Abstract
Snake venoms are known to have different venom compositions and toxicity, but differences can also be found within populations of the same species contributing to the complexity of treatment of envenomated victims. One of the first well-documented intraspecies venom variations comes from the Mohave rattlesnake (Crotalus scutulatus scutulatus). Initially, three types of venoms were described; type A venom is the most toxic as a result of ~45% Mojave toxin in the venom composition, type B lacks the Mojave toxin but contains over 50% of snake venom metalloproteases (SVMPs). Also, type A + B venom contains a combination of Mojave toxin and SVMP. The use of an anti-disintegrin antibody in a simple Enzyme-Linked Immunosorbent Assay (ELISA) can be used to identify the difference between the venoms of the type A, B, and A+B Mohave rattlesnakes. This study implements the use of an anti-recombinant disintegrin polyclonal antibody (ARDPA) for the detection of disintegrins and ADAMs (a disintegrin and metalloproteases) in individual crude snake venoms of Mohave rattlesnakes (Crotalus scutulatus scutulatus) of varying geographical locations. After correlation with Western blots, coagulation activity and LD50 data, it was determined that the antibody allows for a quick and cost-efficient identification of venom types.
Keywords: Crotalus scutulatus, Venom, Disintegrin, Antibodies, ELISA
1. Introduction
Interspecific and intraspecific venom differences within and among populations of a single species are generally present in snakes around the world (Aguilar et al., 2007; Girón et al., 2008; Alape-Girón et al., 2008; Gibbs and Mackessy, 2009; Massey et al., 2012). These venom differences may be a result of many factors such as geographic location (Barrio and Brazil, 1951; Glenn and Straight, 1978; Irwin et al., 1970; Schenberg, 1959), time of year (Gubensek et al., 1974), gender (Marsh and Latston, 1974), age (Fiero et al., 1972; Jimenez-Porras, 1964), and conceivably diet (Pifano and Rodriguez-Acosta, 1996; Daltry et al., 1996). Venom disparity may have serious implications for envenomed humans since antivenoms may have difficulty neutralizing the heterogeneous toxins described in the different venoms.
Geographical differences in the venom of the Mohave rattlesnakes have been found to associate with increased health severity outcomes (Massey et al., 2012). Type A Mohave venom contains Mojave toxin, a pre-synaptically active neurotoxin, displaying oral and facial paresthesias 5 min after a bite, then after 30 min, fasciculation and general weakness (arms, hands and legs) and dysphasia (Clark et al., 1997; Hardy, 1983) occur. This type of venom presents poor or non-proteolytic or hemorrhagic activities (Glenn et al., 1983), but serious rhabdomyolysis with myoglobinuric renal failure has been described (Jansen et al., 1992). Type B venom does not contain Mojave toxin, but does have important proteolytic and hemorrhagic activities (Glenn et al., 1983) as a result of SVMPs (Massey et al., 2012). Patients bitten by this type of snakes present severe local pain in the bitten site (Rhoten and Gennaro, 1968), migrating edema through the affected extremities and to the thorax or abdomen, ecchymosis, blistering, necrosis (Hardy, 1983) and local adenomegaly (Rhoten and Gennaro, 1968; Clark et al., 1997). There are many implications that contribute to toxin composition in venoms (Calvete, 2011) and understanding its importance in antivenom preparation is essential for effective treatments of snakebite (Williams et al., 2011).
In the current work, anti-recombinant disintegrin polyclonal antibodies (ARDPAs) were produced against r-mojastin 1, a recombinant disintegrin cloned from the venom gland of a type B Mohave rattlesnake. These ARDPAs were able to recognize disintegrins and ADAMs (a disintegrin and metalloproteases) in venoms of 12 individual Mohave rattlesnake (Crotalus scutulatus scutulatus) venoms ranging from California to Texas using both Western blots and ELISAs. Metalloproteases are the most copious toxins found in venoms of the Viperidae family. These snake venom metalloproteases (SVMPs) are the main components responsible for the hemorrhage and the interference of the hemostatic system. The SVMPs are classified into P-I, P-II, and P-III classes. P-Is are the simplest classes containing only a metalloprotease domain; P-IIs have a metalloprotease domain followed by a disintegrin domain (ADAMs); and P-IIIs contain a metalloprotease, disintegrin-like and cysteine-rich domains. The P-IIIs are separated into subclasses P-IIIa to P-IIId centered on their distinct post-translational modification (Takeda et al., 2012). The disintegrins are produced as the C-terminal domain of the P-II class of metalloproteases and are released into the venom as an effect of proteolytic processing. The disintegrin name is used to describe a function of this domain, which is its association with integrins found on cell surfaces. Integrins are transmembrane receptors responsible for cell-cell and cell-extracellular matrix interactions. There are ~24 types of integrins, and different types can be found on a single cell surface. The integrin αIIbβ3 is a common target for snake venom disintegrins preventing the binding of fibrinogen or fibronectin thus inhibiting platelet aggregation, which contributes to the typical hemorrhagic effect related to snakebites (Fox and Bjarnason, 1995).
The correlation between toxicity and the presence of Mojave toxin, SVMPs and myotoxin-A was very well described by Massey et al. (2012). In that study, three venom phenotypes were identified by LC-MS and LD50s. The most common, types A, B, and A + B, as described by Glenn and Straight (1989) followed the simple tendency in which a low LD50 signifies type A, a high LD50 is type B, and an intermediate LD50 is type A + B. The new phenotypes involved the presence of myotoxin-A. The myotoxin-A has a tendency to decrease the LD50s of both type B and A + B venoms; however, type A containing myotoxin-A did not seem to have a decrease in venom potency.
In today's research endeavors involving snake comparative genomics and evolution of protein structure and function (Brust et al., 2013), there is an increased demand of individual snake venom and tissue. Phenotypically, one cannot determine a type A venom snake from a type B without doing mass spectrometry or LD50s on their venoms, which requires either expensive equipment or the use of live animals, respectively. The ARDPAs will allow to differentiate Mohave rattlesnake venoms as well as other venoms classified as types A and B (e.g. Southern Pacific rattlesnakes, Tiger rattlesnakes) by using a simple ELISA; thus, saving time, money, and animals. In addition to identifying these venoms by ELISA, disintegrin quantitation in other species of venoms or venom fractions also can be determined by Western blots allowing rapid identification and purification (Borja et al., 2016) of these therapeutically important molecules.
2. Materials and methods
2.1. Collection of crude venoms
Snakes were housed at the National Natural Toxins Research Center at Texas A&M University-Kingsville in compliance with IACUC (Approval # 2015-12-09-A3). Venom was extracted by allowing the snakes to bite into a para-film stretched over a disposable plastic cup. The venom sample was centrifuged (500g for 10 min), and filtered through 0.45 µm filter. The venoms were centrifuged for 5 min at 23 °C at 12,800g to remove cellular debris. The venom supernatant was then transferred to vials with the proper labels and stored individually at −90 °C until lyophilized.
2.2. Purification of r-mojastin 1 and production of the anti-r-mojastin 1 antibody
r-Mojastin 1 was purified according to the methods of Sánchez et al. (2010). Briefly, bacterial cells (BL21) expressing r-mojastin-1 were centrifuged at 3800g for 15 min (4 °C) and resuspended with 80 mL of ice cold 1× PBS buffer pH 7.4. Cell distribution was done with a Branson Sonifier 450. The cell debris was removed by centrifugation at 1200g for 10 min at 4 °C. The crude lysate was incubated with 2 mL of 50% slurry glutathione Sepharose 4B (GS4B) for 30 min at room temperature using gentle agitation. r-Mojastin 1 proteins were cleaved and eluted from glutathione S-transferase (GST) bound to GS4B by thrombin cleavage. Thrombin was removed from r-mojastin 1 using a 1 mL HiTrap™ Benzamidine Sepharose 4 Fast Flow column. Four New Zealand rabbits housed at the National Natural Toxins Research Center (NNTRC) were immunized with r mojastin-1. A total of 7 immunizations consisting of three doses of the antigen in different skin sites at concentrations of 67 µg/100 µL (0.67 mg/mL) were administered subcutaneously over a period of seven months. The primary immunization consisted of the GST fusion protein with complete Freund's adjuvant. Remaining booster injections were administered with incomplete Freund's adjuvant. The final two immunizations were done without the GST tag.
The antibody level in sera from the immunized rabbits was evaluated every first week after immunizations, with an indirect Enzyme-Linked Immunosorbent Assay. Briefly, 96-well plates (Corning) were coated with the antigens r-mojastin 1-GST, r-mojastin 1, native mojastin, and GST (0.5 µg/well) in phosphate buffer saline, pH 7.4 (PBS). The plate was incubated overnight at 4 °C. The plates were washed three times with PBS and blocked for 1 h with 5% non-fat powdered milk in PBS at 37 °C. Individual rabbit serum samples were diluted 1:1000 with PBS and applied for 1 h at 37 °C. The plate was washed three times with PBS and a goat anti-rabbit antibody conjugated with alkaline phosphatase (SIGMA), diluted to 1:40,000 with PBS, was added for 1 h at 37 °C. The plates were washed with 0.05% Tween 20 in PBS and alkaline phosphatase yellow (pNPP) liquid (SIGMA) was added as a substrate and incubated for 30 min at 37 °C. The optical density was read at 405 nm using a microplate reader (Beckman, USA). All experiments were performed in triplicates.
2.3. Venoms
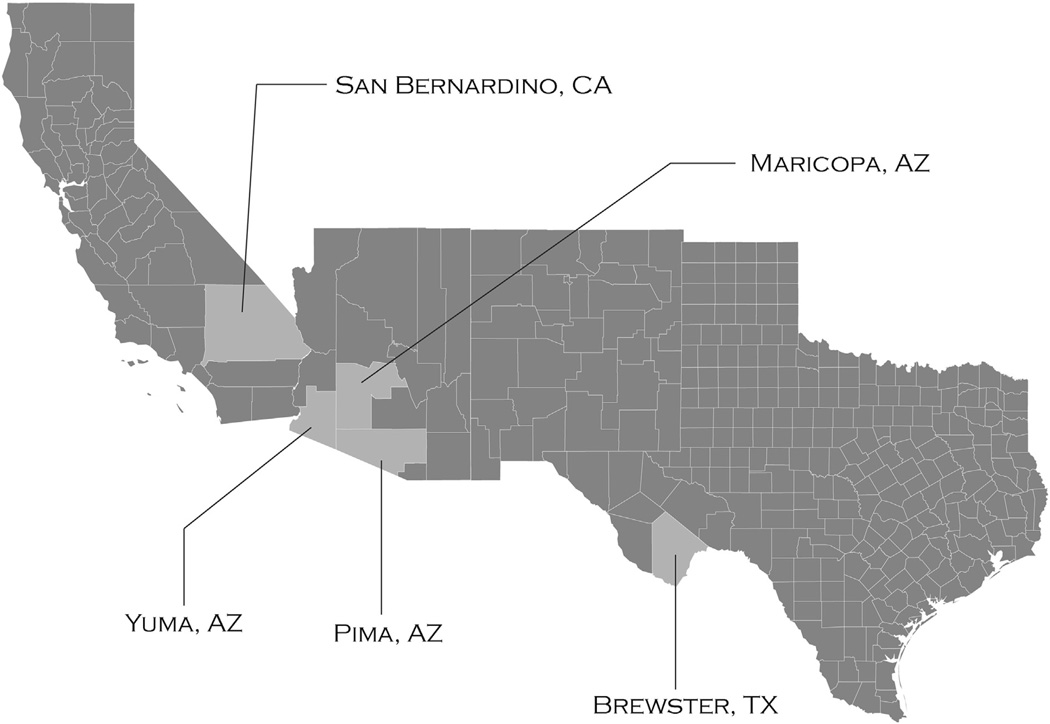
Twelve individual venoms from the species Crotalus scutulatus scutulatus from Texas, Arizona, and California (Fig. 1)were used to evaluate the validity of the anti-disintegrin polyclonal antibody. The venoms of this species vary quite significantly in that they are classified as type A (neurotoxic; ~46% Mojave toxin, <0.1% SVMP, 0% Myotoxin (Massey et al., 2012)), type B (hemorrhagic; ~56% SVMP, 0% Mojave toxin, and 0% Myotoxin) and type A + B (~13–53% SVMP, ~27–7% Mojave toxin, and 0–22% Myotoxin). Venoms 103 (type B), 109 (type A + B) and 307 (type A) have been previously characterized by mass spectrometry and LD50 (Massey et al., 2012) and were used as controls.
Fig. 1.
Geographical distribution by county and state of collected Crotalus scutulatus scutulatus.
2.4. Protein concentration
Protein concentration of venoms was spectrophotometrically assessed by assuming that 1 unit of absorbance/cm of path length at 280 nm corresponds to 1 mg protein/mL (Stoscheck, 1990).
2.5. Western blot and N-terminal sequencing
A total of 25 µg of crude venom of each individual was separated on a non-reduced 4–20% Tricine Gel for 90 min at a voltage of 125 using an XCell SureLock® Mini-Cell system(Invitrogen). Current was moderated using a Bio-Rad PowerPack power supply. Protein was transferred onto a 0.2 µm nitrocellulose membrane (Millipore) using a Trans Blot SD system (Bio-Rad) at 100 mA for 1 h and allowed to set overnight. Duplicate gels under the same conditions were stained with Simply Blue (Invitrogen) for 1 h. The membrane was then blocked with 5% BSA in TBST for 1 h, washed with PBS and incubated with the anti-r-mojastin 1 antibody (1:1000 dilution) overnight at room temperature. The membrane was washed three times with TBS and incubated with a biotinylated goat anti-rabbit antibody (1:30,000 dilution) for 1 h at room temperature. The membrane was washed three times with 0.05% Tween 20 in PBS and incubated with ExtrAvidin-peroxidase (SIGMA, USA) diluted to 1:2000 in PBS for 1 h at room temperature. After washing off the unbound avidin with TBST, the antigen-bound antibody was visualized with SigmaFAST™ 3,3′-diaminobenzidine tablets. Bands of interest were transferred to nitrocellulose using the same conditions, as previously described, excised, and sent for N-terminal sequencing at the Iowa State Sequencing Facility.
2.6. LD50 of crude venoms
The LD50s of Mohave rattlesnake crude venoms were determined using the Spearman-Karber (1964) method. Venoms were reconstituted in 0.85% saline, and a series of 1/2 serial dilutions were made using the highest test dosage with additional four concentrations. The temperature of all solutions was maintained at 0 °C and pre-warmed to room temperature prior to injection. A total of 0.2 mL of venom was injected into the tail vein of 18–20 g, BALB/c mice. The injections were administered using a 1-mL syringe fitted with a 30-gauge, 0.5-in needle. As a control 0.85% saline was used. Five groups consisting of 8 mice/group/venom were monitored for 48 h.
2.7. Coagulation activity
Activated Clot Time (ACT), Clot Rate (CR) and Platelet Function (PF) of whole human blood were measured according to Sánchez et al. (2010). The Sonoclot® signatures display the measurement of the blood's ACT in seconds, the CR in clot signals/min and PF as a function of clot retraction. The ACT is the time in which fibrin formation begins, and the reference values for healthy individuals range from 100 to 240 s; the CR is the kinetic measurement of fibrin formation and clot development, and this ranges from 10 to 35 for healthy individuals; and the PF is obtained from the timing and quality of the clot retraction. The PF values range from 0 to 5, where 0 represents no clot retraction. A PF above 1 signifies normal clot retraction and fluctuates from individual to individual. It is relevant to note that these reference values for a healthy population may differ from the values of a specific healthy patient. The intended use of the Sonoclot® Analyzer System with the gbACT + Kit is for general purpose global hemostasis monitoring in human patients. However, this system has proven useful in identifying crude venoms as well as purified venom toxins that act as hypo- or hypercoagulants on human blood. It is useful in identifying molecules that affect platelets as well as other blood factors involved in the hemostasis process. It can be used with whole blood or plasma, which can be either normal or void of specific blood factors. In our study, we were comparing the activities of individual venoms of the same species on the overall hemostasis profile. A total of 10 µL (1 mg/mL) of crude venom was incubated with 330 µL of citrated human whole blood using glass bead activated cuvettes (gbACT + Kit) on a Sonoclot® Analyzer System. Citrated human blood was pre-incubated to 37 °C and maintained prior to use. The blood was re-calcified using 10 µL of 0.3 M CaCl2 at the start of every analysis. Phosphate buffer saline was used as a normal control. Data was obtained and stored by the Signature Viewer v.4.0 software.
2.8. Detection of type A, B, and A + B venoms by ELISA
The detection and level of disintegrins and ADAMs in the snake venoms were evaluated with an indirect ELISA. Briefly, 96-well plates (Corning) were coated with 100 µL of snake venoms (0.1 mg/well) diluted in PBS, pH 7.4. The plate was incubated overnight at 4 °C. The plate was washed three times with PBS and blocked for 30 min with 1% bovine serum albumin in PBS at 37 °C. Anti r-mojastin 1 polyclonal antibody at 1:1000 was added to individual wells and incubated for 30 min at 37 °C. The plate was washed three times with PBS and a goat anti-rabbit antibody conjugated with alkaline phosphatase (SIGMA), diluted to 1:20,000 with PBS, was added for 30 min at 37 °C. The plates were washed with 0.05% Tween 20 in PBS and alkaline phosphatase yellow(pNPP) liquid (SIGMA)was added as a substrate and incubated for 30 min at 37 °C. The optical density was read at 405 nm using a microplate reader (Beckman, USA). All experiments were performed in triplicates.
2.9. Statistical analyses
The results were expressed as the mean ± standard deviation (SD). Their significance was analyzed by Student's t-test using Microsoft® Excel® for Mac 2011, Version 14.6.8. The level of significance was at P < 0.05.
2.10. Ethical procedures
All animal handling procedures were approved by the Texas A&M University-Kingsville Institute of Animal Care and Use Committee (IACUC approval #s 2009-11-19A-01 and 2015-12-09-A3).
3. Results
3.1. Venom lethality
The lethality of twelve Mohave rattlesnake venoms was determined through intravenous injections (Table 1). The LD50 values ranged from 0.47 mg/kg (993) to 4.7 mg/kg (983). Of the venoms tested, seven (990, 993, 996, 997, 1043, 1049, 252) were classified as belonging to type A, having low LD50s (<1.0 mg/kg). One (988) was identified as type A + B (LD50 1.54 mg/kg). Only one of the venoms (983) was identified as type B, having an LD50 of 4.7 mg/kg. These values were comparable to the LD50s of the control venoms 307-type A, 103-type B and 109-type A + B (Massey et al., 2012), the lethality values of which are 0.5, 4.0, and 1.4 mg/kg, respectively.
Table 1.
Coagulation and lethality characterization of crude C. scutulatus scutulatus venoms.
| Venom ID | Location County, state |
LD50 (mg/kg) |
ELISA (405 nm) |
ACTb |
P-value ACT |
CRc |
P-value CR |
Platelet Function |
P-value PF |
|---|---|---|---|---|---|---|---|---|---|
| Control | 3.63 ± 0.48 | 1 | 18.34 ± 4.36 | 1 | 3.96 ± 0.31 | 1 | |||
| 990 | San Bernardino, CA | 0.58 | 0.43 ± 0.07 | 7.47 ± 0.11 | 3.85E–05 | 4.14 ± 0.24 | 3.38E–03 | 2.17 ± 0.19 | 2.89E–04 |
| 993 | San Bernardino, CA | 0.47 | 0.40 ± 0.04 | 5.48 ± 0.66 | 4.09E–03 | 5.94 ± 0.34 | 6.17E–03 | 2.70 ± 0.85 | 1.36E–02 |
| 996 | Maricopa, AZ | 1.10 | 0.50 ± 0.05 | 9.04 ± 0.77 | 8.71E–07 | 4.31 ± 0.32 | 3.36E–04 | 2.03 ± 0.12 | 1.94E–05 |
| 997 | San Bernardino, CA | 0.71 | 0.45 ± 0.07 | 5.31 ± 0.53 | 4.56E–03 | 6.55 ± 0.15 | 7.62E–03 | 2.04 ± 0.19 | 1.96E–04 |
| 983 | Pima, AZ | 4.70 | 1.40 ± 0.22 | 7.63 ± 0.44 | 5.29E–05 | 3.87 ± 0.14 | 3.09E–03 | 0.49 ± 0.02 | 5.38E–06 |
| 988 | Pima, AZ | 1.54 | 0.70 ± 0.05 | 10.49 ± 1.29 | 9.11E–06 | 3.63 ± 0.57 | 6.89E–03 | 1.60 ± 0.36 | 1.78E–05 |
| 1043 | Yuma, AZ | 0.57 | 0.45 ± 0.02 | 7.52 ± 0.75 | 1.45E–04 | 3.30 ± 0.46 | 2.61E–03 | 1.90 ± 0.37 | 2.22E–04 |
| 1049 | Brewster, TX | 0.62 | 0.44 ± 0.05 | 1.09 ± 0.14 | 8.95E–04 | 5.25 ± 0.00 | 4.89E–03 | 3.31 ± 0.27 | 1.20E–05 |
| 252 | Maricopa, AZ | 0.68 | 0.42 ± 0.07 | 4.25 ± 1.13 | 2.08E–01 | 9.07 ± 1.26 | 2.08E–02 | 2.33 ± 0.71 | 2.59E–03 |
| 307a | Cochise, AZ | 0.54 | 0.46 ± 0.09 | 7.06 ± 0.37 | 8.97E–05 | 4.69 ± 1.79 | 4.15E–03 | 0.97 ± 0.05 | 1.30E–05 |
| 103a | Pima, AZ | 4.00 | 1.70 ± 0.12 | 9.48 ± 1.58 | 8.33E–05 | 1.17 ± 0.37 | 1.38E–03 | 0.10 ± 0.00 | 2.89E–06 |
| 109a | Pima, AZ | 1.40 | 1.21 ± 0.11 | 7.59 ± 1.15 | 2.36E–04 | 5.22 ± 0.54 | 4.83E–03 | 1.29 ± 0.12 | 2.68E–05 |
The levels of significance were compared to the control values, which were analyzed by Student's t-test.
The levels of significance were at P < 0.05.
LD50s were obtained from Massey et al. (2012).
Activated Clot Time was measured in minutes.
Clot Rate was measured in clot signals/min.
3.2. Coagulation activity
Significant variations in coagulation activity (ACT and CR) were observed among venoms as indicated by prolonged ACT values and decreased values of CR (Table 1). The majority of the venoms tested had an anticoagulant effect on whole human blood. Activated Clot Time (ACT) was delayed by an average of approximately 3 min. Of the venoms tested, four had no statistically significant effect on coagulation. Only venom 1049 exhibited pro-coagulant activity (ACT = 1.09 min). Additionally, venoms 983 and 103 inhibited PF with values of 0.49±0.02 and 0.1±0.00, respectively. There was no apparent correlation between measured LD50 values and collected ACT and CR data, but there appears to be a correlation between the LD50, ELISA, and PF values. Type B venoms tend to show high LD50s and ELISA values and low (<0.5) PF values, whereas type A venoms had low LD50s and ELISA values and high (>1.0) PF values.
3.3. SDS-PAGE, Western blot, and N-terminal sequencing
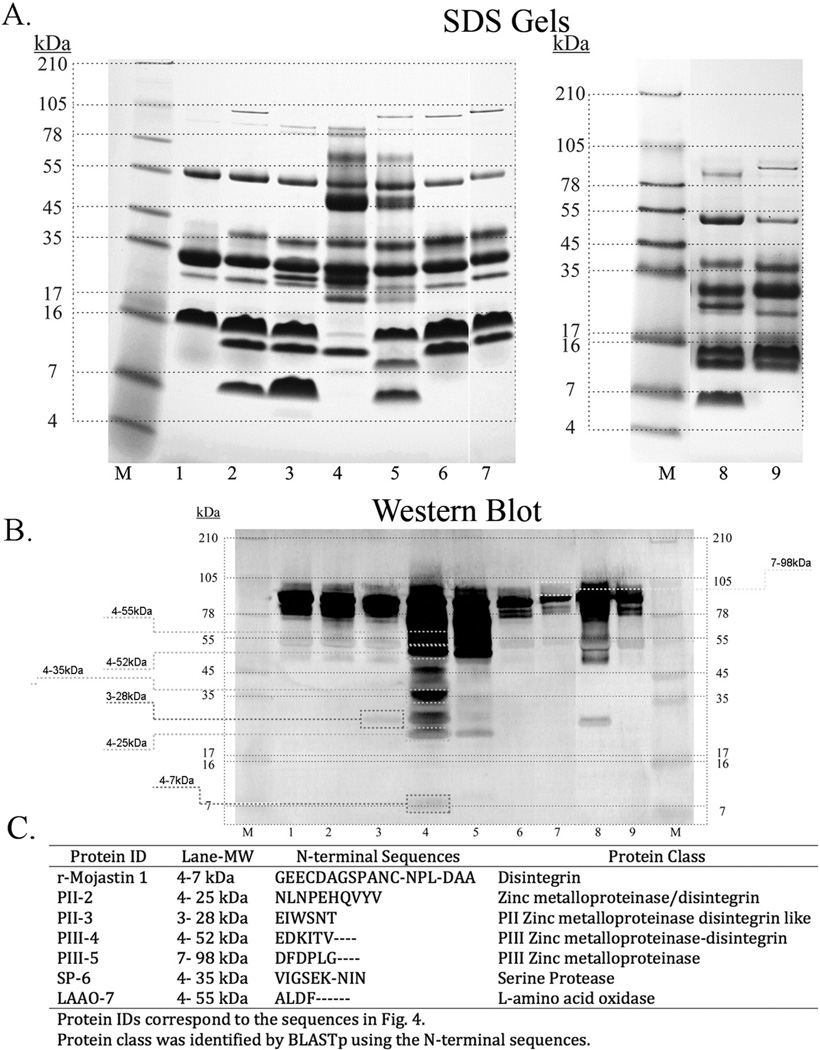
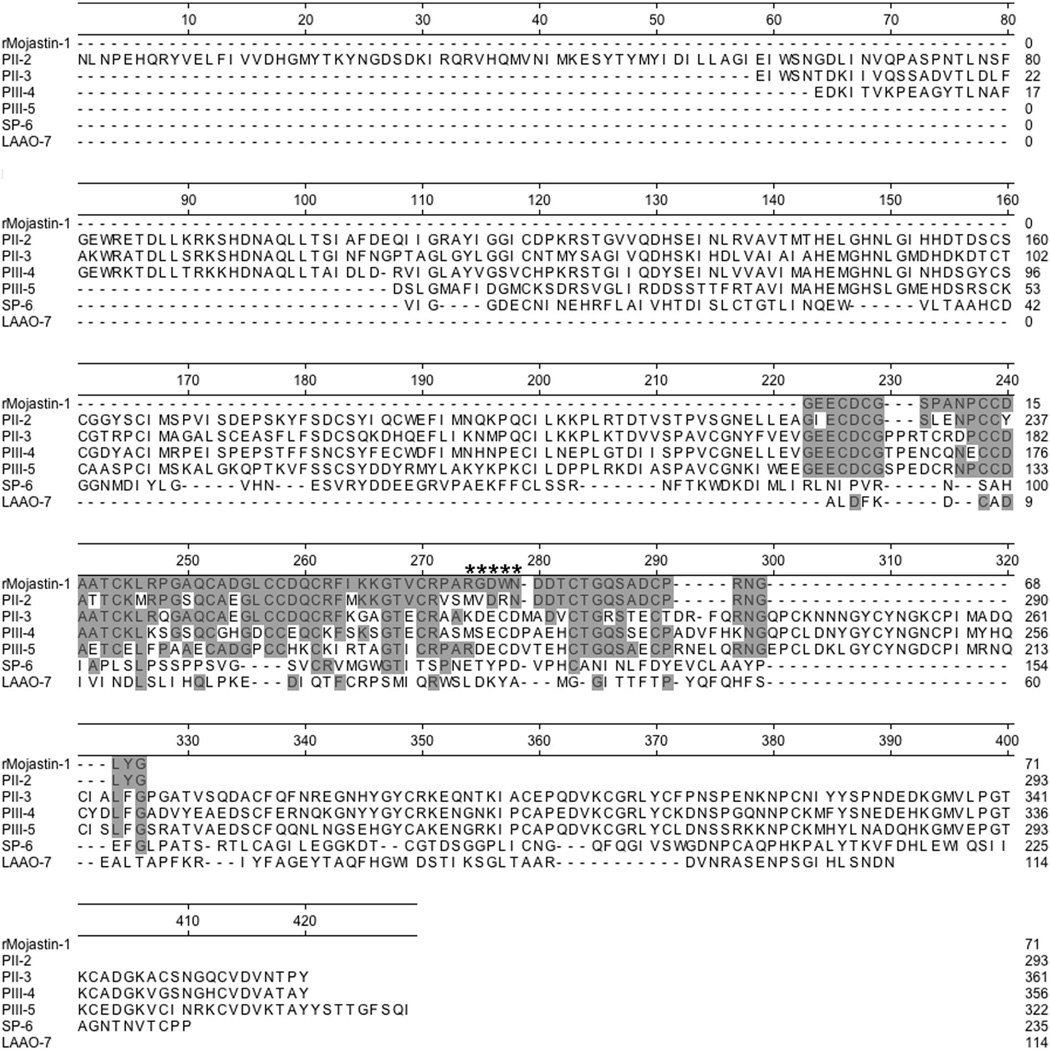
The strong binding of ARDPA correlated with measured LD50s. Venoms having a high LD50 reacted strongly with the antibody over a wide range of molecular weights (Fig. 2A and B). The strongest binding activity was observed within venoms 983 and 103, which had the lowest lethality and suppressed PF (Table 1; Figs. 2B and 3). Reacting bands were distributed over a molecular weight range of approximately 8 kDa to 150 kDa. Binding activity was most intense between the ranges of 35 kDa to 98 kDa. Similar binding activity was observed to varying degrees among all the venoms tested. N-terminal sequencing data was obtained for several representative bands of varying molecular weights. Sequencing data revealed a strong binding correlation of the antibody to several classes of metalloproteases with disintegrin containing domains. According to alignment data, antibody recognition was centered on variations of the PIII-SVMP RGD ancestral sequence, RDECD (approximately 50 amino acids upstream, and 30 amino acids downstream) (Fig. 4). Autolysis resulted in a series of truncated sequences that were observable as an almost seamless continuum of bands occurring within the range where the binding activity was most intense (Figs. 2 and 3).
Fig. 2.
SDS PAGE and Western blots of Mohave rattlesnake venoms. A.) SDS electrophoresis of Mohave rattlesnake venoms. A total of 25 µg of venom protein was run on a Novex® 10–20% Tricine gel using an XCell SureLock® Mini-Cell for 90 min at 125 V. Lane 1: 1049, lane 2: 1043, lane 3: 252, lane 4: 983, lane 5: 988, lane 6: 990, lane 7: 993, lane 8: 996, lane 9: 997. B.) Western blot of Mohave venoms reacting with the ARDPA. Two identical gels were run, and one was used to transfer proteins onto a nitrocellulose membrane using BioRad Trans-Blot SD Semi-Dry Transfer Cell at 100 V for 1 h and then left overnight. Lane 1: 1049, lane 2: 1043, lane 3: 252, lane 4: 983, lane 5: 988, lane 6: 990, lane 7: 993, lane 8: 996, lane 9: 997. C.) N-terminal sequences of selected protein bands recognized by the ARDPA. Identical gels were obtained, and one was utilized for Western blot while the other one was used for a protein transfer blot. The transfer protein bands, which corresponded to those protein bands recognized by the ARDPA, were excised from the blot and sent for N-terminal sequencing. The bands used are represented by the dashed boxes on the Western blot. Protein classification was determined by the NIH Basic Logical Alignment Search Tool for proteins (BLASTp; https://blast.ncbi.nlm.nih.gov).
Fig. 4.
Amino acid sequence comparison of representative venom peptides recognized by the antibodies (ARDPA) with r-mojastin 1 sequence. The sequences represent the N-terminal sequences obtained by Western blot (Fig. 2). Alignment was constructed by Lasergene 12.0 using a Clustal W method. The amino acids denoted by the ***** represent the ancestral subgroup of PIII-SVMPs in which the disintegrin binding sites “RGD” originate.
Fig. 3.
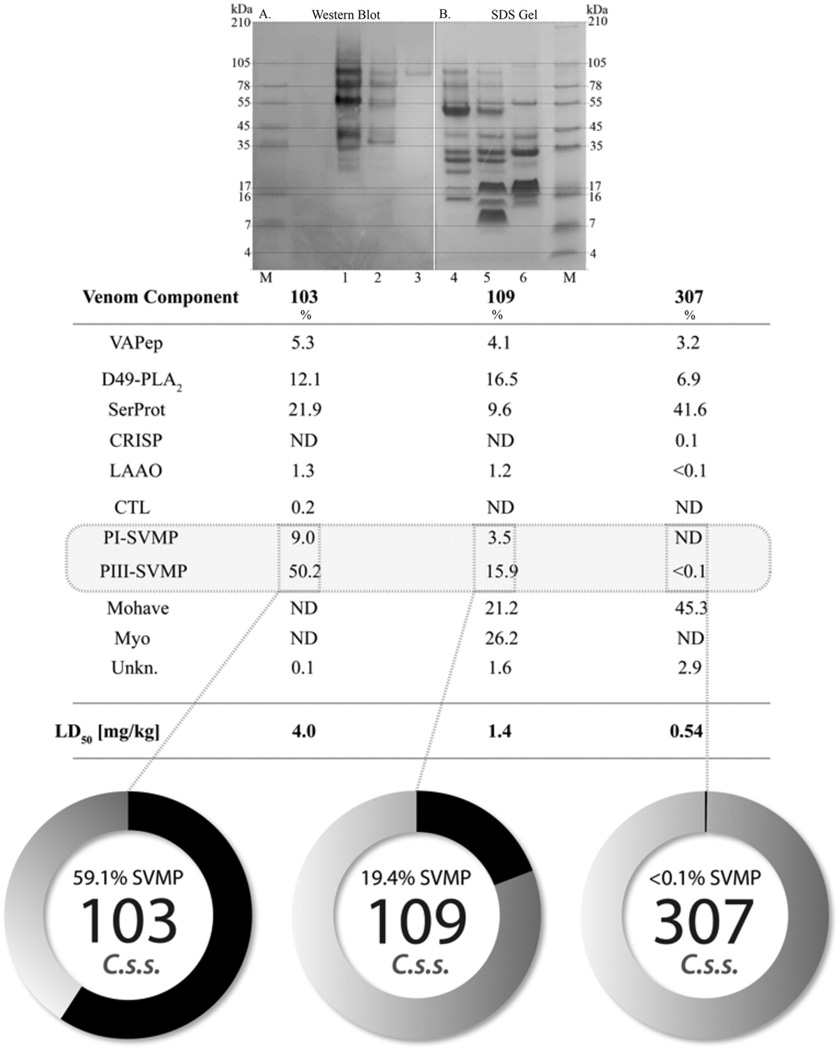
SDS PAGE and Western blot of control Mohave venoms (103-Type B, 109-Type AB, & 307-Type A) using r mojastin-1 antibody. A) A total of 25 µg of each venom was run on a Novex® 10–20% Tricine gel using an XCell SureLock® Mini-Cell for 90 min at 125 V. Lane 1: 103, lane 2: 109, and lane 3: 307. B) Western blot using the ARDPA. Lane 4: 307, lane 5: 109, and lane 6: 103. Data in table represents the relative abundances in % of the different protein families in the venoms as determined by Massey et al. (2012). The black areas on the pie charts represent the percentage of SVMPs present in those venoms.
Furthermore, antibody reactivity was established using venoms for which proteomics data was reported (Fig. 3). Antibody binding and binding density increased with recorded venom lethality. The antibody recognized bands over a wide range of molecular weights in the type B and type A + B venoms, 103 and 109, respectively. Reacting bands were visualized with an approximate weight distribution from 24 kDa to 150 kDa. Binding effectiveness and intensity correlated with the reported metalloprotease content within each of the control venoms. Control venom 307, which had the lowest LD50 and metalloprotease content, did not react significantly with the antibody. Only a single band of approximately 98 kDa was observed.
3.4. ELISA reactivities of anti-recombinant disintegrin polyclonal antibodies (ARDPAs) with various venoms
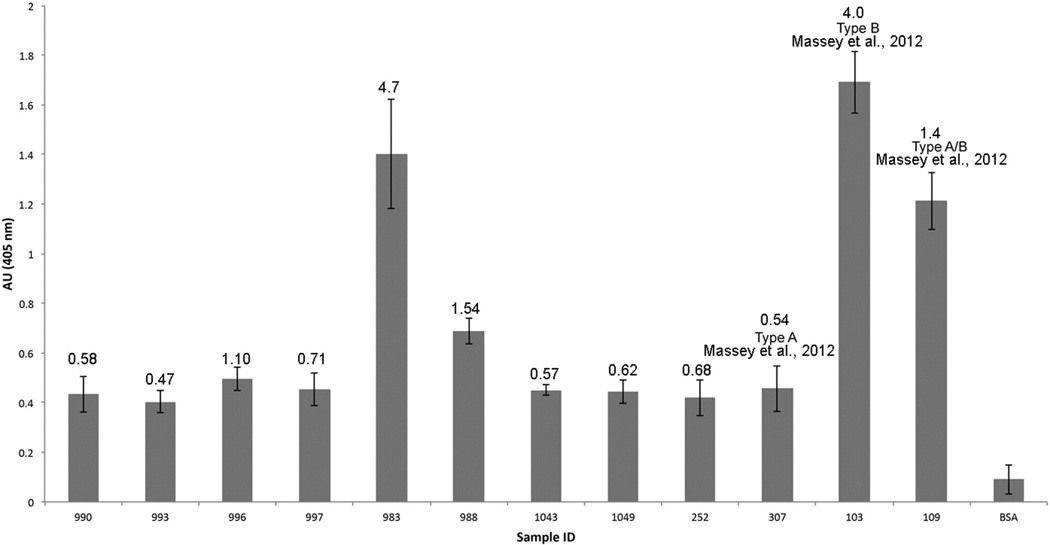
Indirect ELISA showed that ARDPA reacted strongly with type B, 983 and 103, (1.4 and 1.7 A405 nm) venoms and partially with type A+B, 988 and 109 (1.54 and 1.21 A405 nm) venoms. Hence, reactivity of the ARDPA with various venoms on indirect ELISA was widespread: most reactive with type B venoms, moderate with type A + B venoms, and least with type A (990, 993, 996, 997, 1043, 1049, 252) (from 0.40 to 0.50 A405 nm) venoms (Fig. 5).
Fig. 5.
Determination of Mohave type A, B and A + B venoms using a disintegrin antibody in an enzyme linked immunosorbent assay. The higher the absorbancies, the stronger the reactivity of ARDPA with the venoms. The numbers above the bars represent the LD50 (mg/kg) for each venom. In general, the potencies of the venoms are inversely proportional to absorbancies. The most potent venom is 993 with an LD50 of 0.47 mg/kg, and the least potent venom is 983 with an LD50 4.7 mg/kg.
4. Discussion
Intrapopulational venom variation within the Mohave rattlesnake venom was previously established using a combination of lethality testing and hemostatic assays (Glenn and Straight, 1978; Glenn et al., 1983). By correlating LD50 values with hemorrhagic activity, it was possible to designate venoms as venom type A (neurotoxic) or B (hemotoxic). The LD50 remains as one of the primary determinations of venom toxicity (Sánchez et al., 2003, 2008, 2011). This is especially true for venoms in which significant variability influenced by the geographical distribution of populations exists. However, the use of LD50 requires a significant amount of laboratory animals, and may be impractical when screening large numbers of venoms. The use of polyclonal antibodies against a recombinant disintegrins (r-mojastin 1) produced from the venom gland of a Mohave rattlesnake is evaluated in this study for its effectiveness in distinguishing between Mohave venoms, type A, B, and A + B, both qualitatively and quantitatively.
The cloned recombinant disintegrin designated as r-mojastin 1 is a single-chain disintegrin, which codes for 71 amino acids, including 12 cysteines, and an RGD binding motif (Sánchez et al., 2010). This platelet aggregation inhibitor, which binds with high affinity to the αIIbβ3 integrin, has potential use as the basis for tracing the presence of the native disintegrin in Mohave rattlesnake venoms as well as determining venom types, using Western blot and/or ELISA assays.
In evolving a system for typing C. s. scutulatus venoms via ARDPA recognition, 12 venoms of Mojave rattlesnake of varying geographic distributions were selected (Fig. 1). Venoms were tested for their lethality and coagulation activity. Venom lethality varied significantly depending on geographical distribution, from 0.47 mg/kg to 4.70 mg/kg (Table 1). This observation has been reported extensively in the literature (Glenn and Straight, 1978; Glenn et al., 1983; Wilkinson et al., 1991; Sánchez et al., 2006; Massey et al., 2012). There appears to be a strong correlation between ARDPA binding and LD50 data. The ARDPA bound consistently and with greater intensity to venoms having higher LD50s (Figs. 2, 3 and 5). In order to establish a system for designating ARDPA reactivity, three venoms with known lethality and proteomics data were used as controls (Massey et al., 2012). The ARDPA binding and binding density increased with reported values of lower venom lethality (those having higher LD50). Furthermore, ARDPA binding also increased with the measured amount of metalloproteases within each of the venoms (Fig. 3). The amount of metalloproteases in the venoms affects Platelet Function by decreasing the PF values which is due to disintegrins and disintegrin-like proteins associated with P-II to P-III SVMPs (Takeda et al., 2012). These disintegrins bind to the αIIbβ3 integrins on platelet receptors inhibiting normal platelet aggregation, and thus, decrease PF. The PF values are indirectly proportional to the LD50 values (Table 1); the lower the PF values, the less potent the venom in the lethality assay. These observations indicate that even though metalloproteases are responsible for a plethora of activities (Markland and Swenson, 2013) affecting hemostasis and extracellular matrices, they are not the main components contributing to death. N-terminal sequencing data confirmed ARDPA recognition of several P-II and P-III metalloproteases (Fig. 2). Binding intensity and density were greatest within the molecular weight range corresponding to these molecules, which often result in a continuous “streaking” of several bands when analyzing venoms with high metalloprotease content. This “streaking” is likely due to the presence of various truncated metalloproteases, resulting from autolysis processes, from which venom variability and complexity ultimately arise (Moura-da-Silva et al., 2003; Peichoto et al., 2010). Alignment data indicated that the most probable antigen recognition sites were the amino acids forming the disintegrin domain flanking the integrin-binding motif (Fig. 4). The RGD tripeptide sequence that is common to many disintegrins, including mojastin-1, originated from the ancestral subgroup of PIII-SVMPs containing the RDECD sequence (Calvete, 2005, 2013). Although the RDECD sequence may not be recognized in its entirety, aside from those amino acids matching the RGD sequence, the ability for the ARDPA to recognize the surrounding amino acids composing the disintegrin domain is highly likely, considering the significant structural homology between various disintegrins and PII- and PIII-SVMP bearing disintegrin domains (McLane et al., 2004). It is, therefore, possible to utilize the ARDPA as a tracking tool for the purpose of detecting the presence of various disintegrins occurring in a multitude of crude venoms. Most importantly, the ELISA results indicate that the venoms of the Mojave rattlesnake can be classified by their types, and thus this economical test can be used to monitor patients more carefully in addition to selecting venoms to be used for better anti-venom preparation.
The production of polyclonal antibodies against a recombinant disintegrin (r-mojastin-1) can distinguish between Mohave rattle-snake venom types A, B, and A + B using inexpensive and non-animal techniques such as ELISAs and Western blots. These assays will have a significant impact in the study of venom evolution and biochemistry as well as in treating snakebites, since patients can be dealt on a more physio pathological and scientific basis, paying added attention to the neurotoxic phenomena in some cases and hemotoxic in others.
Acknowledgments
Funding for this project was provided by NCRR/BMRG, Viper Resource Grant #s 8P40OD01960-10 and 3P40OD010960-13 (NNTRC, Texas A&M University-Kingsville, Dr. E.E. Sánchez), Special Programs, and the Robert A. Welch Foundation Department, Grant # AC-0006 (TAMUK-Department of Chemistry); Grant from the Science and Technology Fund (FONACIT) programs (PEI 201400352 Grant) (Universidad Central de Venezuela, Dr. A. Rodríguez-Acosta). We would also like to thank, Nora Diaz DeLeon and Mark HockMüller (NNTRC Serpentarium curator) and all the NNTRC personnel.
Abbreviations
- SVMP
snake venom metalloprotease
- ELISA
Enzyme-Linked Immunosorbent Assay
- ARDPA
anti-recombinant disintegrin polyclonal antibody
- r- Mojastin 1
recombinant mojastin 1
- ADAM
a disintegrin and metalloprotease
- LD50
Lethal Dose 50
- LC-MS
Liquid Chromatography-Mass Spectrometry
- IACUC
Institutional Animal Care and Use Committee
- ACT
Activated Clot Time
- CR
Clot Rate
- PF
Platelet Function
- gbACT + Kit
glass bead activated cuvette kit.
References
- Aguilar I, Guerrero B, Maria Salazar A, Girón ME, Pérez JC, Sánchez EE, Rodríguez-Acosta A. Individual venom variability in the South American rattlesnake Crotalus durissus cumanensis . Toxicon. 2007;50:214–224. doi: 10.1016/j.toxicon.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Alape-Girón A, Sanz L, Escolano J, Flores-Díaz M, Madrigal M, Sasa M, Calvete JJ. Snake venomics of the lancehead pitviper Bothrops asper: geographic, individual, and ontogenetic variations. J. Proteome Res. 2008;7:3556–3571. doi: 10.1021/pr800332p. [DOI] [PubMed] [Google Scholar]
- Barrio A, Brazil OV. Neuromuscular action of the Crotalus terrificus terrificus poisons. Acta Physiol. Lat. Am. 1951;1:291–308. [PubMed] [Google Scholar]
- Borja M, Galan JA, Cantu E, Zugasti-Cruz A, Rodriguez-Acosta A, Lazcano D, Lucena S, Suntravat M, Sánchez EE. Morulustatin, a disintegrins that inhibits ADP-induced platelet aggregation, isolated from the Mexican Tamaulipan rock rattlesnake (Crotalus lepidus morulus) Rev Cient. FCV-LUZ. 2016;26:86–94. [PMC free article] [PubMed] [Google Scholar]
- Brust A, Sunagar K, Undheim EA, Vetter I, Yang DC, Casewell NR, Jackson TN, Koludarov I, Alewood PF, Hodgson WC, Lewis RJ, King GF, Antunes A, Hendrikx I, Fry BG. Differential evolution and neofunctionalization of snake venom metalloprotease domains. Mol. Cell. Proteomics. 2013;12:651–663. doi: 10.1074/mcp.M112.023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete JJ. Structure-function correlations of snake venom disintegrins. Curr. Pharm. Des. 2005;11:829–835. doi: 10.2174/1381612053381783. [DOI] [PubMed] [Google Scholar]
- Calvete JJ. Proteomic tools against the neglected pathology of snake bite envenoming. Expert Rev. Proteomics. 2011;8:739–758. doi: 10.1586/epr.11.61. [DOI] [PubMed] [Google Scholar]
- Calvete JJ. The continuing saga of snake venom disintegrins. Toxicon. 2013;62:40–49. doi: 10.1016/j.toxicon.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Clark RF, Williams SR, Nordt SP, Boyer-Hassen LV. Successful treatment of crotalid-induced neurotoxicity with a new polyspecific crotalid Fab antivenom. Ann. Emerg. Med. 1997;30:54–57. doi: 10.1016/s0196-0644(97)70111-2. [DOI] [PubMed] [Google Scholar]
- Daltry JC, Wuster W, Thorpe RS. Diet and snake venom evolution. Nature. 1996;379:537–540. doi: 10.1038/379537a0. [DOI] [PubMed] [Google Scholar]
- Fiero MK, Seifert MW, Weaver JJ, Bonilla CA. Comparative study of juvenile and adult prairie rattlesnake (Crotalus viridis viridis) venoms. Toxicon. 1972;10:81–82. doi: 10.1016/0041-0101(72)90095-5. [DOI] [PubMed] [Google Scholar]
- Fox JW, Bjarnason JB. Atrolysins: metalloproteinases from Crotalus atrox venom . Methods Enzymol. 1995;248:368–387. doi: 10.1016/0076-6879(95)48024-2. [DOI] [PubMed] [Google Scholar]
- Gibbs HL, Mackessy SP. Functional basis of a molecular adaptation: prey-specific toxic effects of venom from Sistrurus rattlesnakes. Toxicon. 2009;53:672–679. doi: 10.1016/j.toxicon.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Girón ME, Salazar AM, Aguilar I, Pérez JC, Sánchez EE, Arocha-Piñango CL, Rodríguez-Acosta A, Guerrero B. Hemorrhagic, coagulant and fibrino(geno)lytic activities of crude venom and fractions from mapanare (Bothrops colombiensis) snakes. Comp. Biochem. Physiol. C Toxicol Pharmacol. 2008;147:113–121. doi: 10.1016/j.cbpc.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Glenn JL, Straight R. Mojave rattlesnake Crotalus scutulatus scutulatus venom: variation in toxicity with geographical origin. Toxicon. 1978;16:81–84. doi: 10.1016/0041-0101(78)90065-x. [DOI] [PubMed] [Google Scholar]
- Glenn JL, Straight R. Intergradation of two different venom populations of the Mojave rattlesnake (Crotalus scutulatus scutulatus) in Arizona. Toxicon. 1989;27:411–418. doi: 10.1016/0041-0101(89)90203-1. [DOI] [PubMed] [Google Scholar]
- Glenn JL, Straight RC, Wolfe MC, Hardy DL. Geographical variation in Crotalus scutulatus scutulatus (Mojave rattlesnake) venom properties. Toxicon. 1983;21:119–130. doi: 10.1016/0041-0101(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Gubensek F, Sket D, Turk V, Lebez D. Fractionation of Vipera ammodytes venom and seasonal variation of its composition. Toxicon. 1974;12:167–171. doi: 10.1016/0041-0101(74)90241-4. [DOI] [PubMed] [Google Scholar]
- Hardy DL. Envenomation by the Mojave rattlesnake (Crotalus scutulatus scutulatus) in southern Arizona. U.S.A. Toxicon. 1983;21:111–118. doi: 10.1016/0041-0101(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Irwin RL, Olivier KL, Mohamed AH, Haast WE. Toxicity of elapid venoms and an observation in relation to geographical location. Toxicon. 1970;8(51):54. doi: 10.1016/0041-0101(70)90173-x. [DOI] [PubMed] [Google Scholar]
- Jansen PW, Perkin RM, Van Stralen D. Mojave rattlesnake envenomation: prolonged neurotoxicity and rhabdomyolysis. Ann. Emerg. Med. 1992;21:322–325. doi: 10.1016/s0196-0644(05)80898-4. [DOI] [PubMed] [Google Scholar]
- Jimenez-Porras JM. Intraspecific variations in composition of venom of the jumping viper, Bothrops mummifera. Toxicon. 1964;2:187–196. doi: 10.1016/0041-0101(64)90021-2. [DOI] [PubMed] [Google Scholar]
- Markland FS, Jr, Swenson S. Snake venom metalloproteinases. Toxicon. 2013;62:3–18. doi: 10.1016/j.toxicon.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Marsh NA, Latston A. Some observations on the venom of the rhinoceros horned viper, Bitis nasicornis (Shaw) Toxicon. 1974;12:621–628. doi: 10.1016/0041-0101(74)90196-2. [DOI] [PubMed] [Google Scholar]
- Massey DJ, Calvete JJ, Sánchez EE, Sanz L, Richards K, Curtis R, Boesen K. Venom variability and envenoming severity outcomes of the Crotalus scutulatus scutulatus (Mojave rattlesnake) from Southern Arizona. J. Proteome. 2012;75:2576–2587. doi: 10.1016/j.jprot.2012.02.035. [DOI] [PubMed] [Google Scholar]
- McLane MA, Sanchez EE, Wong A, Paquette-Straub C, Perez JC. Disintegrins. Curr Drug Targets Cardiovasc. Haematol. Disord. 2004;4:327–355. doi: 10.2174/1568006043335880. [DOI] [PubMed] [Google Scholar]
- Moura-da-Silva AM, Della-Casa MS, David AS, Assakura MT, Butera D, Lebrun I, Shannon JD, Serrano SM, Fox JW. Evidence for heterogeneous forms of the snake venom metalloproteinase jararhagin: a factor contributing to snake venom variability. Arch. Biochem. Biophys. 2003;409:395–401. doi: 10.1016/s0003-9861(02)00598-2. [DOI] [PubMed] [Google Scholar]
- Peichoto ME, Paes Leme AF, Pauletti BA, Batista IC, Mackessy SP, Acosta O, Santoro ML. Autolysis at the disintegrin domain of patagonfibrase, a metallo-proteinase from Philodryas patagoniensis (Patagonia Green Racer; Dipsadidae) venom. Biochim. Biophys. Acta. 2010;1804:1937–1942. doi: 10.1016/j.bbapap.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Pifano F, Rodriguez-Acosta A. Ecological niche and redescription of Crotalus vegrandis (Serpentes: Crotalidae) in Venezuela. Brenesia. 1996;45(46):169–175. [Google Scholar]
- Rhoten WB, Gennaro JF., Jr Treatment of the bite of a Mojave rattlesnake. J. Fla Med. Assoc. 1968;55:324–326. [PubMed] [Google Scholar]
- Sánchez EE, Galan JA, Russell WK, Soto JG, Russell DH, Pérez JC. Isolation and characterization of two disintegrins inhibiting ADP-induced human platelet aggregation from the venom of Crotalus scutulatus scutulatus (Mohave rattlesnake) Toxicol. Appl. Pharmacol. 2006;212:59–68. doi: 10.1016/j.taap.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Galan JA, Perez JC, Rodríguez-Acosta A, Pérez JC. The efficacy of two antivenoms against the venom of North American snakes. Toxicon. 2003;41:357–365. doi: 10.1016/s0041-0101(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Lopez-Johnston JC, Rodríguez-Acosta A, Chase PB, Pérez JC. Neutralization of two North American coral snake venoms with United States and Mexican antivenoms. Toxicon. 2008;51:297–303. doi: 10.1016/j.toxicon.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez EE, Lucena SE, Reyes S, Soto JG, Cantu E, Lopez-Johnston JC, Guerrero B, Salazar AM, Rodríguez-Acosta A, Galan JA, Tao WA, Pérez JC. Cloning, expression, and hemostatic activities of a disintegrin, r-mojastin 1, from the Mohave rattlesnake (Crotalus scutulatus scutulatus) Thromb. Res. 2010;126:e211–e219. doi: 10.1016/j.thromres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez EE, Hotle D, Rodríguez-Acosta A. Neutralization of Bitis parviocula (Ethiopian mountain adder) venom by the South African Institute of Medical Research (SAIMR) antivenom. Rev. Inst. Med. Trop. Sao Paulo. 2011;53:213–217. doi: 10.1590/s0036-46652011000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenberg S. Geographical pattern of crotamine distribution in the same rattle-snake subspecies. Science. 1959;129:1361–1363. doi: 10.1126/science.129.3359.1361. [DOI] [PubMed] [Google Scholar]
- Spearman-Karber R. Alternative methods of analysis for quantal responses. In: Finney D, editor. Statistical Method in Biological Assay. 2nd. Charles Griffin, London volume II: University of California Press, Berkeley, CA; 1964. [Google Scholar]
- Stoscheck CM. Quantitation of protein. Methods in Enzymology. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Takeda S, Takeya H, Iwanaga S. Snake venom metalloproteinases: structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim. Biophys. Acta. 2012;1824:164–176. doi: 10.1016/j.bbapap.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Wilkinson JA, Glenn JL, Straight RC, Sites JW. Distribution and genetic variation in venom A and B populations of the Mojave rattlesnake (Crotalus scutulatus scutulatus) in Arizona. Herpetologica. 1991;47:54–68. [Google Scholar]
- Williams DJ, Gutierrez JM, Calvete JJ, Wüster W, Ratanabanangkoon K, Paiva O, Brown NI, Casewell NR, Harrison RA, Rowley PD, O'Shea M, Jensen SD, Winkel KD, Warrell DA. Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteome. 2011;74:1735–1767. doi: 10.1016/j.jprot.2011.05.027. [DOI] [PubMed] [Google Scholar]