Abstract
Purpose
Despite potential clinical benefits, implementation of pharmacogenomics (PGx) faces many technical and clinical challenges. These challenges can be overcome by a comprehensive and systematic implementation model.
Methods
The development and implementation of PGx was organized into eight interdependent components addressing resources, governance, clinical practice, education, testing, knowledge translation, clinical decision support (CDS) and maintenance. Several aspects of the implementation were assessed including adherence to the model, production of PGx-CDS interventions and access to educational resources.
Results
Between 8/2012 and 6/2015, 21 specific drug-gene interactions were reviewed and 18 of them were implemented in the electronic medical record as PGx-CDS interventions. There was complete adherence to the model with variable production time (98 to 392 days) and delay time (0 to 148 days). The implementation impacted approximately 1247 unique providers and 3788 unique patients. A total of 11 educational resources complementary to the drug-gene interactions and 5 modules specific for pharmacists were developed and implemented.
Conclusion
A comprehensive operational model can support PGx implementation into routine prescribing. Institutions can use this model as a roadmap to support similar efforts. However, we also identified challenges that will require major multidisciplinary and multi-institutional efforts to make PGx a universal reality.
Keywords: precision medicine, pharmacogenetics, medical informatics, clinical decision support systems, delivery of health care
INTRODUCTION
Pharmacogenomics (PGx) has the potential to improve clinical outcomes by using an individual’s genotype to personalize and optimize the selection of drug therapy.1 A large number of PGx variants with demonstrated clinical utility are known and have been incorporated into drug labeling by the US Food and Drug Administration.2 As the availability of high throughput genomics technology becomes more widespread and the associated cost of genetic testing more economical, opportunities for patients to have precision genomic information available will increase. Integration of these genetic data into the clinical decision making process has the potential to significantly advance the practice of precision medicine and in the case of PGx, ultimately affect every patient.
Despite its potential to improve drug efficacy and reduce adverse drug reactions, the incorporation of PGx data into routine clinical practice has been slow. Several significant challenges surround the implementation of PGx-based medicine on a wider scale, including reimbursement for genetic testing, development of infrastructure and standardized processes for storing, accessing, and interpreting genomic data, evidence of clinical utility, ethical and legal concerns, and prescriber uncertainty about the clinical and financial benefits of genome-guided therapy.3–6 Furthermore, the dynamic nature of discovering new clinically actionable variants increases the complexity of the implementation.3,7 Therefore, relying on the cognition of clinicians to integrate this increasingly complex knowledge into already busy clinical workflows is not a sustainable or practical strategy.
Leveraging health information technology, including clinical decision support (CDS) tools and electronic health records (EHR), will be essential to overcoming many of the barriers associated with the translation of PGx guidelines into clinical practice. To this end, a collaboration between the National Institutes of Health-funded Pharmacogenomic Research Network (PGRN) and the Electronic Medical Records and Genomics Network (eMERGE) has supported several pilot projects focused on exploring the utility of integrating genomic data within EHRs.8–14 These initial projects were successful in integrating PGx-CDS into EHR-based models at each member institution. However, current EHRs and CDS tools alone are not likely to be able to handle the influx of genomic data expected in the near future. Therefore, additional infrastructure in combination with a comprehensive strategy will be required, involving all aspects of PGx medicine, from the laboratory, to data migration and clinical participation, to multidisciplinary governance.
Here we describe and evaluate a comprehensive, reproducible, and adaptable model used by Mayo Clinic to implement PGx in the clinical setting. We define the model based on the highly interrelated multidisciplinary components, all needed equally to implement PGx. Our experience with this model provides insight into the challenges and strategies for optimizing the translation of PGx knowledge and test results into actionable prescribing decisions on a larger scale.
METHODS
Study Setting
Mayo Clinic, a large academic medical center located in Rochester, MN, established the Center for Individualized Medicine in order to improve patient care through genomic medicine. This center had several programs, including a PGx program to promote PGx research and translation to clinical practice. PGx testing was performed by the CLIA-approved and CAP-accredited Personalized Genomics and Clinical Genome Sequencing Laboratories, both of which were part of the Mayo Clinic Department of Laboratory Medicine and Pathology. The clinical practice and the Office of Information and Knowledge Management supported the Clinical Decision Support Program, which oversees all aspects related to implementation of CDS integrated in the EHRs used at Mayo Clinic. This study was reviewed and approved by the Mayo Clinic Institutional Review Board.
Operational Model for Pharmacogenomics Implementation
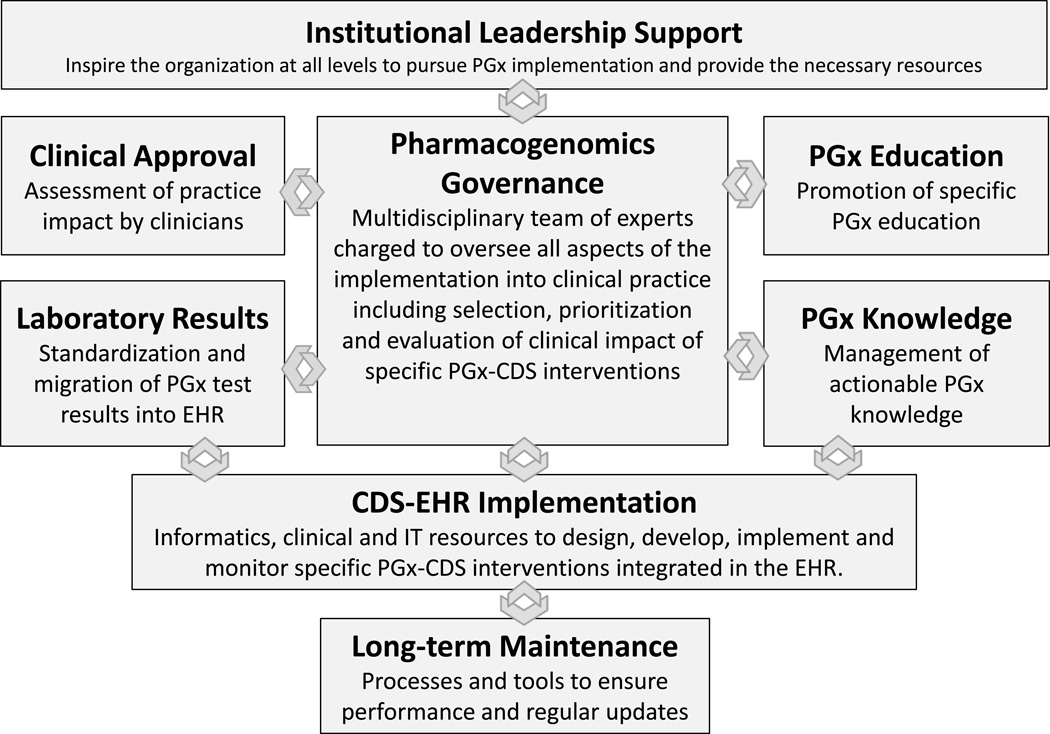
The PGx implementation model used by Mayo Clinic was organized into eight highly interrelated functional components (figure).
Figure.
Operational model to implement pharmacogenomic clinical decision support at the point of care. The model is represented as eight main functional components and their bidirectional relationships (arrows). PGx = pharmacogenomics, CDS = clinical decision support, EHR = electronic health record, IT = information technology.
1. Institutional Leadership Support
Multiple significant challenges, including the existence of organizational silos, had impaired large-scale PGx implementation. Consistent and vocal high-level institutional leadership support was critical to initiating, driving, and maintaining a successful implementation program. As PGx testing was not widely reimbursable, institutional leadership regarded PGx implementation as an investment in good patient care and the future of medicine. Therefore,, the main goals of the leadership were to ensure coordination among the many clinical areas, drive prioritization of the projects and provide the necessary resources.
2. Pharmacogenomics Governance
Formation of a multidisciplinary task force of experts overseeing all aspects of the implementation and coordinating efforts and resources was essential for PGx implementation. The team had representation from all areas involved in the implementation, including genomic medicine, primary and specialty care clinics, pharmacy, laboratory, education, research, informatics, information technology (IT), and administration. This PGx Task Force coordinated implementation efforts across multiple departments and committees and reported directly to the PGx Program of the Center for Individualized Medicine. Routine meetings provided a structured forum to facilitate communication and decision-making with regard to the selection, prioritization, development, and implementation of specific drug-gene interventions. The team developed a systematic approach to review available evidence using clear criteria for selecting and approving specific drug-gene interactions. Some of the primary sources of PGx evidence were: FDA PGx biomarkers,2 PharmGKB,15 Indiana University Drug Interactions,16 and original research articles. The selection criteria were: 1) drug toxicity/risk to patient, 2) strength of support in the literature (i.e. quality and quantity of articles, number of subjects, presence of prospective studies, and presence of studies involving medical and economic benefit), 3) range of use among medical specialties, 4) volume of drug use, 5) existence of protocol/practice guidelines (i.e. PGRN Clinical Pharmacogenetics Implementation Consortium,17 Dutch Pharmacogenetics Working Group18 and other medical society guidelines), and 6) reimbursement criteria.
3. Clinical Approval
Identification and participation of clinical champions was very important to securing clinical acceptance. Their feedback related to the traditional use of the target medications and potential impact of PGx implementation among clinical users was extremely important at the time to approving, developing, and monitoring specific drug-gene interactions.
4. Laboratory Results
The evolving science of PGx testing and reporting represented one of the main challenges to the implementation. Significant effort was needed to coordinate standard definitions for different genotypes and phenotypes among different laboratories and to optimize delivery of structured PGx test results from the laboratories to the EHR. We implemented electronic interfaces between the laboratory systems and the EHR when possible. We also implemented a manual review and data entry process when the electronic interfaces failed or were not feasible (i.e. PDF reports). Extensive translational tables were developed inside the EHR to standardize genotype-phenotype definitions and facilitate the use of structured data by the EHR applications.
The model targeted a comprehensive view of PGx testing available in clinical practice and addressed not only the technical issues but also the knowledge and educational issues surrounding better ordering and interpreting of results within the clinical context. Among the different testing approaches, we found that the most commonly used was reactive testing, which was performed based on clinical guidelines or focused clinical studies (i.e. TPMT for thiopurines, HLA-B*57:01 for abacavir) before using a medication. Preemptive testing was available in a small proportion of patients enrolled in previous studies or from individual patients with previous PGx testing.
5. Pharmacogenomics Education
We implemented a systematic approach to provide needed PGx education as a complement to the overall implementation strategy. 19–23 The education was designed not only for busy clinicians (physician, residents, nurse practitioners, physician’s assistants, etc.) but importantly, also for pharmacists who were responsible for responding to inquiries from both clinicians and patients.24 While general information about genomic medicine and PGx principles delivered via conferences, newsletters, and other means was important, it was often insufficient to change provider decision-making. More specific and actionable education delivered “just in time” and embedded in the clinician and pharmacist workflows at the point of care was preferred. We achieved this by linking relevant online educational resources for specific drug-gene interactions to the PGx-CDS interventions in the EHR.
6. Pharmacogenomics Knowledge
We used the Clinical Pharmacogenetics Implementation Consortium (CPIC) as the main source of peer-reviewed clinical guidelines addressing specific drug-gene interactions.25 These guidelines assume that the PGx test results are available, and they do not provide recommendations regarding testing indications. To complement them, we used clinical guidelines published by medical societies and other professional groups and original publications. If we found discrepancies between them (i.e. clinical utility, population at risk, different phenotypes), we used input from the clinical champions and other experts to achieve consensus on specific recommendations. The recommendations were then structured in paragraphs and transferred to translational tables (genotype/phenotype/recommendation) in the EHR where they were used by the CDS interventions. We also made readily available all the online references as an attempt to facilitate compliance with the recommendations. We used processes and infrastructure available in the institution to implement and manage other types of clinical knowledge, which should facilitate long-term maintenance.
7. CDS-EHR Implementation
Despite the lack of specific functionality in commercially available EHR to manage genomic data, we were able to adapt existent functionality to deliver synchronous interventions as a clinician is interacting with the EHR (i.e. pop-up alert in the order entry system advising the clinician to order a PGx Lab test based on a drug order or a pop-up alert prompted by a specific drug-gene interaction) and asynchronous interventions (i.e. inbox message or email when new PGx test results are available). We avoid custom code changes and used stablished CDS-IT staff and processes to streamline development, testing, implementation and long-term maintenance of the system.
We implemented a variety of interventions in the EHR designed to: 1) remind clinicians if PGx testing was required based on current clinical guidelines (i.e. HLA-B*57:01 for abacavir, TPMT for azathioprine, HLA-B*15:02 for carbamazepine in Asian populations), 2) detect unreadable PGx test results and trigger a manual review process to validate discrete data (i.e. novel variant, transcription error), 3) document relevant genotypes/phenotypes in the problem list (preferred method) or allergy module (only for abacavir-HLA-B*57:01 interaction as advised by current guidelines), 4) notify ordering clinicians of new PGx test result(s) with an inbox message containing specific drug-gene information, 5) use available PGx results to alert prescribers of potential drug-gene interactions and suggest changes to the order (pop-up alert advising drug change, dose change or a calculated dose in the case of warfarin), and 6) provide links in the CDS interventions to facilitate access to web-based, easy-to-use educational resources in a workflow-friendly format. Furthermore, all transactional data was stored to facilitate analytics.
8. Long-term Maintenance
As part of the initial implementation, we developed a strategy to maintain and update the data, knowledge, interfaces, and CDS-EHR applications. The strategy was based on establishing clinical ownership (champions) and operational ownership (collaboration between PGx governance and CDS governance). Additionally, dashboards and reports were developed to monitor performance of the system over time.
Evaluation of the Implementation
To assess the implementation and integrity of our model, we considered the production and implementation of drug-gene CDS interventions integrated in the EHR during the study period, August 2012 to June 31, 2015, as the main study outcome. We assessed several aspects of the implementation, including adherence to the model, implementation time (time between clinical approval and EHR implementation), delay time (time between targeted implementation and EHR implementation), clinical and technical challenges to implementation, and the unique number of providers and patients that interacted with the PGx-CDS interventions. To assess the overall burden of the CDS interventions, we calculated the number of events (PGx-CDS interventions) by provider-patient-drug interaction over 24 hours. This definition helped to standardize the measure of system interaction between providers that triggered the same alert (event) for the same patient-drug multiple times when trying to validate the CDS message and those providers that triggered the alert only one time. We also assessed how frequently the online educational resources were accessed. As source data, we used the extensive records (minutes) kept during the implementation and the electronic logs of the CDS system/EHR and online resources.
RESULTS
Implementation Model
Between August 2012 and June 2015, the PGx Governance team reviewed and approved 21 specific drug-gene interactions. Of these, 18 were implemented as PGx-CDS interventions at the point of care with complete adherence to the model (Table 1). One drug-gene interaction, peginterferon-IL28B, was not endorsed by clinicians due to the existence of very robust clinical protocols to comply with PGx testing before treatment and the expectation that a new drug treatment would soon substitute for the use of interferon. Two other drug-gene interactions (5-fluorouracil-DPYD and tacrolimus-CYP3A5) were approved at the end of the study period, and implementation was pending.
Table 1.
Drug-gene interactions reviewed and approved by the pharmacogenomics governance and implemented in the EHR as pharmacogenomics clinical decision support.
| Drug-gene interaction | Number of days in production |
Implementation time |
Delay time |
Main challengesa |
Clinical review and approval |
|
|---|---|---|---|---|---|---|
| 1 | Abacavir – HLA-B*57:01 | 885 | 157 | 26 | A | Infection Disease (HIV Clinic) |
| 2 | Peginterferon – IL28B | - | - | - | - | Hepatology. It was not approved for implementation |
| 3 | Carbamazepine – HLA- B*15:02 |
807 | 226 | 14 | B | Neurology |
| 4 | Azathioprine - TPMT | 740 | 293 | 0 | B, C, D | Gastroenterology, Dermatology, Rheumatology, Hematology |
| 5 | 6-Mercaptopurine - TPMT | 740 | 293 | 0 | B, C, D | Gastroenterology, Dermatology, Rheumatology, Hematology |
| 6 | Thioguanine - TPMT | 740 | 293 | 0 | B, C, D | Gastroenterology, Dermatology, Rheumatology, Hematology |
| 7 | Codeine - CYP2D6 | 612 | 134 | 46 | F, G | Anesthesia. Pain Clinic |
| 8 | Tramadol - CYP2D6 | 612 | 134 | 46 | F, G | Anesthesia. Pain Clinic |
| 9 | Tamoxifen - CYP2D6 | 558 | 175 | 23 | F, G | Oncology (Breast Clinic) |
| 10 | Clopidogrel – CYP2C9 | 285 | 392 | 148 | C, E, G | Cardiology |
| 11 | Simvastatin – SLCO1B1 | 432 | 231 | 78 | F, G | Cardiology |
| 12 | Allopurinol - HLA-B*58:01 | 222 | 189 | 92 | E | Internal Medicine |
| 13 | Warfarin – CYP2C9/VKORC1 |
285 | 98 | 29 | C, F, G | Hematology (Anticoagulation Clinic) |
| 14 | Fluoxetine – CYP2D6 | 40 | 203 | 113 | D, E, H | Psychiatry |
| 15 | Fluvoxamine – CYP2D6 | 40 | 203 | 113 | D, E, H | Psychiatry |
| 16 | Paroxetine – CYP2D6 | 40 | 203 | 113 | D, E, H | Psychiatry |
| 17 | Venlafaxine – CYP2D6 | 40 | 203 | 113 | D, E, H | Psychiatry |
| 18 | Citalopram – CYP2C19 | 40 | 203 | 113 | D, E, H | Psychiatry |
| 19 | Escitalopram – CYP2C19 | 40 | 203 | 113 | D, E, H | Psychiatry |
| 20 | 5-fluorouracil - DPYD | - | - | - | D | Hematology-Oncology. Approved, pending implementation |
| 21 | Tacrolimus -CYP3A5 | - | - | - | D | Transplant. Approved, pending implementation |
Main challenges: A = Coordination with EHR software update. B =Identification of clinical champions. C = Approval by clinical practice. D = Availability of PGx results in the EHR. E = Limited IT staff and/or conflict with other IT priorities. F = Complexity of rule development. G = Interpretation of clinical guidelines and PGx recommendations. H = Coordination between PGx governance and clinical practice.
Number of days in production = time between EHR implementation and 6/30/2015. Implementation time = time between clinical approval and EHR implementation. Delay time = time between targeted implementation and EHR implementation
EHR = Electronic health record. IT = Information technology. PGx = Pharmacogenomics
There were variable implementation times (range from 98 to 392 days) and delay times (range 0 to 148 days). The implementation times and delays were influenced by several clinical and technical challenges. Table 1 describes the specific challenges for each drug-gene interaction. In general, the most important clinical challenge was clinician resistance to provide approval, in part based on the lack of support by clinical practice guidelines to implement PGx testing. For example, the guideline for management of anticoagulant therapy recommends against routine PGx testing before initiating warfarin, while the guideline for the use of clopidogrel found no studies that demonstrate a correlation between the use of PGx testing and better clinical outcomes.26,27 The most difficult technical challenges were the availability and format of the PGx Lab results in the EHR and issues associated with programming the CDS intervention in the EHR. Additional resources and time were necessary to develop or enhance interfaces, define new elements in the databases, and develop, implement and test novel algorithms using the expert rule engine of the EHR.
Pharmacogenomics Clinical Decision Support Interventions
A total of 1247 unique providers, including staff physicians, residents/fellows, physician assistants, nurse practitioners, and pharmacists from multiple clinical areas, interacted with the PGx-CDS interventions during the study period. These interventions were triggered for 3788 unique patients (mean age 47 years, SD 19; range from <1 to 101; 58% female). Two main types of interventions were implemented: a popup alert at the time a drug order is attempted in a patient with actionable genotype/phenotype(s) documented in the EHR, and a notification (inbox) to the ordering provider of a new actionable PGx test result documented in the EHR. Table 2 contains the specific PGx-CDS interventions and the relative frequency of activation (monthly frequency of PGx-CDS interventions for the same provider, same patient, and same drug in 24 hours) during the study period. Some PGx-CDS interventions (i.e. interventions involving antidepressant medications) were not included in the table, because they were implemented at the end of the study period and did not have enough data to report. The most common events were those related to TPMT (thiopurine methyltransferase). The use of PGx testing before using drugs metabolized by TPMT is widely supported by the clinical practice. On the other hand, the least frequent events were those related to simvastatin and warfarin. Although, these drugs are frequently used in clinical practice, PGx testing is rarely performed as part of routine care.
Table 2.
Pharmacogenomics clinical decision support interventions implemented in the electronic health record.
| Pharmacogenomics clinical decision support interventions | Number of months in production |
Monthly ratesa |
|---|---|---|
| Abacavir - HLA-B*57:01 | ||
| Drug Order Attempted, Total Popup Alerts | 3.7 | |
| Drug order attempted, alerted patient has positive HLA-B*5701 | 0.9 | |
| Drug order attempted, alerted patient should be tested for HLA-B*5701 | 30 | 2.8 |
| Patient Tested, Result Positive, Physician Notified, Allergy Added | 0.3 | |
| Carbamazepine - HLA-B*15:02 | ||
| Drug Order Attempted, Total Popup Alerts | 1.3 | |
| Drug order attempted, alerted patient has positive HLA-B*1502 | 27 | 0.1 |
| Drug order attempted, alerted patient should be tested for HLA-B*1502 | 1.2 | |
| Patient Tested, Result Positive, Physician Notified, Problem Added | 0.0 | |
| Thiopurine - TPMT | ||
| Drug Order Attempted, Total Popup Alerts | 77.6 | |
| Drug order attempted, alerted patient has intermediate or low TPMT test results | 25 | 11.4 |
| Drug order attempted, alerted to consider patient be tested for TPMT | 66.2 | |
| Patient Tested, Result Positive, Physician Notified, Problem Added | 54.7 | |
| Codeine/Tramadol/Tamoxifen - CYP2D6 | ||
| Drug Order Attempted, Total PopUp Alerts | 15.0 | |
| Drug order attempted, alerted patient at risk with Extensive to Ultra Rapid test result | 6.0 | |
| Drug order attempted, alerted patient at risk with Ultra Rapid test result | 21 | 3.9 |
| Drug order attempted, alerted patient at risk with Poor to Intermediate test result | 1.5 | |
| Drug order attempted, alerted patient at risk with Poor test result | 3.6 | |
| Drug order attempted, alerted patient at risk with Intermediate to Ultra Rapid test result | 0.0 | |
| Patient Tested, Result At RISK, Physician Notified, Problem Added | 25.1 | |
| Simvastatin - SLCO1B1 | ||
| Drug Order Attempted, Total PopUp Alerts | 0.7 | |
| Drug order attempted, alerted for TC Genotype | 14 | 0.7 |
| Drug order attempted, alerted for CC Genotype | 0.0 | |
| Patient Tested, Result At RISK, Physician Notified, Problem Added | 0.6 | |
| Warfarin - CYP2C9/VKORC1 | ||
| Drug Order Attempted, Total PopUp Alerts | 10 | 0.7 |
| Drug order attempted, dosing algorithm recommendations presented for warfarin order | 0.7 | |
| Drug order attempted, unable to display dosing algorithm due to missing data | 0.0 | |
| Clopidogrel - CYP2C19 | ||
| Drug Order Attempted, Total PopUp Alerts | 5.6 | |
| Drug order attempted, alerted patient at risk with Intermediate test result | 4.6 | |
| Drug order attempted, alerted patient at risk with Poor to Intermediate test result | 10 | 0.2 |
| Drug order attempted, alerted patient at risk with Poor test result | 0.8 | |
| Patient Tested, Result At RISK, Physician Notified, Problem Added | 28.2 | |
| Allopurinol - HLA-B*58:01 | ||
| Drug Order Attempted, Total PopUp Alerts | 4.7 | |
| Drug order attempted, alerted patient at risk with Positive result | 6 | 0.0 |
| Drug order attempted, alerted patient should be tested for HLA-B*5801 | 4.7 | |
| Patient Tested, Result At RISK, Physician Notified, Problem Added | 0.0 | |
Monthly rate of events calculated as same provider, same patient, same drug order within 24 hours.
Educational Resources
A total of 11 educational resources were developed and implemented to complement the selected drug-gene interactions. They were developed in an internal online medical information system (AskMayoExpert) used by Mayo Clinic to deliver evidence-based information, care process models and frequently asked questions (FAQ) on numerous topics. The PGx education was designed in a FAQ format to inform providers of the nature of the drug-gene interaction and appropriate actions based on the patient’s genotype/phenotype. Table 3 shows the specific resources and the number of time they were accessed (online sessions) during the study period. Approximately 9.3% of the online sessions originated from the links provided by the PGx-CDS interventions in the EHR, while the other 90.7% originated from several other sources including direct access, intranet, and other applications. This difference can be explained based on the relatively small proportion of prescribers able to interact with the PGx-CDS interventions when compared with all the clinicians able to access the educational resources on line. Access to the educational resources was not limited to direct patient care, but also used for education, training and testing. Approximately, 44% of the online sessions were from members of the healthcare team (staff physicians 11.9%, residents 7.4%, nurses 12.1%, and others, including pharmacists, 12.7%).
Table 3.
Online pharmacogenomics educational resources developed and implemented as part of the implementation model.
| Online pharmacogenomics educational resources | Number of months in production |
Number of online sessions by source page |
||
|---|---|---|---|---|
| EHR | Other | Total | ||
| Abacavir and HLA-B* 5701 | 30 | 122 | 454 | 576 |
| Carbamazepine and HLA-B*1502 | 27 | 5 | 456 | 461 |
| Thiopurines and TPMT | 25 | 194 | 671 | 865 |
| Codeine and CYP2D6 | 21 | 10 | 557 | 567 |
| Tramadol and CYP2D6 | 21 | 7 | 422 | 429 |
| Tamoxifen and CYP2D6 | 21 | 4 | 302 | 306 |
| Simvastatin and SLCO1B1 | 14 | 2 | 253 | 255 |
| Clopidogrel and CYP2C19 | 10 | 42 | 274 | 316 |
| Warfarin and CYP2C9 and VKORC1 | 10 | 8 | 177 | 185 |
| Allopurinol and HLA-B*5801 | 6 | - | 139 | 139 |
| Antidepressant Medications and Pharmacogenomics | 1 | - | 137 | 137 |
| Total | 394 | 3842 | 4236 | |
Additionally, five competency-based modules were developed specifically for pharmacists: Pharmacogenomics 101; Cardiovascular: Clopidogrel and Simvastatin; Codeine, Tramadol and Tamoxifen (CYP2D6); Hypersensitivity with Abacavir and Carbamazepine; and Thiopurine Methyltransferase (TPMT); they were completed by 422, 341, 247, 415 and 387 pharmacists, respectively, out of approximately 500 pharmacists in the institution.
DISCUSSION
Our aim was to develop a generalizable implementation model consisting of core components for initial use by Mayo Clinic but one that would also be applicable and transferable to other institutions regardless of size or available infrastructure. To this end, we have created a comprehensive model that incorporates all the necessary components to implement PGx at the point of care. In general, the implementation of this model has proved to be successful based on the number of drug-gene interactions that have been reviewed, approved and implemented in the EHR. The scope of the implementation includes multiple clinics and patients with various clinical conditions, involves CDS integration into commercially-available EHRs, contains access to educational resources at the point of care, and was designed to evaluate the impact of both preemptive and reactive PGx testing. Moreover, the educational component of this model has been well received by clinicians and pharmacists and represents a feasible solution to the challenges associated with the lack of practical PGx knowledge and the barriers imposed by busy clinical workflows.28.
In response to the collaboration between the PGRN and eMERGE Networks, several other institutions published their experiences in developing and integrating active PGx-CDS within the EHR. Through the integration of CDS into a locally-developed EHR, the Pharmacogenomic Resource for Enhanced Decisions in Care and Treatment (PREDICT) project at Vanderbilt University Medical Center involved the successful implementation of a model to deliver PGx-CDS in the clinic.29,30 This model relies on extensive preemptive testing, which was not available in our institution. St. Jude Children’s Research Hospital also successfully implemented a CDS system capable of providing point of care pharmacogenetic alerts.8 This model relies in preemptive testing and on pharmacists to act as an interface between the genotyping laboratory, the EHR and prescribing clinicians. In our model, the CDS system serves as this interface, triggering a manual intervention if any errors are encountered. Other institutions have since followed suit, using CDS to integrate select drug-gene rules into an EHR and monitoring the impact of this integration on patient care and clinical practice.7,9,10.
Despite these initial successes, it must be recognized that the number of drug-gene interventions and the amount of PGx data that can be supported by our model, or any model currently in use, is limited, and challenges related to the scalability of these models may ultimately limit their longevity. One related challenge identified by our PGx governance is how to continuously identify and prioritize the implementation of newly discovered drug-gene interactions into the practice. While currently implemented drug-gene interactions were chosen either based on current clinical guidelines or overwhelming clinical evidence, the selection process was highly manual and time consuming, as it required careful and rigorous review and discussion of all clinical evidence. For some drug-gene interventions, we encountered disagreements between members of the expert panels regarding differences between the CPIC guidelines and guidelines published by medical organizations. These differences usually arose from the need to order PGx testing vs. preemptive testing and the lack of studies showing associated clinical outcomes. Our model successfully helps to solve the disagreement, but we still could not avoid delays in the implementation process (table 1, main challenges). Similarly, while clinicians have extensive knowledge on the traditional use of target medications, some lack a clear understanding of how PGx knowledge may positively impact clinical outcomes. This can often make it challenging to obtain clinical support and approval of new drug-gene interventions, which, for our model (clinical approval module) was required, and without which, it would be difficult to make changes to the practice. We therefore need a national consensus between PGx experts and medical societies in charge of the clinical guidelines to widely disseminate standardized PGx knowledge that can be easily accepted by clinicians and quickly implemented in clinical practice.
One of our major technical challenges was to define how best to integrate PGx test results from the laboratory into the EHR (Table 1, main challenges). Structured test results are required to trigger specific CDS interventions. However, to date, unstructured text reports, usually user-friendly PDF files, have been the preferred way to report PGx test results to clinicians. These reports, while useful for immediate clinical decision-making, are lost to future providers as current commercial EHR are not designed to store genomic information in this format over the long-term. Another problem was the current lack of standardization between different laboratories in reporting PGx nomenclature as well as genotype-phenotype interpretations. Our comprehensive implementation model facilitated coordination of tasks and resources among different departments to implement solutions to these problems. We created electronic interfaces capable of transferring structured results into the EHR but which also allowed for manual data entry when an electronic solution was not available. We used extensive translation tables to standardize the phenotypical interpretation of the PGx test results. We then utilized the current functionality within commercially-available EHRs, namely the allergy module, problem list, inbox messages and alerts, to make patient-specific PGx information relevant to all clinicians. However, we recognize that scaling of the model will ultimately become a challenge, as the amount of genetic data managed in this way is finite. As more clinically-actionable variants are recognized and incorporated into clinical guidelines, and as whole genome and exome sequencing becomes more readily available, the capacity of current EHR to store relevant genotyping results may be exceeded. A future solution may be found external to the EHR, perhaps with the data generated by genetic testing existing in an ancillary system specifically designed for storing and querying genomic data upon demand from the clinician.31,32 The lack of standardization among reports from different laboratories will also require an internationally-coordinated effort to create standardized nomenclature for PGx test results and unambiguous genotype-phenotype interpretations. In this regard, there are several promising efforts including collaboration between the Regenstrief Institute and CPIC to create Logical Observation Identifiers Names and Codes (LOINC) for reporting PGx test results in a standard format 33 and recommendations from the international workgroup for test result reporting organized by the Centers for Disease Control and Prevention.34.
Current research suggests that providers lack PGx knowledge leading to problems with ordering and understanding the results of PGx testing and communicating the clinical impact of these results to their patients.19–23 These challenges were also evident during our implementation. Our model has addressed these issues by emphasizing practical PGx education and helping providers to implement PGx knowledge at the point of care by providing CDS-driven actionable alerts linked to online PGx educational resources available in a straight forward and easy-to-use format. As the number of alerts received per clinician at this time is still relatively small, this method for educating clinicians at the point of care remains feasible. The availability of online resources on demand at any time and outside of the EHR seems to facilitate access to education and may help to overcome the many limitations related to clinical workflows. In fact, our results show that the large majority of online sessions originated outside of the EHR (Table 3). Additionally, our model promotes other means of PGx education institution-wide that are not always related to the CDS alerts or the EHR.24 These include lectures, recorded grand rounds, short educational videos, blended learning courses, videoconferences, targeted emails, and competency-based online training for pharmacists.
In conclusion, we have described our experience implementing a model for PGx-based patient care at Mayo Clinic. A coordinated and dedicated multidisciplinary effort was critical to successfully facilitate the clinical adoption of this model and to ensure the technical feasibility of EHR-driven, PGx-guided therapy. This process has provided significant insight into the current challenges associated with PGx implementation and has highlighted several opportunities for future research and optimization.
Acknowledgments
We acknowledge the support of the Center for Individualized Medicine, the Clinical Decision Support Program and the Office of Information and Knowledge Management of Mayo Clinic.
Financial support
This work was funded in part by the National Institutes of Health grants U19 GM61388, RO1 GM28157 and U01 HG006379.
REFERENCES
- 1.Weinshilboum R, Wang L. Pharmacogenomics: bench to bedside. Nat Rev Drug Discov. 2004;3:739–748. doi: 10.1038/nrd1497. [DOI] [PubMed] [Google Scholar]
- 2.U. S. Food and Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labeling. [Accessed Nov 15, 2015]; http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm.
- 3.Farrugia G, Weinshilboum R. Challenges in implementing genomic medicine: the Mayo Clinic Center for Individualized Medicine. Clin Pharmacol Ther. 2013;94:204–206. doi: 10.1038/clpt.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15:258–267. doi: 10.1038/gim.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott SA. Clinical pharmacogenomics: Opportunities and challenges at point-of-care. Clin Pharmacol Ther. 2013;93:33–35. doi: 10.1038/clpt.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy JJ, McLeod HL, Ginsburg GS. Genomic Medicine: A Decade of Successes, Challenges, and Opportunities. Sci Transl Med. 2013;5:189sr184–189sr184. doi: 10.1126/scitranslmed.3005785. [DOI] [PubMed] [Google Scholar]
- 7.Weitzel KW, Elsey AR, Langaee TY, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet. 2014;166:56–67. doi: 10.1002/ajmg.c.31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell GC, Crews KR, Wilkinson MR, et al. Development and use of active clinical decision support for preemptive pharmacogenomics. J Am Med Inform Assoc. 2014;21:e93–e99. doi: 10.1136/amiajnl-2013-001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Driest SL, Shi Y, Bowton EA, et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014;95:423–431. doi: 10.1038/clpt.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuldiner AR, Palmer K, Pakyz RE, et al. Implementation of pharmacogenetics: The University of Maryland personalized anti-platelet pharmacogenetics program. Am J Med Genet C Semin Med Genet. 2014;166:76–84. doi: 10.1002/ajmg.c.31396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen-Torvik LJ, Stallings SC, Gordon AS, et al. Design and Anticipated Outcomes of the eMERGE-PGx Project: A Multicenter Pilot for Preemptive Pharmacogenomics in Electronic Health Record Systems. Clin Pharmacol Ther. 2014;96:482–489. doi: 10.1038/clpt.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pharmacogenomics Research Network. [Accessed November 15, 2015]; http://www.pgrn.org/. [Google Scholar]
- 13.eMERGE Network. [Accessed November 15, 2015]; https://emerge.mc.vanderbilt.edu/. [Google Scholar]
- 14.Bielinski SJ, Olson JE, Pathak J, et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time—using genomic data to individualize treatment protocol. Mayo Clin Proc. 2014;89:25–33. doi: 10.1016/j.mayocp.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altman RB. PharmGKB: a logical home for knowledge relating genotype to drug response phenotype. Nat Genet. 2007;39:426–426. doi: 10.1038/ng0407-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drug Interactions: Cytochrome P450 Drug Interaction Table. [Accessed November 15, 2015];Indiana University School of Medicine. http://medicine.iupui.edu/clinpharm/ddis/clinical-table/. [Google Scholar]
- 17.Relling M, Klein T. CPIC: clinical pharmacogenetics implementation consortium of the pharmacogenomics research network. Clin Pharmacol Ther. 2011;89:464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: From Bench to Byte— An Update of Guidelines. Clin Pharmacol Ther. 2011;89:662–673. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- 19.Stanek EJ, Sanders CL, Taber KAJ, et al. Adoption of Pharmacogenomic Testing by US Physicians: Results of a Nationwide Survey. Clin Pharmacol Ther. 2012;91:450–458. doi: 10.1038/clpt.2011.306. [DOI] [PubMed] [Google Scholar]
- 20.Haga SB, Burke W, Ginsburg GS, Mills R, Agans R. Primary Care Physicians’ Knowledge of and Experience with Pharmacogenetic Testing. Clin Genet. 2012;82:388–394. doi: 10.1111/j.1399-0004.2012.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calzone KA, Cashion A, Feetham S, et al. Nurses Transforming Health Care Using Genetics and Genomics. Nurs Outlook. 2010;58:26–35. doi: 10.1016/j.outlook.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansen Taber KA, Dickinson BD. Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties. Pharmgenomics Pers Med. 2014;7:145–162. doi: 10.2147/PGPM.S63715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullough KB, Formea CM, Berg KD, et al. Assessment of the Pharmacogenomics Educational Needs of Pharmacists. Am J Pharm Educ. 2011;75:51. doi: 10.5688/ajpe75351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Formea CM, Nicholson WT, Vitek CR. An inter-professional approach to personalized medicine education: one institution's experience. Per Med. 2015;12:129–138. doi: 10.2217/pme.14.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whirl?Carrillo M, McDonagh E, Hebert J, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92:414–417. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial InfarctionA Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: american college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e152S–e184S. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohrer Vitek CR, Nicholson WT, Schultz C, Caraballo PJ. Evaluation of the use of clinical decision support and online resources for pharmacogenomics education. Pharmacogenomics. 2015;16:1595–1603. doi: 10.2217/pgs.15.100. [DOI] [PubMed] [Google Scholar]
- 29.Peterson JF, Bowton E, Field JR, et al. Electronic health record design and implementation for pharmacogenomics: a local perspective. Genet Med. 2013;15:833–841. doi: 10.1038/gim.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulley JM, Denny JC, Peterson JF, et al. Operational implementation of prospective genotyping for personalized medicine: The design of the Vanderbilt PREDICT project. Clin Pharmacol Ther. 2012;92:87–95. doi: 10.1038/clpt.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starren J, Williams MS, Bottinger EP. Crossing the omic chasm: a time for omic ancillary systems. JAMA. 2013;309:1237–1238. doi: 10.1001/jama.2013.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kullo IJ, Jarvik GP, Manolio TA, Williams MS, Roden DM. Leveraging the electronic health record to implement genomic medicine. Genet Med. 2013;15:270–271. doi: 10.1038/gim.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CIPIC. Term Standardization for Clinical Pharmacogenetic Test Results Project. [Accessed June 1, 2016]; https://cpicpgx.org/resources/term-standardization/. [Google Scholar]
- 34.Kalman LV, Agundez J, Appell ML, et al. Pharmacogenetic allele nomenclature: International workgroup recommendations for test result reporting. Clin Pharmacol Ther. 2016;99:172–185. doi: 10.1002/cpt.280. [DOI] [PMC free article] [PubMed] [Google Scholar]