Abstract
Background & aims
Acetaminophen (APAP)-induced liver injury is coupled to activation of the blood coagulation cascade and fibrin(ogen) accumulation within APAP-injured livers of experimental mice. We sought to define the precise role of fibrin(ogen) deposition in APAP-induced liver injury and repair.
Methods
Fasted mice were injected with 300 mg/kg APAP i.p. and evaluated various times later.
Results
In wild-type mice APAP overdose increased intrahepatic levels of high molecular weight cross-linked fibrin(ogen). Anticoagulation reduced early APAP hepatotoxicity (6 hours), but surprisingly, increased hepatic injury at 24 hours, implying a protective role for coagulation at the onset of repair. Complete fibrin(ogen) deficiency delayed liver repair after APAP overdose, evidenced by a reduction of proliferating hepatocytes (24 hours) and unresolved hepatocellular necrosis (48 and 72 hours). Mutant mice with fibrin(ogen) incapable of binding leukocyte αMβ2 integrin (Fibγ390-396A mice) had decreased hepatocyte proliferation and increases in multiple indices of liver injury, suggesting a mechanism related to fibrin(ogen)-leukocyte interaction. Induction of the macrophage-associated gene, matrix metalloproteinase 12 (Mmp12), was dramatically reduced in APAP-treated Fibγ390-396A mice, and mice lacking Mmp12 displayed exacerbated APAP-induced liver injury, resembling Fibγ390-396A mice. In contrast, administration of the αMβ2 integrin allosteric agonist leukadherin-1 enhanced hepatic MMP12 mRNA and reduced necrosis in APAP-treated mice. Further, administration of recombinant MMP12 protein to APAP-treated Fibγ390-396A mice restored hepatocyte proliferation.
Conclusions
Collectively, these studies highlight an entirely novel pathway of liver repair after APAP overdose, mediated by fibrin(ogen)-αMβ2 integrin engagement and demonstrate for the first time a protective role of Mmp12 expression after APAP overdose.
Keywords: coagulation, hemostasis, fibrinogen, liver repair, macrophage, inflammation, αMβ2, metalloproteinase
Graphical abstract

INTRODUCTION
Acute liver injury caused by acetaminophen (APAP) overdose in experimental animals is associated with activation of the coagulation cascade. Strong evidence also connects APAP-induced liver injury in humans to changes in the hemostatic system including thrombocytopenia, elevated plasma levels of thrombin-antithrombin (TAT), increased levels of procoagulant microvesicles, and a reduction in plasma fibrinogen concentration [1–3]. Prolongation of the prothrombin time-international normalized ratio (PT-INR) is linked with increased morbidity in patients with acute liver failure whereas preservation of plasma fibrinogen is coupled to improved outcome [2, 4–6]. Many of these changes are perceived as a direct consequence of acute liver damage, potentially reflecting failed synthesis of coagulation factors, such as fibrinogen, by the injured liver. However, there is accumulating experimental evidence that coagulation factors themselves are powerful modifiers of acute APAP hepatotoxicity.
A hepatotoxic dose of APAP administered experimentally to mice recapitulates many of the perceived changes in coagulation observed in APAP-poisoned patients. Although early experimental APAP hepatotoxicity is independent of fibrin(ogen), we and others have observed profound deposition of fibrin(ogen) within areas of hepatocellular necrosis [7, 8]. The fibrin(ogen) gamma chain is capable of engaging the leukocyte integrin αMβ2 (Mac-1, CD11b/CD18) and through this mechanism, fibrin(ogen) can define the activity of inflammatory cells such as macrophages [9, 10]. Notably, macrophages infiltrate the liver after APAP overdose in humans and mice [11, 12]. Further, strong evidence suggests that macrophages play a central role in liver repair after APAP overdose [13, 14]. However, to date the precise signals required to trigger leukocyte-driven liver repair after APAP overdose have not been identified, and the potential for fibrin(ogen) to act in this capacity has largely been overlooked.
We tested the hypothesis that fibrin(ogen)-integrin αMβ2 interaction contributes to liver repair after APAP overdose utilizing the thrombin inhibitor dabigatran etexilate, mice completely lacking fibrin(ogen), mice expressing a mutant fibrin(ogen) incapable of binding leukocyte integrin αMβ2 and an allosteric agonist of αMβ2. Moreover, we investigated matrix metalloproteinase 12 (Mmp12) induction as a fibrin(ogen)-integrin αMβ2 driven event central to liver repair after APAP overdose.
MATERIALS AND METHODS
Mice
Fibrinogen Aα-deficient (Fib−/−) and fibrinogen heterozygous control mice (Fib+/−) have been described previously [15]. Because bleeding is observed in pregnant Fib−/− female mice, these mice were maintained by breeding male Fib−/− mice with female Fib+/− mice, a strategy employed by numerous previous studies to compare fibrin(ogen)-sufficient mice with mice lacking circulating fibrin(ogen) [8, 16–18]. Mice expressing a mutant form of fibrinogen γ390-396A (Fibγ390-396A) lacking a binding motif for integrin receptor αMβ2 expressed on leukocytes [9, 10, 19] and age-matched wild-type mice on an identical C57BL/6 background were maintained by homozygous breeding. Mmp12-deficient mice (B6.129X-Mmp12tm1Sds/J) and age-matched C57BL/6 wild-type mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained by homozygous breeding. Mice lacking the factor XIII catalytic A subunit (FXIII-deficient mice) have been described previously [20]. Mice were housed in Association for Assessment and Accreditation of Laboratory Animal Care-approved facilities at Michigan State University (MSU). Mice were housed at an ambient temperature of 22 ± 2°C with alternating 12 hour light/dark cycles and provided ad libitum access to standard rodent chow diet and purified drinking water. All animal procedures were approved by the MSU Institutional Animal Care and Use Committee.
Experimental treatment paradigms
To model APAP-induced liver injury, mice were fasted for approximately 16 hours (overnight) and then treated i.p. with 300 mg/kg APAP (at 30 μl/g body weight) or vehicle (sterile saline, 0.9% sodium chloride), at which time food was returned. To examine the impact of thrombin inhibition on APAP-induced liver injury, wild-type mice were fed either a control chow containing 10 mg/g of the direct thrombin inhibitor dabigatran etexilate or identical chow without drug, a formulation described previously [21], for 1 week prior to APAP challenge. Following the fast and APAP challenge, each diet was returned. For studies in which Fibγ390-396A mice were rescued with exogenous MMP12, wild-type and Fibγ390-396A mice were treated with APAP, and 6 hours later injected i.p. with 2 μg recombinant mouse MMP12 (rMMP12) (3467-MP, R&D Systems, Minneapolis, MN) or saline vehicle. To examine the effect of the allosteric leukocyte integrin αMβ2 activator leukadherin-1 (LA-1), mice were treated with APAP and then given i.p. injections of LA-1 (0.4 mg/kg) or its vehicle (0.4% DMSO in saline) at 6 and 12 hours after APAP administration. For details about sample collection and processing, determination of serum cytokines, alanine aminotransferase, plasma hyaluronic acid, plasma fibrinogen, histopathology, immunohistochemistry, immunofluorescence, detection of intrahepatic fibrin(ogen) by standard and capillary western blotting, detection of fibrin(ogen) species in plasma in vitro clots, and RNA isolation, CDNA synthesis and quantitative real-time PCR see Supplementary Material.
Statistical analyses
Comparison of 2 groups was performed using Student’s t-test. Comparison of 3 or more groups was performed using one- or two-way analysis of variance (ANOVA) and Student-Newman-Keuls post-hoc test. Data were considered significant when the p-value was less than 0.05.
RESULTS
Intrahepatic deposition of LMW and HMW urea-insoluble cross-linked fibrin(ogen) after APAP overdose. Plasma fibrinogen levels decreased over time in mice administered a hepatotoxic dose of APAP (300 mg/kg) relative to vehicle-treated animals (Fig. 1A). This was paralleled by fibrin(ogen) deposition in the livers of APAP-treated mice at 24 hours, which immunohistochemistry detected primarily within areas of hepatocellular necrosis (Fig. 1B). Analysis of mouse hepatic extracts revealed that levels of thrombin-cleaved fibrinogen beta chain increased 24 hours after APAP challenge, suggesting that the observed deposits were in the form of thrombin-cleaved fibrin matrix (Fig. 1C).
Figure 1. Plasma fibrinogen levels and hepatic fibrin(ogen) deposition after APAP overdose.
Wild-type (WT) mice were treated i.p. with saline vehicle (Veh) or 300 mg/kg APAP and (A) plasma fibrinogen levels were assessed at 24 and 48 hours. (B) Representative photomicrographs (200X) show hepatic fibrin(ogen) staining (brown) in APAP-treated livers at 24 hours. (C) Thrombin-cleaved fibrinogen beta chain protein levels (~50 kDa) were detected in hepatic extracts from saline- and APAP-treated WT mice at 24 hours. Each lane represents hepatic extract from one treated animal. (D) Fibrin(ogen) species were detected in clots formed in vitro using plasma from untreated WT and FXIII-deficient mice using a capillary western blot approach, as described in Materials and Methods. (E) Levels of fibrin(ogen) species in hepatic extracts from saline- and APAP-treated WT mice were detected using capillary western blot at 24 hours with (F) corresponding area quantification of low molecular weight (LMW, 50–66 kDa) and high molecular weight (HMW, >350 kDa) fibrin(ogen) bands. (G–H) Representative digital capillary image and area quantification depicting the absence of HMW cross-linked fibrin in the livers of fibrinogen-deficient (Fib−/−) mice compared to heterozygous control (Fib+/−) mice treated with 300 mg/kg APAP for 24 hours. N=3–10 mice/group. Data expressed as mean+SEM. *Significantly (p<0.05) different from saline vehicle-treated mice within a group (A & F) or different from APAP-exposed Fib−/− mice (H) as determined by on-way ANOVA (A) or Student’s t-test (F, H).
To define whether intrahepatic fibrin(ogen) deposits after APAP overdose represent true cross-linked fibrin polymers or accumulated fibrin(ogen), we utilized capillary gel automated western blotting (Wes, Protein Simple) to determine the levels of urea-insoluble (i.e., cross-linked fibrin polymers) and urea soluble fibrin(ogen) [25, 26]. Illustrating the specificity of this approach, HMW fibrin(ogen) species (>350 kDa) were detected in wild-type mouse plasma clotted with human α-thrombin (Fig. 1D). However, when coagulation FXIII-deficient plasma was utilized, these HMW species were absent, definitively identifying these as cross-linked fibrin (Fig. 1D). Deposition of LMW fibrin(ogen) species (50–66 kDa) was detectable in both wild-type and FXIII-deficient plasma (Fig. 1D). Utilizing this approach to analyze liver samples, we identified a robust increase in urea-insoluble HMW cross-linked fibrin species in APAP-treated wild-type mice relative to livers from vehicle-treated mice at 24 hours (Fig. 1E–F). Hepatic levels of LMW fibrin(ogen) species also increased in APAP-treated mice (Fig. 1E–F). As anticipated, owing to a complete lack of plasma fibrinogen, no HMW fibrin species were observed in APAP-treated Fib−/− mice (Fig. 1G–H). Although Fib−/− mice have no circulating plasma fibrinogen, fibrinogen beta and gamma chains, still expressed by hepatocytes in Fib−/− mice [15], were detected as weak LMW bands in liver homogenates of APAP-treated Fib−/− mice after 24 hours (Fig. 1G–H). The results indicate that APAP-induced liver injury in mice is associated with accumulation of non-cross-linked LMW fibrin(ogen) and cross-linked HMW fibrin species in the liver.
Fibrinogen deficiency delays liver repair after APAP overdose
Our previous study showed that early APAP-induced hepatotoxicity, indicated by serum ALT activity, was not significantly affected by complete fibrin(ogen) deficiency [8]. To evaluate a potential connection between fibrin(ogen) and resolution of injury after APAP overdose, we examined the time course of liver repair in APAP-treated Fib−/− mice and Fib+/− mice, which express 70% of normal fibrinogen levels. Compared to heterozygous control (Fib+/−) mice, APAP-induced hepatocellular necrosis was not significantly increased in APAP-treated Fib−/− mice 24 hours after APAP overdose (Fig. 2A–B). Interestingly, when the time course was extended, we noted approximately 30% mortality prior to 48 hours in both APAP-treated Fib+/− mice and in APAP-treated Fib−/− mice. Although the basis for elevated mortality is not known, and requires additional experimentation, because Fib+/− mice do not express wild-type fibrin(ogen) levels [15], this suggests that even mild fibrin(ogen) insufficiency could impact liver repair after APAP overdose. The area of hepatic necrosis decreased significantly between 24 and 48 hours in APAP-treated Fib+/− mice. However, no evidence of repair was evident in this same time frame in Fib−/− mice (Fig. 2A–B). This persistent necrosis in Fib−/− mice was associated with marked hemorrhage/congestion both within necrotic areas and neighboring parenchymal tissue (Fig. 2A). Serum ALT levels were quite variable, and were not significantly different between Fib+/− and Fib−/− mice at 48 hours (Supplementary Fig. 1A). Whereas centrilobular hepatic necrosis was essentially absent by 72 hours in APAP-treated Fib+/− control mice, 3 of 5 APAP-treated Fib−/− mice still displayed marked necrosis, accounting for approximately 30% of the total examined area. The delay in resolving necrosis in Fib−/− mice was coupled to an early, but transient decrease in hepatocyte proliferation, as the number of PCNA-positive hepatocyte nuclei was significantly decreased in APAP-treated Fib−/− mice at 24 hours (Fig. 2C–D). The number of PCNA-positive hepatocyte nuclei was not affected by complete fibrin(ogen) deficiency 48 hours after APAP administration (Fig. 2D). Collectively, the results indicate that fibrin(ogen) deficiency delays liver repair after APAP overdose in mice.
Figure 2. Delayed liver repair in fibrin(ogen)-deficient mice after APAP overdose.
Fibrin(ogen)-deficient mice (Fib−/− mice) and heterozygous control mice (Fib+/− mice) were treated i.p. with 300 mg/kg APAP. (A) Representative photomicrographs (100X) of H&E-stained liver sections taken 24, 48, and 72 hours after APAP administration. (B) The area of centrilobular hepatic necrosis was quantified as described in Materials and Methods. (C) Representative hepatic photomicrographs (200X) of PCNA-positive hepatocytes 24 hours after APAP administration. (D) Quantification of PCNA-positive hepatocyte nuclei at 24 and 48 hours after APAP challenge. N=4–8 mice/group. Data expressed as mean+SEM. Data were analyzed using a two-way ANOVA. *Significantly (p<0.05) different from APAP-treated mice of the same genotype at 24 hours. #Significantly (p<0.05) different from APAP-treated Fib+/− control mice at that time. aSignificantly (p<0.05) different from APAP-treated Fib+/− control mice at that time.
Time-dependent effects of thrombin inhibition on hepatocyte proliferation and liver injury after APAP overdose
Discovery of a putative role for fibrin(ogen) in liver repair is somewhat at odds with prior publications indicating that anticoagulation (i.e., heparin, lepirudin) reduced early APAP-induced hepatotoxicity [7, 28]. However, these previous studies only examined the effect of anticoagulants at early times (≤ 6 hours post-APAP), well before peak injury and likely preceding profound activation of liver repair pathways. Thus, while capable of detecting early damaging effects of thrombin mediated by protease activated receptors (PARs) [7, 28], these studies were not suited to define the effect of anticoagulation on liver repair at later time points.
To directly address the role of thrombin in both early APAP hepatotoxicity and initiation of repair of the injured liver, we administered the direct thrombin inhibitor dabigatran etexilate (DABI), as described previously [21]. In agreement with previous studies [7], anticoagulation with DABI reduced hepatic HMW fibrin deposition (Supplementary Fig. 2A–B) and tended to reduce early hepatotoxicity (Supplementary Fig. 2C–E). In contrast to the protection observed at 6 hours, DABI treatment significantly attenuated hepatocyte proliferation 24 hours after APAP administration (Fig. 3A–B), and this was associated with a significant increase in hepatic necrosis and serum ALT activity, coincident with excess hemorrhage/congestion (Fig. 3C–D, Supplementary Fig. 1B). The results indicate that despite multiple studies pointing to anticoagulation attenuating early APAP hepatotoxicity, anticoagulation appears to also restrict initiation of liver repair, ultimately exacerbating liver injury. Because PAR-1- and PAR-4-deficient mice are protected from APAP hepatotoxicity [7, 28] and do not display a defect in liver repair, this suggests that fibrin(ogen) is the putative thrombin target driving liver repair after APAP overdose.
Figure 3. Effect of thrombin inhibition on hepatic injury and repair after APAP overdose.
Wild-type mice were fed a chow diet formulated with 10 mg/g dabigatran etexilate (DABI) or identical diet without the drug (control) for 1 week and were afterwards treated i.p. with 300 mg/kg APAP and evaluated 24 hours later (A) Representative hepatic photomicrographs (100X) of PCNA-positive hepatocytes. (B) Quantification of PCNA-positive hepatocytes. (C) Representative photomicrographs (100X) of H&E-stained liver sections from APAP-treated mice fed control diet or DABI diet. (D) The area of centrilobular necrosis in liver sections of APAP-treated control diet and DABI diet fed mice. N=5–10 mice/group. Data expressed as mean+SEM. *Significantly (p<0.05) different from APAP-treated control diet mice as determined by a Student’s t-test.
Fibrin(ogen) engagement of leukocyte αMβ2 integrin stimulates liver repair after APAP overdose
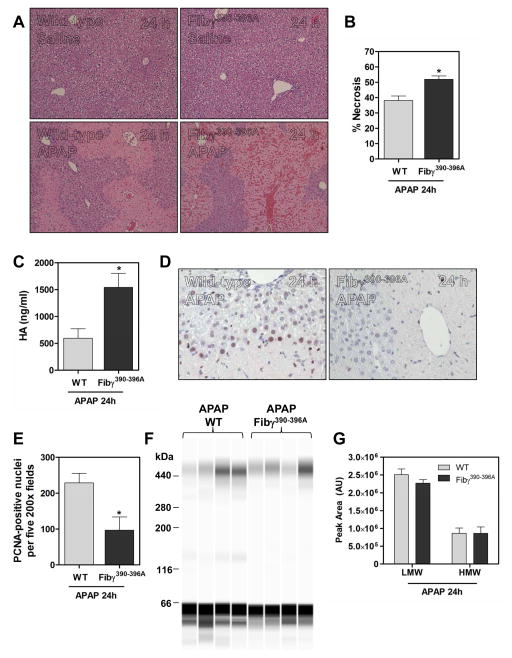
Multiple studies have documented the importance of leukocytes, particularly macrophages, in liver repair after APAP overdose [11, 13, 29, 30]. A central non-hemostatic function of fibrin(ogen) is engagement of the leukocyte integrin αMβ2 [9, 19], potentially linking fibrin(ogen) deposits with inflammatory cell functions required for liver repair. To determine if this fibrin(ogen)-integrin interaction could account for the importance of fibrin(ogen) in liver repair after APAP overdose, we utilized Fibγ390-396A mice, which lack the fibrinogen gamma chain binding motif required to engage αMβ2 integrin on leukocytes [9, 10, 19]. Vehicle-treated mice did not exhibit noticeable changes in liver histology regardless of genotype (Fig. 4A). As with Fib−/− mice, ~30% of Fibγ390-396A mice died following APAP challenge. Hepatocellular necrosis was increased in APAP-treated Fibγ390-396A mice at 24 hours, and this was accompanied by marked hemorrhage/congestion (Fig. 4A–B, Supplementary Fig. 1C), features shared by mice lacking intrahepatic macrophages after APAP overdose [13, 30]. Consistent with increased sinusoidal endothelial cell (SEC) injury observed in macrophage-depleted mice after APAP overdose [13, 30], plasma levels of hyaluronic acid (HA), an extracellular matrix component cleared by SECs, were almost 3 times higher in Fibγ390-396A mice relative to wild-type mice (Fig. 4C). Analogous to fibrin(ogen)-deficient mice, assessment of hepatocyte proliferation at 24 hours in APAP overdose mice revealed significantly less PCNA-positive hepatocytes in Fibγ390-396A mice relative to wild-type mice receiving APAP (Fig. 4D–E).
Figure 4. Impact of disrupting fibrin(ogen)- αMβ2 integrin engagement on hepatic injury and repair after APAP overdose.
Wild-type (WT) and Fibγ390-396A mice were treated i.p. with saline vehicle or 300 mg/kg APAP and evaluated 24 hours later. (A) Representative photomicrographs (100X) of H&E-stained liver sections from vehicle- and APAP-treated mice. (B) The area of centrilobular necrosis in liver sections of APAP-treated WT and Fibγ390-396A mice. (C) Plasma hyaluronic acid levels. (D) Representative hepatic photomicrographs (200X) of PCNA-positive hepatocytes. (E) Quantification of PCNA staining. (F–G) Representative digital capillary image and quantification of low molecular weight (LWM) and high molecular weight (HMW) cross-linked fibrin(ogen) species in livers of APAP-treated WT and Fibγ390-396A mice. N=4–10 mice/group. Data expressed as mean+SEM. *Significantly (p<0.05) different from APAP-treated WT mice as determined by a Student’s t-test.
Fibγ390-396A mice have a well-documented normal hemostatic profile and arterial thrombi in these mice are comparable to wild-type mice [9]. Notably, a recent study found that FXIII-mediated cross-linking of γ390-396A fibrinogen was delayed, leading to decreased retention of red blood cells in a model of venous thrombosis [27]. To address the possibility that delayed fibrin(ogen) cross-linking could partially account for the phenotype of APAP-treated Fibγ390-396A mice, we measured intrahepatic levels of HMW fibrin polymers. Importantly, there was no difference in levels of cross-linked HMW and LMW fibrin(ogen) species in livers of wild-type and Fibγ390-396A mice treated with APAP (Fig. 4F–G). Preservation of HMW urea-insoluble fibrin(ogen) species in APAP-treated Fibγ390-396A mice suggests that the phenotype of these mice after APAP exposure is unlikely to be explained by a delay in fibrin(ogen) cross-linking. Indeed, the similarity of histopathological changes observed in livers of mice lacking intrahepatic macrophages given APAP [13, 30], and in APAP-treated Fibγ390-396A mice, strongly suggests a mechanism related to altered leukocyte accumulation and/or function.
Fibrin(ogen) engagement of leukocyte αMβ2 integrin contributes to localization of intrahepatic macrophages after APAP overdose
Neutrophils and macrophages are two cell types known to express integrin αMβ2 and accumulate in the injured liver after APAP overdose [11, 29, 31, 32]. Hepatic neutrophil accumulation after APAP administration was similar in wild-type and Fibγ390-396A mice (Fig. 5A–B). The number and distribution of resident hepatic macrophages was similar in saline-treated mice regardless of genotype (Fig. 5C–D). Livers from wild-type mice given APAP showed uniquely organized macrophages that clearly demarcated the borders of necrotic lesions (Fig. 5C). In contrast, macrophages lacked this organization in APAP-treated Fibγ390-396A mice (Fig. 5C). Similar patterns of macrophage localization were observed when hepatic sections were co-stained for fibrin(ogen). Macrophages lined the borders of fibrin(ogen)-filled necrotic lesions only in APAP-treated wild-type mice and showed a disorganized arrangement in Fibγ390-396A mice (Fig. 5E). There was not a significant difference in overall macrophage number between vehicle- and APAP-treated mice of either genotype (Fig. 5D). These findings suggest that fibrin(ogen) engagement of integrin αMβ2 contributes to macrophage localization to the borders of necrotic injury in livers of mice after APAP overdose.
Figure 5. Impact of disrupting fibrin(ogen)- αMβ2 integrin engagement on hepatic neutrophil and macrophage accumulation after APAP overdose.
Wild-type (WT) and Fibγ390-396A mice were treated i.p. with saline vehicle or 300 mg/kg APAP and immune cell infiltration was evaluated 24 hours later. (A) Representative photomicrographs (200X) of hepatic neutrophil staining (red). (B) Quantification of neutrophil accumulation in livers of vehicle- and APAP-treated mice. (C) Representative photomicrographs (200X) showing hepatic macrophage accumulation (F4/80+CD68, red) in vehicle- and APAP-treated mice, co-stained with nuclear DAPI stain (blue). (D) Number of F4/80+CD68-positive macrophages per 200X image. (E) Representative photomicrographs (200X) of fibrin(ogen) (green) and F4/80+CD68-positive (red) macrophages in APAP-treated mice. N=3–5 mice/group. Data expressed as mean+SEM. *Significantly (p<0.05) different from genotype-matched vehicle groups using a two-way ANOVA.
Fibrin(ogen) engagement of leukocyte αMβ2 integrin drives hepatic macrophage-associated gene induction after APAP overdose
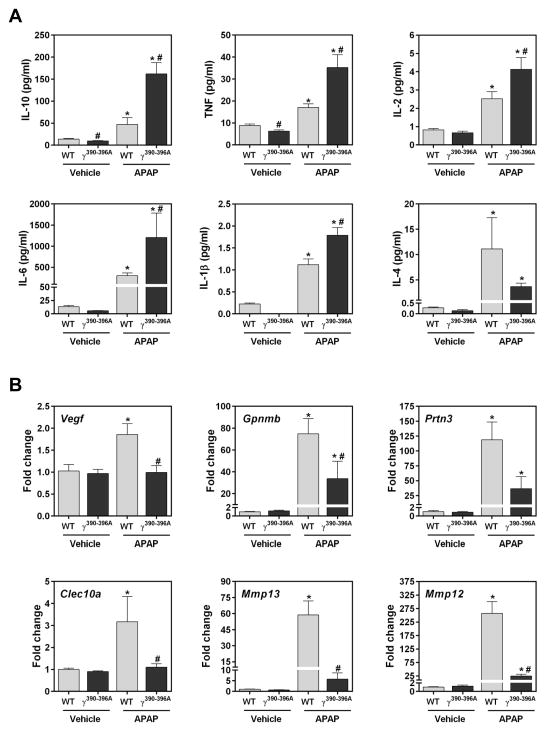
Fibγ390-396A mice are protected in multiple experimental settings of chronic and acute disease owing to reduced inflammation [9, 10]. To our surprise, with the exception of IL-4, APAP-driven increases in plasma cytokine levels were significantly exacerbated in Fibγ390-396A mice at 24 hours, suggesting a dysregulated proinflammatory response (Fig. 6A). Despite this unexpected increase in systemic inflammation, hepatic expression of several genes induced in macrophages after APAP overdose, including Vegf, Gpnmb, Prtn3, Clec10a, Mmp13 and Mmp12 (Fig. 6B) was significantly reduced in Fibγ390-396A mice treated with APAP relative to wild-type mice after APAP overdose. Mmp12, previously shown to be induced in macrophages from APAP-treated mice [11], exhibited the greatest induction among all investigated genes, with expression reaching ~250-fold in APAP-treated wild-type mice, compared to only ~25-fold induction in APAP-treated Fibγ390-396A mice. Notably, Mmp12 expression was also reduced by approximately 50% in Fib−/− mice compared to Fib+/− mice (not shown). In agreement with mRNA levels, immunohistochemistry showed marked decreases in Mmp12-positive inflammatory cells in APAP-treated Fibγ390-396A livers relative to wild-type livers 24 hours after APAP administration (Supplementary Fig. 3A–B).
Figure 6. Impact of disrupting fibrin(ogen)- αMβ2 integrin engagement cytokine levels and hepatic macrophage-associated gene induction after APAP overdose.
Wild-type (WT) and Fibγ390-396A mice were treated i.p. with saline vehicle or 300 mg/kg APAP and (A) plasma levels of cytokines and (B) hepatic gene expression levels were assessed 24 hours later. N=3–10 mice/group. Data expressed as mean+SEM. Data were analyzed using a two-way ANOVA. *Significantly (p<0.05) different from genotype-matched vehicle mice. #Significantly (p<0.05) different from treatment-matched WT mice.
Mmp12-deficiency worsens liver injury and inhibits hepatocyte proliferation after APAP overdose
The aforementioned results prompted us to investigate whether fibrin(ogen) engagement of αMβ2 integrin expressed on macrophages regulates Mmp12 expression, in turn driving liver repair after APAP overdose. To test this hypothesis, we utilized Mmp12-deficient (Mmp12−/−) mice. Liver histology was unremarkable in vehicle-treated wild-type and Mmp12−/− mice (Fig. 7A). APAP-induced liver necrosis and serum ALT levels were increased in Mmp12−/− mice compared to wild-type mice (Fig. 7A–B, Supplementary Fig. 1D), and reminiscent of Fibγ390-396A mice. Congestion and hemorrhage were also clear features of livers from APAP-treated Mmp12−/− mice (Fig. 7A). Moreover, there was evidence of SEC injury, noted by increased plasma HA levels (Fig. 7C). Increases in necrosis, ALT levels and congestion/hemorrhage persisted in Mmp12−/− mice 48 hours after APAP administration (Fig. 7D, Supplementary Fig. 1E). Hepatocyte proliferation, marked by the number of PCNA-positive hepatocytes, was significantly reduced in APAP-treated Mmp12−/− mice compared to wild-type mice at 24 hours, but not different between the genotypes at 48 hours (Fig. 7E–F). Next, we determined whether administration of mouse recombinant MMP12 protein (rMMP12) could rescue the defect in liver repair in APAP-treated Fibγ390-396A mice. Compared to wild-type mice given vehicle (PBS), the number of PCNA-positive hepatocytes was significantly reduced in APAP-treated Fibγ390-396A mice (Fig. 8A–B). In contrast, administration of 2 μg rMMP12 partially rescued this defect in hepatocyte proliferation, restoring hepatic PCNA staining to levels not significantly different from wild-type mice (Fig. 8A–B). Administration of rMMP12 also markedly reduced hemorrhage/congestion in APAP-treated Fibγ390-396A mice, reduced serum ALT levels and slightly reduced necrotic area (Fig. 8C–D, Supplementary Fig. 1F). Collectively, the results suggest that fibrin(ogen)- αMβ2 integrin-dependent induction of Mmp12 contributes to liver repair in mice after APAP overdose.
Figure 7. Impact of Mmp12 deficiency on hepatic injury and repair after APAP overdose.
Wild-type (WT) and Mmp12−/− mice were treated i.p. with saline vehicle or 300 mg/kg APAP. (A) Representative photomicrographs (100X) of H&E-stained liver sections from vehicle- and APAP-treated mice at 24 hours. (B) The area of centrilobular necrosis in liver sections of APAP-treated WT and Mmp12−/− mice at 24 hours. (C) Plasma hyaluronic acid levels at 24 hours. (D) Representative photomicrographs showing H&E-stained cross-sections of the left lateral lobes from APAP-treated WT and Mmp12−/− mice at 48 hours. (E) Hepatic photomicrographs (200X) showing PCNA-positive hepatocytes 24 hours after APAP administration. (F) Quantification of PCNA-positive hepatocytes at 24 and 48 hours. N=9–20 mice/group. Data expressed as mean+SEM. Data were analyzed using a Student’s t-test (B, C) or using a two-way ANOVA (F). *Significantly (p<0.05) different from time-matched APAP-treated WT mice.
Figure 8. Effect of recombinant MMP12 administration on hepatic injury and repair after APAP overdose in Fibγ390-396A mice.
Wild-type (WT) and Fibγ390-396A mice were treated i.p. with 300 mg/kg APAP and 6 hours later injected with PBS vehicle or 2 μg of recombinant mouse MMP12 protein (rMMP12) and evaluated 24 hours after APAP challenge. (A) Representative photomicrographs (100X) of PCNA-positive hepatocytes. (B) Quantification of PCNA-positive hepatocytes. (C) Representative photomicrographs (40X) showing H&E-stained liver sections. (D) The area of centrilobular necrosis in liver sections of APAP-treated mice. N=4 mice/group. Data expressed as mean+SEM. *Significantly (p<0.05) different from WT mice treated with PBS as determined by a one-way ANOVA.
Administration of the allosteric integrin αMβ2 agonist induces hepatic Mmp12 gene expression and attenuates early hepatotoxicity
Because multiple independent “loss of function” approaches indicated fibrin(ogen)-leukocyte integrin αMβ2 interaction and Mmp12 induction to be a critical mechanism of liver repair after APAP overdose, we sought to perform a basic, proof-of-principle experiment evaluating whether enhancing this pathway could reduce APAP-induced liver injury. This approach is attractive, in particular, because in principle this function of fibrin(ogen) can be modified without affecting hemostasis. LA-1 is a small-molecule allosteric agonist of leukocyte integrin αMβ2 that promotes activation by ligands including fibrin(ogen) [33, 34]. Previous studies have demonstrated beneficial effects of LA-1 in a variety of experimental settings, including bile duct fibrosis [34–36]. Application of LA-1 in the APAP overdose model is complex, and interpretation should be cautious, as the routinely applied vehicle for this compound is DMSO, which is not an innocuous vehicle in the APAP overdose model [37–39]. Acknowledging this caveat, we sought to examine the effect of LA-1 treatment on APAP-induced liver injury by initiating LA-1 treatment after APAP hepatotoxicity had occurred (6 hours) (Supplementary Fig. 4A), which 1) largely mitigates the potential for LA-1 to impact APAP metabolism, and 2) represents a likely clinical scenario, where a therapeutic goal is to limit progression of liver damage. Administration of LA-1 (0.4 mg/kg, bid) does not produce an overt liver phenotype in naïve mice [35]. Of importance, LA-1 administration significantly enhanced hepatic Mmp12 levels in APAP-treated mice (Supplementary Fig. 4B), consistent with Mmp12 induction being controlled by a fibrin(ogen)-αMβ2 integrin pathway. Enhanced Mmp12 induction was associated with reduced APAP-induced liver injury in LA-1-treated mice, as indicated by reductions in serum ALT activity and hepatic necrosis (Supplementary Fig. 4C–D). Hepatocyte proliferation was also attenuated by LA-1 treatment (Supplementary Fig. 4E), a compensatory reduction likely reflecting reduced liver damage. Although limited to a single treatment paradigm and dose, these promising results suggest that LA-1 treatment reduces the progression of APAP-induced liver injury in mice.
DISCUSSION
Several studies link coagulation cascade activation to exacerbation of early (6 hours) APAP hepatotoxicity in mice with thrombin activation of PAR-1 and PAR-4 being identified as the primary mechanism [7, 8, 28]. However, despite a profound reduction in coagulation, tissue factor (TF) deficiency offered only transient protection from APAP-induced liver damage [7], and here we identified that prolonged anticoagulation with DABI ultimately reduced hepatocyte proliferation and increased liver damage. The present study offers a plausible explanation for this result. By reducing thrombin activity, TF deficiency or DABI also limit the deposition of fibrin(ogen) [7], which we identify for the first time here as playing a key role in stimulating early hepatocyte proliferation and liver repair. Our studies also offer important insight into the nature of fibrin(ogen) deposits in liver after APAP-induced liver injury, as previous methods have not identified the form of accumulated fibrin(ogen). We found that both cross-linked fibrin and fibrinogen (or non-cross-linked lateral aggregates of fibrin) accumulate in the livers of APAP-treated mice. Because the molecular form of fibrin(ogen) has potential to alter activities of fibrin beyond its widely-appreciated “clotting” function, such as integrin binding, this approach may pave the way for defining more precisely the function of fibrin(ogen) deposits in different experimental settings of hepatotoxicity and liver disease.
Fibrin polymers and surface-adhered fibrinogen are capable of engaging leukocyte αMβ2 integrin. We found that specifically disrupting the capacity of fibrin(ogen) to serve as an αMβ2 ligand produced a severe phenotype after APAP overdose, characterized by reduced hepatocyte proliferation, excess congestion/hemorrhage, and evidence of SEC injury. This implies that the integrin αMβ2 binding function of fibrin(ogen) deposits is a pivotal mechanism in place to limit these pathologic features of APAP-induced liver damage. Multiple studies have documented a role for the interaction of the fibrinogen gamma chain with leukocytes in modulating leukocyte function in experimental models of inflammatory disease [9, 10, 19]. Plasma coagulation analyses and assessment of bleeding have concluded the hemostatic profile of Fibγ390-396A mice is similar to wild-type mice and the timing and outcome in ferric chloride-triggered arterial thrombosis is not affected by this fibrin(ogen) mutation [9]. However, a previous study noted a delay in FXIII-dependent cross-linking of γ390-396A fibrinogen, which in vivo, resulted in decreased red cell retention within venous thrombi [27]. Of importance, the exaggerated liver pathology in APAP-treated Fibγ390-396A mice could not be attributed to a reduction in intrahepatic deposition of cross-linked fibrin(ogen). Histopathological observations in APAP-treated Fibγ390-396A mice are very similar to macrophage-insufficient mice [13, 30]. Although it is difficult to completely exclude a role for the reduced rate of fibrin(ogen) cross-linking in the pathology we observe in Fibγ390-396A mice, collectively these results strongly suggest the mechanism underlying the phenotype of Fibγ390-396A mice is largely attributed to failed activation of leukocytes through engagement of integrin αMβ2. This should not preclude interest in future studies that determine the overall role of FXIII transglutaminase activity in APAP-induced liver injury, through fibrin(ogen) cross-linking or cross-linking of other potential FXIII targets.
It is plausible that fibrin(ogen) promotes liver repair after APAP through integrin αMβ2 activation on multiple different cell types [9, 19]. Importantly, many studies demonstrate a role for macrophages in these elements of hepatic repair after APAP overdose [11, 13, 29]; however the mechanistic cues for macrophage repair function in toxic liver injury are not completely understood. Notably, macrophages infiltrating livers of mice after APAP overdose express αMβ2 [11], as do circulating neutrophils in patients after APAP overdose [40]. Interestingly, whereas neutrophil accumulation appeared unaffected, the loss of fibrinogen-αMβ2 interaction in Fibγ390-396A mice left macrophages seemingly incapable of localizing to the borders of necrotic foci. Moreover, we identified defective induction of multiple macrophage-associated genes in APAP-treated Fibγ390-396A mice. Collectively, the results suggest that fibrin(ogen) engagement of αMβ2 integrin is an important trigger of macrophage-driven liver repair after APAP overdose in mice.
Fibrin(ogen) appears to contribute to multiple facets of liver pathology and repair after APAP overdose, perhaps through distinct mechanisms. Resolution of injury in fibrin(ogen)-deficient mice was preceded at 24 hours by a reduction in PCNA-positive hepatocytes, also observed when fibrin(ogen) binding to integrin αMβ2 was disrupted. Notably, this reduction was transient and no difference was observed at 48 hours, despite an obvious inability to resolve liver necrosis at this time. Mirroring the effect of fibrin(ogen) deficiency or dysfunction, a deficiency in hepatic macrophages also had no impact on PCNA-positive cells 48 hours after APAP administration [13]. It is conceivable that macrophage-derived mediators contribute to early hepatocyte proliferation, but this effect diminishes despite engagement of these cells in other processes. For example, similarly to mice lacking intrahepatic macrophages, Fibγ390-396A mice developed exacerbated SEC injury which was coupled to obvious histological congestion/hemorrhage, suggesting failed vascular integrity. The absence of Kupffer cells and infiltrating macrophages after APAP overdose impaired sinusoidal integrity, as measured by increased Evans blue dye extravasation into the liver and increased hepatic hemorrhage [13, 30]. The hepatoprotective effects of macrophages in APAP-induced liver injury have been postulated to be mediated, at least in part, via regulation of SEC integrity [13, 30]. Indeed, intrahepatic macrophages express mediators such as VEGF after APAP overdose [13]. We observed that failed hepatic induction of VEGF mRNA in Fibγ390-396A mice was coupled to increased SEC injury. Strong evidence supports a protective role for VEGF and its receptor in maintaining vascular integrity and limiting injury after APAP overdose [41, 42]. This suggests that stimulating macrophage VEGF induction is one potential mechanism whereby fibrinogen-αMβ2 interaction limits vascular injury after APAP overdose.
Macrophage expression of Mmp12 and its function have been studied extensively in the context of acute and chronic pulmonary inflammatory diseases, atherosclerosis and cancers [43–45]. Importantly, robust expression of Mmp12 by both resident Kupffer cells and infiltrating macrophages has been shown in mice following APAP overdose [11]. Here, we discovered that the robust hepatic induction of Mmp12 after APAP overdose is mediated by fibrin(ogen) engagement of αMβ2 integrin. The similar phenotypes of Fibγ390-396A and Mmp12−/− mice after APAP overdose suggest that failed Mmp12 induction accounts in part for the protective effects of fibrin(ogen) in this model. Indeed, rMMP12 administration provided a substantial level of correction for both hepatocyte proliferation and necrosis toward severity observed in wild-type mice. The mechanism whereby Mmp12 activity is protective after APAP is not understood. Similar to other metalloproteinases, Mmp12 is able to degrade extracellular matrix components allowing for tissue remodeling [44, 46]. However, the protective action of Mmp12 is not necessarily shared by other MMPs, as Mmp9 deficiency, for example, had no effect on APAP hepatotoxicity [8]. With respect to its role in tissue repair, Mmp12 plays a central role in proteolytic degradation of extracellular matrix components required for effective macrophage migration [46]. Additional studies are required to more clearly delineate the role of Mmp12 in APAP-induced liver injury. However, the discovery that Mmp12 induction is driven by fibrin(ogen) deposits in the injured liver suggest a highly plausible mechanism connecting a rapid and sensitive indicator of tissue injury (i.e., coagulation) to cellular processes central to tissue repair, such as macrophage migration and extracellular matrix degradation.
Collectively, our studies have identified an entirely novel pathway whereby crosstalk between intrahepatic coagulation and inflammation stimulates liver repair after APAP overdose. The results suggest that fibrin(ogen) engagement of αMβ2 is a pivotal catalyst of leukocyte-driven liver repair after APAP overdose, and we identify Mmp12 as one of several potentially novel macrophage-derived products involved in liver repair. Detection in blood or urine of Mmp12 or other mediators derived from fibrin(ogen)-activated macrophages could offer new promising biomarkers of liver repair after APAP overdose. Potential translational opportunities could also include fibrin(ogen) supplementation to stimulate liver repair in patients with acute liver failure, although clearly additional analysis is needed to weigh the risks and benefits of this intervention. Our proof-of-principle studies with LA-1 suggest another alternative approach to stimulating liver repair through allosteric activation of fibrin(ogen)- αMβ2 integrin engagement. Additional studies targeting the fibrin(ogen)- αMβ2 integrin interaction with LA-1 or other compounds may be promising, given that our studies suggest interventions that can be achieved without compromising hemostasis or instigating pathological thrombosis. Thus, the results suggest that novel interventions maximizing liver repair stimulated by intrahepatic fibrin(ogen) could form an innovative strategy to stimulate liver repair in patients with APAP-induced acute liver failure.
Supplementary Material
LAY SUMMARY.
Acetaminophen overdose leads to activation of coagulation cascade and deposition of high molecular weight cross-linked fibrin(ogen) species in the liver.
Fibrin(ogen) is required for stimulating liver repair after acetaminophen overdose.
The mechanism whereby fibrin(ogen) drives liver repair after acetaminophen overdose requires engagement of leukocyte αMβ2 integrin and subsequent induction of matrix metalloproteinase 12.
ABBREVIATIONS
- APAP
acetaminophen
- αMβ2
alphaMbeta2 integrin
- ALT
alanine aminotransferase
- Clec10a
C-type lectin domain family 10, member A
- cDNA
complementary DNA
- DABI
dabigatran etexilate
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- FXIII
factor XIII
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- Gpnmb
glycoprotein (transmembrane) nmb
- H&E
hematoxylin & eosin
- HMW
high molecular weight
- IL-1β
interleukin 1β
- IL-2
interleukin 2
- IL-4
interleukin 4
- IL-6
interleukin 6
- IL-10
interleukin 10
- LA-1
leukadherin-1
- LMW
low molecular weight
- Mmp9
matrix metalloproteinase 9
- Mmp12
matrix metalloproteinase 12
- Mmp13
matrix metalloproteinase 13
- PBS
phosphate buffered saline
- PCNA
proliferating cell nuclear antigen
- PAR-1
protease activated receptor-1
- PAR-4
protease activated receptor-4
- Prtn3
proteinase 3
- PT-INR
prothrombin time-international normalized ratio
- qPCR
quantitative real-time PCR
- rMMP12
recombinant MMP12
- SEC
sinusoidal endothelial cell
- TAT
thrombin-antithrombin
- TF
tissue factor
- TNF
tumor necrosis factor
- Vegf
vascular growth endothelial factor
- WT
wild-type
Footnotes
AUTHOR CONTRIBUTIONS
Designed and conducted experiments: AKK, NJ, HC-F, AVW, JLR, BPS, JEF, BFJ, MJF, JPL
Analyzed data: AKK, JEF, BFJ, MJF, JPL
Drafted initial sections of the manuscript: AKK, MJF, JPL
Approved final version of the manuscript: AKK, NJ, HC-F, AVW, JLR, BPS, JEF, BFJ, MJF, JPL
CONFLICT OF INTEREST STATEMENT
JPL has a sponsored research grant from Boehringer-Ingelheim, which is unrelated to the manuscript. Boehringer-Ingelheim did not provide material or financial support for the work described.
FINANCIAL SUPPORT STATEMENT
This work was supported by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [R01 DK087886 and R01 DK105099]. JEF and BFJ were supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy through grant no. DE–FG02–91ER20021 (to JEF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stravitz RT, Bowling R, Bradford RL, Key NS, Glover S, Thacker LR, et al. Role of procoagulant microparticles in mediating complications and outcome of acute liver injury/acute liver failure. Hepatology. 2013;58:304–313. doi: 10.1002/hep.26307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison PM, O’Grady JG, Keays RT, Alexander GJ, Williams R. Serial prothrombin time as prognostic indicator in paracetamol induced fulminant hepatic failure. BMJ. 1990;301:964–966. doi: 10.1136/bmj.301.6758.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr R, Newsome P, Germain L, Thomson E, Dawson P, Stirling D, et al. Effects of acute liver injury on blood coagulation. J Thromb Haemost. 2003;1:754–759. doi: 10.1046/j.1538-7836.2003.00194.x. [DOI] [PubMed] [Google Scholar]
- 4.Anand AC, Nightingale P, Neuberger JM. Early indicators of prognosis in fulminant hepatic failure: an assessment of the King’s criteria. J Hepatol. 1997;26:62–68. doi: 10.1016/s0168-8278(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 5.Borlak J, Chatterji B, Londhe KB, Watkins PB. Serum acute phase reactants hallmark healthy individuals at risk for acetaminophen-induced liver injury. Genome Med. 2013;5:86. doi: 10.1186/gm493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izumi S, Hughes RD, Langley PG, Pernambuco JR, Williams R. Extent of the acute phase response in fulminant hepatic failure. Gut. 1994;35:982–986. doi: 10.1136/gut.35.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganey PE, Luyendyk JP, Newport SW, Eagle TM, Maddox JF, Mackman N, et al. Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology. 2007;46:1177–1186. doi: 10.1002/hep.21779. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan BP, Kassel KM, Jone A, Flick MJ, Luyendyk JP. Fibrin(ogen)-independent role of plasminogen activators in acetaminophen-induced liver injury. Am J Pathol. 2012;180:2321–2329. doi: 10.1016/j.ajpath.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flick MJ, Du X, Witte DP, Jirousková M, Soloviev DA, Busuttil SJ, et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flick MJ, LaJeunesse CM, Talmage KE, Witte DP, Palumbo JS, Pinkerton MD, et al. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J Clin Invest. 2007;117:3224–3235. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56:735–746. doi: 10.1002/hep.25657. [DOI] [PubMed] [Google Scholar]
- 13.You Q, Holt M, Yin H, Li G, Hu CJ, Ju C. Role of hepatic resident and infiltrating macrophages in liver repair after acute injury. Biochem Pharmacol. 2013;86:836–843. doi: 10.1016/j.bcp.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- 15.Suh TT, Holmbäck K, Jensen NJ, Daugherty CC, Small K, Simon DI, et al. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 1995;9:2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- 16.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, et al. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drew AF, Liu H, Davidson JM, Daugherty CC, Degen JL. Wound-healing defects in mice lacking fibrinogen. Blood. 2001;97:3691–3698. doi: 10.1182/blood.v97.12.3691. [DOI] [PubMed] [Google Scholar]
- 18.Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002;62:6966–6972. [PubMed] [Google Scholar]
- 19.Flick MJ, Du X, Degen JL. Fibrin(ogen)-alpha M beta 2 interactions regulate leukocyte function and innate immunity in vivo. Exp Biol Med (Maywood) 2004;229:1105–1110. doi: 10.1177/153537020422901104. [DOI] [PubMed] [Google Scholar]
- 20.Souri M, Koseki-Kuno S, Takeda N, Degen JL, Ichinose A. Administration of factor XIII B subunit increased plasma factor XIII A subunit levels in factor XIII B subunit knock-out mice. Int J Hematol. 2008;87:60–68. doi: 10.1007/s12185-007-0005-z. [DOI] [PubMed] [Google Scholar]
- 21.Antoniak S, Owens AP, Baunacke M, Williams JC, Lee RD, Weithäuser A, et al. PAR-1 contributes to the innate immune response during viral infection. J Clin Invest. 2013;123:1310–1322. doi: 10.1172/JCI66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopec AK, Boverhof DR, Nault R, Harkema JR, Tashiro C, Potter D, et al. Toxicogenomic evaluation of long-term hepatic effects of TCDD in immature, ovariectomized C57BL/6 mice. Toxicol Sci. 2013;135:465–475. doi: 10.1093/toxsci/kft156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luyendyk JP, Cantor GH, Kirchhofer D, Mackman N, Copple BL, Wang R. Tissue factor-dependent coagulation contributes to alpha-naphthylisothiocyanate-induced cholestatic liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G840–849. doi: 10.1152/ajpgi.90639.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiler-Guettler H, Christie PD, Beeler DL, Healy AM, Hancock WW, Rayburn H, et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest. 1998;101:1983–1991. doi: 10.1172/JCI2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laki K, Lorand L. On the Solubility of Fibrin Clots. Science. 1948;108:280. doi: 10.1126/science.108.2802.280. [DOI] [PubMed] [Google Scholar]
- 26.Lorand L. A study on the solubility of fibrin clots in urea. Hung Acta Physiol. 1948;1:192–196. [PubMed] [Google Scholar]
- 27.Aleman MM, Byrnes JR, Wang JG, Tran R, Lam WA, Di Paola J, et al. Factor XIII activity mediates red blood cell retention in venous thrombi. J Clin Invest. 2014;124:3590–3600. doi: 10.1172/JCI75386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyakawa K, Joshi N, Sullivan BP, Albee R, Brandenberger C, Jaeschke H, et al. Platelets and protease-activated receptor-4 contribute to acetaminophen-induced liver injury in mice. Blood. 2015;126:1835–1843. doi: 10.1182/blood-2014-09-598656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, et al. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- 30.Holt MP, Yin H, Ju C. Exacerbation of acetaminophen-induced disturbances of liver sinusoidal endothelial cells in the absence of Kupffer cells in mice. Toxicol Lett. 2010;194:34–41. doi: 10.1016/j.toxlet.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Jaeschke H, McGill MR, Williams CD. Pathophysiological relevance of neutrophils in acetaminophen hepatotoxicity. Hepatology. 2013;57:419. doi: 10.1002/hep.25877. [DOI] [PubMed] [Google Scholar]
- 32.Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celik E, Faridi MH, Kumar V, Deep S, Moy VT, Gupta V. Agonist leukadherin-1 increases CD11b/CD18-dependent adhesion via membrane tethers. Biophys J. 2013;105:2517–2527. doi: 10.1016/j.bpj.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, et al. Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci Signal. 2011;4:ra57. doi: 10.1126/scisignal.2001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi N, Kopec AK, Ray JL, Cline-Fedewa H, Nawabi A, Schmitt T, et al. Fibrin deposition following bile duct injury limits fibrosis through an αMβ2-dependent mechanism. Blood. 2016;127:2751–2762. doi: 10.1182/blood-2015-09-670703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan SQ, Guo L, Cimbaluk DJ, Elshabrawy H, Faridi MH, Jolly M, et al. A Small Molecule β2 Integrin Agonist Improves Chronic Kidney Allograft Survival by Reducing Leukocyte Recruitment and Accompanying Vasculopathy. Front Med (Lausanne) 2014;1:45. doi: 10.3389/fmed.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park Y, Smith RD, Combs AB, Kehrer JP. Prevention of acetaminophen-induced hepatotoxicity by dimethyl sulfoxide. Toxicology. 1988;52:165–175. doi: 10.1016/0300-483x(88)90202-8. [DOI] [PubMed] [Google Scholar]
- 38.Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Masson MJ, Carpenter LD, Graf ML, Pohl LR. Pathogenic role of natural killer T and natural killer cells in acetaminophen-induced liver injury in mice is dependent on the presence of dimethyl sulfoxide. Hepatology. 2008;48:889–897. doi: 10.1002/hep.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol. 2014;275:122–133. doi: 10.1016/j.taap.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato T, Ito Y, Hosono K, Suzuki T, Tamaki H, Minamino T, et al. Vascular endothelial growth factor receptor-1 signaling promotes liver repair through restoration of liver microvasculature after acetaminophen hepatotoxicity. Toxicol Sci. 2011;120:218–229. doi: 10.1093/toxsci/kfq366. [DOI] [PubMed] [Google Scholar]
- 42.Donahower B, McCullough SS, Kurten R, Lamps LW, Simpson P, Hinson JA, et al. Vascular endothelial growth factor and hepatocyte regeneration in acetaminophen toxicity. Am J Physiol Gastrointest Liver Physiol. 2006;291:G102–109. doi: 10.1152/ajpgi.00575.2005. [DOI] [PubMed] [Google Scholar]
- 43.Goncalves I, Bengtsson E, Colhoun HM, Shore AC, Palombo C, Natali A, et al. Elevated Plasma Levels of MMP-12 Are Associated With Atherosclerotic Burden and Symptomatic Cardiovascular Disease in Subjects With Type 2 Diabetes. Arterioscler Thromb Vasc Biol. 2015;35:1723–1731. doi: 10.1161/ATVBAHA.115.305631. [DOI] [PubMed] [Google Scholar]
- 44.Lagente V, Le Quement C, Boichot E. Macrophage metalloelastase (MMP-12) as a target for inflammatory respiratory diseases. Expert Opin Ther Targets. 2009;13:287–295. doi: 10.1517/14728220902751632. [DOI] [PubMed] [Google Scholar]
- 45.Kerkelä E, Ala-Aho R, Jeskanen L, Rechardt O, Grénman R, Shapiro SD, et al. Expression of human macrophage metalloelastase (MMP-12) by tumor cells in skin cancer. J Invest Dermatol. 2000;114:1113–1119. doi: 10.1046/j.1523-1747.2000.00993.x. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.