55 | 1.81 | 1.75-1.86 | 1.86 | 1.79-1.93 |
| Disability (ref=none) | ||||||||||
| 0-20% | 0.93 | 0.91-0.95 | 0.94 | 0.92-0.96 | 0.93 | 0.90-0.97 | 0.94 | 0.91-0.97 | 0.81 | 0.78-0.85 |
| 30-60% | 1.03 | 1.01-1.06 | 1.07 | 1.04-1.10 | 0.97 | 0.94-1.00 | 1.06 | 1.02-1.10 | 0.82 | 0.78-0.85 |
| 70-100% | 1.35 | 1.32-1.39 | 1.46 | 1.42-1.50 | 1.18 | 1.14-1.22 | 1.45 | 1.40-1.50 | 1.31 | 1.25-1.37 |
N=1,463,003. Cox proportional hazards models controlled for all variables listed as well as osteoarthritis, distance from a patient’s ZIP code to a facility offering MOVE, marital status, kidney disease, sleep apnea, mental health conditions (depression, psychoses, and post traumatic stress disorder), number of primary care visits per year, and years of care in the VA system. Two measures were considered for inclusion (drug abuse and substance abuse), but were suspected to be poorly coded in administrative data. For these two measures, backward elimination model selection was used to determine that they would not improve model fit based on the Akaike information criterion (AIC), and they were not included in final models. Models were stratified by baseline diabetes status using the strata option in the SAS phreg procedure. Models were adjusted for clustering by clinic, and included propensity scores to adjust for likelihood of MOVE! participation.
CVD, cardiovascular disease; VA, Veterans Health Administration; HR, hazard ratio; ref, reference category; COPD, chronic obstructive pulmonary disease
Other factors associated with greater incidence of CVD included male sex, current smoking status, higher baseline age, and hypertension (Table 2). In 701,930 patients with available data for baseline SBP, HDL cholesterol, non-HDL cholesterol, and RPG (Table 3), MOVE! participation was associated with 13% lower incidence of total CVD, indicating an attenuated, but still statistically significant, association after further adjustment for these clinical measures (HR=0.87, 95% CI=0.83, 0.91).
Table 3.
CVD Incidence in Subset With Additional Baseline Risk Factor Data, VA 2005-2012a
| Characteristic | Total CVD | Coronary artery disease | Cerebrovascular disease | Peripheral vascular disease | Heart failure | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| MOVE! participation | 0.87 | 0.83-0.91 | 0.86 | 0.81-0.91 | 0.91 | 0.85-0.98 | 0.94 | 0.87-1.01 | 0.83 | 0.77-0.89 |
| Systolic BP, 10mm Hg | 1.05 | 1.04-1.05 | 1.03 | 1.03-1.04 | 1.07 | 1.06-1.08 | 1.07 | 1.06-1.08 | 1.08 | 1.07-1.09 |
| HDL cholesterol, 10mg/dL | 0.94 | 0.93-0.95 | 0.92 | 0.91-0.93 | 0.96 | 0.94-0.97 | 0.94 | 0.92-0.96 | 1.01 | 0.99-1.02 |
| Non-HDL cholesterol, 10mg/dL | 1.01 | 1.01-1.01 | 1.02 | 1.01-1.02 | 1.01 | 1.01-1.02 | 1.01 | 1.01-1.02 | 0.99 | 0.99-1.00 |
| Random plasma glucose, 10mg/dL | 1.01 | 1.01-1.01 | 1.01 | 1.01-1.01 | 1.01 | 1.01-1.01 | 1.02 | 1.01-1.02 | 1.02 | 1.02-1.03 |
Multivariable models identical to Table 2 above, but including additional adjustment for systolic blood pressure, HDL cholesterol, non-HDL cholesterol, and random plasma glucose, and restricted to patients with available data for these laboratory and clinical measures (N=701,930). Hazard ratios for clinical measures are per 10-unit increase.
CVD, cardiovascular disease; VA, Veterans Health Administration; HR, hazard ratio; HDL
Subgroup analyses were performed to examine possible heterogeneity of effects across sociodemographic and clinical characteristics (Figure 1). Across nearly all categories for sex, age, race/ethnicity, BMI, smoking status, hypertension, and diabetes, an inverse association between MOVE! participation and CVD incidence was observed. No subgroups indicated harm (HR >1). There were variations in degree of benefit associated with MOVE! participation in some subgroups. The inverse association between MOVE! and CVD incidence appeared to be stronger for men (HR=0.82, 95% CI=0.79, 0.85) than women (HR=0.94, 95% CI=0.86, 1.02), among whom the association was not statistically significant. MOVE! appeared to be less effective among younger people aged <55 years (HR=0.90, 95% CI=0.85, 0.95), compared with older adults aged 55–64 years (HR=0.80, 95% CI=0.77, 0.83) or 65–70 years (HR=0.79, 95% CI=0.75, 0.83). A stronger association was observed among current smokers (HR=0.77, 95% CI=0.70, 0.86) and former smokers (HR=0.83, 95% CI=0.75, 0.93) compared with nonsmokers (HR=0.88, 95% CI=0.84, 0.94).
Figure 1.
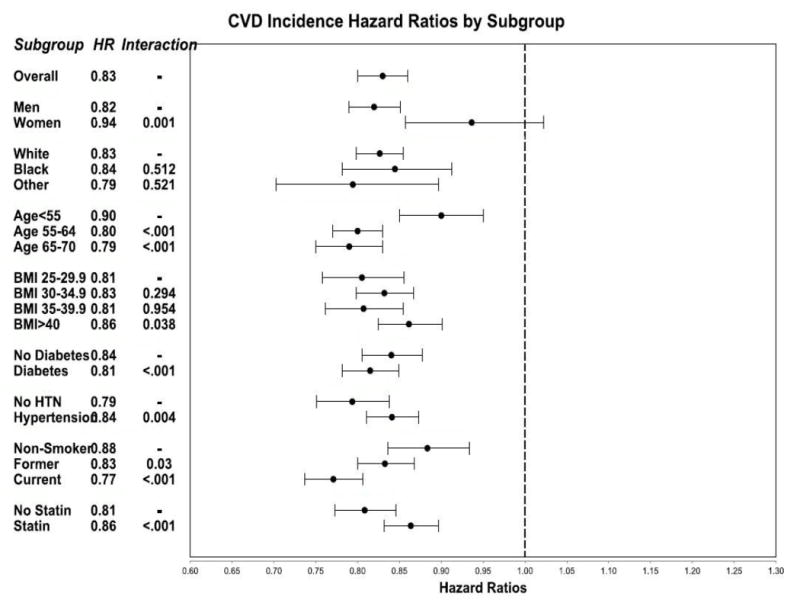
Hazard ratios for total CVD incidence among participants compared to non-participants, by subgroup, VA 2005-2012.
Notes: *N=1,463,003. Multivariable Cox proportional hazards models included covariates, in addition to those listed above: Charlson Comorbidity Index, dyslipidemia, chronic obstructive pulmonary disease, prescriptions for weight loss, prescriptions with a risk of weight gain, disability status, osteoarthritis, kidney disease, sleep apnea, mental health conditions, marital status, distance to MOVE! clinic, number of primary care visits per year, and years of care in the VA system. Wald p-values for interaction terms are shown. Hazard ratios less than 1 (to the left of the dashed axis) indicate that MOVE! participation was associated with reduced CVD incidence. Significant p-values indicate possible heterogeneity of effects across subgroups.
VA, Veterans Health Administration; CVD, cardiovascular disease; HR, hazard ratio; HTN, hypertension
Clinical and laboratory data, including SBP, cholesterol, and RPG, are not readily available as ICD-9 codes and are not cleaned in standardized ways across VA facilities. These data were analyzed for a subset of participants with available data, to attempt to examine the biological factors underlying the observed association between MOVE! participation and CVD incidence. At 6 months, participation was associated with slightly lower SBP (−0.63 mmHg), non-HDL cholesterol (−1.59 mg/dL), and RPG (−1.49 mg/dL), after controlling for baseline value, BMI, age, sex, race/ethnicity, and diabetes status (all p<0.001, Appendix Table 2), but HDL did not differ significantly between participants and non-participants. Differences between participants and non-participants generally decreased over time between 6 and 36 months. As baseline diabetes was strongly related to risk factors (particularly RPG and non-HDL cholesterol), risk factor results were stratified by diabetes status. “Intense and sustained” participants had lower SBP (−2.72 mmHg), non-HDL cholesterol (−4.98 mg/dL), and RPG (−5.61 mg/dL) at 6 months compared with non-participants.
DISCUSSION
The authors observed a significant inverse association between CVD incidence and participation in a national lifestyle change program implemented in a healthcare setting. Participation in MOVE! was associated with 17% lower total CVD incidence after adjustment for clinical and demographic characteristics. For individual CVD outcomes, participation was associated with 19% lower CAD incidence, 13% lower CBD incidence, 11% lower PVD incidence, and 22% lower HF incidence. The association of MOVE! participation with reduced CVD incidence was attenuated, but remained significant, after adjusting for baseline CVD risk factors: SBP, RPG, and cholesterol.
These results are consistent with clinical trials demonstrating modest improvements in cardiovascular risk factors among lifestyle change program participants.31, 32 The Diabetes Prevention Program Outcomes Study, conducted among people with prediabetes, demonstrated improved CVD risk factors, including SBP and diastolic BP, low-density lipoprotein cholesterol, triglycerides, and HDL cholesterol among all groups, despite lower lipid and BP medication use among lifestyle change program participants.12 Four-year results of the Action for Health in Diabetes (Look AHEAD) study in diabetes patients revealed improved hemoglobin A1c, blood pressure, and HDL cholesterol.8
Despite apparent benefit for cardiovascular risk factors, few studies have shown an association between lifestyle change program participation and CVD incidence. Specifically, the present results contrast with those of the Look AHEAD study, in which participants achieved substantial weight loss of 8.6%, and improved their CVD risk factor levels, but did not have reduced CVD incidence.13 It has been suggested that Look AHEAD findings may have been confounded by differential statin use, and weight loss in the controls (3.5% by the end of the trial).13, 33 In contrast to the Look AHEAD results, it could be that there was a greater effect among MOVE! participants because non-participants in this population actually gained weight (as opposed to Look AHEAD controls, who lost weight). Although weight loss among participants was very modest in the present study, there may be a substantial metabolic difference inherent in the states of weight loss versus weight gain, reflected in the observed cardiovascular effects.
A recent Cochrane review questioned the ability of multifactorial lifestyle change programs to affect total or CVD mortality among a general population, but did find benefit among trials restricted to high-risk participants with diabetes or hypertension (for fatal and nonfatal CVD events, OR=0.78, 95% CI=0.68, 0.89).31 The present study population was also high risk based on mean BMI of 32 and approximately 50% with diagnosed hypertension, so results are consistent with this finding. The present results are also consistent with the reduction in cardiovascular mortality over the 17 years following participation in the Da Qing study.34
The authors observed less benefit among the youngest participants (aged younger than 55 years). This could be due to scarcity of events among both participants and non-participants in this age group. Benefits among younger participants may occur in later years, and thus require a longer period of follow-up. In addition, younger participants generally attended fewer sessions than older participants, which may have lessened the effects of the program. MOVE! sessions are often offered mid-day, which may make it difficult for working adults to participate.
Weight loss was limited among participants, and it is difficult to determine whether the cardiovascular benefit observed herein may be due to unmeasured confounding (discussed below), or other factors such as increase in cardiorespiratory fitness, reduced sedentary time, improved nutrition, avoidance of weight gain, or even mental health benefits such as reduced depression—each of which may have independent cardiovascular benefit.35 Most of these factors are not captured by the data. However, a large proportion of the population reported depression and other mental illnesses at baseline, and mental health status may affect engagement in MOVE!, weight loss outcomes, and CVD. Weight loss appears to be comparable in MOVE! participants with and without serious mental illness, but participants with depression may lose less weight than others.36 Future studies could investigate whether MOVE! participation was associated with reduced depression or reduced medication use. Methods to improve participation warrant further study, as more than half of MOVE! participants attended only one or two sessions, and CVD risk factor changes were better among participants who engaged more actively with the program.
Limitations
The strengths of this study include a population large enough to evaluate CVD as an outcome, as well as the use of national data to examine the largest lifestyle change program implemented in a healthcare setting in the U.S. One limitation is the potential for confounding, due to the observational nature of the data. Although it is impossible to rule out confounding by unmeasured factors, detailed electronic health record data allowed for adjustment of known cardiovascular risk factors, and a propensity score approach was used to further minimize confounding by measured variables. A second limitation is many veterans receive care outside of the VA,37 so some cardiovascular events may not be recorded in VA databases. This is especially true of the Medicare-eligible population, as there is widespread dual use of both VA and Medicare services.38, 39 However, in analyses restricted to veterans aged younger than 65 years, findings remained consistent. Third, bias could occur if there was differential reporting of CVD events among participants versus non-participants, owing to Medicare eligibility or other factors such as proximity or access to VA services. MOVE! participants interacted slightly more frequently with the VA system, which may have increased their relative likelihood of having CVD events recorded; this could substantially bias results toward the null. To minimize this potential source of misclassification of outcomes, analyses were restricted to veterans receiving at least 3 continuous years of outpatient care in the VA, to ensure that all included patients had substantial and consistent contact with the VA system. Fourth, a continuing challenge of the MOVE! program is participation, and the small percentage of the eligible population that participated in MOVE! likely introduced selection bias into the present study, as those who chose to participate may be more health-conscious than non-participants. Further research would be needed to determine whether the program is still associated with reduced CVD incidence if a larger proportion of the eligible population participated.
CONCLUSIONS
This large observational study indicates that participation in a national lifestyle change program, implemented in a routine healthcare setting, was associated with reduced CVD incidence. Lifestyle change programs may be an attractive strategy for healthcare systems to consider as an adjunct to conventional pharmacotherapy approaches for control of CVD risk factors.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contributions of Ms. Christine Jasien and Dr. Alyson Littman, as well as Ms. Kathleen McGinnis and her colleagues at the Veterans Aging Cohort Study Team (funding source: NIAAA 5U10AA013566 and 1U24AA020794), who shared their expertise with VA data and informed the present work.
This work was supported in part by U.S. Food and Drug Administration award RO1FD003527 (LSP), VA award HSR&D IIR 07-138 (LSP and SLJ), NIH awards R21DK099716 (LSP, QL, LS, and SLJ), DK066204 (LSP), U01 DK091958 (LSP and MKR), U01 DK098246 (LSP and DEO), R21NS091630 (QL), K12 award 5K12HD085850 (SS), PCORI award ME-1303-5840 (SS and QL), and a Cystic Fibrosis Foundation award PHILLI12A0 (LSP). It is also supported in part by the National Center for Advancing Translational Sciences of NIH under award number UL1TR000454. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Drs. Phillips, Rhee, Olson, and Tomolo receive support from the VA, and Dr. Jackson conducted analyses using VA resources and data. This work is not intended to reflect the official opinion of the VA or the U.S. government.
Footnotes
Sandra Jackson conducted data analyses and drafted the manuscript, including literature search, production of figures, data interpretation, and writing. Lawrence Phillips provided guidance and input across all stages of study planning, analysis, and manuscript development and revisions. Qi Long and Sandra Safo provided statistical expertise for analyses and edited the manuscript. Mary Rhee, Lisa Staimez, Darin Olson, Anne Tomolo, Solveig Cunningham, Usha Ramakrishnan, and K.M. Venkat Narayan contributed conceptually to study planning and edited manuscript revisions. Sandra Jackson, Sandra Safo, and Lawrence Phillips had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. There was no medical writer or editor.
The authors declare that there is no duality of interest associated with this manuscript. With regard to potential conflicts of interest, within the past several years, LSP has served on Scientific Advisory Boards for Boehringer Ingelheim and Janssen, and has or had research support from Merck, Amylin, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, Glaxo SmithKline, and the Cystic Fibrosis Foundation. In the past, he was a speaker for Novartis and Merck, but not for the last several years. QL receives support from NIH, PCORI, NSF, AHA, and the Cystic Fibrosis Foundation and was a consultant for Eisai. SLJ received support from Amylin. SAC receives support from NIH. KMVN receives support from NIH, the Robert Wood Johnson Foundation, and Novo Nordisk. Listed activities involve diabetes, but have nothing to do with this manuscript. Other authors have no potential conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics—2011 Update: A Report From the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. http://dx.doi.org/10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. http://dx.doi.org/10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary Prevention of Coronary Heart Disease in Women through Diet and Lifestyle. N Engl J Med. 2000;343(1):16–22. doi: 10.1056/NEJM200007063430103. http://dx.doi.org/10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 4.Schieb LJ, Greer SA, Ritchey MD, George MG, Casper ML. Vital Signs: Avoidable Deaths from Heart Disease, Stroke, and Hypertensive Disease — United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2013;62(35):721–727. [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. http://dx.doi.org/10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan X-R, Li G-W, Hu Y-H, et al. Effects of Diet and Exercise in Preventing NIDDM in People With Impaired Glucose Tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. http://dx.doi.org/10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 7.Lindström J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS) Diabetes Care. 2003;26(12):3230–3236. doi: 10.2337/diacare.26.12.3230. http://dx.doi.org/10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 8.The Look AHEAD Research Group. Long-term Effects of a Lifestyle Intervention on Weight and Cardiovascular Risk Factors in Individuals With Type 2 Diabetes Mellitus: Four-Year Results of the Look AHEAD Trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. http://dx.doi.org/10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ornish D, Scherwitz LW, Billings JH et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–2007. doi: 10.1001/jama.280.23.2001. http://dx.doi.org/10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 10.Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(8):543–551. doi: 10.7326/0003-4819-159-8-201310150-00007. http://dx.doi.org/10.7326/0003-4819-159-8-201310150-00007. [DOI] [PubMed] [Google Scholar]
- 11.de Waure C, Lauret GJ, Ricciardi W, et al. Lifestyle interventions in patients with coronary heart disease: a systematic review. Am J Prev Med. 2013;45(2):207–216. doi: 10.1016/j.amepre.2013.03.020. http://dx.doi.org/10.1016/j.amepre.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Orchard TJ, Temprosa M, Barrett-Connor E, et al. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med. 2013;30(1):46–55. doi: 10.1111/j.1464-5491.2012.03750.x. http://dx.doi.org/10.1111/j.1464-5491.2012.03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Look AHEAD Research Group. Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. N Engl J Med. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. http://dx.doi.org/10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Center for Veterans Analysis and Statistics. Trends in the Utilization of VA Programs and Services. [November 29, 2013];2012 www.va.gov/vetdata/docs/quickfacts/Utilization-slideshow.pdf.
- 15.Nelson KM. The Burden of Obesity Among a National Probability Sample of Veterans. J Gen Intern Med. 2006;21(9):915–919. doi: 10.1111/j.1525-1497.2006.00526.x. http://dx.doi.org/10.1007/BF02743137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson ML, Pietz K, Battleman DS, Beyth RJ. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Am J Manag Care. 2004;10(12):926–932. [PubMed] [Google Scholar]
- 17.NHLBI Obesity Education Initiative Expert Panel on the Identification Evaluation and Treatment of Obesity in Adults (U.S.) Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: National Heart, Lung, and Blood Institute; 1998. [Google Scholar]
- 18.Littman AJ, Boyko EJ, McDonell MB, Fihn SD. Evaluation of a Weight Management Program for Veterans. Prev Chronic Dis. 2012;9 doi: 10.5888/pcd9.110267. http://dx.doi.org/10.5888/pcd9.110267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson SL, Long Q, Rhee MK, et al. Weight loss and incidence of diabetes with the Veterans Health Administration MOVE! lifestyle change programme: an observational study. Lancet Diabetes Endocrinol. 2015;3(3):173–180. doi: 10.1016/S2213-8587(14)70267-0. http://dx.doi.org/10.1016/S2213-8587(14)70267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VA Health Services Research and Development Service. [February 3, 2013];VA Informatics and Computing Infrastructure. 2012 www.hsrd.research.va.gov/for_researchers/vinci/#.UupYerT4smA.
- 21.Kinsinger LS, Jones KR, Kahwati L, et al. Design and dissemination of the MOVE! Weight-Management Program for Veterans. Prev Chronic Dis. 2009;6(3):A98. [PMC free article] [PubMed] [Google Scholar]
- 22.Kahwati LC, Lewis MA, Kane H, et al. Best practices in the Veterans Health Administration’s MOVE’ Weight management program. Am J Prev Med. 2011;41(5):457–464. doi: 10.1016/j.amepre.2011.06.047. http://dx.doi.org/10.1016/j.amepre.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 23.Flicker L, McCaul KA, Hankey GJ, et al. Body Mass Index and Survival in Men and Women Aged 70 to 75. J Am Geriatr Soc. 2010;58(2):234–241. doi: 10.1111/j.1532-5415.2009.02677.x. http://dx.doi.org/10.1111/j.1532-5415.2009.02677.x. [DOI] [PubMed] [Google Scholar]
- 24.Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005;29(9):1011–1029. doi: 10.1038/sj.ijo.0803005. http://dx.doi.org/10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 25.Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: Opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739–750. doi: 10.1682/jrrd.2009.08.0110. http://dx.doi.org/10.1682/JRRD.2009.08.0110. [DOI] [PubMed] [Google Scholar]
- 26.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233–1239. doi: 10.1093/ntr/ntr206. http://dx.doi.org/10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. http://dx.doi.org/10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 28.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26(8):2392–2399. doi: 10.2337/diacare.26.8.2392. http://dx.doi.org/10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- 29.Lee EW, Wei LJ, Amato D. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. Netherlands: Kluwer Academic; 1992. http://dx.doi.org/10.1007/978-94-015-7983-4_14. [Google Scholar]
- 30.D’Agostino RB. Propensity Scores in Cardiovascular Research. Circulation. 2007;115(17):2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 31.Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Davey Smith G. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev. 2011;(1) doi: 10.1002/14651858.CD001561.pub3. CD001561. http://dx.doi.org/10.1002/14651858.cd001561.pub3. [DOI] [PMC free article] [PubMed]
- 32.Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA. 2010;304(16):1795–1802. doi: 10.1001/jama.2010.1505. http://dx.doi.org/10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerstein HC. Do Lifestyle Changes Reduce Serious Outcomes in Diabetes? N Engl J Med. 2013;369(2):189–190. doi: 10.1056/NEJMe1306987. http://dx.doi.org/10.1056/NEJMe1306987. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2(6):474–480. doi: 10.1016/S2213-8587(14)70057-9. http://dx.doi.org/10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 35.Després J-P. Obesity and Cardiovascular Disease: Weight Loss Is Not the Only Target. Can J Cardiol. 2015;31(2):216–222. doi: 10.1016/j.cjca.2014.12.009. http://dx.doi.org/10.1016/j.cjca.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Littman AJ, Damschroder LJ, Verchinina L, et al. National evaluation of obesity screening and treatment among veterans with and without mental health disorders. Gen Hosp Psychiatry. 2015;37(1):7–13. doi: 10.1016/j.genhosppsych.2014.11.005. http://dx.doi.org/10.1016/j.genhosppsych.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Borowsky SJ, Cowper DC. Dual use of VA and non-VA primary care. J Gen Intern Med. 1999;14(5):274–280. doi: 10.1046/j.1525-1497.1999.00335.x. http://dx.doi.org/10.1046/j.1525-1497.1999.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C-F, Manning WG, Burgess JFJ, et al. Reliance on Veterans Affairs Outpatient Care by Medicare-eligible Veterans. Medical Care. 2011;49(10):911–917. doi: 10.1097/MLR.0b013e31822396c5. http://dx.doi.org/10.1097/MLR.0b013e31822396c5. [DOI] [PubMed] [Google Scholar]
- 39.Liu CF, Chapko M, Bryson CL, et al. Use of outpatient care in Veterans Health Administration and Medicare among veterans receiving primary care in community-based and hospital outpatient clinics. Health Serv Res. 2010;45(5 Pt 1):1268–1286. doi: 10.1111/j.1475-6773.2010.01123.x. http://dx.doi.org/10.1111/j.1475-6773.2010.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


