INTRODUCTION
A recent rise in the rates of new and recurrent Clostridium difficile infections (rCDI) has prompted interest in novel therapies including fecal microbiota transplantation (FMT). Recent studies attest to the safety and efficacy of FMT in treating rCDI with similar response rates according to routes of administration (i.e. colonoscopy, naso-gastric/duodenal tube, and capsules)1, 2. Patients with inflammatory bowel disease (IBD) are at particularly high risk of developing rCDI3, however little is known about the safety and efficacy of FMT in this population. Here, we present a single center experience on the use of FMT in IBD patients with rCDI.
METHODS
We prospectively studied IBD patients who underwent FMT for rCDI, defined by at least 2 CDI episodes at Massachusetts General Hospital. Information on donor selection, stool preparation and transplantation using colonoscopy, naso-gastric/duodenal tube, or frozen capsules has previously been reported2. Briefly, patients undergoing FMT received follow up phone calls at 1–3 days, 2 weeks, 8 weeks, and 6 months to collect information on potential side effects and recurrence of symptoms (i.e. abdominal pain, frequency/consistency of bowel movements). We excluded patients with less than 2 months of follow-up. Baseline and follow-up information on demographics, medications, disease-related surgery, and microbiology data were collected through medical record review. We evaluated rates of rCDI (at 2 months) and IBD treatment escalation defined by medication changes and surgery.
RESULTS
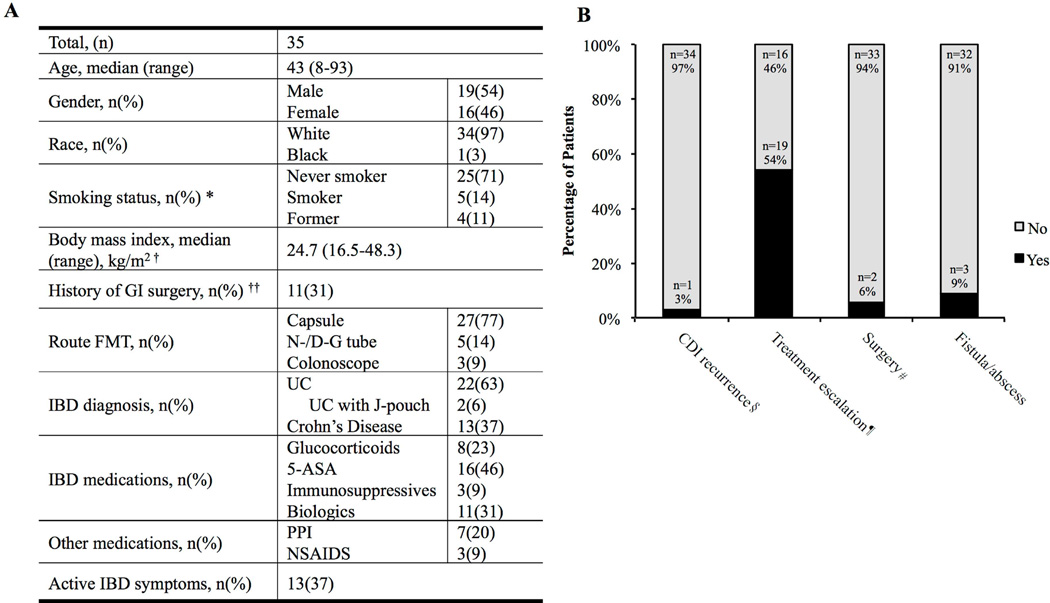
A total of 35 patients with median age of 43 years (range: 8–93 years) were included in this study, of which 22 had ulcerative colitis (UC) and 13 had Crohn’s disease (CD) (Figure 1). The most common route of FMT administration was oral via capsule (n= 27, 77%) with 5(14%) patients had previously undergone FMT. At the time of FMT, 28 (80%) patients were receiving medications for IBD including 8 patients (23%) on glucocorticoids (10–20 mg prednisone). FMT was well tolerated in all patients. Of 13 (37%) patients that underwent C. diff testing within 2 months of FMT, one tested positive. In regards to their IBD, 19 (54%) patients required treatment escalation: glucocorticoids (n = 5, 14%), anti-TNF therapy (n = 7, 20%), and vedolizumab therapy (n = 3, 9%). Two patients required surgery (i.e. diverting ileostomy, total proctocolectomy). Interestingly, two patients with no history of perianal disease were diagnosed with perianal abscess or fistula within 64 and 85 days of FMT; additionally, one patient with a history of perianal disease developed perianal abscess 47 days after FMT. Patients who had no symptoms of active IBD at the time of FMT (n = 22, 63%) in general did not require treatment escalation (64%).
Figure 1. Characteristics and outcomes of patients with IBD undergoing FMT for rCDI.
A) Baseline characteristics of patients with IBD that underwent FMT. *Smoking information was missing for 1 (3%) patient. † Children less than 12 years old (n=1, 3%) were not included; BMI was missing for 6 (17%) patients. †† Previous GI surgeries included colectomy with IPAA, colostomy, diverting colostomy, small bowel resection, fistula repair, cholecystectomy, and appendectomy. B) Outcomes of patients with IBD that underwent FMT for recurrent CDI. § CDI recurrence within 2 months of FMT treatment. ¶ Treatment escalation included new IBD medications or an increase in dosage. # Surgical interventions included a diverting ileostomy and total proctocolectomy.
DISCUSSION
In this brief report, we described a single center experience of FMT for rCDI in patients with established IBD. Although FMT appeared to be safe and effective for IBD patients with rCDI, more than half of patients in our study required IBD treatment escalation shortly after FMT. Restoration of intestinal microbiota is thought to be the primary mechanism by which FMT prevents future CDI. Although FMT is highly effective in preventing rCDI, recent data in IBD, where dysbiosis has been widely reported, have demonstrated significantly lower efficacy4, 5. Nevertheless, among IBD patients, FMT may be a promising therapeutic option by remedying dysbiosis partially induced by CDI. Despite this compelling rationale, we did not observe a consistent improvement in disease activity following FMT among IBD patients with rCDI. Our data is supported by a recently published multicenter study of nearly 65 patients6. Fischer and colleagues reported that among 54 IBD patients with available data after FMT, nearly 54% of patients had either no change in their symptoms or experienced worsening disease activity. Similarly, Khoruts and colleagues reported that over a quarter of IBD patients undergoing FMT through colonoscopy experienced a clinically significant flare7. Interestingly, similar to our observation that three patients developed rectal abscess/fistula, Moayeddi and colleagues reported 2 cases of rectal abscess in their randomized trials of FMT in treatment of ulcerative colitis4. Our finding that FMT is effective in treating rCDI among IBD patients is reassuring particularly since our study represents the largest experience with use of stool capsules. Nevertheless, the limited sample size, variations in donor/recipients gut microbiota, and presence of significant heterogeneity within IBD population limit the generalizability of our findings. In conclusion, we demonstrate that FMT is safe and effective in treating rCDI in IBD patients, however there were no significant improvements in disease-specific activity following FMT. Future studies focusing on the timing of FMT with respect to IBD disease activity may further optimize its potential benefit in this population.
Acknowledgments
Grant Support: Dr. Khalili is supported by a career development award from the American Gastroenterological Association (AGA) and by the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681).
Financial Disclosures: Dr. Khalili has received a consulting fee from Abbvie Inc. and Samsung Bioepis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None to declare
Ethical Approval: The study was approved by Partners Human Research Committee (Institutional Review Board).
Authors Contributions
SMC- acquisition of data; drafting of the manuscript
JS - acquisition of data; analysis; critical revision of the manuscript
JM- acquisition of data; analysis; critical revision of the manuscript
JLK- acquisition of data; analysis; critical revision of the manuscript
ELH- acquisition of data; analysis; critical revision of the manuscript
HK- Study concept and design; acquisition of data; drafting of the manuscript
References
- 1.Youngster I, Mahabamunuge J, Systrom HK, et al. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14:134. doi: 10.1186/s12916-016-0680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515–1522. doi: 10.1093/cid/ciu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen GC, Kaplan GG, Harris ML, et al. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443–1450. doi: 10.1111/j.1572-0241.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 4.Moayyedi P, Surette MG, Kim PT, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149:102–109. doi: 10.1053/j.gastro.2015.04.001. e6. [DOI] [PubMed] [Google Scholar]
- 5.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology. 2015;149:110–118. doi: 10.1053/j.gastro.2015.03.045. e4. [DOI] [PubMed] [Google Scholar]
- 6.Fischer M, Kao D, Kelly C, et al. Fecal Microbiota Transplantation is Safe and Efficacious for Recurrent or Refractory Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:2402–2409. doi: 10.1097/MIB.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 7.Khoruts A, Rank KM, Newman KM, et al. Inflammatory Bowel Disease Affects the Outcome of Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection. Clin Gastroenterol Hepatol. 2016;14:1433–1438. doi: 10.1016/j.cgh.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]