Abstract
Receptive language (e.g., reading) is largely preserved in the aging brain, and semantic processes in particular may continue to develop throughout the lifespan. We investigated the neural underpinnings of phonological and semantic retrieval in older and younger adults during receptive language tasks (rhyme and semantic similarity judgments). In particular, we were interested in the role of competition on language retrieval and varied the similarities between a cue, target, and distractor that were hypothesized to affect the mental process of competition. Behaviorally, all participants responded faster and more accurately during the rhyme task compared to the semantic task. Moreover, older adults demonstrated higher response accuracy than younger adults during the semantic task. Although there were no overall age-related differences in the neuroimaging results, an Age × Task interaction was found in left inferior frontal gyrus (IFG), with older adults producing greater activation than younger adults during the semantic condition. These results suggest that at lower levels of task difficulty, older and younger adults engaged similar neural networks that benefited behavioral performance. As task difficulty increased during the semantic task, older adults relied more heavily on largely left hemisphere language regions, as well as regions involved in perception and internal monitoring. Our results are consistent with the stability of language comprehension across the adult lifespan and illustrate how the preservation of semantic representations with aging may influence performance under conditions of increased task difficulty.
Keywords: aging, language comprehension, fMRI, semantics, phonology, lexical competition
Introduction
Normal aging is characterized by significant declines in gray matter volume and white matter integrity across the brain, with the prefrontal cortex among the most vulnerable regions (Barrick, Charlton, Clark, & Markus, 2010; Good, et al., 2001; Raz, 2005; Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003; Salat, et al., 2005; Sowell, et al., 2003). Some cognitive functions also show age-related decline, such as memory, perception and executive control (e.g., Craik & Bialystok, 2006; Park, et al., 2002), while other cognitive functions such as language comprehension are relatively spared (Burke & Shafto, 2008). The relation between age-related neural and cognitive decline, however, is complex and requires further investigation. With respect to language, some processes, such as basic syntactic parsing and language comprehension appear to be well preserved despite neural atrophy because of functional reorganization of the neural language system (Shafto & Tyler, 2014; Tyler, et al., 2010; Wingfield & Grossman, 2006).
Moreover, core language processes interact with attentional control systems that may be affected by aging (e.g., Braver & West, 2008; Kramer & Madden, 2008), and as core language processes increase in complexity this may further increase demands on attentional control. For example, studies of comprehension of sentences of varying syntactic complexity found consistent left-hemisphere lateralization for older and younger adults, especially when task demands were minimized (Davis, Zhuang, Wright, & Tyler, 2014). However, when task-demands increased, age-related increases in functional activation in task-control regions were observed, suggesting that such age-related increases in activation that are commonly seen in many studies may be in response to task-demands as opposed to natural language comprehension (Davis, et al., 2014). We investigate here the neural basis of semantic and phonological processes in young and older adults during language comprehension and how it is affected by variation in task demands for attentional processes.
Cognitive models postulate that word recognition involves activation of multiple candidates that share sensory, phonological, or semantic properties with the auditory or visual input. Competition between candidates continues until the best-matching candidate is selected (Allopenna, Magnuson, & Tanenhaus, 1998; Marslen-Wilson, 1987; McClelland & Elman, 1986; Norris, 1994). One critical brain region involved in such selection processes includes left inferior frontal gyrus (IFG). Some neural models propose that left IFG subserves language selection processes, but with distinct subregions involved in different processes, such as dorsal left IFG’s (BA 44) involvement in processing sentential syntax and language production, and ventral region’s (BA 47) involvement in semantic processing (Dapretto & Bookheimer, 1999; Friederici, Meyer, & von Cramon, 2000; Poldrack, et al., 1999). Other models have argued that left IFG has a domain-general role in selecting among competing candidates (Humphreys & Gennari, 2014; January, Trueswell, & Thompson-Schill, 2009; Moss, et al., 2005; Schnur, et al., 2009; Thompson-Schill, Bedny, & Goldberg, 2005; Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997). A recent study in younger adults further suggests that right IFG may play a role similar to left IFG in resolving competition at the word level (Bozic, Tyler, Ives, Randall, & Marslen-Wilson, 2010). Bozic and colleagues investigated embedded phonological word stems (e.g. “clay, /klei/” within “claim, /kleim/”) as a source of lexical competition in spoken word recognition, and found that both left IFG and right IFG were activated similarly to solve lexical competition from embedded stems. In contrast, only left inferior frontal regions were engaged in higher-level syntactic competition processes. While these results demonstrate bilateral IFG involvement in language tasks, non-linguistic stimuli were not examined, so the specificity of IFG for language processes remains unresolved.
While left-lateralized regions, such as IFG and temporal cortex, have established roles supporting core language processes, the potential contribution of right hemisphere regions, particularly frontal cortex, is of special relevance to studies of aging. Age-related increases in right hemisphere activation are frequently observed (e.g., Cabeza, Anderson, Locantore, & McIntosh, 2002), but there is considerable debate about whether these age-related neural differences reflect mechanisms of compensation (Cabeza, et al., 2002; Park & Reuter-Lorenz, 2009; Peelle, Troiani, Wingfield, & Grossman, 2010; Wierenga, et al., 2008) or a more diffuse and less efficient neural response that is related to natural age-related decline of gray and white matter (Li, Lindenberger, & Sikstrom, 2001; Park, et al., 2004). Evidence in support of compensation includes reports of increased functional activation in right IFG associated with higher accuracy during picture naming for older adults (Wierenga, et al., 2008). Other studies have reported increased activation in right inferior frontal regions (BA 45/47) for older adults during an auditory syntactic comprehension task where their performance was comparable to younger adults’ (Tyler et al., 2010). Moreover, this increased right IFG recruitment was related to gray matter loss in left IFG regions, suggesting that increases in right hemisphere activation are compensatory and may be a response to neuroanatomical changes in the left frontal regions. Such decreases in gray matter within the language network may lead to decreased connectivity within specific areas of the language network but increased functional connectivity overall (Meunier, Stamatakis, & Tyler, 2013).
However, increased functional activation has not always been associated with better performance (Logan, Sanders, Snyder, Morris, & Buckner, 2002; Meinzer, et al., 2009). In a semantic fluency task, Meinzer and colleagues found negative correlations between language production and neural activity in right IFG in older adults, suggesting that the increased involvement of right IFG in older adults did not contribute to better performance. Others have observed increases in right frontal activation for older adults performing word and picture rhyme judgment tasks when they had comparable performance to younger adults (Geva, et al., 2012). However, in both groups there was a positive relation between error rate and activation in right frontal regions suggesting that the worst performers recruited right hemisphere resources more (Geva, et al., 2012). Our own work has shown that older adults produced more errors than young adults in a phonological judgment task that required covert production of picture names, and although older adults elicited greater activation than younger adults, this was not related to behavioral performance (Diaz, Johnson, Burke, & Madden, 2014). Thus, it is clear that in some instances, recruitment of right hemisphere PFC regions aids language performance, while in other cases, it does not.
The relation between age-related neural differences and language processes is even more complex because normal aging may differentially affect specific language processes. For example, semantic aspects of receptive and productive language abilities are largely preserved: Semantic knowledge, as indexed by vocabulary, increases across the life span (Alwin & McCammon, 2001; Verhaeghen, 2003), and many studies have provided convergent evidence in support of preserved semantic function in older adults across various tasks, such as semantic priming (Madden, Pierce, & Allen, 1993), semantic judgment (Little, Prentice, & Wingfield, 2004), and word associations (Burke & Peters, 1986). Likewise, phonological processes are preserved during language comprehension, but there are notable age-related declines in language production, such as increased retrieval failures in producing spoken or written words (Burke & MacKay, 1997; MacKay & Abrams, 1998; MacKay, Abrams, & Pedroza, 1999; MacKay & James, 2004; Shafto & Tyler, 2014). During a tip-of-the-tongue (TOT) state in which individuals know they know a word corresponding to a meaning but cannot retrieve the word itself (Brown & McNeill, 1966), older adults show less activation in the left anterior insula compared to young adults. This is consistent with older adults having weaker phonological retrieval, especially during more challenging word retrieval states (Shafto, Stamatakis, Tam, & Tyler, 2010). Older adults showed a negative correlation between TOT rates and activation in left insula, suggesting that while overall older adults elicited less left insula activation than younger adults, those older adults who could produce more activation had fewer phonological retrieval failures. Thus we may expect different brain-behavior relations depending on the aspect of language being examined (e.g., comprehension – compensation, phonological aspects of production – dedifferentiation).
While several previous studies have examined the effects of age on neural activation during language production (Bergerbest, et al., 2009; Diaz, et al., 2014; Geva, et al., 2012; Meinzer, et al., 2009; Nagels, et al., 2012; Tyler, et al., 2010), previous research has not investigated how the frontal control system functions during different types of lexical competition in normal aging. In this study we manipulated executive control demands in both phonological and semantic aspects of word comprehension to investigate brain mechanisms involved in resolving semantic and phonological competition. In the present study, participants were instructed to make rhyme and semantic similarity judgments about word triplets, and perceptual similarity judgments about dot clusters. Each trial consisted of three items (either words or dot clusters) displayed with one item (the cue) presented above two other items (the target and distractor) and participants judged which of these items was more similar to the cue. In the language tasks, we manipulated the phonological or semantic overlap between the cue and distractor and between the cue and target. We calculated a composite measure of selection demands based on ratings of the similarities of the cue and target and of the cue and distractor to yield a single measure of phonological or semantic competition. We also included a perceptual similarity judgment task as a baseline condition, in which spatial dot pattern similarity was manipulated between the cue and target and between the cue and distractor dot displays. Across the three tasks, we parametrically modulated selection demands by directly correlating the neural activity of each stimulus trial with the corresponding selection demands of the trial. This method provided a more fine-grained tool than canonical factorial contrasts to investigate these brain-behavior relationships.
We hypothesized that inasmuch as older adults are able to maintain their language systems through neural compensation, there would be a significant relation between behavioral performance and functional activation for a given condition. The likely pattern of age differences is either increased activation for older adults in a region that supports language in both younger and older adults (i.e., over-activation), or selective activation for older adults in a region outside of the typical language network. In either case, compensatory accounts predict that fMRI activations should positively correlate with improvements in behavioral performance. Dedifferentiation accounts, however, would predict increases in activation that do not correspond to maintained or improved behavioral performance. Moreover, if older adults maintain their language processes in these tasks via cognitive control then these patterns of over activation or selective activation should occur for older adults in control regions such as anterior cingulate or superior frontal regions. We also expect patterns of activation to be associated with task difficulty. Inasmuch as older adults show patterns of hemispheric asymmetry similar to younger adults’ (e.g., Bozic et al., 2010) we would expect bilateral frontal activation during low levels of task difficulty and left inferior frontal engagement as difficulty increases.
Methods
Participants
Twenty healthy younger adults (19–34 years, mean age = 23.7; 10 males) and 20 healthy older adults (ages 60–78 years, mean age = 66.6; 8 males) participated in this study. All were community-dwelling, right-handed (Edinburgh Handedness Inventory, Oldfield (1971)), native speakers of American English. All participants had normal or corrected to normal vision and none reported a history of neurological or psychological disorders, major medical conditions (e.g., diabetes, heart disease), or taking medication that might affect the brain or blood flow (Christensen, Moye, Armson, & Kern, 1992). All participants underwent neuropsychological testing to assess basic cognitive skills such as speed, memory, executive function, and language. Demographic characteristics and scores from neuropsychological testing are reported in Table 1. Consistent with prior research, older adults had larger vocabularies, as assessed by the WAIS-III, and were slower to respond on speed, Stroop, and digit-symbol tasks, compared to younger adults. There were no age differences in verbal fluency, recall (immediate or delayed), or forward or backward digit span. All participants provided informed consent and were compensated for their time. The Duke University Medical Center Institutional Review Board approved all experimental procedures.
Table 1.
Participant Demographics
| Younger | Older | |
|---|---|---|
| N | 20 | 20 |
| Age*** | 23.70 (3.45) | 66.55 (4.47) |
| Education | 15.90 (2.07) | 17.05 (2.16) |
| MMSE | 29.35 (0.93) | 29.30 (0.86) |
| BDI | 0.79 (1.04) | 1.32 (1.25) |
| Vocabulary (WAIS III)* | 57.4 (6.68) | 61.3 (5.05) |
| Verbal Fluency (total) | 77.45 (15.29) | 71.70 (12.70) |
| Digit Symbol RT*** | 1246.68 (265.19) | 1778.86 (330.53) |
| Stroop Task | ||
| Congruent RT* | 498.45 (76.73) | 558.24 (82.85) |
| Incongruent RT** | 535.59 (102.70) | 629.18 (113.77) |
| Speed RT** | 277.49 (27.57) | 315.30 (44.23) |
| Immediate Recall | 11.68 (1.80) | 11.10 (2.79) |
| Delayed Recall | 10.63 (2.19) | 9.65 (3.48) |
| Digit Span | ||
| Forward | 11.65 (1.84) | 12.65 (2.06) |
| Backward | 8.35 (2.52) | 7.65 (1.84) |
Note. Values provided are means, with standard deviation in parentheses; RT = reaction time.
= p < .05;
= p < .01;
= p < .001
Stimuli
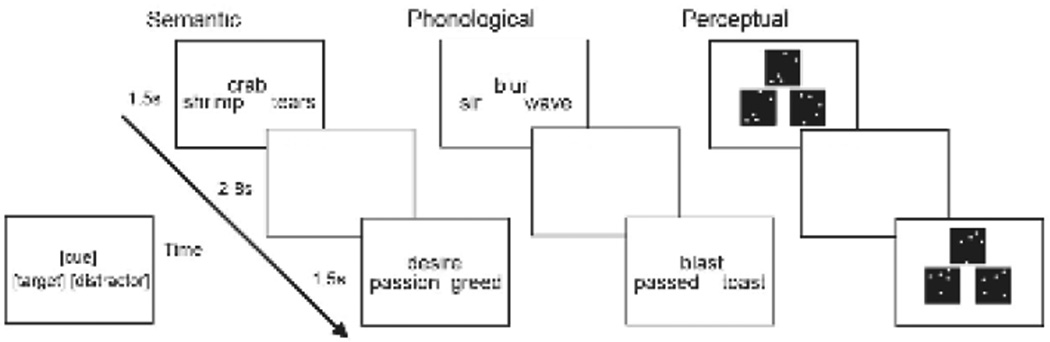
For each of the three task types (rhyme judgment, semantic similarity judgment, perceptual similarity judgment) we selected a set of 165 word or dot pattern triplets that varied respectively in rhyme, semantic, or perceptual similarity between the cue and target and the cue and distractor (see Figure 1 for stimuli examples). All words were high frequency English nouns, verbs, or adjectives that did not differ significantly in length or frequency across conditions (length: rhyme M = 5.12, SD = 1.30, Min = 2, Max = 9, semantic M = 5.04 SD = 1.28, Min = 3, Max = 10; Log HAL frequency: phonological M = 8.49, SD = 2.12, Min = 3.33, Max = 13.58, semantic M = 8.69, SD =1.63, Min = 3.43, Max = 13.89). Dot patterns consisted of five white dots on a black background, and the positions of the dots varied to generate visual dot patterns differing in spatial similarity.
Figure 1.
Examples of stimuli from each condition and an overview of the experimental procedure are presented.
An independent group of 20 native English-speaking participants (M age = 38.1, 8 females) recruited from Amazon M-Turk who did not participate in the fMRI study were instructed to evaluate the similarity of the cue-target pairs and the cue-distractor pairs using a 1–7 scale, where 1 indicated no similarity and 7 indicated very similar or almost identical. The similarity ratings were averaged across participants for each stimulus pair, and then multiplied by 100. Additional behavioral piloting was conducted on a separate group of 14 young participants (Mean Age = 25.3, 3 males). This was done to ensure that participants could perform the task. Data from the fMRI participants indicated that increasing similarity values for cue-target pairs, indicated decreasing selection demands as measured by RT/accuracy (r = −0.34, p < 0.001), whereas for cue-distractor pairs increasing similarity values indicated increasing selection demands (r = 0.54, p < 0.001). To create a common selection demand measure, we transformed cue-target values by subtracting each value from 800 so that increases in this variable reflected increasing selection demands. We then generated a composite measure of selection demands by averaging values for the transformed cue-target similarity ratings and cue-distractor similarity ratings for each triad. We chose to use this composite measure in our analyses because the correlation between the composite selection measure and RT/accuracy (Townsend & Ashby, 1978, 1983) was stronger than the correlations of the individual measures, r = −0.59, p < 0.001. Moreover the composite selection measure was strongly correlated with each measure individually (cue-target similarity, r = 0.74, p < 0.001; cue-distractor similarity, r = −0.75, p < 0.001), indicating that this measure represented both individual measures well.
For the rhyme triplets only, the composite selection measure was significantly positively correlated with the number of phonemes in the words, r = 0.28, p < 0.05, and because of this, number of phonemes was included as a nuisance regressor in the fMRI model for this task only. The rhyme selection measure did not correlate with any other variables, such as word imageability, word length, or frequency, after partialling out the number of phonemes. The composite selection measure for the semantic triplets did not correlate with the number of phonemes, word length, imageability, or frequency so none of these nuisance regressors were needed. Ratings for these lexical stimulus properties were obtained from the English Lexicon Project (Balota, et al., 2007).
Procedure
The experiment consisted of three tasks: rhyme judgment, semantic similarity judgment, and perceptual similarity judgment (Figure 1). On each trial, three words or dot patterns were simultaneously displayed in the center of the screen and arranged as a triad with the cue presented above the target and distractor (duration = 1500 ms). Participants were instructed to decide which of the two bottom stimuli (target, distractor) best matched the top stimulus (cue) in terms of rhyme, semantic, or perceptual similarity, depending on the task and could respond while the triad was on the screen or during the variable inter-stimulus-interval (ISI) that followed each trial. Across trials, the locations of the target and distractor were varied equally across left and right positions, and across participants these locations were counterbalanced to control for any remaining effects of stimulus location on task performance. The ordering of the three tasks was counterbalanced order across participants. Each task was presented across two, 5-minute runs of 82 or 83 trials, and within each task, the two runs were always presented together (e.g., rhyme, rhyme, semantic, semantic, perceptual, perceptual or semantic, semantic, perceptual, perceptual, rhyme, rhyme, etc.). No stimuli were repeated within or across runs.
Participants were asked to respond as quickly and accurately as possible on each trial by pressing the button that corresponded to the item that they judged to be most similar to the cue. All participants were provided with extensive instructions on how to make the rhyme, semantic, and perceptual judgments and performed practice runs with 30 trials of each condition to ensure that they understood the tasks. Practice runs were repeated as needed. Additional details on the instructions can be found in the supplemental material. Response times (RTs) were calculated from the onset of each word/dot triplet to the button press, and recorded with a hand-held fiber optic response box (Current Designs, Philadelphia, PA, USA). A variable inter-stimulus-interval was used (interval = 2 – 8s, M = 3 s) and the trial order was randomized and optimized using the Optseq2 program (Dale, 1999). A fixation cross was presented at the beginning and the end of each run, and a blank screen was presented between trials. All stimuli were presented via a projector using E-Prime presentation software.
MRI acquisition and statistical analysis
MRI scanning was completed on a 3.0 Tesla GE MR 750 whole-body 60 cm bore human scanner equipped with 50 mT/m gradients and a 200 T/m/s slew rate. An eight-channel head coil was used for radio frequency (RF) reception (General Electric, Milwaukee Wisconsin, USA). Sagittal T-1 weighted localizer images were acquired and used to define a volume for data collection and high order shimming. A semi-automated high-order shimming program was used to ensure global field homogeneity. High-resolution structural images were acquired using a 3D fSPGR pulse sequence (TR = 7.64 ms; TE = 2.94 ms; ti = 450 ms; FOV = 25.6 cm2; flip angle =12°; voxel size = 1 × 1 × 1 mm; 162 contiguous slices, sense factor = 2). Functional images sensitive to blood oxygen level-dependent (BOLD) contrast were acquired using a gradient-echo EPI sequence (TR = 2 s; TE = 23 ms; FOV = 19.2 cm2; flip angle = 77°; SENSE factor = 1; voxel size = 3 mm3; 40 contiguous oblique axial slices, parallel to the AC-PC line, interleaved acquisition). Each of the six runs consisted of the acquisition of a time series of 198 brain volumes. Three initial RF excitations were performed to achieve steady state equilibrium and were subsequently discarded. These factors resulted in individual functional runs that were 6.5 minutes each.
We incorporated a quality assurance protocol that assessed the acquired images for the number of potentially clipped voxels, mean signal fluctuation to noise ratio (SFNR), and per-slice variation (Glover, et al., 2012). Pre-processing and statistical analysis were carried out in SPM8 (Wellcome Institute of Cognitive Neurology, London, UK. www.fil.ion.ucl.ac.uk), under MATLAB (Mathworks Inc., Natick, MA, USA). All images in the six runs were concatenated together as a single run before the pre-processing analysis, following the procedures in (Kriegeskorte, Mur, & Bandettini, 2008). EPI images were realigned to the first EPI image (excluding 3 initial discarded and deleted acquisitions) to correct for head motion, slice time corrected, and spatially normalized to a standard MNI (Montreal Neurological Institute) EPI template, using a cutoff of 25 mm for the discrete cosine transform functions. Statistical modeling was done in the context of the general linear model as implemented in SPM8 (GLM, Friston, et al. (1995), using a 8mm full-width half-maximal Gaussian smoothing kernel.
In the individual analysis for each participant, we used a parametric modulation design to model the experimental conditions and variables of interest such that the value of the variable of interest (e.g., selection demands) was used to model the hemodynamic response (Buchel, Wise, Mummery, Poline, & Friston, 1996; Henson, 2004). The advantage of this method is that it provides a more sensitive measurement of the relationships between neural activity and the continuous variables of interest in contrast to categorical analyses that combine many different stimuli in a single condition average. In the analysis of task-related data, the six runs (with two runs for each of the three tasks) were concatenated into a single analysis session, and the design matrix consisted of three independent events (critical stimulus, i.e., word or dot triplet, fixation, and errors), together with six head motion parameters, a set of linear trend predictors, and a confound-mean predictor. Four task-related parametric modulators modulated the first event: a nuisance variable (number of phonemes for the rhyme triplets) and three parametrically modulated composite selection measures for each condition (rhyme triplets, semantic triplets and dot triplets, respectively). These selection measures are perceptual/lexical competition scores that were computed from a norming study. The raw values, ranging from 100 to 700, were used as parametric modulators for each task without any transformation. These modulators were treated in parallel so that no shared variance among them was allocated to any of these modulators. The number of phonemes variable was partialed out from the rhyme selection measure because of their significant correlation. However, because number of phonemes was not significantly correlated with the semantic selection measure we did not include this as a nuisance regressor in the semantic analysis. Errors (incorrect responses and omitted responses) were modeled as a separate event (regressor). RT outliers were defined as responses faster than 300 ms or greater than 3 SDs from that individual's overall mean. Combined, these trials accounted for approximately 15.9% of the trials (13.6% incorrect, 1.5% no response, 0.8% outliers). A significant modulator effect means that neural activity in some brain regions significantly correlated with increasing or decreasing values of the modulator (i.e., selection demands).
Trials were modeled using a canonical hemodynamic response function (HRF), and the offset of each stimulus was taken as the onset of the trial in the SPM analysis model with duration as 0, so as to detect the activation peak in processing each word or dot triplet1. The data for each participant were analyzed at the participant level using a fixed effects model, and the activity maps corresponding to positive effects of rhyme, semantic and perceptual tasks in each participant were input into a 2 (younger/older adults) × 3 (rhyme/semantic/perceptual) random effects ANOVA at the group level. Follow up t-tests were conducted to further characterize significant main effects and interactions from the ANOVA. Additionally, the t-test results were masked by the relevant ANOVA main effect or interaction result (i.e., a conjunction) to limit the possibility of reporting false-positives (i.e., regions that were only significant in the t-test, but not the ANOVA). Activations were thresholded at p < 0.005, uncorrected, at the voxel level, and significant clusters were reported only when they also survived a p < 0.05, cluster-level correction for multiple comparisons. Coordinates of significant clusters peaks and sub-peaks for all analyses were reported in the Tables in MNI space. The extents of activations are further verbally characterized in the text. Regions were identified by using the AAL atlas (Tzourio-Mazoyer, et al., 2002) and Brodmann templates as implemented in MRIcron (http://www.MRicro.com/MRicron).
We also correlated several of the neuroimaging measures with the behavioral measures. For these analyses, we used non-parametric statistics to accommodate slight deviations from normality (RT: mean = 1492 ms, median = 1470 ms, skew = 0.50, kurtosis = 0.02, Shapiro-Wilk statistic = .978, p = .05; Accuracy: mean = 84.1%, median = 85.7%, skew = −0.57, kurtosis: −0.19, Shapiro-Wilk statistic = .958, p < .01. To further supplement and confirm these analyses between activity and performance on a trial-by-trial basis, we conducted a logistic mixed effects regression extracting left IFG activity from each participant's first-level statistics, combining this with age group and task type, as independent variables to predict accuracy on each trial (0/1). To assess whether the brain-behavior relations were influenced by gray matter volume, we conducted a correlation between individual differences in gray matter and brain activation. In these analyses the structural T1 image of each participant was segmented into three images of gray matter, white matter and CSF, following the standard segmentation analysis procedures in SPM. An ROI mask of the LIFG was created in standard MNI space using the AAL atlas, then inverse-normalized into each participant’s native space (Samanez-Larkin & D'Esposito, 2008). The individualized LIFG ROI was then applied to the segmented gray matter image of each participant to extract the gray matter volume of the LIFG for each participant. We further correlated the extracted LIFG gray matter volume with brain activation.
Results
Behavioral results
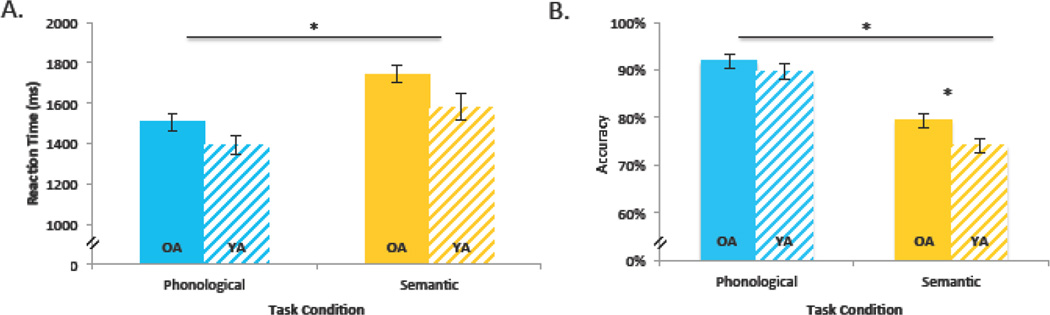
RTs for correct trials and response accuracy were recorded and averaged for each task condition and participant (Figure 2). In a 2 (Age; young, old) × 3 (Task; rhyme, semantic, perceptual) ANOVA on RT we found a significant main effect of task, F(2, 119) = 54.02, p < 0.01, with the fastest responses in the perceptual task, followed by the rhyme task, and the semantic task. Follow-up t-tests indicated that responses in the semantic task (M = 1675 ms) were significantly slower than in the rhyme task (M = 1444 ms), t(78) = 4.14, p <.001, and the perceptual task (M = 1360 ms), t(78) = 5.90, p <.001. Responses to the rhyme task were marginally slower than responses to the perceptual task, t(78) = 1.81, p =.07. The main effect of age was also significant, F(1, 119) = 7.89, p < 0.01, with faster responses for younger adults (M = 1410 ms) than older adults (M = 1576 ms), with no significant age by task interaction. Additional analyses of log-transformed RT data yielded an identical pattern of results. See supplemental Table 1 for additional details on the behavioral data.
Figure 2.
Behavioral Results. Shown are group means for the rhyme and semantic tasks (a: reaction time (RT) and b: accuracy with standard error bars, p < 0.05). There was a main effect of Task in all three behavioral measures with participants responding faster and more accurately to the rhyme trials. There was also a main effect of Age Group in the RT with older adults responding more slowly overall. In the accuracy analyses, there was also a significant interaction between Age Group and Condition with older adults responding more accurately in the semantic task compared to younger adults.
In a similar 2 × 3 ANOVA on response accuracy, we observed a significant main effect of task, F(2, 119) = 123.66, p < 0.01, with the highest accuracy in the rhyme task (90.8%), followed by the perceptual task (84.7%), and semantic task (76.8%). Follow-up t-tests indicated that accuracy in the semantic task was significantly lower than accuracy in the rhyme task, t(78) = 9.30, p <.001, and the perceptual task, t(78) = 5.70, p <.001. Accuracy in the rhyme task was significantly higher than accuracy in the perceptual task, t(78) = 4.35, p < .001. There was no significant main effect of age, F(1, 119) = 2.28, p > 0.1, but age interacted with task, F(2, 119) = 4.04, p < 0.05. Follow-up analyses revealed a significant age effect in the semantic task, F(1, 38) = 6.86, p < 0.05, with higher response accuracy for older adults (79.4%) than younger adults (74.2%), but no age difference in the rhyme F(1, 38) = 1.08, p > 0.1, or perceptual task, F(1, 38) < 1. Additional analyses of log-transformed accuracy data yielded a similar pattern of results with a significant main effect of task, and the main effect of age also reached significance: older = 85.3%, younger = 82.8%. The age × condition interaction did not reach significance, p = 0.16).
Neuroimaging results
Our main analytic approach was to examine the patterns of activation using a 2 × 3 ANOVA to investigate the main effects of age and task and their interaction in specific neural regions. Effects of neural compensation or dedifferentiation were evaluated by examining patterns of brain activation between the age groups. There was no significant main effect of age on brain activation.
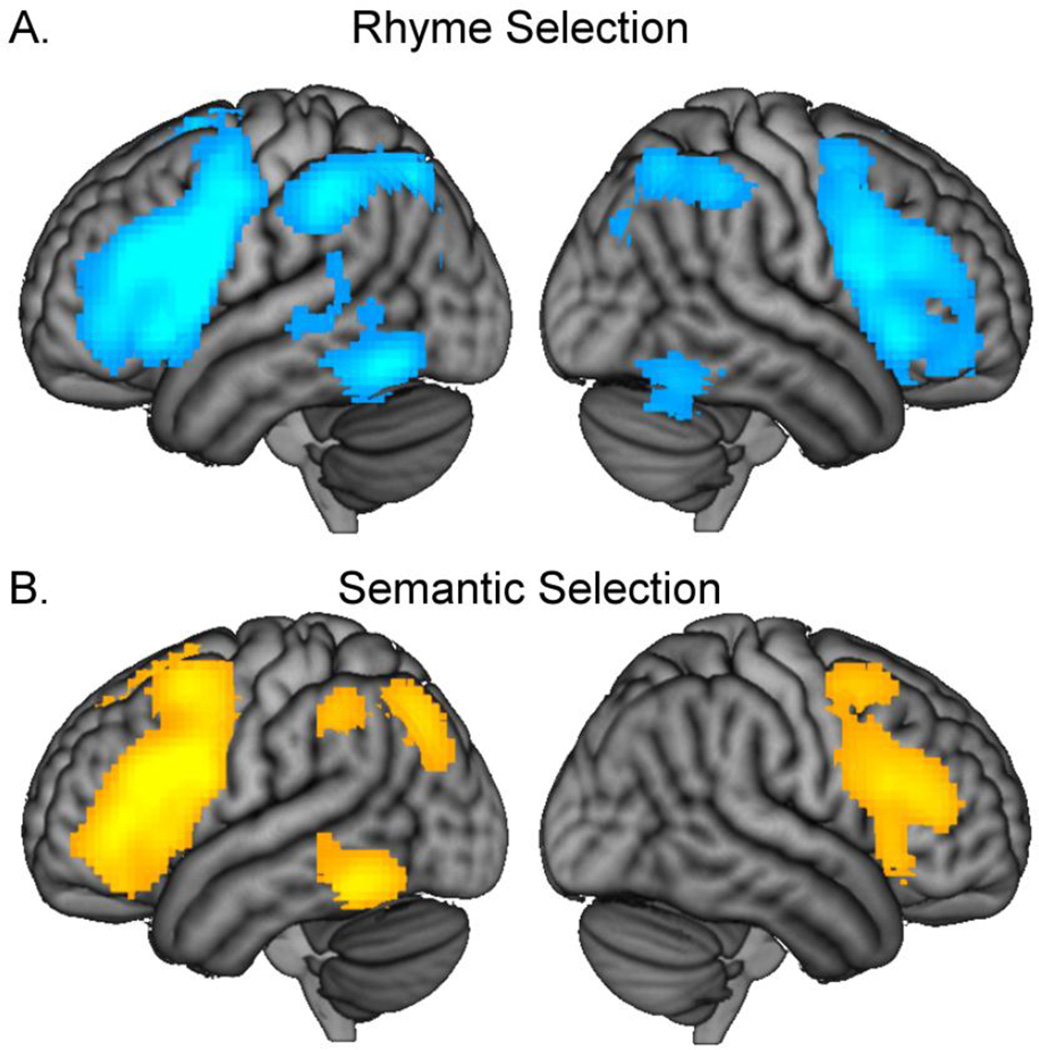
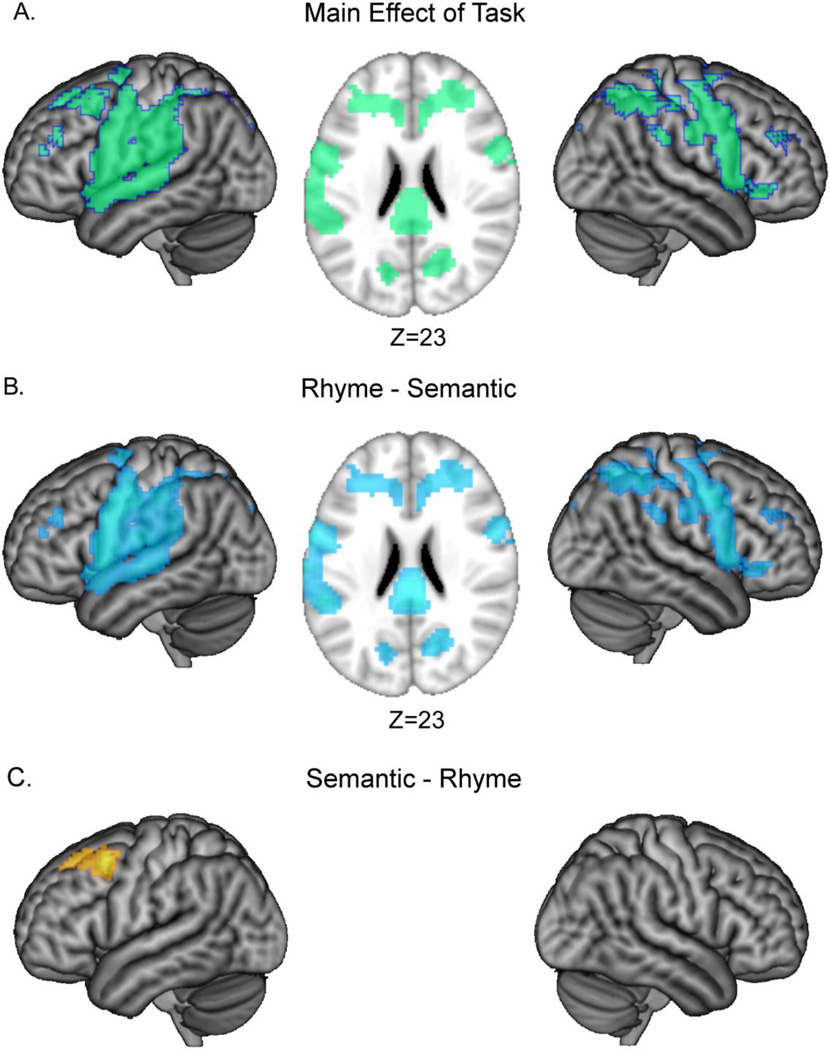
Effects of task were evaluated by examining neural regions that scaled in activation along with our parametric competition manipulations within each task and by comparing differences in neural activations between the tasks. There was a significant main effect of task in bilateral IFG (BA 47), precentral and postcentral gyri (BA 1, 2, 3, 4, 6), middle frontal gyri (BA 6, 8, 9), insula, supramarginal gyri and inferior parietal lobule (BA 40), middle cingulate (BA 24, 32, 23), cuneus, and left superior temporal gyrus (BA 22, see Table 3 & Figure 4A). To further assess where the rhyme and semantic tasks differed, we performed two corresponding t-tests, i.e., rhyme greater than semantic selection, and semantic greater than rhyme selection. The perceptual dots condition was not included in these contrasts because there were no regions that showed a parametric modulation to the dots condition. The contrast of rhyme greater than semantic selection produced significant activation in many of the same brain regions as the main effect of task including bilateral inferior frontal gyri, bilateral supramarginal gyri, middle cingulate, and left superior temporal gyrus (Table 3 & Figure 4B). In contrast, the semantic selection effect was greater than rhyme selection effect only in a region in posterior left middle frontal gyrus (BA 6, 8, 9, Table 3 & Figure 4C). As can be seen from our individual analyses of each condition (Table 2, Figure 3), both rhyme and semantic tasks engaged similar brain regions, thus the differences in the t-test reflect a difference in degree of activation.
Table 3.
Areas of activity for the main effect of task and corresponding t-tests
| Regions | BA | Cluster-level | Voxel-level | Coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| P-corr. | Extent | P-corr. | Z | x | y | z | ||
|
main effect of task |
||||||||
| Bilateral precentral & postcentral gyri, IFG, MFG, SMG, IPL, middle cingulate, cuneus, L STG |
1, 2, 3, 4, 6, 8, 9, 47, 40, 24, 32, 23, 22 |
0.000 | 8301 | 0.000 0.000 0.000 |
6.63 6.3 5.92 |
15 −12 −51 |
−67 −70 5 |
31 31 7 |
| L MFG | 6, 8, 9 | 0.030 | 312 | 0.004 0.006 0.987 |
5.06 4.95 3.09 |
−36 −24 −18 |
11 20 38 |
55 43 49 |
| rhyme minus semantic | ||||||||
| Bilateral precentral & postcentral gyri, IFG, MFG, SMG, IPL, middle cingulate, cuneus, L STG |
1, 2, 3, 4, 6, 8, 9, 47, 40, 24, 32, 23, 22 |
0.000 | 8266 | 0.000 0.000 0.000 |
6.73 6.4 6.02 |
15 −12 −51 |
−67 −70 5 |
31 31 7 |
| semantic minus rhyme | ||||||||
| L MFG | 6, 8, 9 | 0.034 | 312 | 0.002 0.003 0.888 |
5.18 5.08 3.29 |
−36 −24 −18 |
11 20 38 |
55 43 49 |
IFG = Inferior Frontal Gyrus, IPL = Inferior Parietal Lobule, MFG = Middle Frontal Gyrus, STG = Superior Temporal Gyrus, SMG = Supramarginal Gyrus, P-corr = p-value corrected
Figure 4.
Significant activations for (A) the main effect of task in an F-test, (B) significant activation for the contrasts of rhyme minus semantic judgment, and (C) semantic minus rhyme judgment in t-tests are shown. All analyses are presented at a threshold of p < 0.005, voxel-level uncorrected, and p < 0.05, cluster-level corrected.
Table 2.
Areas of activity for selection effects in the rhyme and semantic tasks
| Regions | BA | Cluster-level | Voxel-level | Coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| Pcorrected | Extent | Pcorrected | Z | x | y | z | ||
| rhyme selection effect | ||||||||
| L IFG, precentral gyrus, MFG, IPL, SMG, AG, MTG, bilateral AC, SMA, ITG, fusiform |
44, 45, 47, 6, 46, 39, 40, 21, 20, 37 |
0.000 |
6790 | 0.000 0.000 0.000 |
>20 >20 >20 |
3 −48 −51 |
23 5 11 |
46 25 13 |
| R IFG, precentral gyrus, MFG |
44, 45, 47, 6, 46 |
0.000 | 2258 | 0.000 0.000 0.000 |
>20 >20 7.38 |
33 48 51 |
26 14 11 |
−5 16 31 |
| L hippocampus, R thalamus |
0.000 | 971 | 0.000 0.001 0.017 |
5.68 5.28 4.69 |
−12 15 −9 |
11 5 −16 |
1 1 4 |
|
| R SMG, IPL, AG, cuneus, precuneus |
39, 40, 7 |
0.000 | 900 | 0.000 0.000 0.000 |
6.33 6.24 6.09 |
33 33 48 |
−58 −61 −40 |
43 52 46 |
| semantic selection effect | ||||||||
| L IFG, MFG, precentral gyrus |
44, 45, 47, 6, 46 |
0.000 | 2072 | 0.000 0.000 0.000 |
>20 >20 >20 |
−45 −45 −45 |
11 26 35 |
28 22 7 |
| R IFG, precentral gyrus |
44, 45, 47, 6 |
0.000 | 1132 | 0.000 0.000 0.000 |
6.78 6.52 6.08 |
48 48 30 |
20 29 26 |
19 19 −5 |
| Bilateral SMFG, SMA, SFG, middle cingulate |
32, 24, 6, 8 |
0.000 | 765 | 0.000 0.000 |
>20 >20 |
−3 −6 |
17 26 |
49 43 |
| L SMG, IPL, MOG |
40, 19 | 0.001 | 632 | 0.000 0.01 |
7.2 4.81 |
−27 −45 |
−67 −43 |
40 40 |
| L fusiform, ITG, MTG |
21, 20, 37 |
0.021 | 355 | 0.000 0.228 0.98 |
7.29 3.98 3.06 |
−51 −33 −51 |
−52 −37 −40 |
−17 −23 −2 |
AC = Anterior Cingulate, AG = Angular Gyrus, IFG = Inferior Frontal Gyrus, IPL = Inferior Parietal Lobule, ITG = Inferior Temporal Gyrus, MFG = Middle Frontal Gyrus, MOG = Middle Occipital Gyrus, MTG = Middle Temporal Gyrus, SFG = Superior Frontal Gyrus, SMA = Supplementary Motor Area, SMFG = Superior Medial Frontal Gyrus, SMG = Supramarginal Gyrus
Figure 3.
(A) Significant activation for increasing selection demands in the rhyme judgment, and (B) semantic judgment are shown at a threshold of p < 0.005, voxel-level uncorrected, and p < 0.05, cluster-level corrected. There were no regions that significantly correlated with increasing selection demands in the perceptual condition.
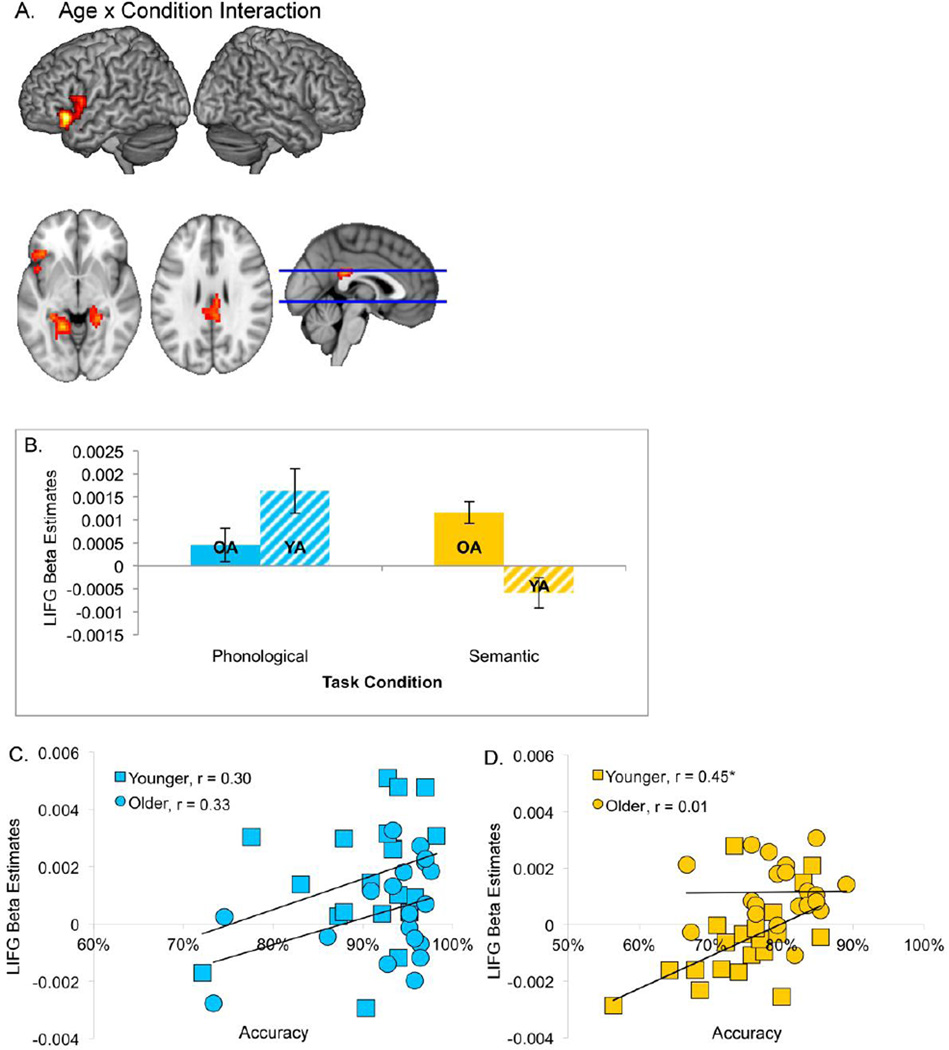
Moreover, there was a significant interaction of age and task in which older adults elicited significantly greater activation than younger adults during the semantic condition. These effects were found in the left IFG (BA 47, 45, 44) that extended slightly into left rolandic operculum, insula, and superior temporal pole (BA 38); and also included left fusiform gyrus and parahippocampus (BA 37); and bilateral posterior cingulate (BA 23), extending into right precuneus and hippocampus (Table 4 & Figure 5). To further understand the nature of this interaction we performed conjunction analyses to find the overlap between regions that were significantly activated in the interaction analysis and in the main effect of task. This further confirmed that there were no significant age differences for the rhyme task but in the semantic task older adults elicited significantly greater activation than younger adults in left IFG, left fusiform gyrus, and bilateral posterior cingulate (Figure 5b).
Table 4.
Areas of activity for the main effect of interaction and conjunction analyses with t-tests
| Regions | BA | Cluster-level | Voxel-level | Coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| Pcorrected | Extent | Pcorrected | Z | x | y | z | ||
| main effect of interaction | ||||||||
| L IFG, insula, rolandic operculum, STG |
45, 44, 47, 38 |
0.021 | 315 | 0.235 0.691 0.972 |
4.01 3.56 3.17 |
−45 −30 −54 |
23 2 8 |
−11 −20 7 |
| L fusiform, PHG | 37 | 0.037 | 278 | 0.562 0.913 0.942 |
3.67 3.31 3.25 |
6 15 21 |
−40 −43 −28 |
25 −2 −8 |
| bilateral posterior cingulate, R precuneus, hippocampus |
23 | 0.032 | 265 | 0.13 0.757 0.800 |
4.19 3.5 3.45 |
−24 −27 −15 |
−19 −31 −49 |
−20 −14 −5 |
| Semantic condition: older > younger adults | ||||||||
| L IFG, PHG, fusiform |
47, 21, 37 |
0.0000 |
725 |
0.125 0.252 0.501 |
4.17 3.95 3.68 |
−30 −21 −57 |
−13 −1 −4 |
−14 −17 −14 |
| R posterior cingulate, PHG, thalamus, fusiform |
23 |
0.035 |
309 |
0.007 0.204 0.887 |
4.88 4.02 3.29 |
69 69 51 |
−31 −43 −55 |
−2 4 19 |
IFG = Inferior Frontal Gyrus, PHG = ParaHippocampal Gyrus, STG = Superior Temporal Gyrus
Figure 5.
(A) Significant activations for the interaction effect of Condition × Age Group in an F-test are shown at a threshold of p < 0.005, voxel-level uncorrected, and p < 0.05, cluster-level corrected. (B) Mean activity in the significant cluster in left inferior frontal gyrus was extracted and plotted across the four conditions and age combinations. (C) The relation between neural activity in the left IFG and behavioral performance was numerically positive but non-significant for both younger and older adults in the rhyme judgment and (D) was significant in younger, but not older adults, for the semantic judgment. Spearman’s rho values are provided for each group in each analysis.
To further investigate the function of these clusters, we used non-parametric correlations between the functional activation elicited by each condition and behavioral performance (accuracy). In inferior frontal gyrus, for the semantic task, there was an overall significant positive correlation, Spearman’s rho = .42, p < .01. Further analyses revealed that this correlation was driven by the younger, but not older, adults (younger: Spearman’s rho = .45, p < .05; older: Spearman’s rho = .01, n.s., Figure 5d). To further confirm the relation between left IFG activation and accuracy, we conducted a logistic mixed effects regression using single-trial task activity. In the logistic regression analysis, we examined the extent to which accuracy as the dependent variable (coding correct and error responses were coded as “1s” and “0s”, respectively), was predicted by three independent variables: neural activity in the LIFG ROI, age group (younger and older adults were coded as “1s” and “0s”, respectively), and task type (phonological and semantic trials were coded as “1s” and “0s”, respectively). The measure of neural activity in the LIFG ROI was composed of continuous data, and was not mean-centered. The results showed that all three independent variables were significant predictors of response accuracy of participants, β = 0.02, χ2 = 15.44, p < 0.0001, for the LIFG ROI activity predictor; β = 0.31, χ2 = 41.07, p < 0.0001, for the age group predictor; and β = − 1.12, χ2 = 456.21, p < 0.0001, for the task type predictor. Increasing neural activity in the LIFG is significantly associated with higher response accuracy.
For the rhyme task, overall and individually, groups showed numerically positive, but non-significant correlations between accuracy and activation in left IFG (overall: Spearman’s rho = .19; younger: Spearman’s rho = .30; older: Spearman’s rho = .33, p = .15, Figure 5c). There were no statistically significant differences between these correlations (Steiger’s Z = −0.1). No correlations between accuracy and fMRI activation in the fusiform or posterior cingulate were significant. One potential concern is whether age-related differences in brain volume are driving these correlations. To assess this, we calculated the correlation between individual gray matter volumes in the left IFG and brain activation; there were no significant relations between these factors (collapsing across both conditions: r = 0.17, p > 0.1; rhyme: r = 0.26, p > 0.1; semantic: r = −0.06, p > 0.1). This finding suggests that potential brain volume differences among participants did not affect our experimental results substantially.
Discussion
We investigated the functional activation associated with a parametric modulation of rhyme, semantic, and perceptual selection processes in older and younger adults during a receptive language task. Although receptive language is well maintained with increased age, it is unclear how executive control processes interact with language comprehension. We measured this here by examining the relation between age and selection difficulty in rhyme, semantic, and perceptual tasks. Our main findings centered on the significant Age × Task interactions in which older adults engaged left IFG to a greater extent than younger adults in the semantic task. Behavioral results indicated that, compared to younger adults, older adults performed significantly more accurately in the semantic task. These results support the idea that semantic representation and retrieval are preserved with aging.
In contrast, the rhyme task was the easier language task for all participants to complete, and performance was characterized by similar accuracies and response times for younger and older adults. In turn, both groups elicited similar levels of activation in bilateral IFG, bilateral supramarginal gyri, cingulate, and left superior temporal gyrus to support performance. Combined, these results show that the age-related differences only emerged during the more challenging semantic task and behavioral performance in this condition was supported by increased recruitment of left inferior frontal regions for older adults, as well as bilateral posterior cingulate, and left fusiform regions.
Prior work in younger adults demonstrated that bilateral inferior frontal resources were recruited for non-grammatical increases in complexity during speech comprehension, while unilateral left inferior frontal regions were sensitive to grammatical complexity (Bozic, et al., 2010). Here we extend those findings and demonstrate that for both older and younger adults, rhyme judgments engaged bilateral frontal regions, while age-related semantic differences emerged in left IFG only. Engagement of left frontal regions for both rhyme and semantic tasks supports the left IFG’s involvement in a broad spectrum of language-related processes. Moreover, our results parallel prior work by demonstrating that in the present study our relatively easier rhyme task engaged bilateral frontal regions for both older and younger adults, while the more difficult semantic task, elicited age-related differences in left IFG.
Of note, our parametric neuroimaging main effect of Task revealed increases in activation for the rhyme task in regions typically associated with phonological processing: bilateral frontal and supramarginal gyri, as well as left superior temporal gyrus. In contrast, increases in semantic selection demands were associated with activation in left middle frontal gyrus. While this is not a traditional ‘language’ region, effects of task difficulty are often found in this region (e.g., Huettel & McCarthy, 2004). Alternatively, this region (bilaterally) has also been implicated in the use of semantic organizational strategies (Miotto, et al., 2006; Savage, et al., 2001). Either interpretation supports the recruitment of additional control regions beyond the typical language network during the semantic task for all adults. Because there were significant differences in task difficulty between the rhyme and semantic conditions, it is also possible that difficulty in general rather than semantic processing per se, may be contributing to these results.
As previously mentioned, although there was not a significant fMRI main effect of age, we found a significant Age × Task interaction in left inferior frontal gyrus, bilateral posterior cingulate, and left fusiform that was driven by age differences in resolving semantic competition (older > younger). It is interesting to note how this fMRI result intersects with the behavioral results. Behavioral performance suggests that overall, the rhyme task was the easier language task for all participants to complete, and both groups performed similarly behaviorally and engaged similar brain regions. Although aging has been associated with declines in phonological processing, this is typically found in language production. Here we examined reading, in which orthography drives access to phonological representations. For skilled readers, such as the participants in our study, letters provide a salient cue to the sounds of the words (e.g., Bonin, Peereman, & Fayol, 2001) and older adults showed no deficit in retrieving the phonology of written words. The semantic condition was overall the most difficult condition, and older adults performed more accurately than younger adults. Moreover, older adults elicited significantly greater activation in left inferior frontal gyrus than younger adults while performing the semantic task. Although the correlation between individual levels of activation and behavior for the older adults was not significant, the overall group pattern suggests that increases in activation were associated with better behavioral performance. Overall these results support the preservation and enhancement of semantic representations and retrieval with aging.
Taken into context with neurocognitive models of aging, our results are not consistent with a typical right-hemisphere compensation account (e.g., Cabeza, et al., 2002), but rather suggest that when faced with a challenging semantic task, older adults continued to rely on left inferior frontal gyrus. In addition to left inferior frontal gyrus, our Age × Task interaction was also significant in left fusiform/parahippocampus and bilateral posterior cingulate. Even though the activation-behavior correlations were not significant in these regions for either group, recruitment of these additional regions may suggest an increased reliance on perceptual processing (e.g., Grill-Spector, 2003) and internal monitoring (e.g., Vogt, Finch, & Olson, 1992) during difficult tasks.
Although our findings are somewhat at odds with previous language studies that found right inferior frontal gyrus activation that was associated with matched or improved performance in older adults during object naming (Wierenga, et al., 2008) and verb generation (Persson, et al., 2004), part of this discrepancy in the recruitment of homologous right frontal regions may be due to the type of language process being engaged. The aforementioned studies were tasks of language production, while this was a language comprehension task. Although there were task difficulty differences in the present study, language comprehension in general is largely preserved with age. It is possible that further increases in difficulty are required for recruitment of homologous right frontal regions, (although see Diaz, et al., 2014; Meinzer, et al., 2009 for examples of non-compensatory increased activation in language production in older adults).
Part of the challenge in distinguishing neural accounts of compensation from dedifferentiation rests in their association with behavior and cognition. One of the limitations of the present study is that we found numerically positive, but non-significant, correlations between accuracy and left IFG activation for the rhyme task. Although we feel that the task was reflective of phonological processing, and both groups performed well on this task reflecting the idea that phonological aspects of reading are well preserved with age. It is possible that with a more challenging phonological task, brain-behavior correlations and age-effects may emerge. Moreover, although older adults outperformed younger adults on the semantic task, and elicited significantly greater activation in left IFG than younger adults, within the older adults there was not a significant correlation between activation and behavior. The lack of a significant correlation could reflect the more limited power of the typical sample sizes in neuroimaging studies and perhaps the increased variability in behavioral and neural measures for older adults.
In summary, we investigated the brain mechanisms of healthy aging in language comprehension using rhyme, semantic, and perceptual similarity judgment tasks. Overall older and younger adults performed the rhyme task similarly, engaging bilateral IFG, supramarginal gyri, and left STG. In contrast, older adults had higher accuracy on the semantic task than younger adults and a significant Age × Task interaction in left IFG, left fusiform, and bilateral cingulate. In these regions, older adults elicited greater activation than younger adults, and across all adults increases in activation during the semantic task were associated with higher accuracies. Our results suggest that at lower levels of task difficulty, older and younger adults engaged similar neural networks that benefited behavioral performance. During the more difficult semantic task, older adults engaged largely left hemisphere regions both within the language network and in perceptual and monitoring regions to complete the task. The recruitment of these perceptual, monitoring regions in the more difficult semantic task suggests an increased reliance on control processes that may not be required for younger adults. Moreover, our results support the general stability of the language comprehension system in aging and emphasize how the preservation of semantic representations with aging can benefit performance under conditions of increased task difficulty.
Supplementary Material
Highlights.
All adults performed best and engaged similar brain regions during the rhyme judgment.
A Task × Age interaction was found in left inferior frontal gyrus.
Older adults elicited greater activation than younger adults in the semantic condition.
Results support the general stability of language comprehension in aging.
Age-related preservation of semantics benefits performance in harder tasks.
Acknowledgments
This project was funded by NIA grant R01 AG034138 (MTD). DJM was also supported by R01 AG039684. We thank Avery Rizio and Anna Epps for feedback on the manuscript and assistance with figure preparation. We also thank the staff and scientists at the Duke University Brain Imaging and Analysis Center, especially the center director Allen W. Song for their support of this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors declare no conflicts of interest.
Different parameters, such as the onset of each stimulus as the trial onset with the stimulus duration as the trial duration, were also tested in other models and produced the same patterns of results as in the reported analysis.
References
- Allopenna PD, Magnuson JS, Tanenhaus MK. Tracking the Time Course of Spoken Word Recognition Using Eye Movements: Evidence for Continuous Mapping Models. Journal of Memory & Language. 1998;38:419–439. [Google Scholar]
- Alwin DF, McCammon RJ. Aging, cohorts, and verbal ability. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2001;56:S151–S161. doi: 10.1093/geronb/56.3.s151. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, Treiman R. The English Lexicon Project. Behav Res Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010;51:565–577. doi: 10.1016/j.neuroimage.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Bergerbest D, Gabrieli JDE, Whitfield-Gabrieli S, Kim H, Stebbins GT, Bennett DA, Fleischman DA. Age-associated reduction of asymmetry in prefrontal function and preservation of conceptual repetition priming. Neuroimage. 2009;45:237–246. doi: 10.1016/j.neuroimage.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin P, Peereman R, Fayol M. Do phonological codes constrain the selection of orthographic codes in written picture naming? Journal of Memory & Language. 2001;45:688–720. [Google Scholar]
- Bozic M, Tyler LK, Ives DT, Randall B, Marslen-Wilson WD. Bihemispheric foundations for human speech comprehension. Proceedings of the National Academy of Science. 2010;107:17439–17444. doi: 10.1073/pnas.1000531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, West R. working memory, executive processes, and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. New York: Psychology Press; 2008. pp. 311–372. [Google Scholar]
- Brown R, McNeill D. The “tip of the tongue” phenomenon. Journal of Verbal Learning and Verbal Behavior. 1966;5:325–337. [Google Scholar]
- Buchel C, Wise RJ, Mummery CJ, Poline JB, Friston KJ. Nonlinear regression in parametric activation studies. Neuroimage. 1996;4:60–66. doi: 10.1006/nimg.1996.0029. [DOI] [PubMed] [Google Scholar]
- Burke DM, MacKay DG. Memory, language, and ageing. Philosophical Transactions of the Royal Society: Biological Sciences. 1997;352:1845–1856. doi: 10.1098/rstb.1997.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, Peters L. Word associations in old age: Evidence for consistency in semantic encoding during adulthood. Psychology and Aging. 1986;1:283–292. doi: 10.1037//0882-7974.1.4.283. [DOI] [PubMed] [Google Scholar]
- Burke DM, Shafto MA. Language and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd. New York: Psychology Press; 2008. pp. 373–443. [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Christensen KJ, Moye J, Armson RR, Kern TM. Health screening and random recruitment for cognitive aging research. Psychology & Aging. 1992;7:204–208. doi: 10.1037//0882-7974.7.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Bialystok E. Planning and task management in older adults: cooking breakfast. Mem Cognit. 2006;34:1236–1249. doi: 10.3758/bf03193268. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Davis SW, Zhuang J, Wright P, Tyler LK. Age-related sensitivity to task-related modulation of language-processing networks. Neuropsychologia. 2014;63:107–115. doi: 10.1016/j.neuropsychologia.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, Madden DJ. Age-related differences in the neural bases of phonological and semantic processes. Journal of Cognitive Neuroscience. 2014:1–14. doi: 10.1162/jocn_a_00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Meyer M, von Cramon DY. Auditory language comprehension: an event-related fMRI study on the processing of syntactic and lexical information. Brain Lang. 2000;75:289–300. [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Geva S, Jones PS, Crinion JT, Price CJ, Baron JC, Warburton EA. The effect of aging on the neural correlates of phonological word retrieval. Journal of Cognitive Neuroscience. 2012;24:2135–2146. doi: 10.1162/jocn_a_00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Mueller B, Van Erp T, Liu TT, Greve D, Voyvodic J, Rasmussen J, Turner J, Brown GG, Keator DB, Calhoun VD, Lee HJ, Ford J, Diaz MT, O’Leary DS, Potkin SG FBIRN. Function biomedical informatics research network recommendations for prospective multi-center functional neuroimaging studies. Journal of Magnetic Resonance Imaging. 2012;36:39–54. doi: 10.1002/jmri.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K. The neural basis of object perception. Current Opinion in Neurobiology. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Henson RN. Analysis of fMRI timeseries: linear timer-invariant models, event-related fMRI and optimal experimental design. Elsevier. 2004:793–822. [Google Scholar]
- Huettel SA, McCarthy G. What is odd in the oddball task? Prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia. 2004;42:379–386. doi: 10.1016/j.neuropsychologia.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Humphreys GF, Gennari SP. Competitive mechanisms in sentence processing: Common and distinct production and reading comprehension networks linked to the prefrontal cortex. Neuroimage. 2014;84:354–366. doi: 10.1016/j.neuroimage.2013.08.059. [DOI] [PubMed] [Google Scholar]
- January D, Trueswell JC, Thompson-Schill SL. Co-localization of stroop and syntactic ambiguity resolution in Broca's area: implications for the neural basis of sentence processing. J Cogn Neurosci. 2009;21:2434–2444. doi: 10.1162/jocn.2008.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd. New York: Psychology Press; 2008. pp. 189–249. [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P. Representational Similarity Analysis – connecting the branches of systems neuroscience. Frontiers in Systems Neuroscience. 2008;2:1–28. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikstrom S. Aging cognition: from neuromodulation to representation. Trends in Cognitive Sciences. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Little DM, Prentice KJ, Wingfield A. Adult age differences in judgments of semantic fit. Applied Psycholinguistics. 2004;25:135–143. [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- MacKay DG, Abrams L. Age-linked declines in retrieving orthographic knowledge: empirical, practical, and theoretical implications. Psychol Aging. 1998;13:647–662. doi: 10.1037//0882-7974.13.4.647. [DOI] [PubMed] [Google Scholar]
- MacKay DG, Abrams L, Pedroza MJ. Aging on the input versus output side: theoretical implications of age-linked asymmetries between detecting versus retrieving orthographic information. Psychol Aging. 1999;14:3–17. doi: 10.1037//0882-7974.14.1.3. [DOI] [PubMed] [Google Scholar]
- MacKay DG, James LE. Sequencing, speech production, and selective effects of aging on phonological and morphological speech errors. Psychology & Aging. 2004;19:93–107. doi: 10.1037/0882-7974.19.1.93. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Pierce TW, Allen PA. Age-related slowing and the time course of semantic priming in visual word identification. Psychology and Aging. 1993;8:490–507. doi: 10.1037//0882-7974.8.4.490. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD. Functional parallelism in spoken word-recognition. Cognition. 1987;25:71–102. doi: 10.1016/0010-0277(87)90005-9. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Elman JL. The TRACE model of speech perception. Cogn Psychol. 1986;18:1–86. doi: 10.1016/0010-0285(86)90015-0. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Euitz C, Rockstroh B, Conway T, Gonzalez Rothi LJ, Crosson B. Neural signatures of semantic and phonemic fluency in young and old adults. Journal of Cognitive Neuroscience. 2009;21:2007–2018. doi: 10.1162/jocn.2009.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D, Stamatakis EA, Tyler LK. Age-related functional reorganization, structural changes, and preserved cognition. Neurobiology of Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Miotto EC, Savage CR, Evans JJ, Wilson BA, Martins MG, Iaki S, Amaro E., Jr Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Human Brain Mapping. 2006;27:288–295. doi: 10.1002/hbm.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, Tyler LK. Selecting among competing alternatives: selection and retrieval in the left inferior frontal gyrus. Cereb Cortex. 2005;15:1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagels A, Kircher T, Dietsche B, Backes H, Marquetand J, Krug A. Neural processing of overt word generation in healthy individuals: The effect of age and word knowledge. Neuroimage. 2012;61:832–840. doi: 10.1016/j.neuroimage.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Norris D. Shortlist: a connectionist model of continuous speech recognition. Cognition. 1994;52:189–234. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology & Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences U S A. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Wingfield A, Grossman M. Neural processing during older adults' comprehension of spoken sentences: age differences in resource allocation and connectivity. Cerebral Cortex. 2010;20:773–782. doi: 10.1093/cercor/bhp142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Sylvester CYC, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: Differential recruitment in older and young adults. Neuroimage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Oxford: Oxford University Press; 2005. pp. 19–57. [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. Journal of Neuroscience. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, D'Esposito M. Group comparisons: imaging the aging brain. Social, Cognitive, and Affective Neuroscience. 2008;3:290–297. doi: 10.1093/scan/nsn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, Fischman AJ, Rauch SL. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: evidence from PET. Brain. 2001;124:219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca's area. Proc Natl Acad Sci U S A. 2009;106:322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Stamatakis EA, Tam PP, Tyler LK. Word retrieval failures in old age: The relationship between structure and function. Journal of Cognitive Neuroscience. 2010;22:1530–1540. doi: 10.1162/jocn.2009.21321. [DOI] [PubMed] [Google Scholar]
- Shafto MA, Tyler LK. Language in the aging brain: the network dynamics of cognitive decline and preservation. Science. 2014;346:583–587. doi: 10.1126/science.1254404. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Current Opinion in Neurobiology. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Science. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JT, Ashby FG. Methods of modeling capacity in simple processing systems. In: Castellan JNJ, Restle F, editors. Cognitive Theory. Vol. 3. Hillsdale, NJ: Lawrence Erlbaum Associates Inc.; 1978. pp. 199–239. [Google Scholar]
- Townsend JT, Ashby FG. The stochastic modeling of elementary psychological processes. Cambridge, UK: Cambridge University Press; 1983. [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cerebral Cortex. 2010;20:352–364. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. Aging and vocabulary score: A meta-analysis. Psychology and Aging. 2003;18:332–339. doi: 10.1037/0882-7974.18.2.332. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Gonzalez Rothi LJ, Conway T, Cato MA, Briggs R, Crosson B. Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks. Neurobiology of Aging. 2008;29:436–451. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. Journal of Neurophysiology. 2006;96:2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.