Abstract
Background
Although hypertension guidelines define treatment resistant hypertension as blood pressure uncontrolled by ≥3 antihypertensive medications, including a diuretic, it is unknown whether patient prognosis differs when a diuretic is included.
Methods
Participants in the Antihypertensive and Lipid-Lowering to Prevent Heart Attack Trial were randomly assigned to first-step therapy with chlorthalidone, amlodipine, or lisinopril. At a Year 2 follow-up visit, those with average BP≥140 mmHg systolic or ≥90 mmHg diastolic on ≥3 antihypertensive medications, or BP<140/90 mmHg on ≥4 antihypertensive medications, were identified as having apparent treatment resistant hypertension. The prevalence of treatment resistant hypertension and its association with ALLHAT primary (combined fatal coronary heart disease or nonfatal myocardial infarction) and secondary (all-cause mortality, stroke, heart failure, combined coronary heart disease, and combined cardiovascular disease) outcomes were identified for each treatment group.
Results
Of participants assigned to chlorthalidone, amlodipine and lisinopril, 9.6%, 11.4% and 19.7%, respectively, had treatment resistant hypertension. During mean follow-up of 2.9 years, primary outcome incidence was similar for those assigned to chlorthalidone compared to amlodipine or lisinopril (amlodipine vs. chlorthalidone adjusted HR=0.86; 95% CI 0.53–1.39; P=0.53; lisinopril vs. chlorthalidone adjusted HR=1.06; 95% CI 0.70–1.60; P=0.78). Secondary outcome risks were similar for most comparisons except coronary revascularization, which was higher with amlodipine than with chlorthalidone (HR=1.86; 95% CI 1.11–3.11; P=0.02). An as-treated analysis based on diuretic use produced similar results.
Conclusions
In this study, which titrated medications to a goal, participants assigned to chlorthalidone were less likely to develop treatment resistant hypertension. However, prognoses in those with treatment resistant hypertension were similar across treatment groups.
Clinical Trial Registration
Keywords: hypertension, resistance, diuretics, calcium channel blocker, angiotensin-converting enzyme inhibitor
Introduction
The 2008 American Heart Association (AHA) scientific statement defines treatment resistant hypertension as: “blood pressure that remains above goal in spite of concurrent use of 3 antihypertensive agents of different classes. Patients whose blood pressure is controlled with 4 or more medications should be considered to have resistant hypertension.” (1) The scientific statement indicates that the antihypertensive medications prescribed should include, if possible, a diuretic. (1) Likewise, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), (2) European Society of Cardiology (ESC)/European Society of Hypertension (ESH) (3) and the British National Institute for Health and Care Excellence (NICE) (4) guidelines on hypertension all require that the BP remain uncontrolled on at least 3 antihypertensive agents including a diuretic to qualify as TRH. Despite this, the reason for requiring that the definition of TRH mandate one of the 3 medications is a diuretic has not been clearly demonstrated. In a recent analysis from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), we have shown a significant increase in the risk for coronary heart disease, stroke, all-cause mortality, heart failure, peripheral artery disease and end-stage renal disease comparing participants with versus without TRH.(5) However, it is not clear if this risk of adverse outcomes would be different whether TRH is defined with or without a diuretic.
Our objectives were therefore two-fold: (1) to evaluate the prevalence of apparent treatment resistant hypertension in participants randomized to first-step therapy with the thiazide-type diuretic chlorthalidone, the calcium channel blocker (CCB) amlodipine, or the angiotensin converting enzyme inhibitor (ACEi) lisinopril in ALLHAT; (2) to assess whether the outcomes of patients with treatment resistant hypertension differed based on whether their first-step therapy was with chlorthalidone, amlodipine, or lisinopril.
Methods
Study Design
Our study was based on a non-prespecified post hoc analysis of the ALLHAT dataset. The rationale, design, and main results of ALLHAT have been published previously.(6–8) In brief, ALLHAT was a randomized, double-blind, multicenter clinical trial designed to determine whether first-step treatment with amlodipine, lisinopril, or the α-blocker doxazosin would significantly reduce the incidence of fatal coronary heart disease or nonfatal myocardial infarction (primary outcome) compared to treatment with chlorthalidone in 42,418 high-risk hypertensive individuals. The doxazosin treatment arm was discontinued in 2000.(7) In the remaining 33,357 participants, incidence of the primary outcome was not significantly different during an average follow-up of 4.9 years between those assigned to chlorthalidone and those assigned to amlodipine or lisinopril. However, chlorthalidone was superior to amlodipine and lisinopril in preventing one or more additional forms of cardiovascular disease.(8) The outcomes comparing ALLHAT participants with and without treatment resistant hypertension have been described previously.(5)
Blood Pressure Measurements
All the BP measurements were obtained by trained observers using a standardized technique. Measurements were taken in the seated position, with back supported and with the arm at the level of the heart after participants had rested quietly for at least 5 minutes. Two BP readings, separated by at least 30 seconds, were obtained and the measurements were recorded to the nearest even number. Visit BP was the average of the two readings.
Treatment
The BP goal for participants in ALLHAT was <140/90 mm Hg. Up-titration of double-blind assigned study medications to achieve the BP goal occurred at monthly titration visits (step one-chlorthalidone 12.5 to 25 mg; lisinopril 10 to 40 mg; amlodipine 2.5 to 10 mg), followed by addition of open-label agents (step two medications-atenolol, reserpine, clonidine; step 3 medication-hydralazine) as needed.(6, 7)
Apparent Treatment Resistant Hypertension
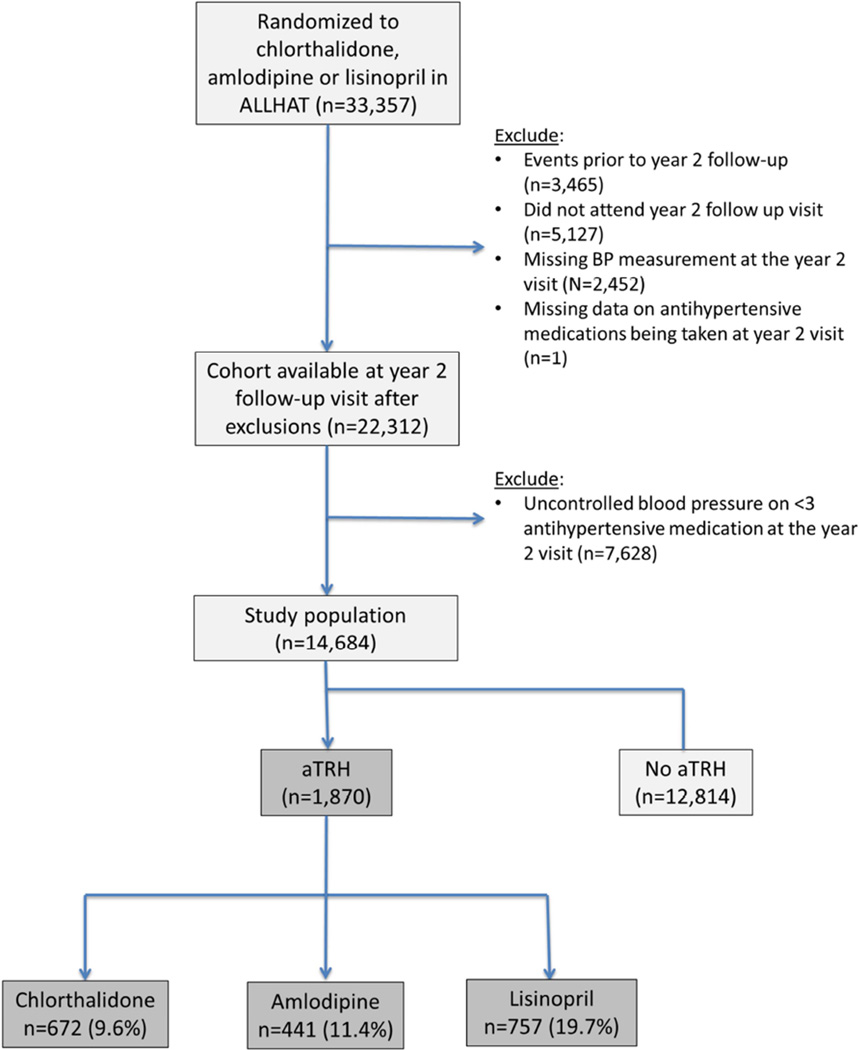
We used the following definition of apparent treatment resistant hypertension: participants with an average BP≥140 mm Hg systolic or ≥90 mm Hg diastolic on ≥3 antihypertensive medications or BP<140/90 mm Hg on ≥4 antihypertensive medications at their Year-2 follow-up visit.(1) The rationale for use of this visit was to provide an adequate balance between allowing sufficient time for titration of the antihypertensive agents while maximizing the period of follow-up for recognition of study outcomes once the diagnosis of treatment resistant hypertension was established. Participants randomized to doxazosin had limited follow-up beyond their Year-2 study visit and they were therefore omitted from the current analyses. A total of 14,864 participants were available for inclusion in the current analyses (Figure 1).
Figure 1.
Patient flow.
Follow-up and Outcomes
After their initial monthly titration visits, participants were examined every 3 months during the first year and every 4 months thereafter. The mean period of follow-up during the treatment phase of the trial was 4.9 years. For this analysis, participants with treatment resistant hypertension were followed from the date of their Year-2 visit (i.e., when treatment resistant hypertension status was determined) to the date of each study outcome, with censoring on their date of death or the end of active follow-up.
The primary outcome was combined fatal coronary heart disease or nonfatal myocardial infarction. Secondary outcomes included all-cause mortality, stroke, combined coronary heart disease (primary outcome, coronary revascularization, or angina with hospitalization), combined cardiovascular disease (combined coronary heart disease, stroke, treated angina without hospitalization, heart failure, and peripheral arterial disease) and hospitalization for gastrointestinal bleeding.
Statistical Analysis
Data were analyzed according to each participant’s treatment assignment (chlorthalidone, amlodipine, or lisinopril) regardless of their subsequent therapy (intention-to-treat analysis). The prevalence of treatment resistant hypertension in each of the three treatment groups was calculated at the Year-2 visit. The risk of treatment resistant hypertension was calculated using a logistic regression model with treatment resistant hypertension as the dependent variable and adjusted for baseline characteristics as discussed in models 2–5 below. In addition, the risk of treatment resistant hypertension was compared across the three groups after adjusting for low treatment adherence (defined as<80% adherence by pill count). Additional models were created to evaluate whether the risk of treatment resistant hypertension across the three groups differed in blacks vs. non-blacks. Cox proportional hazards regression models were used to evaluate the risk of outcomes for the amlodipine and lisinopril groups in comparison with the chlorthalidone group. Five models were used for adjustment: 1) Model 1: unadjusted; 2) Model 2: adjusted for age, sex, race/ethnicity, and region of residence; 3) Model 3: adjusted for variables in model 2 plus practice setting, education level, smoking status, and body mass index (BMI); 4) Model 4: adjusted for variables in models 2 and 3 along with estimated glomerular filtration rate (eGFR), diabetes, low density lipoprotein (LDL)-cholesterol, high density lipoprotein (HDL)-cholesterol, history of coronary heart disease, left ventricular hypertrophy, and taking blood pressure medications prior to randomization; and 5) Model 5: adjusted for variables in models 2, 3 and 4 along with baseline and Year-2 blood pressure.
Sensitivity analyses were performed based on components of the definition for treatment resistant hypertension. Specifically, the hazard ratios for outcomes associated with being in the amlodipine and lisinopril groups, each versus the chlorthalidone group, were calculated separately for the cohort with uncontrolled BP on ≥3 medications and for the cohort with controlled BP on ≥4 medications. In addition, sensitivity analyses were performed based on the actual status of thiazide or thiazide like diuretic use (on-treatment analysis) at or before the Year-2 visit rather than the intention-to-treat analysis. Moreover, sensitivity analyses were performed defining treatment resistant hypertension at 1 year. In addition, further sensitivity analyses were performed on an alternate/expanded cohort where patients with an event other than death were not excluded and in patients with a missing BP value at Year-2 visit, BP values were replaced by 20 month or 28 month values when available, with preference given to 20 month values. All statistical analyses were performed using STATA version 12.0 (STATA Corp. College Station, TX), with a P-value <0.05 considered to reflect statistical significance.
Results
Baseline Characteristics
Among the 14,684 ALLHAT participants who met our inclusion criteria, (672) 9.6%, (441) 11.4% and (757) 19.7% of those who had been assigned to chlorthalidone, amlodipine, and lisinopril, respectively, had treatment resistant hypertension (Figure 1). The increased odds of treatment resistant hypertension with lisinopril and amlodipine when compared with chlorthalidone was seen in blacks (lisinopril vs. chlorthalidone: OR=3.42; 95% CI 2.86–4.09; P<0.0001; amlodipine vs. chlorthalidone: OR=1.30; 95% CI 1.06–1.60; P=0.01) as well as non-blacks (lisinopril vs. chlorthalidone: OR=1.80; 95% CI 1.55–2.09; P<0.0001; amlodipine vs. chlorthalidone: OR=1.30; 95% CI 0.98–1.35; P=0.09). When compared with the chlorthalidone group (reference OR=1.0), the odds of treatment resistant hypertension were significantly increased in the lisinopril group (adjusted OR=2.32; 95% CI 1.86–2.90; P<0.0001) and numerically increased in the amlodipine group (adjusted OR=1.24; 95% CI 0.98–1.56; P=0.07) after adjusting for baseline characteristics. The odds of treatment resistant hypertension were significantly increased in the lisinopril group (adjusted OR=2.39; 95% CI 1.88–3.04; P<0.0001) when compared with chlorthalidone group even after adjustment for low treatment adherence. Baseline characteristics of the group with treatment resistant hypertension by treatment assignment are listed in Table 1. When compared with those assigned to chlorthalidone, a lower proportion of participants assigned to amlodipine were Hispanic whereas the lisinopril group was younger, with higher percent of Blacks, those with left ventricular hypertrophy on ECG but a smaller percentage had an eGFR <60 ml/min/1.73 m2, atherosclerotic vascular disease or diabetes with lower mean systolic BP (Table 1).
Table 1.
Baseline characteristics of study participants with treatment resistant hypertension* by randomized
| Chlorthalidone (N=672) |
Amlodipine (N=441) |
Lisinopril (N=757) |
P value (Amlodipine vs. Chlorthalidone) |
P value (Lisinopril vs. Chlorthalidone) |
|
|---|---|---|---|---|---|
| Age, years, mean (sd) | 67.0 (7.7) | 66.7 (7.4) | 66.1 (7.5) | 0.58 | 0.04 |
| Men, % | 55.5 | 58.7 | 58.0 | 0.29 | 0.34 |
| Race-ethnicity, % | |||||
| Black | 38.2 | 39.9 | 48.3 | 0.58 | <0.001 |
| Non-Black | 61.8 | 60.1 | 51.7 | ||
| Hispanic | 10.7 | 6.6 | 9.1 | 0.02 | 0.31 |
| Non-Hispanic | 89.3 | 93.4 | 90.9 | ||
| Region of residence, % | 0.45 | 0.84 | |||
| Northeast | 14.0 | 12.7 | 13.7 | ||
| Midwest | 19.8 | 17.9 | 18.2 | ||
| South | 50.1 | 51.9 | 52.2 | ||
| West | 10.4 | 12.9 | 10.2 | ||
| Canada | 1.3 | 1.8 | 0.8 | ||
| Practice setting, % | 0.07 | 0.74 | |||
| Private | 22.9 | 22.7 | 22.9 | ||
| Group | 16.7 | 19.3 | 16.5 | ||
| Health Maintenance | 2.4 | 2.3 | 2.4 | ||
| Organization | |||||
| Community Health Center | 11.2 | 9.3 | 12.5 | ||
| University | 8.0 | 10.2 | 9.5 | ||
| Other | 9.2 | 9.3 | 8.6 | ||
| Veterans Affairs Medical | 24.7 | 25.6 | 24.4 | ||
| Center | |||||
| Unknown | 4.9 | 1.4 | 3.2 | ||
| Education less than high school, % | 43.6 | 45.8 | 44.6 | 0.49 | 0.72 |
| BMI, kg/m2, mean(sd) | 30.5 (6.2) | 31.2 (9.1) | 30.6 (6.4) | 0.11 | 0.63 |
| eGFR <60 , ml/min/1.73 m2, % | 29.6 | 26.6 | 22.6 | 0.28 | 0.003 |
| Diabetes, % | 51.0 | 49.9 | 42.7 | 0.73 | 0.003 |
| Cholesterol, mg/dL, mean(sd) | |||||
| Total | 217.5 (42.0) | 217.3 (44.9) | 212.4 (42.3) | 0.95 | 0.03 |
| LDL | 135.8 (36.6) | 135.1 (35.9) | 133.5 (35.9) | 0.76 | 0.27 |
| HDL | 46.7 (15.3) | 46.9 (15.4) | 46.7 (14.9) | 0.83 | 0.93 |
| Left ventricular hypertrophy on ECG, % |
18.6 | 20.4 | 24.6 | 0.45 | 0.006 |
| ASCVD, % | 51.5 | 50.6 | 43.2 | 0.76 | 0.002 |
| On blood pressure medications prior to randomization, % |
96.4 | 96.8 | 95.0 | 0.72 | 0.18 |
| Systolic BP, mm Hg, mean (sd) | 153.9 (14.6) | 154.1 (15.5) | 151.0 (15.2) | 0.78 | <0.001 |
| Diastolic BP, mm Hg, mean (sd) | 85.0 (11.0) | 84.1 (10.7) | 85.6 (10.3) | 0.18 | 0.30 |
| Antihypertensive agents at 2 years, n (%) |
|||||
| On 3 or more agents at 2 years |
672 (100.0) | 441 (100.0) | 757 (100.0) | - | - |
| On 4 or more agents at 2 years |
180 (26.8) | 111 (25.2) | 237 (31.3) | 0.55 | 0.06 |
| On 5 or more agents at 2 years |
27 (4.0) | 8 (1.8) | 40 (5.3) | 0.04 | 0.26 |
| On 6 or more agents at 2 years |
5 (0.7) | 1 (0.2) | 4 (0.5) | 0.25 | 0.61 |
Abbreviations: ASCVD, atherosclerotic vascular disease; ; BMI, body mass index; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate;.
Treatment resistant hypertension was defined as having uncontrolled hypertension despite the use of antihypertensive medications from 3 or more classes or the use of 4 or more antihypertensive medication classes to achieve blood pressure control.
Blood Pressure and Antihypertensive Agents
In the participants with treatment resistant hypertension, systolic BP at the Year-2 visit in the chlorthalidone group (153.9 mm Hg) was similar to the amlodipine group (154.1 mm Hg) but higher than in the lisinopril group (151.0 mm Hg) (Table 1). At the Year-2 study visit, 25–30% of the participants with treatment resistant hypertension were taking 4 or more antihypertensive agents, with a smaller percent taking 5 or more antihypertensive agents (Table 1).
Outcomes
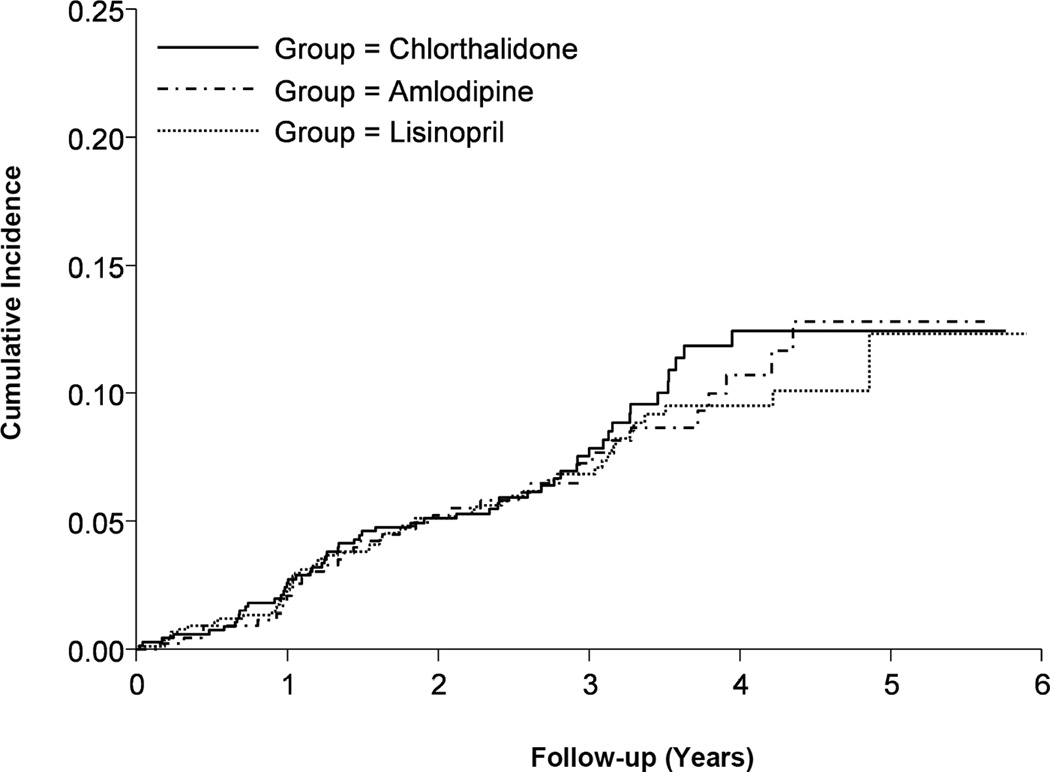
Incidence of the primary outcome was similar among the 3 groups (Figure 2). When compared with the chlorthalidone group, there were no significant differences in the adjusted risk of the primary outcome with amlodipine (fully adjusted model HR=0.86; 95% CI 0.53–1.39; P=0.53) or lisinopril (fully adjusted model HR=1.06; 95% CI 0.70–1.60; P=0.78) across all the models tested (Table 2).
Figure 2.
Cumulative incidence of primary outcome (combined fatal coronary heart disease or nonfatal myocardial infarction) by treatment group in those with treatment resistant hypertension.
Table 2.
Clinical outcomes in the antihypertensive treatment groups in patient with treatment resistant hypertension*
| Chlorthalidone (N=672) |
Amlodipine (N=441) |
Lisinopril (N=757) |
Amlodipine vs. Chlorthalidone |
Lisinopril vs. Chlorthalidone |
|||
|---|---|---|---|---|---|---|---|
| No. of events (%) |
No. of events (%) |
No. of events (%) |
HR (95% CI) | P–Value | HR (95% CI) | P–Value | |
| Primary outcome coronary heart disease† |
55 (8.2) | 36 (8.3) | 57 (7.6) | ||||
| Model 1 | 0.95 (0.63–1.45) | 0.82 | 0.89 (0.62–1.29) | 0.55 | |||
| Model 2 | 0.96 (0.63–1.47) | 0.86 | 0.94 (0.65–1.36) | 0.74 | |||
| Model 3 | 0.95 (0.61–1.48) | 0.83 | 1.00 (0.68–1.47) | 0.99 | |||
| Model 4 | 0.86 (0.53–1.39) | 0.53 | 1.06 (0.70–1.60) | 0.78 | |||
| Model 5 | 0.86 (0.53–1.40) | 0.54 | 1.04 (0.69–1.58) | 0.83 | |||
| Secondary outcomes | |||||||
| All-cause mortality | 81 (12.1) | 60 (13.6) | 92 (12.2) | ||||
| Model 1 | 1.07 (0.76–1.49) | 0.71 | 0.97 (0.72–1.31) | 0.85 | |||
| Model 2 | 1.12 (0.80–1.56) | 0.52 | 1.05 (0.77–1.41) | 0.77 | |||
| Model 3 | 1.12 (0.79–1.58) | 0.52 | 1.05 (0.77–1.43) | 0.75 | |||
| Model 4 | 1.06 (0.73–1.54) | 0.77 | 1.12 (0.81–1.55) | 0.50 | |||
| Model 5 | 1.14 (0.78–1.66) | 0.50 | 1.13 (0.82–1.58) | 0.45 | |||
| Combined coronary heart disease‡ |
102 (15.3) | 71 (16.4) | 93 (12.5) | ||||
| Model 1 | 1.04 (0.77–1.40) | 0.81 | 0.78 (0.59–1.03) | 0.08 | |||
| Model 2 | 1.04 (0.77–1.41) | 0.80 | 0.80 (0.61–1.07) | 0.13 | |||
| Model 3 | 1.10 (0.80–1.50) | 0.56 | 0.85 (0.63–1.13) | 0.26 | |||
| Model 4 | 1.08 (0.76–1.54) | 0.67 | 0.96 (0.70–1.32) | 0.80 | |||
| Model 5 | 1.08 (0.75–1.54) | 0.68 | 0.95 (0.69–1.32) | 0.77 | |||
| Stroke | 23 (3.5) | 24 (5.5) | 30 (4.0) | ||||
| Model 1 | 1.54 (0.87–2.73) | 0.14 | 1.12 (0.65–1.93) | 0.68 | |||
| Model 2 | 1.60 (0.90–2.85) | 0.11 | 1.18 (0.68–2.05) | 0.54 | |||
| Model 3 | 1.68 (0.93–3.04) | 0.09 | 1.19 (0.68–2.10) | 0.54 | |||
| Model 4 | 1.63 (0.86–3.12) | 0.14 | 1.33 (0.72–2.45) | 0.37 | |||
| Model 5 | 1.71 (0.89–3.30) | 0.11 | 1.34 (0.72–2.49) | 0.35 | |||
| Combined cardiovascular disease§ |
162 (24.3) | 120 (27.6) | 154 (20.6) | ||||
| Model 1 | 1.12 (0.88–1.41) | 0.37 | 0.81 (0.65–1.01) | 0.06 | |||
| Model 2 | 1.11 (0.87–1.40) | 0.40 | 0.83 (0.67–1.04) | 0.10 | |||
| Model 3 | 1.16 (0.91–1.48) | 0.24 | 0.86 (0.68–1.08) | 0.19 | |||
| Model 4 | 1.21 (0.92–1.58) | 0.17 | 0.95 (0.74–1.22) | 0.69 | |||
| Model 5 | 1.19 (0.91–1.56) | 0.21 | 0.95 (0.74–1.22) | 0.68 | |||
| End-stage renal disease‖ |
13 (2.0) | 7 (1.6) | 9 (1.2) | ||||
| Model 1 | 0.84 (0.33–2.10) | 0.70 | 0.69 (0.29–1.62) | 0.39 | |||
| Model 2 | 0.86 (0.34–2.21) | 0.76 | 0.67 (0.28–1.61) | 0.37 | |||
| Model 3 | 1.01 (0.37–2.72) | 0.99 | 0.88 (0.34–2.26) | 0.80 | |||
| Model 4 | 1.58 (0.52–4.84) | 0.42 | 1.08 (0.37–3.13) | 0.89 | |||
| Model 5 | 1.87 (0.59–5.94) | 0.29 | 1.26 (0.42–3.75) | 0.68 | |||
| Cancer | 39 (5.9) | 21 (5.0) | 44 (6.0) | ||||
| Model 1 | 0.80 (0.47–1.37) | 0.42 | 0.97 (0.63–1.49) | 0.89 | |||
| Model 2 | 0.82 (0.48–1.40) | 0.47 | 1.00 (0.65–1.54) | 0.99 | |||
| Model 3 | 0.83 (0.49–1.43) | 0.51 | 0.97 (0.63–1.50) | 0.89 | |||
| Model 4 | 0.95 (0.54–1.67) | 0.85 | 1.03 (0.64–1.65) | 0.91 | |||
| Model 5 | 0.90 (0.50–1.59) | 0.71 | 1.05 (0.65–1.69) | 0.84 | |||
| Hospitalized for gastrointestinal bleeding |
32 (6.0) | 19 (5.6) | 35 (6.3) | ||||
| Model 1 | 0.91 (0.51–1.60) | 0.73 | 1.00 (0.62–1.62) | 0.99 | |||
| Model 2 | 0.91 (0.51–1.60) | 0.73 | 1.01 (0.62–1.63) | 0.98 | |||
| Model 3 | 0.98 (0.55–1.77) | 0.95 | 1.09 (0.66–1.80) | 0.75 | |||
| Model 4 | 0.88 (0.46–1.70) | 0.71 | 1.17 (0.68–2.01) | 0.57 | |||
| Model 5 | 0.83 (0.43–1.62) | 0.59 | 1.22 (0.71–2.09) | 0.48 | |||
| Components of secondary outcomes |
|||||||
| Heart failure | 47 (7.0) | 43 (9.9) | 39 (5.3) | ||||
| Model 1 | 1.37 (0.91–2.07) | 0.14 | 0.71 (0.47–1.09) | 0.12 | |||
| Model 2 | 1.40 (0.92–2.12) | 0.11 | 0.78 (0.51–1.20) | 0.26 | |||
| Model 3 | 1.29 (0.84–1.98) | 0.24 | 0.76 (0.49–1.16) | 0.20 | |||
| Model 4 | 1.38 (0.88–2.17) | 0.16 | 0.81 (0.51–1.27) | 0.35 | |||
| Model 5 | 1.35 (0.85–2.14) | 0.20 | 0.79 (0.50–1.25) | 0.32 | |||
| Hospitalized/fatal heart failure |
41 (6.1) | 33 (7.6) | 28 (3.8) | ||||
| Model 1 | 1.20 (0.76–1.90) | 0.43 | 0.58 (0.36–0.94) | 0.03 | |||
| Model 2 | 1.24 (0.78–1.96) | 0.37 | 0.64 (0.40–1.04) | 0.07 | |||
| Model 3 | 1.17 (0.73–1.88) | 0.50 | 0.62 (0.38–1.01) | 0.06 | |||
| Model 4 | 1.27 (0.77–2.11) | 0.35 | 0.70 (0.42–1.18) | 0.18 | |||
| Model 5 | 1.23 (0.73–2.05) | 0.43 | 0.69 (0.41–1.16) | 0.16 | |||
| Angina (hospitalized or treated) |
50 (7.5) | 41 (9.4) | 46 (6.2) | ||||
| Model 1 | 1.24 (0.82–1.87) | 0.31 | 0.79 (0.53–1.18) | 0.26 | |||
| Model 2 | 1.21 (0.80–1.83) | 0.36 | 0.78 (0.52–1.16) | 0.22 | |||
| Model 3 | 1.38 (0.90–2.11) | 0.14 | 0.81 (0.53–1.24) | 0.34 | |||
| Model 4 | 1.43 (0.88–2.31) | 0.15 | 0.92 (0.58–1.48) | 0.74 | |||
| Model 5 | 1.39 (0.85–2.27) | 0.19 | 0.91 (0.56–1.46) | 0.68 | |||
| Angina (hospitalized) |
40 (6.0) | 31 (7.1) | 32 (4.3) | ||||
| Model 1 | 1.16 (0.73–1.86) | 0.53 | 0.69 (0.43–1.09) | 0.11 | |||
| Model 2 | 1.15 (0.72–1.85) | 0.55 | 0.68 (0.43–1.08) | 0.10 | |||
| Model 3 | 1.26 (0.78–2.03) | 0.35 | 0.68 (0.42–1.11) | 0.13 | |||
| Model 4 | 1.32 (0.76–2.30) | 0.32 | 0.79 (0.46–1.37) | 0.41 | |||
| Model 5 | 1.33 (0.75–2.33) | 0.33 | 0.78 (0.45–1.35) | 0.37 | |||
| Coronary revascularizations |
44 (6.6) | 41 (9.4) | 32 (4.3) | ||||
| Model 1 | 1.41 (0.92–2.16) | 0.11 | 0.62 (0.39–0.98) | 0.04 | |||
| Model 2 | 1.43 (0.93–2.19) | 0.10 | 0.66 (0.42–1.04) | 0.08 | |||
| Model 3 | 1.57 (1.01–2.43) | 0.05 | 0.71 (0.44–1.13) | 0.15 | |||
| Model 4 | 1.86 (1.11–3.11) | 0.02 | 0.94 (0.55–1.61) | 0.82 | |||
| Model 5 | 1.84 (1.09–3.13) | 0.02 | 0.94 (0.55–1.60) | 0.81 | |||
| Peripheral arterial disease (hospitalized or treated) |
27 (4.1) | 9 (2.1) | 14 (1.9) | ||||
| Model 1 | 0.49 (0.23–1.05) | 0.07 | 0.45 (0.24–0.86) | 0.02 | |||
| Model 2 | 0.48 (0.23–1.02) | 0.06 | 0.48 (0.25–0.91) | 0.03 | |||
| Model 3 | 0.49 (0.23–1.05) | 0.07 | 0.49 (0.25–0.96) | 0.04 | |||
| Model 4 | 0.66 (0.28–1.56) | 0.35 | 0.50 (0.22–1.11) | 0.09 | |||
| Model 5 | 0.60 (0.25–1.43) | 0.25 | 0.51 (0.23–1.14) | 0.10 | |||
Abbreviations: treatment resistant hypertension, apparent treatment resistant hypertension; HR indicates Hazard Ratio; CI, confidence interval;
treatment resistant hypertension was defined as having uncontrolled hypertension despite the use of antihypertensive medications from 3 or more classes or the use of 4 or more antihypertensive medication classes to achieve blood pressure control.
coronary heart disease includes nonfatal myocardial infarction and fatal coronary heart disease; end-stage renal disease: kidney disease death, kidney transplant, or start of chronic renal dialysis; and heart failure: fatal, nonfatal hospitalized, or treated.
Combined coronary heart disease indicates coronary heart disease death, nonfatal myocardial infarction, coronary revascularization procedures, and hospitalized angina.
Combined cardiovascular disease indicates coronary heart disease death, nonfatal myocardial infarction, stroke, coronary revascularization procedures, hospitalized or treated angina, treated or hospitalized heart failure, and peripheral arterial disease (hospitalized or outpatient revascularization).
For end stage renal disease all models include baseline eGFR
Model 1: unadjusted;
Model 2: adjusted for age, sex, race/ethnicity and region of residence;
Model 3: adjusted for variables in model 2 plus practice setting, education level, smoking status and body mass index (BMI);
Model 4: adjusted for variables in model 2 and 3 along with estimated glomerular filtration rate (eGFR), diabetes, low density lipoprotein (LDL)-cholesterol, high density lipoprotein (HDL)-cholesterol, history of coronary heart disease, left ventricular hypertrophy, and taking blood pressure medications prior to randomization
Model 5: adjusted for variables in model 2, 3 and 4 along with baseline and Year-2 blood pressure
Similarly, there were no significant differences for the secondary outcomes of all-cause mortality (HR=1.06; 95% CI 0.73–1.54; P=0.77 and HR=1.12; 95% CI 0.81–1.55; P=0.50), combined coronary heart disease (HR=1.08; 95% CI 0.76–1.54; P=0.67 and HR=0.96; 95% CI 0.70–1.32; P=0.80), stroke (HR=1.63; 95% CI 0.86–3.12; P=0.14 and HR=1.33; 95% CI 0.72–2.45; P=0.37), combined cardiovascular disease (HR=1.21; 95% CI 0.92–1.58; P=0.17 and HR=0.95; 95% CI 0.74–1.22; P=0.69), end-stage renal disease (HR=1.58; 95% CI 0.52–4.84; P=0.42 and HR=1.08; 95% CI 0.37–3.13; P=0.89), heart failure (HR=1.38; 95% CI 0.88–2.17; P=0.16 and HR=0.81; 95% CI 0.51–1.27; P=0.35) and other secondary outcomes (Table 2) in the fully adjusted models comparing amlodipine vs. chlorthalidone and lisinopril vs. chlorthalidone respectively. However, the risk of coronary revascularization was higher with amlodipine when compared with chlorthalidone (HR=1.86; 95% CI 1.11–3.11; P=0.02) in the fully adjusted model and the risk of peripheral artery disease was lower with lisinopril compared with chlorthalidone, although this did not reach statistical significance in the fully adjusted model (P=0.09) (Table 2).
Sensitivity Analysis
The results were largely similar in a number of sensitivity analyses performed: 1) cohort with uncontrolled BP while taking ≥3 antihypertensive agents (eTable 1); 2) cohort with controlled BP on ≥4 antihypertensive agents (eTable 2); 3) on-treatment analysis after dividing the cohort into those with treatment resistant hypertension on a thiazide-type diuretic vs. those not on a thiazide-type diuretic (eTable 3); 4) cohort where treatment resistant hypertension was defined at Year-1 visit rather than Year-2 visit (eTable 4) and 5)Alternate/expanded cohort of patients (n=2359 patients) (eTable 5).
Discussion
The study evaluated the prevalence of treatment resistant hypertension and outcomes in patients with treatment resistant hypertension based on treatment assignment in the ALLHAT trial. The study showed that the prevalence of treatment resistant hypertension was significantly lower in the group allocated to thiazide-type diuretic-based treatment when compared with the non-diuretic groups with consistent results in blacks and non-blacks. Despite this, the incidences of primary and secondary outcomes were largely similar across all 3 groups, indicating worse outcomes with treatment resistant hypertension regardless of the randomized treatment group in ALLHAT.
Definition of TRH
There has been an exponential increase in the number of publications on TRH, especially in the last decade.(9) Yet, there is no consensus on the definition of TRH. The JNC-7, ESC/ESH and NICE guidelines require uncontrolled BP on at least 3 agents including a diuretic to qualify as TRH. This, along with differences in implementation of the definition and the population studied has led to wide variability in the reported prevalence of TRH with reported rates of 1.9% to 30%.<sup>RW.ERROR - Unable to find reference:1444</sup> (10–12) In the REduction of Atherothrombosis for Continued Health (REACH) Registry, the prevalence of TRH was 12.7% using the JNC-7/ESC/ESH definition, 21.6% using the AHA definition,(13) and 6.0% using the definition that is commonly employed for identification of patients for renal artery denervation (14) (systolic BP of at least 160 mm Hg despite being on 3 antihypertensive agents including a diuretic).(13) The AHA scientific statement on TRH therefore notes that the exact prevalence of treatment resistant hypertension is unknown.(1) The definition is important as it aids in the identification of patients for advanced therapeutics, including aldosterone antagonists, or assessment for secondary causes of hypertension.
The ALLHAT trial provides an opportunity to answer the question as to whether the prevalence of treatment resistant hypertension would be different for a diuretic-based strategy versus a non-diuretic-based strategy, as this is a prospective trial where medications were titrated to a goal. The results show that the prevalence of treatment resistant hypertension varied from 9.6% to 19.7% based on the randomized groups in ALLHAT, with the lowest prevalence in the diuretic arm of the trial. Moreover, previous analysis from ALLHAT has shown that blacks treated with lisinopril demonstrated poorer blood pressure (BP) control (5/2mm Hg higher BP), and worse outcomes than those randomized to diuretics.(15) In order to account for this, we performed separate analysis for blacks vs. non-blacks to evaluate the odds of treatment resistant hypertension with lisinopril when compared with chlorthalidone. Our analysis showed increased odds of treatment resistant hypertension with lisinopril in both blacks and non-blacks when compared with chlorthalidone.
Outcomes in Patients with TRH
Several studies have reported that outcomes of patients with TRH are worse than those without TRH.(10, 13) In an analysis from the REACH registry, an increased risk of cardiac death/myocardial infarction/stroke, non-fatal stroke, and heart failure hospitalization was observed in patients with TRH, using a TRH definition that was similar to that of JNC-7 and ESC/ESH (diuretic based).(13) Moreover, when the AHA definition was used, there was increase in all cardiovascular outcomes, including all-cause mortality, cardiovascular mortality, non-fatal myocardial infarction, and hospitalization for heart failure. Similarly, in an analysis from the Treating to New Targets trial, treatment resistant hypertension (using the AHA definition without the need for diuretic) was associated with significant increase in cardiovascular events when compared with patients without treatment resistant hypertension.(16) In a recent analysis from ALLHAT we have shown a significant increase in the risk of cardiovascular and renal events in patients with treatment resistant hypertension (using the AHA definition without the need for diuretic) when compared with patients without treatment resistant hypertension.(5)
The results of the present study show that the incidence of cardiovascular outcomes was similar whether a diuretic-based or a non-diuretic–based definition was used. It is therefore interesting to note that although the prevalence of treatment resistant hypertension was lowest on a diuretic (chlorthalidone), the prognosis was similar across all randomized groups. These relationships are important to consider when the diuretic-based definitions cannot be used, such as in patients intolerant to a diuretic. However, it is possible that, as the diuretic controlled blood pressure in a higher proportion of participants than the other agents, those meeting criteria for treatment resistant hypertension on a diuretic may have been a higher risk group on average than those on the other drugs. In addition, there were a greater proportion of black patients in those with treatment resistant hypertension assigned to lisinopril when compared to those assigned to the chlorthalidone group. Prior studies and analyses have shown that angiotensin converting enzyme inhibitors are less effective in blacks when compared with non-blacks.(15, 17)
Study Limitations
Although the analyses were performed based on the randomized treatment groups in ALLHAT, this post hoc analysis loses the benefit and balance of randomization given the definition of treatment resistant hypertension at Year-2 follow-up and inclusion of a subset of the overall patients randomized. Our various exclusion criteria led to exclusion of a consideration number of patients who were originally randomized in the ALLHAT trial with only 14864 patients out of the 33357 randomized included. In order to minimize this large number of excluded patients, we performed sensitivity analyses in an alternate/expanded cohort and the results were largely similar. In our definition of TRH we did not have data to rule out secondary causes of TRH (including medication noncompliance, white coat hypertension, etc.) and hence the definition conforms to the definition of treatment resistant hypertension used by Egan et al.(11) However, we do not believe the lack of out-of-office BP measurements to rule out secondary causes would differentially affect the three treatment groups. In addition, the small number of outcomes for certain endpoints (such as stroke) may have limited statistical power to detect differences among the groups. Moreover, we did not account for multiple testing.
Conclusions
In patients randomized in the ALLHAT trial, a prospective trial where medications were being titrated to a goal, the prevalence of treatment resistant hypertension using the AHA definition was lowest in the group of patients randomized to chlorthalidone when compared with the groups randomized to amlodipine or lisinopril. Yet, the risk of cardiovascular outcomes was largely similar for patients with treatment resistant hypertension across all 3 groups. These associations should be tested in future trials and be taken into consideration for the design of future trials with treatment resistant hypertension.
Supplementary Material
Patients who were in the diuretic treatment group of the ALLHAT trial were less likely to develop treatment resistant hypertension than were patients in the calcium channel blocker or angiotensin converting enzyme inhibitor groups.
For patients who had treatment-resistant hypertension, cardiovascular outcomes were similar across all treatment groups.
Acknowledgments
Funding Sources
This study was supported by contracts NO1-HC-35130 and HHSN268201100036C with the National Heart, Lung, and Blood Institute. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial investigators acknowledge study medications contributed by Pfizer, Inc., (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors had access to the data and a role in writing the manuscript
Disclosures
S Bangalore has received honoraria from Daiichi Sankyo, Boerhinger Ingelheim, Merck, Abbott, and Pfizer. H.R. Black has received honoraria from Bayer, Novartis, Pfizer, Phase Bio, Servier, and Takeda. J.B. Kostis has received honoraria from Bristol-Myers Squibb. J.L. Probstfield has received honoraria from Sanofi.
The other authors report no conflicts.
Contributor Information
Sripal Bangalore, New York University School of Medicine, New York, NY.
Barry R. Davis, The University of Texas School of Public Health, Houston, TX.
William C. Cushman, Memphis Veterans Affairs Medical Center, University of Tennessee College of Medicine, Memphis, TN.
Sara L. Pressel, The University of Texas School of Public Health, Houston, TX.
Paul M. Muntner, University of Alabama, Birmingham, AL.
David A. Calhoun, University of Alabama, Birmingham, AL.
John B. Kostis, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
Paul K. Whelton, Tulane University School of Public Health and Tropical Medicine, New Orleans, LA.
Jeffrey L. Probstfield, The University of Washington Medical Center, Seattle, WA.
Mahboob Rahman, Case Western Reserve University, Cleveland, OH.
Henry R. Black, New York University School of Medicine, New York, NY.
References
- 1.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA: The Journal of the American Medical Association. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 4.National Clinical Guideline Centre. The clinical management of primary hypertension in adults. http://www.nice.org.uk/guidance/index.jsp?action=download&o=53228. London: National Clinical Guideline Centre; 2011. [Accessed Dec 03, 2011]. [cited 2011 December 03]; Available from: http://www.nice.org.uk/guidance/index.jsp?action=download&o=53228. [Google Scholar]
- 5.Muntner P, Davis BR, Cushman WC, et al. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2014;64(5):1012–1021. doi: 10.1161/HYPERTENSIONAHA.114.03850. [DOI] [PubMed] [Google Scholar]
- 6.Davis BR, Cutler JA, Gordon DJ, et al. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. AmJ Hypertens. 1996;9(4 Pt 1):342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 7.Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Collaborative Research Group. JAMA. 2000;283(15):1967–1975. [PubMed] [Google Scholar]
- 8.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid- Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 9.Messerli FH, Bangalore S. Treatment-resistant hypertension: another Cinderella story. Eur Heart J. 2013;34(16):1175–1177. doi: 10.1093/eurheartj/eht028. [DOI] [PubMed] [Google Scholar]
- 10.Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan BM, Zhao Y, Axon RN, et al. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124(9):1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57(6):1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 13.Kumbhani DJ, Steg PG, Cannon CP, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2012;34(16):1204–1214. doi: 10.1093/eurheartj/ehs368. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 15.Wright JT, Jr, Dunn JK, Cutler JA, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293(13):1595–1608. doi: 10.1001/jama.293.13.1595. [DOI] [PubMed] [Google Scholar]
- 16.Bangalore S, Fayyad R, Laskey R, et al. Prevalence, predictors, and outcomes in treatment-resistant hypertension in patients with coronary disease. Am J Med. 2014;127(1):71–81. e1. doi: 10.1016/j.amjmed.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 17.Ogedegbe G, Shah NR, Phillips C, et al. Comparative Effectiveness of Angiotensin- Converting Enzyme Inhibitor-Based Treatment on Cardiovascular Outcomes in Hypertensive Blacks Versus Whites. J Am Coll Cardiol. 2015;66(11):1224–1233. doi: 10.1016/j.jacc.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.