Abstract
Programmed cell death protein 1 (PD-1) acts on PD-1 ligands (PD-L1 and PD-L2) to suppress activation of cytotoxic T lymphocytes. Interleukin-17 (IL-17) and tumor necrosis factor-α (TNF-α) are co-expressed by T helper 17 (TH17) cells in many tumors. The purpose of this study was to test if IL-17 and TNF-α may synergistically induce PD-L1 expression in human prostate cancer LNCaP and human colon cancer HCT116 cell lines. We found that IL-17 did not induce PD-L1 mRNA expression, but up-regulated PD-L1 protein expression in HCT116 and LNCaP cells. TNF-α induced PD-L1 mRNA and protein expression in both cell lines. Neither IL-17 nor TNF-α induced PD-L2 mRNA or protein expression. IL-17 and TNF-α acted individually rather than cooperatively in induction of PD-L1 expression. IL-17 and/or TNF-α activated AKT, nuclear factor-κB (NF-κB), and extracellular signal-regulated kinases 1/2 (ERK1/2) signaling pathways in HCT116 cells, whereas only NF-κB signaling was activated in LNCaP cells. NF-κB inhibitor could diminish PD-L1 protein expression induced by IL-17 and/or TNF-α in both HCT116 and LNCaP cell lines. ERK1/2 inhibitor could also reduce PD-L1 protein expression induced by IL-17 and/or TNF-α in HCT116 cells, while AKT inhibitor could abolish PD-L1 protein expression induced by IL-17 and/or TNF-α in LNCaP cells. These results suggest that IL-17 and TNF-α act individually rather than cooperatively through activation of NF-κB and ERK1/2 signaling to up-regulate PD-L1 expression in HCT116 cells, while the two inflammatory cytokines act through activation of NF-κB signaling, in the presence of AKT activity, to up-regulate PD-L1 expression in LNCaP cells.
Keywords: Interleukin-17, Tumor necrosis factor-α, Programmed cell death protein 1 ligand 1, Prostate cancer, Colon cancer
1. Introduction
Cancer poses a major threat to public health worldwide due to its increasing prevalence and is a leading cause of deaths worldwide. In 2013, there were approximately 14.9 million incident cancer cases and 8.2 million cancer-related deaths [1]. Cancer is a chronic disease with multiple driver and passenger genes involved in the complicated pathogenesis [2]. In recent years, both laboratory and clinical studies revealed that immune escape is one of the most important mechanisms for cancer to avoid immune destruction and acquire resistance to anti-tumor drugs, which becomes a key barrier in cancer therapy [3,4]. Cancer immune escape mechanism involves many factors. One of these factors is programmed cell death protein 1 (PD-1) that acts on PD-1 ligands (PD-L1 and PD-L2) [5,6]. PD-1, encoded by the PDCD1 gene, is a cell surface receptor that belongs to the immunoglobulin superfamily and is expressed on T cells, B cells, monocytes, natural killer cells, dendritic cells, and regulatory T cells (Treg). PD-1 is considered as an immune checkpoint that inhibits activation of T cells to prevent autoimmunity and promote self-tolerance [7]. PD-L1 and PD-L2 belong to the membrane protein B7 family, also called B7-H1 and B7-DC, respectively. PD-L1, also known as cluster of differentiation 274 (CD274), is a 40 kDa type I transmembrane protein encoded by CD274 gene in humans. PD-L1 is expressed in a variety of cancer cells, such as prostate cancer, colorectal cancer, gastric cancer, lung cancer, melanoma, renal cell carcinoma, multiple myeloma, and leukemia [8–11]. Tumor-associated PD-L1 promotes apoptosis of cytotoxic T cells while enhancing survival of Treg cells, thus suppressing the anti-cancer immune system [12]. PD-1/PD-L1 signaling axis has been demonstrated to play a role in regulating tumor microenvironment of prostate cancer and colorectal cancer [13,14]. Lee et al [15] showed that 5% colorectal carcinomas exhibited high PD-L1 expression and 19% had elevated numbers of PD-1-positive tumor infiltrating lymphocytes. Gevensleben et al [10] found that 52.2% of 209 primary prostate cancer samples expressed moderate to high PD-L1 levels and PD-L1 positivity was prognostic for biochemical recurrence. However, how PD-L1 expression is regulated in colorectal cancer and prostate cancer remains elusive. It has been demonstrated that certain pro-inflammatory factors can up-regulate PD-L1 expression, including interferon-γ (IFN-γ), tumor necrosis factor – α (TNF-α), lipopolysaccharide, granulocyte-monocyte colony stimulating factor, vascular endothelial growth factor, interleukin-10 (IL-10), and IL-4, with IFN-γ being the most potent inducer [16]. Interleukin-17 (IL-17, also called IL-17A) is a key pro-inflammatory cytokine that has been shown to promote prostate and colon cancer development [17–20]. IL-17 and TNF-α have been shown to cooperate functionally to induce expression of down-stream genes [21]. Yet, it is unknown if IL-17 and TNF-α may synergistically induce PD-L1 expression. The objective of the present study was to test if IL-17 and TNF-α may synergistically induce PD-L1 expression in human prostate cancer and colon cancer cells.
2. Materials and methods
2.1. Cell culture
Human colorectal cancer cell line HCT116 and human prostate cancer cell line LNCaP were purchased from the American Type Culture Collection (Manassas, VA, USA) and were free of mycoplasma contamination. HCT116 cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM, Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Fisher Scientific) and 100 U/mL penicillin/streptomycin. LNCaP cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Fisher Scientific) supplemented with 10% FBS and 100 U/mL penicillin/streptomycin. Both cell lines were cultured in a humidified incubator with 5% CO2 at 37°C.
2.2. Reagents
Recombinant human IL-17 (also called IL-17A) and recombinant human TNF-α were obtained from Fisher Scientific. Akt inhibitor AZD5363 was obtained from Selleck Chemicals, Inc. (Houston, TX, USA). NF-κB inhibitor Bay11-7082 was obtained from Santa Cruz Biotechnology (Dallas, TX, USA), ERK1/2 inhibitor U0126 was obtained from Promega Corporation (Madison, Wisconsin, USA). The primary antibodies used were: rabbit anti-PD-L1 monoclonal antibody (ab205921, Abcam, Cambridge, MA, USA); rabbit anti-PD-L2 polyclonal antibody (SAB3500395, Sigma-Aldrich, St. Louis, MO, USA); rabbit anti-phospho-AktSer473 polyclonal antibody, rabbit anti-Akt polyclonal antibody, rabbit anti-phospho-IκBα polyclonal antibody, and mouse anti-IκBα polyclonal antibody(Cell Signaling Technology, Danvers, MA, USA); rabbit anti-phospho-ERK1/2 polyclonal antibody and rabbit anti-ERK1/2 polyclonal antibody (Santa Cruz Biotechnology); and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (#MAB374, Millipore, Billerica, MA, USA).
2.3. Quantitative real-time reverse transcription – polymerase chain reaction (qRT-PCR)
After the indicated time of treatment, cells were harvested for total RNA extraction using NucleoSpin RNA kit (Macherey-Nagel, Bethlehem, PA, USA) according to the manufacturer’s instructions. cDNA was synthesized from total RNA using PrimeScriptTM reverse transcription kit (Takara, Mountain View, CA, USA). Human PD-L1, PD-L2, and GAPDH primers were obtained from Eurofins MWG Operon (Huntsville, AL, USA). The sequences are as follows: PD-L1, Forward: 5′-CTCAGGGTGACAGAGAGAGAAG-3′, Reverse: 5′-GACACCAACCACCAGGGTTT-3′; PD-L2, Forward: 5′-TGGCATTTGCTGACGCATTT-3′, Reverse: 5′-TGCAGCCAGGTCTAATTGTTTT-3′; GAPDH, Forward: 5′-CCACATCGCTCAGACACCAT-3′, Reverse: 5′-TAAAAGCAGCCCTGGTGACC-3′. qRT-PCR was performed in triplicates with an iQ5 iCycler and iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) following the manufacturer′s protocols. Results were normalized to GAPDH levels using the formula Δ cycle threshold (Ct) = Ct of target gene - Ct of GAPDH. The mRNA level of the control group (vehicle treatment) was used as the baseline; therefore, ΔΔCt was calculated using the formula ΔΔCt = ΔCt of target gene - ΔCt of the baseline. The fold change of mRNA level was calculated as fold = 2-ΔΔCt.
2.4. Western blot analysis
After the indicated time of treatment, proteins were extracted from the cells in radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM sodium fluoride, 0.5% Igepal CA-630 [NP-40], 10 mM sodium phosphate, 150 mM sodium chloride, 25 mM Tris pH 8.0, 1 mM phenylmethylsulfonyl fluoride, 2 mM ethylenediaminetetraacetic acid [EDTA], 1.2 mM sodium vanadate) supplemented with protease inhibitor cocktail (Sigma-Aldrich). Equal amount of proteins was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membrane. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline – Tween 20 (TBS-T) buffer (25 mM Tris-HCl, 125 mM NaCl, 0.1% Tween-20) for 1 hour and probed with the indicated primary antibodies overnight and then IRDye 800CW- or IRDye 680RD-conjugated secondary antibodies (LI-COR Biosciences, Lincoln, NE, USA) for 1 hour. The results were visualized using an Odyssey Infrared Imager (LICOR Biosciences). For loading control, the membranes were stripped and probed for unphosphorylated proteins and/or GAPDH. Quantification of the Western blot signals was performed using the image analysis software of the Odyssey Infrared Imager system. The integrated density values of signals of targeted genes were normalized by those of GAPDH. The ratio indicates the relative level of target protein.
2.5. Statistical analysis
Statistical analysis was performed using SPSS (v17.0, SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation (SD). Quantitative data were analyzed using analysis of variance (ANOVA, two-tailed) and Fisher’s least significant difference (LSD) method. Statistical significance was reached with a P value less than 0.05.
3. Results
3.1. PD-L1, but not PD-L2, mRNA expression was up-regulated by TNF-α, but not by IL-17
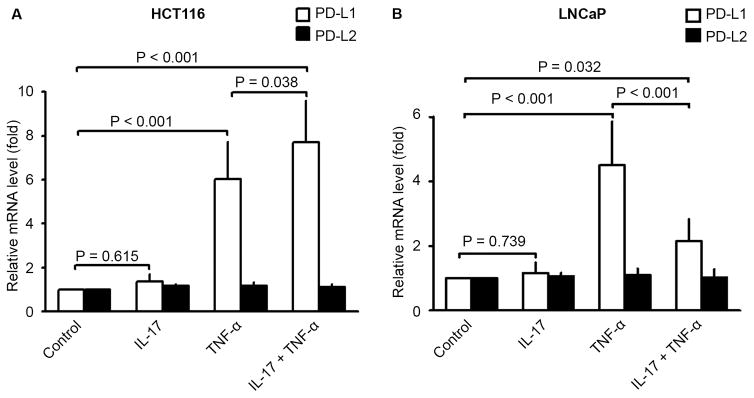
Human colon cancer cell line (HCT116) and human prostate cancer cell line (LNCaP) were treated with IL-17, TNF-α, or a combination of both for 3 hours. qRT-PCR analysis found that IL-17 treatment alone did not affect expression of either PD-L1 or PD-L2 mRNA in HCT116 and LNCaP cells (Fig. 1). TNF-α treatment alone significantly increased expression of PD-L1, but not PD-L2, mRNA in both HCT116 and LNCaP cell lines (Fig. 1, P < 0.001). In HCT116 cells, a combination of IL-17 and TNF-α treatment further increased PD-L1 mRNA expression to a level that was significantly higher than TNF-α alone (Fig. 1A, P = 0.038). In contrast, the combination of IL-17 and TNF-α treatment increased PD-L1 mRNA expression to a level that was significantly lower than TNF-α alone in LNCaP cells (Fig. 1B, P < 0.001).
Fig. 1.
Effects of IL-17 and/or TNF-α on PD-L1 and PD-L2 mRNA expression in human cancer cell lines HCT116 and LNCaP. (A and B) The cells were treated with IL-17 (20 ng/ml), TNF-α (10 ng/ml), or a combination of both for 3 hours. PD-L1 and PD-L2 mRNA expression was determined by qRT-PCR analysis. Data were represented as means ± SD (error bars) of three independent experiments.
3.2. PD-L1, but not PD-L2, protein expression was up-regulated by both IL-17 and TNF-α
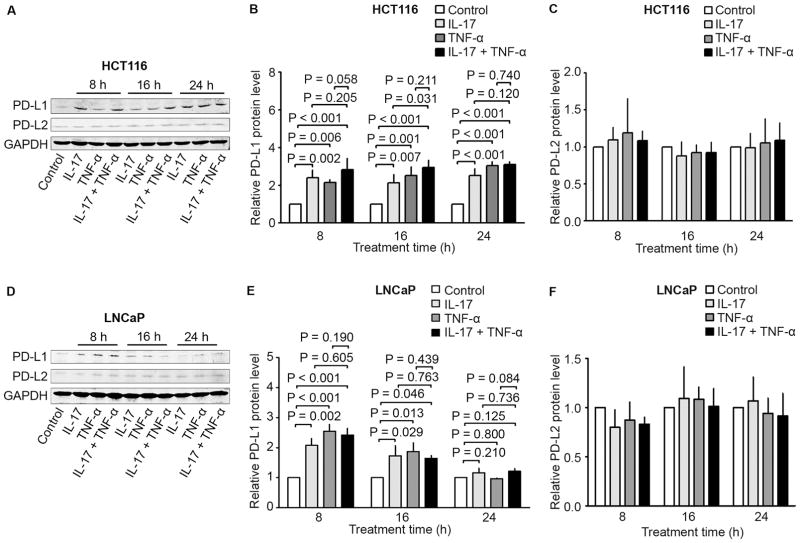
When HCT116 and LNCaP cells were treated with IL-17 and/or TNF-α for 8 hours, IL-17 or TNF-α alone or a combination of both significantly increased PD-L1, but not PD-L2, protein expression in both cell lines (Fig. 2). In HCT116 cells, the increased PD-L1 protein levels sustained up to 24 hours, whereas such increase in PD-L1 levels was diminished at 16 hours and disappeared at 24 hours in LNCaP cells (Fig. 2). Of note, although there was a slight further increase of PD-L1 protein levels in HCT116 cells treated with the combination of IL-17 and TNF-α compared to either cytokine alone, IL-17 and TNF-α did not appear to have any synergistic effects in induction of PD-L1 protein expression (Fig. 2).
Fig. 2.
Effects of IL-17 and/or TNF-α on PD-L1 and PD-L2 protein expression in human cancer cell lines HCT116 and LNCaP. (A to C) HCT116 cells and (D to F) LNCaP cells were treated with IL-17 (20 ng/ml), TNF-α (10 ng/ml), or a combination of both for the indicated time. PD-L1 and PD-L2 protein expression was analyzed using Western blot analysis. GAPDH was probed for protein loading control (A and D). The relative protein levels were presented as the ratio of PD-L1/GAPDH or PD-L2/GAPDH (B–C and E–F), which was normalized against the ratio of the control group (arbitrarily designated as “1”). Data were represented as means ± SD of three independent experiments.
3.3. IL-17 and TNF-α activated different signaling pathways in HCT116 and LNCaP cells
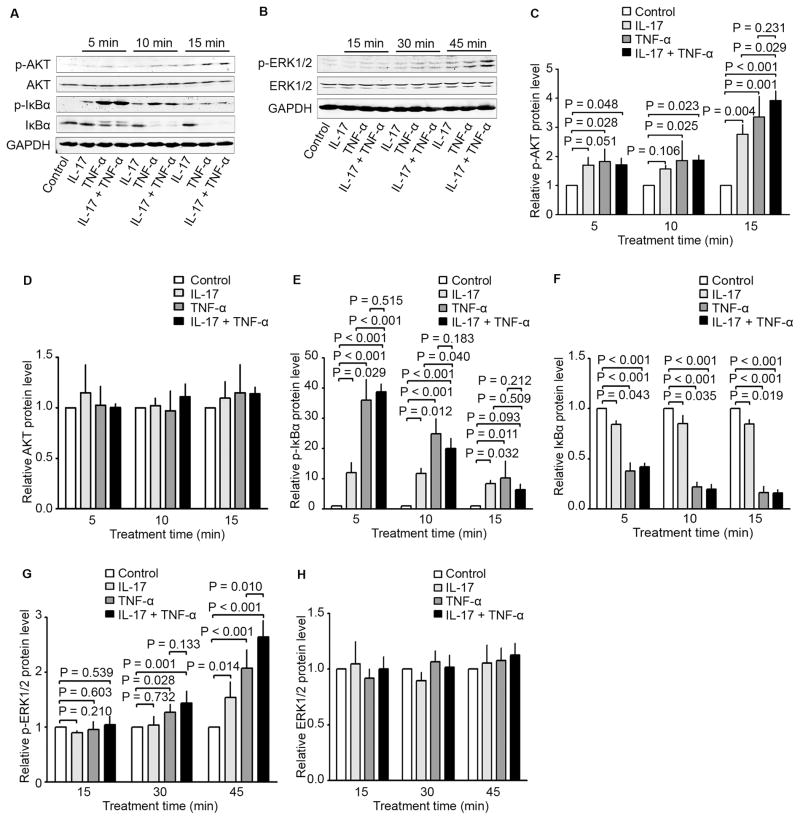
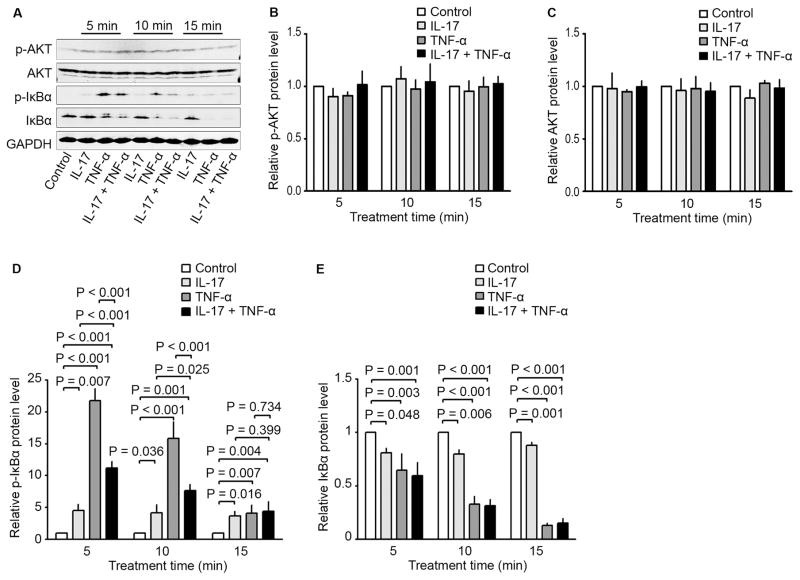
In HCT116 cells, IL-17, TNF-α, and a combination of both increased the phosphorylated AKT (p-AKT) levels in a time-dependent manner with the peak levels at 15 minutes, without affecting the AKT levels (Fig. 3A, C, and D). IL-17, TNF-α, and a combination of both increased the phosphorylated IκBα (p-IκBα) levels in a time-dependent manner with the peak levels at 5 minutes, which was accompanied with a decrease in IκBα levels (Fig. 3A, E, and F). TNF-α was significantly more effective in causing the changes in p-IκBα and IκBα levels than IL-17 (Fig. 3A, E, and F). IL-17, TNF-α, and a combination of both increased the phosphorylated extracellular signal-regulated kinases 1/2 (p-ERK1/2) levels in a time-dependent manner with the peak levels at 45 minutes, without affecting the ERK1/2 levels (Fig. 3B, G, and H). On the other hand, in LNCaP cells, IL-17, TNF-α, or a combination of both did not significantly change the levels of p-AKT or AKT (Fig. 4A to C), though the basal levels of p-AKT were high due to lack of phosphatase and tensin homolog (PTEN) expression in LNCaP cells [22]. IL-17, TNF-α, or a combination of both did not significantly change the levels of p-ERK1/2 or ERK1/2 (data not shown). Nevertheless, IL-17, TNF-α, and a combination of both increased the phosphorylated IκBα (p-IκBα) levels in a time-dependent manner with the peak levels at 5 minutes, which was accompanied with a decrease in IκBα levels (Fig. 4A, D, and E). TNF-α was significantly more effective in causing the changes in p-IκBα and IκBα levels than IL-17 (Fig. 4A, D, and E). Of note, the combination of IL-17 and TNF-α increased p-IκBα levels not as high as TNF-α alone, though IκBα levels were reduced similarly by TNF-α alone or the combination of IL-17 and TNF-α (Fig. 4A, D, and E).
Fig. 3.
Effects of IL-17 and/or TNF-α on activation of signaling pathways in human colon cancer line HCT116. (A to H) HCT116 cells were treated with IL-17 (20 ng/ml), TNF-α (10 ng/ml), or a combination of both for the indicated time. Activation of Akt, IκBα, and ERK1/2 was assessed using Western blot analysis. GAPDH was probed for protein loading control (A and B). Relative protein levels were presented as the ratio of each protein divided by GAPDH, which was normalized against the ratio of the control group (arbitrarily designated as “1”) (C to H). Data were represented as means ± SD of three independent experiments.
Fig. 4.
Effects of IL-17 and/or TNF-α on activation of signaling pathways in human prostate cancer line LNCaP. (A to E) LNCaP cells were treated with IL-17 (20 ng/ml), TNF-α (10 ng/ml), or a combination of both for the indicated time. Activation of Akt, IκBα, and ERK1/2 was assessed using Western blot analysis. GAPDH was probed for protein loading control (A). Relative protein levels were presented as the ratio of each protein divided by GAPDH, which was normalized against the ratio of the control group (arbitrarily designated as “1”) (B to E). Data were represented as means ± SD of three independent experiments.
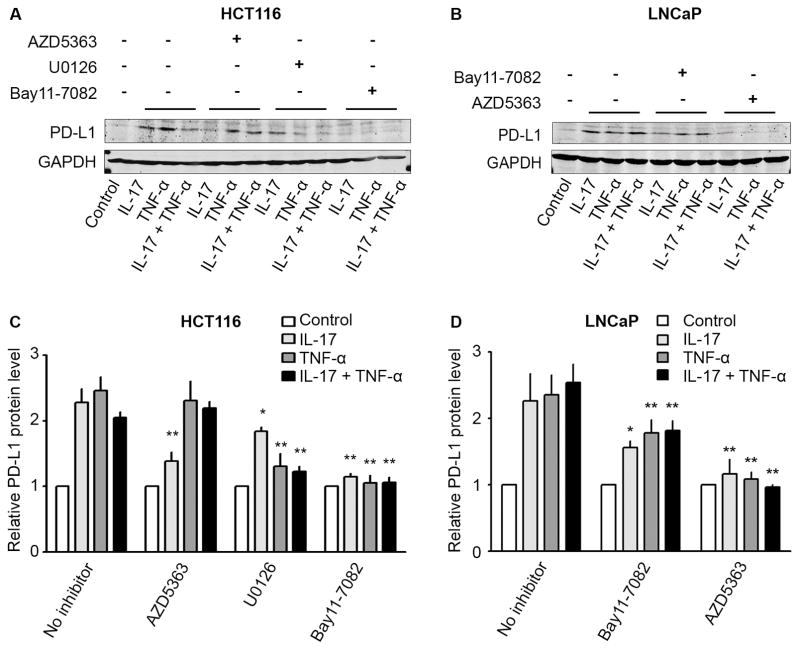
3.4. Blockade of AKT, NF-κB, or ERK1/2 signaling pathways diminished PD-L1 protein expression induced by IL-17 and/or TNF-α
Since AKT, NF-κB, or ERK1/2 signaling pathways were activated in HCT116 cells by IL-17 and/or TNF-α, we tested if inhibitors of AKT (AZD5363), NF-κB (Bay11-7082), or ERK1/2 (U0126) signaling pathways could diminish PD-L1 protein expression induced by IL-17 and/or TNF-α. We found that AZD5363 only significantly reduced PD-L1 protein levels induced by IL-17, but not by TNF-α or a combination of IL-17 and TNF-α in HCT116 cells (Fig. 5A and C). In contrast, both U0126 and Bay11-7082 significantly diminished the increase of PD-L1 levels induced by IL-17, TNF-α, or a combination of both in HCT116 cells (Fig. 5A and C). On the other hand, in LNCaP cells, both Bay11-7082 and AZD5363 significantly diminished the increase of PD-L1 levels induced by IL-17, TNF-α, or a combination of both (Fig. 5B and D).
Fig. 5.
Effects of pan-Akt inhibitor (AZD5363), MEK/ERK1/2 inhibitor (U0126), and NF-κB inhibitor (Bay11-7082) on PD-L1 protein expression induced by IL-17 and/or TNF-α in human cancer cell lines HCT116 and LNCaP. (A and C) HCT116 cells and (B and D) LNCaP cells were first treated with AZD5363 (2 μM), U0126 (10 μM), or Bay11-7082 (5 μM) for 30 minutes. Then, HCT116 cells were treated with IL-17 (20 ng/ml), TNF-α (10 ng/ml), or a combination of both for 24 hours, while LNCaP cells were similarly treated for 8 hours. PD-L1 protein levels were analyzed using Western blot analysis. GAPDH was probed for protein loading control (A and B). Relative protein levels were presented as the ratio of PD-L1/GAPDH, which was normalized against the ratio of the control group (arbitrarily designated as “1”) (C and D). Data were represented as means ± SD of three independent experiments. *P< 0.05 and **P< 0.01, compared to the corresponding group with IL-17 and/or TNF-α treatment in the absence of any inhibitor.
4. Discussion
T helper 17 (TH17) cells are a distinct group of CD4+ T cells that co-express IL-17 and TNF-α [23–25]. IL-17 and TNF-α-secreting TH17 cells have been found to be enriched in colon tumors and prostate tumors [26,27]. Given that IL-17 and TNF-α have been shown to cooperate functionally to induce expression of down-stream genes [21], we investigated if IL-17 and TNF-α could synergistically induce PD-L1 expression in human prostate cancer and colon cancer cells. In the present study, we found that, although only TNF-α induced PD-L1 mRNA expression in human colon cancer HCT116 cell line and human prostate cancer LNCaP cell line, both IL-17 and TNF-α induced PD-L1 protein expression in HCT116 and LNCaP cells. We speculate that the increase of PD-L1 level is likely adequate to inhibit T cell function as shown in our recent study [28]. In that study, we found that estrogen up-regulated PD-L1 expression via PI3K/Akt signaling pathway and the increased PD-L1 expression suppressed interferon-γ and IL-2 expression in Jurkat cells and primary T cells [28]. Given that the amplitude of PD-L1 induction by IL-17 and TNF-α is comparable to or even higher than the amplitude induced by estrogen, we speculate that the two situations are comparable. Neither IL-17 nor TNF-α induced PD-L2 mRNA or protein expression, suggesting that PD-L2 expression may be regulated by different factors.
It is worth pointing out that IL-17 and TNF-α did not appear to have any synergy in inducing PD-L1 expression. Our findings showed that a combination of IL-17 and TNF-α increased PD-L1 mRNA levels to be higher than that induced by TNF-α alone in HCT116 cells, but the combination caused less increase of PD-L1 mRNA levels than TNF-α alone in LNCaP cells. The combined treatment increased PD-L1 protein expression to the levels similar to what was induced by TNF-α alone. We consistently observed that TNF-α was more effective than IL-17 in induction of PD-L1 expression. These findings suggest that IL-17 and TNF-α act individually rather than cooperatively in regulating PD-L1 expression in HCT116 and LNCaP cell lines.
In order to understand how IL-17 and TNF-α induce PD-L1 expression, we checked the signaling pathways that could be activated by IL-17 and TNF-α. We found that NF-κB signaling was activated as early as 5 minutes after treatment with IL-17 and/or TNF-α in both HCT116 and LNCaP cell lines. AKT signaling was activated 5 minutes after treatment with IL-17 and/or TNF-α in HCT116 cells, which became more dramatic at 15 minutes, while ERK1/2 signaling was activated 30 to 45 minutes after treatment with IL-17 and/or TNF-α in HCT116 cells. In contrast, AKT and ERK1/2 signaling pathways were not activated by IL-17 or TNF-α in LNCaP cells. These findings suggest that the responses to IL-17 and TNF-α are cell type-specific. To check if any of these signaling pathways is responsible for induction of PD-L1 expression, we used selective inhibitors to block each signaling pathway. We found that Bay11-7082 (NF-κB inhibitor) significantly diminished PD-L1 protein expression induced by IL-17 and/or TNF-α in both HCT116 and LNCaP cell lines. This finding is consistent with the finding that IL-17 and/or TNF-α activated NF-κB signaling as early as 5 minutes upon the treatment. AZD5363 is a pan-AKT inhibitor [29]. AZD5363 diminished PD-L1 protein expression induced by IL-17 and/or TNF-α in LNCaP cells, whereas it only diminished PD-L1 protein expression induced by IL-17 in HCT116 cells. This finding indicates that AKT signaling is not a major driver in inducing PD-L1 expression by TNF-α in HCT116 cells. Of note, Neither IL-17 or TNF-α activates AKT signaling in LNCaP cells. However, the basal level of AKT activity is high due to inactivation of PTEN in LNCaP cells [22]. It has been demonstrated that human immunodeficiency virus can activate PI3K/Akt to up-regulate PD-L1 expression [30]. We speculate that the basal level of AKT activity is required for IL-17 and TNF-α to induce PD-L1 expression in LNCaP cells, thus AZD5363 is able to diminish the effects of IL-17 and/or TNF-α. In addition, MEK/ERK1/2 inhibitor (U0126) also significantly diminished PD-L1 protein expression induced by IL-17 and/or TNF-α in HCT116 cells. Together, these findings suggest that IL-17 and/or TNF-α act through activation of NF-κB and ERK1/2 signaling to up-regulate PD-L1 expression in HCT116 cells, while the two cytokines act through activation of NF-κB signaling, in the presence of AKT activity, to up-regulate PD-L1 expression in LNCaP cells. Our findings are consistent with several previous studies showing that NF-κB plays a key role in regulating PD-L1 expression [31–34]. Previous studies have also demonstrated that activation of ERK1/2 signaling drives PD-L1 expression in melanomas and lung cancers [35,36]. Activation of ERK1/2 and Akt pathways has been shown to up-regulate PD-L1 expression in melanoma cells [37], colon cancers [38], and breast cancer [39]. The novelty of the present study is the finding that IL-17 and TNF-α act individually rather than cooperatively to up-regulate PD-L1 expression in human colon cancer HCT116 cell line and human prostate cancer LNCaP cell line, which has never been reported before.
5. Conclusion
The present study suggests that IL-17 and TNF-α act individually rather than cooperatively through activation of NF-κB and ERK1/2 signaling to up-regulate PD-L1 expression in HCT116 cells, while the two cytokines act through activation of NF-κB signaling, in the presence of AKT activity, to up-regulate PD-L1 expression in LNCaP cells. Given that IL-17 and TNF-α-secreting TH17 cells have been found to be enriched in colon tumors and prostate tumors [26,27], our findings imply that TH17 cells may create an immunosuppressive tumor microenvironment through up-regulating PD-L1 expression.
Acknowledgments
This work was partially supported by National Institutes of Health (R01CA174714 and P20GM103518); Department of Defense [W81XWH-14-1-0050, W81XWH-14-1-0149, W81XWH-14-1-0458 (PI: Feng Chen; Co-I: Z.Y.), and W81XWH-15-1-0444]. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
Abbreviations
- Ct
cycle threshold
- DMEM
Dulbecco’s Modified Eagles Medium
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IFN-γ
interferon-γ
- IL-17
interleukin-17
- NF-κB
nuclear factor-κB
- PD-1
Programmed cell death protein 1
- PD-L1
PD-1 ligand 1
- PD-L2
PD-1 ligand 2
- qRT-PCR
quantitative real-time reverse transcription – polymerase chain reaction
- SD
standard deviation
- TH17
T helper 17
- Treg
regulatory T cells
- TNF-α
tumor necrosis factor-α
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catala-Lopez F, deVeber G, Gotay C, Khan G, Hosgood HD, 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castaneda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parcesepe P, Giordano G, Laudanna C, Febbraro A, Pancione M. Cancer-Associated Immune Resistance and Evasion of Immune Surveillance in Colorectal Cancer. Gastroenterol Res Pract. 2016;2016:6261721. doi: 10.1155/2016/6261721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A. Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discov. 2015;5:915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 7.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 9.Gentzler R, Hall R, Kunk PR, Gaughan E, Dillon P, Slingluff CL, Jr, Rahma OE. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016;8:583–600. doi: 10.2217/imt-2015-0029. [DOI] [PubMed] [Google Scholar]
- 10.Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, Majores M, Stein J, Uhl B, Muller S, Ellinger J, Stephan C, Jung K, Brossart P, Kristiansen G. The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate Cancer. Clin Cancer Res. 2016;22:1969–1977. doi: 10.1158/1078-0432.CCR-15-2042. [DOI] [PubMed] [Google Scholar]
- 11.Moehler M, Delic M, Goepfert K, Aust D, Grabsch HI, Halama N, Heinrich B, Julie C, Lordick F, Lutz MP, Mauer M, Alsina Maqueda M, Schild H, Schimanski CC, Wagner AD, Roth A, Ducreux M. Immunotherapy in gastrointestinal cancer: Recent results, current studies and future perspectives. Eur J Cancer. 2016;59:160–170. doi: 10.1016/j.ejca.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelova M, Charoentong P, Hackl H, Fischer ML, Snajder R, Krogsdam AM, Waldner MJ, Bindea G, Mlecnik B, Galon J, Trajanoski Z. Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biol. 2015;16:64. doi: 10.1186/s13059-015-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massari F, Ciccarese C, Calio A, Munari E, Cima L, Porcaro AB, Novella G, Artibani W, Sava T, Eccher A, Ghimenton C, Bertoldo F, Scarpa A, Sperandio N, Porta C, Bronte V, Chilosi M, Bogina G, Zamboni G, Tortora G, Samaratunga H, Martignoni G, Brunelli M. Magnitude of PD-1, PD-L1 and T Lymphocyte Expression on Tissue from Castration-Resistant Prostate Adenocarcinoma: An Exploratory Analysis. Target Oncol. 2016;11:345–351. doi: 10.1007/s11523-015-0396-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee LH, Cavalcanti MS, Segal NH, Hechtman JF, Weiser MR, Smith JJ, Garcia-Aguilar J, Sadot E, Ntiamoah P, Markowitz AJ, Shike M, Stadler ZK, Vakiani E, Klimstra DS, Shia J. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016 doi: 10.1038/modpathol.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, Oh S, Shin JG, Yao S, Chen L, Choi IH. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Liu S, Parajuli KR, Zhang W, Zhang K, Mo Z, Liu J, Chen Z, Yang S, Wang AR, Myers L, You Z. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene. 2016 doi: 10.1038/onc.2016.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Liu S, Xiong Z, Wang AR, Myers L, Melamed J, Tang WW, You Z. Interleukin-17 promotes development of castration-resistant prostate cancer potentially through creating an immunotolerant and pro-angiogenic tumor microenvironment. Prostate. 2014;74:869–879. doi: 10.1002/pros.22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Liu S, Ge D, Xue Y, Xiong Z, Abdel-Mageed AB, Myers L, Hill SM, Rowan BG, Sartor O, Melamed J, Chen Z, You Z. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res. 2012;72:2589–2599. doi: 10.1158/0008-5472.CAN-11-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham D, You Z. In vitro and in vivo model systems used in prostate cancer research. J Biol Methods. 2015;2:1–14. doi: 10.14440/jbm.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 25.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunne MR, Ryan C, Nolan B, Tosetto M, Geraghty R, Winter DC, O’Connell PR, Hyland JM, Doherty GA, Sheahan K, Ryan EJ, Fletcher JM. Enrichment of Inflammatory IL-17 and TNF-alpha Secreting CD4(+) T Cells within Colorectal Tumors despite the Presence of Elevated CD39(+) T Regulatory Cells and Increased Expression of the Immune Checkpoint Molecule, PD-1. Front Oncol. 2016;6:50. doi: 10.3389/fonc.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Huang F, Mei J, Wang X, Zhang Q, Wang H, Xi M, You Z. Posttranscriptional Control of PD-L1 Expression by 17beta-Estradiol via PI3K/Akt Signaling Pathway in ERalpha-Positive Cancer Cell Lines. Int J Gynecol Cancer. 2017;27:196–205. doi: 10.1097/IGC.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C, Zhang Q, Liu S, Lambrechts M, Qu Y, You Z. AZD5363 inhibits inflammatory synergy between interleukin-17 and insulin/insulin-like growth factor 1. Front Oncol. 2014;4:343. doi: 10.3389/fonc.2014.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muthumani K, Shedlock DJ, Choo DK, Fagone P, Kawalekar OU, Goodman J, Bian CB, Ramanathan AA, Atman P, Tebas P, Chattergoon MA, Choo AY, Weiner DB. HIV-mediated phosphatidylinositol 3-kinase/serine-threonine kinase activation in APCs leads to programmed death-1 ligand upregulation and suppression of HIV-specific CD8 T cells. Journal of immunology. 2011;187:2932–2943. doi: 10.4049/jimmunol.1100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Zhang J, Li J, Zhao T, Zou L, Tang Y, Zhang X, Wu Y. Triptolide inhibits B7-H1 expression on proinflammatory factor activated renal tubular epithelial cells by decreasing NF-kappaB transcription. Mol Immunol. 2006;43:1088–1098. doi: 10.1016/j.molimm.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 32.Gowrishankar K, Gunatilake D, Gallagher SJ, Tiffen J, Rizos H, Hersey P. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-kappaB. PLoS One. 2015;10:e0123410. doi: 10.1371/journal.pone.0123410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang G, Wen Q, Zhao Y, Gao Q, Bai Y. NF-kappaB plays a key role in inducing CD274 expression in human monocytes after lipopolysaccharide treatment. PLoS One. 2013;8:e61602. doi: 10.1371/journal.pone.0061602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong HY, Ma TT, Wu BT, Lin Y, Tu ZG. IL-12 regulates B7-H1 expression in ovarian cancer-associated macrophages by effects on NF-kappaB signalling. Asian Pac J Cancer Prev. 2014;15:5767–5772. doi: 10.7314/apjcp.2014.15.14.5767. [DOI] [PubMed] [Google Scholar]
- 35.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, Zhang Y, He X, Zhou T, Qin T, Huang Y, Yi X, Zhang L. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol. 2015;10:910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 37.Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 38.Song M, Chen D, Lu B, Wang C, Zhang J, Huang L, Wang X, Timmons CL, Hu J, Liu B. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PloS one. 2013;8:e65821. doi: 10.1371/journal.pone.0065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A, Curran M, Hwu P, Sharma P, Litton JK, Molldrem JJ, Alatrash G. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]