Abstract
Purpose
To review current literature on the renin-angiotensin system (RAS)-mediated pathogenic mechanisms and therapeutic targets in ocular diseases.
Methods
A comprehensive literature survey was performed on PubMed, Scopus, and Google Scholar databases published from 1977 to 2016. The search terms were a RAS, angiotensin, angiotensin receptor, prorenin, pro (renin) receptor, angiotensin converting enzyme inhibitor, angiotensin receptor blocker associated with ocular disorders like cataract, glaucoma, diabetic retinopathy (DR), macular degeneration, and uveitis. Articles were reviewed on the basis of the association between ocular disorders and RAS and relevant articles were discussed.
Results
The literature revealed that the individual RAS components including renin, angiotensins, angiotensin converting enzymes, and RAS receptors have been expressed in the specific ocular tissues like retina, choroid, and ciliary body. The activation of both circulatory and local RAS potentiate the various inflammatory and angiogenic signaling molecules, including vascular endothelial growth factor (VEGF), extracellular signal-regulated kinase, and advanced glycation end products (AGE) in the ocular tissues and leads to several blinding disorders like DR, glaucoma, and macular degeneration. The classical and newer RAS inhibitors have illustrated protective effects on blinding disorders, including DR, glaucoma, macular degeneration, uveitis, and cataract.
Conclusions
The RAS components are present in the extrarenal tissues including ocular tissue and have an imperative role in the ocular pathophysiology. The clinical studies are needed to show the role of therapeutic modalities targeting RAS in the treatment of different ocular disorders.
Keywords: Ocular renin-angiotensin system, Ocular disorders, Angiotensin II, Angiotensin II type 1 receptor, (Pro) renin receptor
Introduction
The circulatory renin-angiotensin system (RAS) plays an important role in the regulation of blood pressure, fluid volume, electrolyte balance, and inflammation.1 The circulatory RAS system initiates with renin which cleaves angiotensinogen to form the decapeptide angiotensin I (Ang-I) is then converted to octapeptide angiotensin II (Ang-II) by the angiotensin-converting enzyme (ACE).2 Ang-II regulates various biological effects through the activation of Angiotensin II type I receptors (AT1R) and Angiotensin II type 2 receptors (AT2R). Ang-II elicits most of its well-known biological effects, including vasoconstriction, electrolyte homeostasis, fibrosis, inflammation, and proliferation through activation of AT1R.3, 4, 5 The actions of the AT2R are not so much defined, but they possibly oppose the actions of the AT1R like vasodilatory effects.6 However, findings indicate that AT2R acts similar to AT1R, like promoting cell growth, apoptosis, and angiogenesis in some tissues.7, 8, 9
Plethora researchers highlighted the significance of the local RAS in various extrarenal tissues, including the adrenal glands,10 thymus,11 and ocular tissues.12 The presence and functional role of the RAS components, including prorenin, renin, ACE, angiotensinogen, Ang-II, (pro)renin receptor ((P)RR), and AT1R in the eye have been established in the several species (Table 1). These findings propose that the local RAS plays an important role in the regulation of the ocular physiology. The aim of our present article is to review the role of the RAS in the regulation of various ocular disorders such as diabetic retinopathy (DR), glaucoma, age-related macular degeneration (AMD), uveitis, and cataract, and beneficial effects of RAS regulation through RAS inhibitors in the therapeutic management of such ocular disorders.
Table 1.
Distribution of renin-angiotensin system (RAS) components in ocular tissues in different species.
| RAS components | Localization | Species | References |
|---|---|---|---|
| Prorenin | Retina, vitreous fluids, iris, ciliary body, choroid, sclera, cornea, conjunctiva | Human | 2, 13, 14, 15 |
| Renin | Retina (Muller cells, RPE), iris, vitreous fluid, choroid | Human, rabbit | 2, 13, 16, 17, 18, 19, 20 |
| Ciliary body | Human, rabbit, rat | ||
| Sclera, cornea | Human | ||
| Aqueous fluid | Rabbit | ||
| Angiotensinogen | Retina (Muller cells, RPE), ciliary body, vitreous fluid, choroid, iris | Human, rabbit | 2, 19, 20 |
| Sclera, cornea, conjunctiva | Human | ||
| Aqueous fluid | Rabbit | ||
| Ang-I | Retina, choroid, subretinal fluid | Porcin | 13, 21 |
| Aqueous fluid | Human | ||
| Vitreous fluid | Human, porcine | ||
| Ang-II | Retina (Muller cells, retinal vessel endothelial cells, ganglion cells, photoreceptor cells, subretinal fluid), vitreous fluid, choroid | Human, rabbit, porcine | 19, 21, 22, 23, 24 |
| Ciliary body, aqueous fluid | Human, rabbit | ||
| Cornea | Human | ||
| Iris | Rabbit | ||
| Ang (1–7) | Retinal Muller cells, aqueous humor | Human | 24, 25 |
| ACE | Retina (Muller cells, ganglion cells, retinal vessel endothelial cells, photoreceptor cells), choroid | Human, monkey, dog, rabbit, porcine | 2, 19, 20, 23, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 |
| Ciliary body | Human, rabbit, rat, porcine | ||
| Aqueous fluid | Human, monkey, dog, rabbit | ||
| Vitreous fluid | Monkey, dog, rabbit | ||
| Tear fluid | Human, rabbit | ||
| Cornea, conjunctiva | Human | ||
| Iris | Human, rabbit, porcine | ||
| Sclera | Human, monkey, dog | ||
| ACE2 | Retina | Human, rodent, porcine | 24, 25, 40, 41 |
| Chymase | Vitreous fluid | Human | 32 |
| (P)RR | Retina (Muller cells, RPE, ganglion cells), choroid, iris, ciliary body, cornea, conjunctiva | Human | 2, 42, 43, 44 |
| AT1R | Retina (Muller cells, amacrine cells, RPE, blood vessels, photoreceptors, ganglion cells), choroid, cornea, ciliary body, iris, conjunctiva | Human | 2, 18, 23, 24, 45, 46, 47, 48 |
| AT2R | Retina (Muller cells, nuclei of some inner nuclear layer neurons, and ganglion cell nuclei) | Human | 9, 24 |
| Mas receptor | Retina, ciliary body | Human, Rabbit, rats | 49, 50, 51 |
ACE: angiotensin-converting enzyme; ACE2: angiotensin-converting enzyme type 2; Ang (1–7): angiotensin (1–7); Ang-I: angiotensin I; Ang-II: angiotensin II; AT1R: angiotensin II type 1 receptor; AT2R: angiotensin II type 2 receptor; (P)RR: (pro)renin receptor; RAS: renin-angiotensin system.
Methods
This narrative review was based on a literature search using PubMed, Scopus, and Google Scholar databases from 1977 to 2016. The search terms were a RAS, angiotensin, angiotensin receptor, prorenin, (P)RR, angiotensin converting enzyme inhibitor, angiotensin receptor blocker associated with ocular disorders like cataract, glaucoma, DR, macular degeneration, and uveitis. All article types, including original research articles, reviews, and case reports that described the role of RAS in ocular disorders were selected and reviewed thoroughly by the authors to review RAS-mediated pathogenic mechanisms and therapeutic targets in ocular diseases.
Results
During the literature survey, 180 articles were retrieved from the databases. 148 articles were found relevant to the discussion in the present review. After extensively examining the plethora of literature on the various aspects of ocular RAS, expected to have a pivotal role in the treatment of various ocular disorders, we reviewed the essentiality of the ocular RAS system along with its role in ocular disorders.
Expression of renin-angiotensin system (RAS) components in the eye
Ocular RAS has been the focus of growing interest in the recent year after finding the RAS components in the ocular tissues. The literature concerning that research on the ocular RAS started with a study by Igic and coworkers on the detection of ACE activity in retinal homogenates.52 Thereafter, RAS components in the eye have been established in various research studies (Table 1).
In early studies, RAS components were found in the eye but there was a lake to identify the origin of the ocular RAS, either local production or selective uptake by ocular tissues from the circulatory RAS.13, 14, 52 This question has been refused after findings of Danser et al that the circulatory RAS components, including angiotensinogen, Ang-I, and Ang-II from plasma could not enter into the eye,21 suggesting that RAS components in the ocular tissues are locally synthesized, which is affirmed by Brandt et al after finding the renin mRNA in the eye.17 These findings suggest that the presence of RAS components in ocular tissues play a pivotal role in the ocular pathophysiology.
Ocular renin-angiotensin system (RAS) signaling cascades
Angiotensin II-dependent signaling cascades
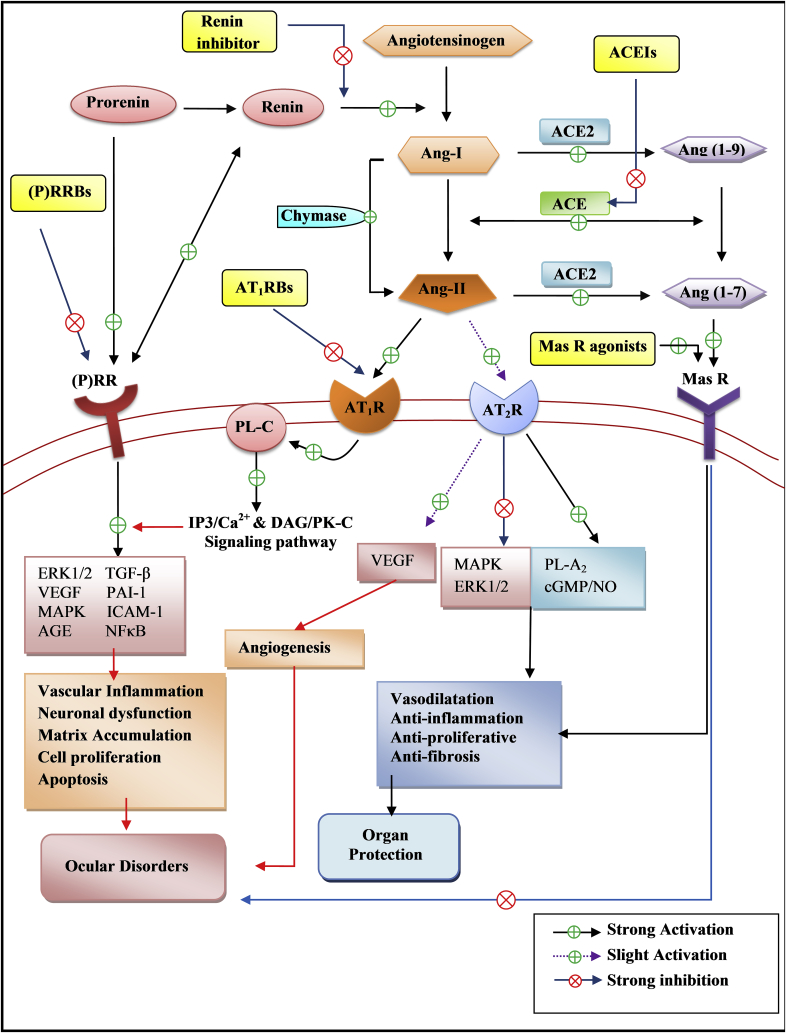
On the basis of literature, RAS signaling cascades in the eye are represented in Fig. 1. The circulatory RAS components are unable to enter into ocular cells,21 but Milenkovic et al found that systemic infusion of Ang-II into mice decreases renin expression in the kidney and reduces the renin mRNA levels in both retinal pigment epithelium (RPE) cells and neuronal retina, whereas systemic application of ACE inhibitors (ACEIs) increased renin expression in RPE by 20-fold, suggesting that the circulatory RAS can modulate the ocular RAS.18 In ocular tissues, Ang-II modulates the ocular physiology either from local production or systemic circulation. Ang-II is produced by classical enzyme ACE and also catalyzed by ACE-independent pathways, e.g. via chymase,53 which is also expressed in the eye.32 In addition, recently angiotensin converting enzyme type 2 (ACE2) is also found in the eye,24, 25, 41 which can catalyze Ang-I to angiotensin (1–9) and Ang-II to angiotensin (1–7), which act oppositely to Ang-II.41 angiotensin (1–7) mainly acts through novel angiotensin receptor type, Mas receptor, a G-protein coupled receptor encoded by Mas proto-oncogene found first in mouse kidney.54 The Mas receptor acts opposite to AT1R to induce vasodilatation, antiproliferation, antifibrosis, and also plays a role in fluid volume homeostasis.55 Mas receptor is also expressed in ocular tissues particularly in the retina and ciliary body.50, 51
Fig. 1.
Schematic representation of ocular renin-angiotensin system signaling cascades on the basis of literature.2, 12, 41, 50, 51, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 ACE: angiotensin-converting enzyme; ACE2: angiotensin-converting enzyme type 2; ACEIs: angiotensin-converting enzyme inhibitors; AGE: advanced glycation end products; Ang (1–7): angiotensin (1–7); Ang (1–9): angiotensin (1–9); Ang-I: angiotensin I; Ang-II: angiotensin II; AT1R: angiotensin II type 1 receptor; AT1RBs: angiotensin II type 1 receptor blockers; AT2R: angiotensin II type 2 receptor; cGMP/NO: cyclic guanosine mono phosphate/nitric oxide; DAG: diacyl glycerol; ERK1/2: extracellular signal regulated kinase 1/2; ICAM-1: Intercellular adhesion molecule-1; IP3: inositole-1,4,5-triphosphate; MAPK: mitogen-activated protein kinase; Mas R: Mas receptor; NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells; PAI-1: plasminogen activator inhibitor-1; PK-C: protein kinase-C; PLA2: phospholipase-A2; PL-C: phospholipase-C; (P)RR: (pro)renin receptor; (P)RRBs: (pro)renin receptor blockers; TGF-β: transforming growth factor- β; VEGF: vascular endothelial growth factor.
In the eye, Ang-II activates AT1R, a G-protein coupled receptor which is associated with Gq protein and triggers inositole-1,4,5-triphosphate (IP3)/Ca2, 56, 57 and diacyl glycerol/protein kinase-C (DAG/PK-C) signaling cascades,58, 59 which leads to increase intracellular Ca2+ through transient release of Ca2+ from endoplasmic reticulum via IP3 receptor and transient receptor potential-V2 (TRPV2) channels, which is present in the RPE cells.56, 57 The Ang-II/AT1R mediated IP3/DAG signaling cascades further potentiates of inflammatory/angiogenic molecules in the diseases conditions, such as vascular endothelial growth factor (VEGF),60, 61, 62, 63, 64 extracellular signal-regulated kinase (ERK),65, 66 mitogen-activated protein kinase (MAPK),66 nuclear factor-kappaB (NF-κB), intracellular adhesion molecule-1 (ICAM-1),67 transforming growth factor-β1 (TGF-β1),68 nicotinamide adenine dinucleotide phosphate (NADP(H)) oxidase69 and advanced glycation end products (AGE) accumulation70, 71 thus leading to disruption of intracellular signaling and cellular growth. These findings provide strong evidence that RAS, especially Ang-II/AT1R signaling, is not just a regulator of cardio-renal physiology but also regulates an inflammatory and ocular physiology.
Angiotensin II independent signaling cascades
Apart from AT1R, (P)RR also regulates blood pressure and cell function, including proliferation, angiogenesis, inflammation, and stimulation of growth factor.72 The (P)RR binds both with renin and prorenin to exert the catalytic efficiency of renin and activate (P)RR without conventional proteolysis of prorenin prosegments,73, 74 thus induces signal transduction pathway that is independent to Ang-II. Renin and prorenin not only activate the (P)RR but also increase the formation of Ang-II, through increase in renin activity.68 Moreover, binding of renin and prorenin also stimulate phosphorylation of (P)RR on serine and tyrosine residues, which associated with phosphorylation of ERK1/2 and an induction of MAPK.74 Huang et al (2007) reported similar findings that prorenin binds with (P)RR, induces TGF-β, fibronectin, and collagen via ERK1/2.75 These findings suggest that inhibition of (P)RR might play an important role in organ protection that cannot be achieved with conventional Ang-II blockade. Thus, (P)RR regulates the organ physiology, including cardiorenal73, 76 and ocular functions.42, 77
All of these possible signaling pathways (Fig. 1) play an important role in the regulation of ocular pathophysiology. The effects of these signaling cascades of the ocular RAS may be controlled with RAS inhibitors such as ACEIs, angiotensin II Type 1 receptor blockers (AT1RBs), and (P)RR blockers ((P)RRBs).
Ocular disorders and renin-angiotensin system (RAS)
The ocular disorders like DR, AMD, glaucoma, and cataract are leading causes of blindness worldwide.78 All of these blinding disorders (except cataract) occur in the retina, which consists of neurons, glia, pigment epithelium and blood vessels. These ocular disorders are associated with the local or systemic neuronal and vascular homeostasis.12 The findings of the ocular RAS in retinal cells imply various physiological functions within the eyes and associated with those blinding disorders. In the eye, Ang-II has an important role in the ocular pathophysiology; the above discussions illustrates that Ang-II and its receptor, AT1R mostly abundant in retinal cells, including Muller cells, RPE, blood vessels, and ganglion cells and are involved in the pathogenesis of those blinding ocular disorders. The RAS signaling pathways in the particular ocular diseases are represented in Table 2.
Table 2.
Renin-angiotensin system (RAS) signaling pathways in ocular disorders.
| Diseases | RAS Signaling pathway | Species | RAS modulators | References |
|---|---|---|---|---|
| Diabetic retinopathy | AT1R and (P)RR signaling potentiate angiogenic and inflammatory action in the eye. | Human, rat, mice, bovine | ACEIs protect DR by reducing the over expression of VEGF in retina. AT1RBs protect DR by reducing inflammatory response and oxidative stress in the eye. (P)RRB abolishes the angiogenic action of ERK signaling molecules. ACE2 protects retinal ganglion cell death. |
61, 62, 63, 67, 69, 70, 79, 80, 81, 82, 83, 84, 85, 86, 87 |
| Glaucoma | AT1R signaling regulates aqueous humor formation, secretion, uveoscleral outflow, and IOP. Mas receptor signaling reduces the IOP. |
Human, monkey, rabbit, rat, bovine | ACEIs reduce IOP by reducing aqueous humor formation and increasing uveoscleral outflow. AT1RBs reduce IOP by increasing uveoscleral outflow. Ang (1–7) reduces IOP via Mas receptor signaling pathway. ACE2 activation reduces IOP. |
49, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97 |
| Age-related macular degeneration | AT1R and (P)RR signaling potentiate macular degeneration in the eye. | Human, rat, mice | ACEIs, AT1RBs, and (P)RRBs prevent progression of choroidal neo-vascularization through suppression of inflammatory response of RAS signaling. | 66, 98, 99, 100, 101 |
| Uveitis | AT1R and (P)RR signaling potentiate ocular inflammation. | Rat, mice | AT1RBs and (P)RRBs downregulate the expression of inflammatory molecules. ACE2 activation protects endotoxin-induced uveitis. |
42, 102, 103, 104, 105, 106, 107 |
| Cataract | RAS activation potentiates oxidative stress and ionic imbalance in the eye lenses. | Rat | ACEIs prevent the progression of cataract by restoring antioxidants defense system and ionic imbalance. | 108, 109, 110 |
ACE2: angiotensin-converting enzyme type 2; ACEIs: angiotensin-converting enzyme inhibitors; Ang (1–7): angiotensin (1–7); AT1R: angiotensin II type 1 receptor; AT1RBs: angiotensin II type 1 receptor blockers; IOP: intra ocular pressure; (P)RR: (pro)renin receptor; (P)RRBs: (pro)renin receptor blockers; RAS: renin-angiotensin system; VEGF: vascular endothelial growth factor.
Diabetic retinopathy
DR is one of the most common microvascular complication of diabetes mellitus.111 Many factors are involved in the pathogenesis of DR such as metabolic disorders like hyperglycemia, high blood pressure, hyperlipidemia, age, and oxidative stress.59, 112 It is reported that hyperglycemia induces the inflammatory response, oxidative stress,12 AGE accumulation,70, 71 expression of growth factors, including VEGF,113, 114 TGF-β, pigment epithelium-derived growth factor,58 insulin-like growth factor-159 in the eye and finally leads to the development of DR.
Several clinical and experimental research have shown that RAS plays an important role in the progression of DR,12, 60, 115 presumably through Ang-II/AT1R mediated actions.116, 117, 118 Ang-II potentiates VEGF/VEGFR-2 mediated angiogenesis63, 114 and increase the permeability of retinal blood vessels, thus, it may increase the risk of neovascularization119 and hyperpermeability.120 Ang-II, VEGF,121, 122 and prorenin13, 123 have found to be overexpressed in the vitreous humor of proliferative diabetic retinopathy (PDR) and DR patients. ACEIs have been shown to produce the protective effect on DR through reduction of retinal VEGF/VEGFR-2 overexpression in various preclinical and clinical studies.61, 62, 79, 80 Whereas, Pradhan et al (2002) found that enalapril, an ACE inhibitor, at low dose did not significantly reduce the progression of moderate to severe DR in normotensive Type 2 diabetic patients,124 suggesting that low dose of ACEIs did not block the ocular RAS sufficiently enough to exert an effect. Moreover, it was also found that AT1RBs effectively block diabetes-induced inflammatory response and oxidative stress in the eye such as VEGF,63, 67, 81 AGE,81 NF-κB, ICAM-1,67 NADP(H) oxidase69, 82 and enhance the neuroprotective markers, including brain-derived neurotrophic factor, ciliary neurotrophic factor, tyrosine hydroxylase, glutathione and caspase activity.69 Furthermore, Miller et al found that AT1RBs also restore the Ang-II mediated downregulation of glyoxalase-I in retinal vascular cells, which is a key regulator of AGE formation.70
Although these multiple events indicate that blockade of AT1R may have beneficial effects on DR, the Diabetic Retinopathy Candesartan Trials (DIRECT) failed to show a beneficial effect of Candesartan on retinopathy progression in the type 1 diabetes patients.125 Failure of the DIRECT programme suggested that the pathogenesis of DR also independent from Ang-II/AT1R signaling. This hypothesis is supported by the biological action of a novel receptor (P)RR, a part of the RAS signaling, which is present in retinal Muller cells,42 which is a site of VEGF synthesis and its tyrosine kinase receptors,126 indicating an independent role in the pathogenesis of DR. Further studies provide the evidence that (P)RR triggers the expression of angiogenic molecules, including VEGF/VEGFR2, ERK1/2 and TGFβ1 in the retinal cells and leads to DR, which was abolished by (P)RR/ERK signaling blockade.83, 84, 85, 86 Recently Foureaux et al found that the activation of ACE2 reduced the death of retinal ganglion cells in hyperglycemic rats.87 The overall findings suggest that the RAS is strongly involved in the pathogenesis of DR and inhibition of these RAS signaling events may have a beneficial effects on the reduction and prevention of DR and improves aspects of vascular and neuroglial injury in diabetic retina.
Glaucoma
Glaucoma, is the multifactorial long-term ocular neuropathy, is generally associated with a progressive loss of retinal nerve fibers and visual field.127 It is characterized by elevated intraocular pressure (IOP) and long-term ocular neuropathy, which is associated with several risk factors, including systemic hypertension, vascular dysfunction, and diabetes.128, 129, 130, 131 The most important pathophysiological feature of the diseases is neurodegeneration of retinal ganglion cells that leads to increasing IOP. Most of the RAS components including ACE, Ang-II, and AT1R are present in retinal ganglion cells and ciliary body, which regulate IOP in the eye. It is known that ciliary body secrete aqueous humor. Cullinane et al found that the RAS components in cultured human non-pigmented ciliary epithelial cells are particularly responsible for aqueous humor formation and secretion.88 It is reported that Ang-II induces cell proliferation in bovine trabecular meshwork cells,89 which is involved in aqueous humor outflow and diminishes uveoscleral out-flow.90 These findings indicate that the ocular RAS may implicate in the formation of aqueous humor, its drainage and regulation of IOP. Therefore, several researchers showed that inhibition of RAS through ACE inhibition91, 92 and AT1R blockade93, 94 have beneficial effects in both normotensive and glaucomatous eyes. ACEIs trigger the synthesis of prostaglandins by preventing the breakdown of bradykinin, which leads to lowering of IOP by increasing uveoscleral outflow.95 Additionally, it also reduces aqueous humor formation by reducing blood flow in the ciliary body.96 AT1RBs may be slightly increased uveoscleral outflow94 and effectively suppresses retinal ganglion cell death.132, 133
Moreover, the Mas receptor50 and ACE225 are also expressed in the ocular tissues, which may also regulate the ocular physiology. Thereby, Mas receptor activator, angiotensin (1–7),49 and ACE2 activator, diminazene aceturate (DIZE)87, 97 showed beneficial effects for glaucoma management via a decrease in IOP. These findings indicate that RAS inhibition may be effective for treatment of glaucoma.
Age-related macular degeneration
The World Health Organization reported that AMD is responsible for 8.7% of blindness worldwide.77 Generally, it is characterized by choroidal neovascularization (CNV; wet AMD), and atrophy of RPE and photoreceptor cells (dry AMD).12 Wet AMD is developed through ocular inflammation, infiltration of macrophages, and AGE formation, and the main mediator is VEGF.134, 135, 136 At present, wet AMD is treated with the VEGF inhibitors.137, 138 The presence of RAS components in the ocular tissues including RPE, choroid, and photoreceptor cells and its inflammatory response, suggesting that deregulation of RAS may enhance the risk of AMD.98, 139, 140 The activation of AT1R and (P)RR in the eye, potentiate the ERK1/2, VEGF, ICAM-1, and monocyte chemoattractant protein-1expression in the ocular tissues and leads to DR and AMD.99, 100 Therefore, AT1RBs,66, 101 ACEIs,98 and (P)RRBs100 prevent progression of CNV through suppression of such inflammatory molecules. These findings suggested that the controlling of RAS may provide a pivotal strategy to reduce the progression of wet AMD. At present, effective therapies are not available for dry AMD, but nutritional supplements may slow the progression of the disease.141 Alcazar et al showed (P)RR is involved in the pathology of dry AMD, suggesting that in the future, RAS may play an important role to manage dry AMD.44
Uveitis
Hyperactivation of RAS involved in the overexpression of the inflammatory response and immune function. It stimulates accumulation of neutrophils,142 differentiation of dendritic cells143 and production of inflammatory chemokines by vascular endothelial cells.144 Endotoxin-induced uveitis42, 102 and experimental autoimmune uveoretinitis103 models upregulate expression of proinflammatory and adhesion molecules like ICAM-1, interferon-γ and interleukin-6. These molecules inhibited by AT1RBs102, 103, 104, 105, 106 and (P)RRBs.42 Recently, Qiu et al reported that activation of endogenous ACE2 by DIZE showed the preventive effects on endotoxin-induced uveitis mouse model.107 All these findings support that the regulation of RAS may play a beneficial role in the treatment of uveitis.
Cataract
Cataractous opacification of the lens is one of leading causes of visual dysfunction and contributes to 50% of blindness worldwide.145 Progression of cataract depends on several risk factors such as diabetes and systemic hypertension.146 At present, there is no evidence to find the presence of RAS components in eye lenses, but hyperactivation of RAS through diabetes and systemic hypertension65, 147, 148 can modulate the production of AGE,70, 71 reactive oxygen species,58 and electrolyte homeostasis,94 which might be responsible for the increase in the incidence of cataract formation. Moreover, we previously reported that RAS activation via two-kidney, one clip model significantly modulates the oxidative stress and ionic imbalance in eye lenses and further leads to the development of cataract in the hypertensive state, which is prevented by administration of angiotensin converting enzyme inhibitor (ramipril).108 Additionally, several researchers found that ACE inhibition showed beneficial effects in reduction of the cataract through the restoration of the ionic balance (Na+/K+), free radical scavenging activity, enhanced the enzymatic and non-enzymatic defense mechanism as well as inhibition of AGE production.109, 110 Therefore, it may be hypothesized that the ocular RAS has an important role to play in regulation of lenticular physiology and blockade of Ang-II mediated action through ACEIs and AT1RBs may reduce the progression of cataract particularly in diabetes and hypertensive conditions.
In conclusion, the classical RAS are known as blood pressure as well as electrolyte homeostasis regulator. Recently, it has been recognized as a proinflammatory mediator and involved in the various age-related ocular disorders through exacerbation of the inflammatory molecules. The findings of the RAS components in the eye initiate a new therapeutic approach to attenuate the ocular disorders through RAS inhibitors such as ACEIs and AT1RBs. The new RAS modulators like renin inhibitors, (P)RRBs, AT2R, Mas receptor have shown potential role in the circulatory as well as local RAS modulation and had beneficial effects on the management of cardio-renal and ocular disorders. The present review describes the ocular RAS in the pathophysiology of such ocular disorders and effects of classical and newer RAS inhibitors in respect of pathogenic inflammatory molecules that elicit the newer approach in ophthalmic research. In future novel RAS components like Ang-III, Ang-IV, and its receptor AT4R may also have an important ocular physiology. Therefore, the work to develop the novel and selective RAS inhibitors may hold great promise to attenuate ocular disorders and help to treat life-threatening blinding disorders.
Footnotes
Peer review under responsibility of the Iranian Society of Ophthalmology.
Funding information: None.
Declaration: The authors declare that the present manuscript has not been published, accepted or under editorial review for publication elsewhere.
Conflict of interest: All authors have none to declare.
References
- 1.Fyhrquist F., Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008;264:224–236. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White A.J.R., Cheruvu S.C., Sarris M. Expression of classical components of the renin-angiotensin system in the human eye. J Renin Angiotensin Aldosterone Syst. 2015;16:59–66. doi: 10.1177/1470320314549791. [DOI] [PubMed] [Google Scholar]
- 3.Cheng Z.J., Vapaatalo H., Mervaala E. Angiotensin II and vascular inflammation. Med Sci Monit. 2005;11:RA194–205. [PubMed] [Google Scholar]
- 4.Culman J., Hohle S., Qadri F. Angiotensin as neuromodulator/neurotransmitter in central control of body fluid and electrolyte homeostasis. Clin Exp Hypertens. 1995;17:281–293. doi: 10.3109/10641969509087071. [DOI] [PubMed] [Google Scholar]
- 5.Qi G.M., Jia L.X., Li X.L., Du J. Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology. 2014;155:2254–2265. doi: 10.1210/en.2013-2011. [DOI] [PubMed] [Google Scholar]
- 6.Chung O., Kuhl H., Stoll M., Unger T. Physiological and pharmacological implications of AT1 versus AT2 receptors. Kidney Int. 1998;67:S95–S99. doi: 10.1046/j.1523-1755.1998.06719.x. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z., Kelly D.J., Cox A. Angiotensin type 2 receptor is expressed in the adult rat kidney and promotes cellular proliferation and apoptosis. Kidney Int. 2000;58:2437–2451. doi: 10.1046/j.1523-1755.2000.00427.x. [DOI] [PubMed] [Google Scholar]
- 8.Levy B.I., Benessiano J., Henrion D. Chronic blockade of AT2-subtype receptors prevents the effect of angiotensin II on the rat vascular structure. J Clin Invest. 1996;98:418–425. doi: 10.1172/JCI118807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarlos S., Rizkalla B., Moravski C.J., Cao Z., Cooper M.E., Wilkinson-Berka J.L. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol. 2003;163:879–887. doi: 10.1016/S0002-9440(10)63448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong P., Wilkinson-Berka J.L., Skinner S.L. Control of renin secretion from adrenal gland in transgenic Ren-2 and normal rats. Mol Cel Endocrinol. 2001;173:203–212. doi: 10.1016/s0303-7207(00)00406-8. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson-Berka J.L., Kelly D.J., Rong P., Campbell D.J., Skinner S.L. Characterization of a thymic renin-angiotensin system in the transgenic m(Ren-2)27 rat. Mol Cel Endocrinol. 2002;194:201–209. doi: 10.1016/s0303-7207(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 12.Kurihara T., Ozawa Y., Ishida S., Okano H., Tsubota K. Renin angiotensin system hyperactivation can induce inflammation and retinal neural dysfunction. Int J Inflam. 2012;581695:14. doi: 10.1155/2012/581695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danser A.H., Van den Dorpel M.A., Deinum J. Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. J Clin Endocrinol Metab. 1989;68:160–167. doi: 10.1210/jcem-68-1-160. [DOI] [PubMed] [Google Scholar]
- 14.Sramek S.J., Wallow I.H., Day R.P., Ehrlich E.N. Ocular renin-angiotensin: immunohistochemical evidence for the presence of prorenin in eye tissue. Invest Ophthalmol Vis Sci. 1988;29:1749–1752. [PubMed] [Google Scholar]
- 15.Wallow I.H.L., Sramek S.J., Bindley C.D., Darjatmoko S.R., Gange S.J. Ocular rennin angiotensin: immunocytochemical localization of prorenin. Curr Eye Res. 1993;12:945–950. doi: 10.3109/02713689309020401. [DOI] [PubMed] [Google Scholar]
- 16.Berka J.L., Stubbs A.J., Wang D.J. Renin-containing Muller cells of the retina display endocrine features. Invest Ophthalmol Vis Sci. 1995;36:1450–1458. [PubMed] [Google Scholar]
- 17.Brandt C.R., Pumfery A.M., Micales B. Renin mRNA is synthesized locally in rat ocular tissues. Curr Eye Res. 1994;13:755–763. doi: 10.3109/02713689409047011. [DOI] [PubMed] [Google Scholar]
- 18.Milenkovic V.M., Brockmann M., Meyer C. Regulation of the renin expression in the retinal pigment epithelium by systemic stimuli. Am J Physiol Ren Physiol. 2010;299:F396–F403. doi: 10.1152/ajprenal.00576.2009. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez M., Davidson E.A., Luttenauer L. J Ocul Pharmacol Ther. 1996;12:299–312. doi: 10.1089/jop.1996.12.299. [DOI] [PubMed] [Google Scholar]
- 20.Wagner J., Jan Danser A.H., Derkx F.H. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol. 1996;80:159–163. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danser A.H., Derkx F.H., Admiraal P.J., Deinum J., De Jong P.T., Schalekamp M.A. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci. 1994;35:1008–1018. [PubMed] [Google Scholar]
- 22.Goel A.K., Jabbour N.M. Vitreous level of angiotensin-II in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 1991;32:1027. [Google Scholar]
- 23.Savaskan E., Loffler K., Meier F., Muller F.S., Flammer J., Meyer P. Immunohistochemical localization of angiotensin-converting enzyme, angiotensin II and angiotensin AT1 receptor in human ocular tissues. Ophthalmic Res. 2004;36:312–320. doi: 10.1159/000081633. [DOI] [PubMed] [Google Scholar]
- 24.Senanayake P., Drazba J., Shadrach K. Angiotensin II and its receptor subtypes in the human retina. Invest Ophthalmol Vis Sci. 2007;48:3301–3311. doi: 10.1167/iovs.06-1024. [DOI] [PubMed] [Google Scholar]
- 25.Holappa M., Valjakka J., Vaajanen A. Angiotensin(1–7) and ACE2, “The hot spots” of renin-angiotensin system, detected in the human aqueous humor. Open Ophthalmol J. 2015;9:28–32. doi: 10.2174/1874364101509010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari D.G., Ryan J.W., Rockwood E.J., Davis E.B., Anderson D.R. Angiotensin-converting enzyme in bovine, feline, and human ocular tissues. Invest Ophthalmol Vis Sci. 1988;29:876–881. [PubMed] [Google Scholar]
- 27.Geng L., Persson K., Nilsson S.F.E. Angiotensin converting enzyme (ACE) activity in porcine ocular tissue: effects of diet and ACE inhibitors. J Pharm Ther. 2003;19:589–596. doi: 10.1089/108076803322660503. [DOI] [PubMed] [Google Scholar]
- 28.Igic R., Kojovic V. Angiotensin I converting enzyme (kininase II) in ocular tissues. Exp Eye Res. 1980;30:299–303. doi: 10.1016/0014-4835(80)90010-x. [DOI] [PubMed] [Google Scholar]
- 29.Ikemoto F., Yamamoto K. Renin angiotensin system in the aqueous humor of rabbits, dogs and monkeys. Exp Eye Res. 1978;27:723–725. doi: 10.1016/0014-4835(78)90042-8. [DOI] [PubMed] [Google Scholar]
- 30.Immonen I., Friberg K., Sorsila R., Fyhrquist F. Concentration of angiotensin-converting enzyme in tears of patients with sarcoidosis. Acta Ophthalmol Copenh. 1987;65:27–29. doi: 10.1111/j.1755-3768.1987.tb08486.x. [DOI] [PubMed] [Google Scholar]
- 31.Kida T., Ikeda T., Nishimura M. Renin-angiotensin system in proliferative diabetic retinopathy and its gene expression in cultured human Muller cells. J Ophthalmol. 2003;47:36–41. doi: 10.1016/s0021-5155(02)00624-x. [DOI] [PubMed] [Google Scholar]
- 32.Maruichi M., Oku H., Takai S. Measurement of activities in two different angiotensin II generating systems, chymase and angiotensin-converting enzyme, in the vitreous fluid of vitreoretinal diseases: a possible involvement of chymase in the pathogenesis of macular hole patients. Curr Eye Res. 2004;29:321–325. doi: 10.1080/02713680490516161. [DOI] [PubMed] [Google Scholar]
- 33.Sharma O.P., Vita J.B. Determination of angiotensin converting enzyme in tears: a noninvasive test for evaluation of ocular sarcoidosis. Arch Ophthalmol. 1983;101:559–561. doi: 10.1001/archopht.1983.01040010559004. [DOI] [PubMed] [Google Scholar]
- 34.Shiota N., Saegusa Y., Nishimura K., Miyazaki M. Angiotensin II generating system in dog and monkey ocular tissues. Clin Exp Pharmacol Physiol. 1997;24:243–248. doi: 10.1111/j.1440-1681.1997.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 35.Strittmatter S.M., Braas K.M., Snyder S.H. Localisation of angiotensin converting enzyme in the ciliary epithelium of the rat eye. Invest Ophthalmol Vis Sci. 1989;30:2209–2214. [PubMed] [Google Scholar]
- 36.Vita J.B., Anderson J.A., Hulem C.D., Irving H.L. Angiotensin converting enzyme activity in ocular fluids. Invest Ophthalmol Vis Sci. 1981;20:255–257. [PubMed] [Google Scholar]
- 37.Ward P.E., Stewart T.A., Hammon K.J., Reynolds R.C., Igic R. Angiotensin I converting enzyme (kininase II) in isolated retinal microvessels. Life Sci. 1979;24:1419–1424. doi: 10.1016/0024-3205(79)90013-4. [DOI] [PubMed] [Google Scholar]
- 38.Weinreb R.N., Sandman R., Ryder M.I., Friberg T.R. Angiotensin converting enzyme activity in human aqueous humor. Arch Ophthalmol. 1985;103:34–36. doi: 10.1001/archopht.1985.01050010038013. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J.Z., Gao L., Windness M., Xi X., Kern T. Captopril inhibits glucose accumulation in retinal cells in diabetes. Invest Ophthalmol Vis Sci. 2003;44:4001–4005. doi: 10.1167/iovs.02-1193. [DOI] [PubMed] [Google Scholar]
- 40.Luhtala S., Vaajanen A., Oksala O., Valjakka J., Vapaatalo H. Activities of angiotensin-converting enzymes ACE1 and ACE2 and inhibition by bioactive peptides in porcine ocular tissues. J Ocul Pharmacol Ther. 2009;25:23–28. doi: 10.1089/jop.2008.0081. [DOI] [PubMed] [Google Scholar]
- 41.Tikellis C., Johnston C.I., Forbes J.M. Identification of angiotensin converting enzyme 2 in the rodent retina. Curr Eye Res. 2004;29:419–427. doi: 10.1080/02713680490517944. [DOI] [PubMed] [Google Scholar]
- 42.Satofuka S., Ichihara A., Nagai N. Suppression of ocular inflammation in endotoxin-induced uveitis by inhibiting nonproteolytic activation of prorenin. Invest Ophthalmol Vis Sci. 2006;47:2686–2692. doi: 10.1167/iovs.05-1458. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson-Berka J.L., Miller A.G., Fletcher E.L. Prorenin and the (pro) renin receptor: do they have a pathogenic role in the retina? Front Biosci Elite Ed. 2010;2:1054–1064. doi: 10.2741/e163. [DOI] [PubMed] [Google Scholar]
- 44.Buschini E., Fea A.M., Lavia C.A. Recent developments in the management of dry age-related macular degeneration. Clin Ophthalmol. 2015;9:563–574. doi: 10.2147/OPTH.S59724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Downie L.E., Vessey K., Miller A. Neuronal and glial cell expression of angiotensin II type 1 (AT1) and type 2 (AT2) receptors in the rat retina. Neuroscience. 2009;161:195–213. doi: 10.1016/j.neuroscience.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 46.Fletcher E.L., Phipps J.A., Ward M.M., Vessey K.A., Wilkinson-Berka J.L. The renin-angiotensin system in retinal health and disease: its influence on neurons, glia and the vasculature. Prog Retin Eye Res. 2010;29:284–311. doi: 10.1016/j.preteyeres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Murata M., Nakagawa M., Takahashi S. Expression and localization of angiotensin II type I receptor mRNA in rat ocular tissues. Ophthalmologica. 1997;211:384–386. doi: 10.1159/000310835. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler-Schilling T.H., Kohler K., Sautter M., Guenther E. Angiotensin II receptor subtype gene expression and cellular localization in the retina and non-neuronal ocular tissues of the rat. Euro J Neurosci. 1999;11:3387–3394. doi: 10.1046/j.1460-9568.1999.00787.x. [DOI] [PubMed] [Google Scholar]
- 49.Vaajanen A., Vapaatalo H., Kautiainen H., Oksala O. Angiotensin (1–7) reduces intraocular pressure in the normotensive rabbit eye. Invest Ophthalmol Vis Sci. 2008;49:2557–2562. doi: 10.1167/iovs.07-1399. [DOI] [PubMed] [Google Scholar]
- 50.Vaajanen A., Lakkisto P., Virtanen I. Angiotensin receptors in the eyes of arterial hypertensive rats. Acta Ophthalmol. 2010;88:431–438. doi: 10.1111/j.1755-3768.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 51.Vaajanen A., Kalesnykas G., Vapaatalo H., Uusitalo H. The expression of Mas-receptor of the renin–angiotensin system in the human eye. Graefes Arch Clin Exp Ophthalmol. 2015;253:1053–1059. doi: 10.1007/s00417-015-2952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Igic R., Robinson C.J.G., Erdos E.G. Angiotensin I converting enzyme activity in the choroid plexus and in the retinal. In: Buckley J.P., Ferrario C.M., editors. Central actions of Angiotensin and Related Hormones. Pergamon Press; New York: 1977. pp. 23–27. [Google Scholar]
- 53.Kramkowski K., Mogielnicki A., Buczko W. The physiological significance of the alternative pathways of angiotensin II production. J Physiol Pharmacol. 2006;57:529–539. [PubMed] [Google Scholar]
- 54.Santos R.A., Simoes e Silva A.C., Maric C. Angiotensin (1–7) is an endogenous ligand for the G-protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;8:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kostenis E., Milligan G., Christopoulos A. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type I receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 56.Barro-Soria R., Stindl J., Muller C., Foeckler R., Todorov V., Castrop H. Angiotensin-2-mediated Ca2+ signaling in the retinal pigment epithelium: role of angiotensin-receptor-associated-protein and TRPV2 channel. PLoS One. 2012;7:e49624. doi: 10.1371/journal.pone.0049624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fellner S.K., Arendshorst W.J. Angiotensin II Ca2+signaling in rat afferent arterioles: stimulation of cyclic ADP ribose and IP3 pathways. Am J Physiol Ren Physiol. 2005;288:F785–F791. doi: 10.1152/ajprenal.00372.2004. [DOI] [PubMed] [Google Scholar]
- 58.Boehm I.H., Sosna T., Andersen H.L., Porta M. The eyes in diabetes and diabetes through the eyes. Diabetes Res Clin Prac. 2007;78S:S51–S58. [Google Scholar]
- 59.Tarr J.M., Kaul K., Chopra M., Kohner E.M., Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;34:35–60. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Funastu H., Yamashita H. Pathogenesis of diabetic retinopathy and the renin angiotensin system. Ophthalmic Physiol Opt. 2003;23:495–501. doi: 10.1046/j.1475-1313.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- 61.Gilbert R.E., Kelly D.J., Cox A.J. Angiotensin converting enzyme inhibition reduces retinal overexpression of vascular endothelial growth factor and hyperpermeability in experimental diabetes. Diabetologia. 2000;43:1360–1367. doi: 10.1007/s001250051539. [DOI] [PubMed] [Google Scholar]
- 62.Moravski C.J., Kelly D.J., Cooper M.E. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000;36:1099–1104. doi: 10.1161/01.hyp.36.6.1099. [DOI] [PubMed] [Google Scholar]
- 63.Nagisa Y., Shintani A., Nakagawa S. The angiotensin II receptor antagonist candesartan cilexetil (TCV-116) ameliorates retinal disorders in rats. Diabetologia. 2001;44:883–888. doi: 10.1007/s001250100556. [DOI] [PubMed] [Google Scholar]
- 64.Sjolie A.K., Chaturvedi N. The retinal renin-angiotensin system: implications for therapy in diabetic retinopathy. J Hum Hypertens. 2002;16:S42–S46. doi: 10.1038/sj.jhh.1001438. [DOI] [PubMed] [Google Scholar]
- 65.Kurihara T., Ozawa Y., Nagai N. Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes. 2008;57:2191–2198. doi: 10.2337/db07-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pons M., Cousins S.W., Alcazar O., Striker G.E., Marin-Castano M.E. Angiotensin II-induced MMP-2 activity and MMP-14 and basigin protein expression are mediated via angiotensin II receptor type 1-mitogen-activated protein kinase 1 pathway in retinal pigment epithelium. Am J Pathol. 2011;178:2665–2681. doi: 10.1016/j.ajpath.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagai N., Izumi-Nagai K., Oike Y. Suppression of diabetes induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci. 2007;48:4342–4350. doi: 10.1167/iovs.06-1473. [DOI] [PubMed] [Google Scholar]
- 68.Wilkinson-Berka J.L., Campbell D.J. (Pro)renin receptor: a treatment target for diabetic retinopathy? Diabetes. 2009;58:1485–1487. doi: 10.2337/db09-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ola M.S., Ahmed M., Abuohashish H.M., Al-Rejaie S.S., Alhomida A.S. Telmisartan ameliorates neurotrophic support and oxidative stress in the retina of streptozotocin-induced diabetic rats. Neurochem Res. 2013;38:1572–1579. doi: 10.1007/s11064-013-1058-4. [DOI] [PubMed] [Google Scholar]
- 70.Miller A.G., Tan G., Binger B.J. Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase-I function. Diabetes. 2010;59:3208–3215. doi: 10.2337/db10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilkinson-Berka J.L. Angiotensin and diabetic retinopathy. Int J Biochem Cell Biol. 2006;38:752–765. doi: 10.1016/j.biocel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Wilkinson-Berka J.L. Prorenin and the (pro)renin receptor in ocular pathology. Am J Pathol. 2008;173:1591–1594. doi: 10.2353/ajpath.2008.080757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ichihara A., Hayashi M., Kaneshiro Y. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114:1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguyen G., Delarue F., Burckle C., Bouzhir L., Giller T., Sraer J.D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Y., Noble N.A., Zhang J., Xu C., Border W.A. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007;72:45–52. doi: 10.1038/sj.ki.5002243. [DOI] [PubMed] [Google Scholar]
- 76.Kaneshiro Y., Ichihara A., Sakoda M. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18:1789–1795. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- 77.Satofuka S., Ichihara A., Nagai N. Role of nonproteolytically activated prorenin in pathologic, but not physiologic, retinal neovascularization. Invest Ophthalmol Vis Sci. 2007;48:422–429. doi: 10.1167/iovs.06-0534. [DOI] [PubMed] [Google Scholar]
- 78.Vision 2020: The Right to Sight. Global Initiative for the Elimination of Avoidable Blindness: Action Plan 2006–2011. World Health Organization. http://www.who.int/blindness/Vision2020_report.pdf.
- 79.Hogeboom B.I.M., Polak B.C., Reichert-Thoen J.W. Angiotensin converting enzyme inhibiting therapy is associated with lower vitreous vascular endothelial growth factor concentrations in patients with proliferative diabetic retinopathy. Diabetologia. 2002;45:203–209. doi: 10.1007/s00125-001-0747-8. [DOI] [PubMed] [Google Scholar]
- 80.Zheng Z., Chen H., Ke G. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to-pigment epithelium-derived factor ratio: involvement of a mitochondria-reactive oxygen species pathway. Diabetes. 2009;58:954–964. doi: 10.2337/db07-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sugiyama T., Okuno T., Fukuhara M. Angiotensin II receptor blocker inhibits abnormal accumulation of advanced glycation end products and retinal damage in a rat model of type 2 diabetes. Exp Eye Res. 2007;85:406–412. doi: 10.1016/j.exer.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 82.White A.J.R., Heller J.P., Leung J., Tassoni A., Martin K.R. Retinal ganglion cell neuroprotection by an angiotensin II blocker in an ex vivo retinal explant model. J Renin Angiotensin Aldosterone Syst. 2015;16:1193–1201. doi: 10.1177/1470320314566018. [DOI] [PubMed] [Google Scholar]
- 83.Haque R., Hur E.H., Farrell A.N., Iuvone P.M., Howell J.C. MicroRNA-152 represses VEGF and TGFβ1 expressions through post-transcriptional inhibition of (Pro)renin receptor in human retinal endothelial cells. Mol Vis. 2015;21:224–235. [PMC free article] [PubMed] [Google Scholar]
- 84.Kanda A., Ishida S. The vitreous renin–angiotensin system is mediated by soluble (pro)renin receptor in diabetic retinopathy: a new implication of the receptor-associated prorenin system. Taiwan J Ophthalmol. 2013;3:51–53. [Google Scholar]
- 85.Kanda A., Noda K., Saito W., Ishida S. (Pro)renin receptor is associated with angiogenic activity in proliferative diabetic retinopathy. Diabetologia. 2012;55:3104–3113. doi: 10.1007/s00125-012-2702-2. [DOI] [PubMed] [Google Scholar]
- 86.Satofuka S., Ichihara A., Nagai N. (Pro)renin receptor–mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes. 2009;58:1625–1633. doi: 10.2337/db08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Foureaux G., Nogueira B.S., Coutinho D.C.O., Raizada M.K., Nogueira J.C., Ferreira A.J. Activation of endogenous angiotensin converting enzyme 2 prevents early injuries induced by hyperglycemia in rat retina. Braz J Med Bio Res. 2015;48:1109–1114. doi: 10.1590/1414-431X20154583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cullinane A.B., Leung P.S., Ortego J., Coca-Prados M., Harvey B.J. Renin-angiotensin system expression and secretory function in cultured human ciliary body non-pigmented epithelium. Br J Ophthalmol. 2002;86:676–683. doi: 10.1136/bjo.86.6.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen F., Zhang L., Liu T. Effects of angiotensin II on the 3H-TdR incorporation and synthesis of collagen in cultured bovine trabecular meshwork cells (article in Chinese) Yan Ke Xue Bao. 2001;17:209–212. [PubMed] [Google Scholar]
- 90.Inoue T., Yokoyoma T., Koike H. The effect of angiotensin II on uveoscleral outflow in rabbits. Curr Eye Res. 2001;23:139–143. doi: 10.1076/ceyr.23.2.139.5470. [DOI] [PubMed] [Google Scholar]
- 91.Mehta A., Iyer L., Parmar S., Shah G., Goyal R. Oculohypotensive effect of perindopril in acute and chronic models of glaucoma in rabbits. Can J Physiol Pharmacol. 2010;88:595–600. doi: 10.1139/y10-026. [DOI] [PubMed] [Google Scholar]
- 92.Shah G.B., Sharma S., Mehta A.A., Goyal R.K. Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma. J Cardiovasc Pharm. 2000;36:169–175. doi: 10.1097/00005344-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 93.Costagliola C., Verolino M., de Rosa M.L., Iaccarino G., Ciancaglini M., Mastropasqua L. Effect of oral losartan potassium on intraocular pressure in normotensive and glaucomatous human subjects. Exp Eye Res. 2000;71:167–171. doi: 10.1006/exer.2000.0866. [DOI] [PubMed] [Google Scholar]
- 94.Wang R.F., Podos S.M., Mittag T.W., Yokoyoma T. Effect of CS-088, an angiotensin AT1 receptor antagonist, on intraocular pressure in glaucomatous monkey eyes. Exp Eye Res. 2005;80:629–632. doi: 10.1016/j.exer.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 95.Lotti V.J., Pawlowski N. Prostaglandins mediate the ocular hypotensive action of the angiotensin converting enzyme inhibitor MK-422 (enalaprilat) in African green monkeys. J Ocul Pharmacol. 1990;6:1–7. doi: 10.1089/jop.1990.6.1. [DOI] [PubMed] [Google Scholar]
- 96.Reitsamer H.A., Kiel J.W. Relationship between ciliary body blood flow and aqueous production in rabbits. Invest Ophthalmol Vis Sci. 2003;44:3967–3971. doi: 10.1167/iovs.03-0088. [DOI] [PubMed] [Google Scholar]
- 97.Sharif N.A. Novel potential treatment modalities for ocular hypertension: focus on angiotensin and bradykinin system axes. J Ocul Pharmacol Ther. 2015;31:131–145. doi: 10.1089/jop.2014.0114. [DOI] [PubMed] [Google Scholar]
- 98.Nagai N., Oike Y., Izumi-Nagai K. Suppression of choroidal neovascularization by inhibiting angiotensin-converting enzyme: minimal role of bradykinin. Invest Ophthalmol Vis Sci. 2007;48:2321–2326. doi: 10.1167/iovs.06-1296. [DOI] [PubMed] [Google Scholar]
- 99.Ishida S. Lifestyle-related diseases and anti-aging ophthalmology: suppression of retinal and choroidal pathologies by inhibiting renin-angiotensin system and inflammation. Nihon Ganka Gakkai Zasshi. 2009;113:403–422. [PubMed] [Google Scholar]
- 100.Satofuka S., Ichihara A., Nagai N. (Pro)renin receptor promotes choroidal neovascularization by activating its signal transduction and tissue renin angiotensin system. Am J Pathol. 2008;173:1911–1918. doi: 10.2353/ajpath.2008.080457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagai N., Oike Y., Izumi-Nagai K. Angiotensin II type 1 receptor-mediated inflammation is required for choroidal neovascularization. Arterioscler Thromb Vasc Bio. 2006;26:2252–2259. doi: 10.1161/01.ATV.0000240050.15321.fe. [DOI] [PubMed] [Google Scholar]
- 102.Nagai N., Oike Y., Noda N. Suppression of ocular inflammation in endotoxin-induced uveitis by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2005;46:2925–2931. doi: 10.1167/iovs.04-1476. [DOI] [PubMed] [Google Scholar]
- 103.Okunuki Y., Usui Y., Nagai N. Suppression of experimental autoimmune uveitis by angiotensin II type 1receptor blocker telmisartan. Invest Ophthalmol Vis Sci. 2009;50:2255–2261. doi: 10.1167/iovs.08-2649. [DOI] [PubMed] [Google Scholar]
- 104.Kurihara T., Ozawa Y., Shinoda K. Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Invest Ophthalmol Vis Sci. 2006;47:5545–5552. doi: 10.1167/iovs.06-0478. [DOI] [PubMed] [Google Scholar]
- 105.Miyazaki A., Kitaichi N., Ohgami K. Anti-inflammatory effect of angiotensin type 1 receptor antagonist on endotoxin-induced uveitis in rats. Graefe's Arch Clin Exp Ophthalmol. 2008;246:747–757. doi: 10.1007/s00417-007-0730-2. [DOI] [PubMed] [Google Scholar]
- 106.Nagai N., Noda K., Urano T. Selective suppression of pathologic, but not physiologic, retinal neovascularization by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2005;46:1078–1084. doi: 10.1167/iovs.04-1101. [DOI] [PubMed] [Google Scholar]
- 107.Qiu Y., Shil P.K., Zhu P. Angiotensin-converting enzyme 2 (ACE2) activator dize ameliorates endotoxin-induced uveitis in mice. Invest Ophthalmol Vis Sci. 2014;55:3809–3818. doi: 10.1167/iovs.14-13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khan S.A., Choudhary R., Singh A., Bodakhe S.H. Hypertension potentiates cataractogenesis in rat eye through modulation of oxidative stress and electrolyte homeostasis. J Curr Ophthalmol. 2016;28:123–130. doi: 10.1016/j.joco.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jablecka A., Czaplicka E., Olszewski J., Bogdanski P., Krauss H., Smolarek I. Influence of selected angiotensin-converting enzyme inhibitors on alloxan-induced diabetic cataract in rabbits. Med Sci Monit. 2009;15:BR334–338. [PubMed] [Google Scholar]
- 110.Langade D.G., Rao G., Girme R.C., Patki P.S., Bulakh P.M. In vitro prevention by ACE inhibitors of cataract induced by glucose. Indian J Pharmacol. 2006;38:107–110. [Google Scholar]
- 111.Harindhanavudhi T., Mauer M., Klein R., Zinman B., Sinaiko A., Caramori M.L. Benefits of renin-angiotensin blockade on retinopathy in type 1 diabetes vary with glycemic control. Diabetes Care. 2011;34:1838–1842. doi: 10.2337/dc11-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sjolie A.K., Dodson P., Hobbs F.R.R. Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. Int J Clin Pract. 2011;65:148–153. doi: 10.1111/j.1742-1241.2010.02552.x. [DOI] [PubMed] [Google Scholar]
- 113.Otani A., Takagi H., Suzuma K., Honda Y. Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res. 1998;82:619–628. doi: 10.1161/01.res.82.5.619. [DOI] [PubMed] [Google Scholar]
- 114.Otani A., Takagi H., Oh H. Angiotensin II-stimulated potentiates vascular endothelial growth factor expression in bovine retinal pericytes. Invest Ophthalmol Vis Sci. 2000;41:1192–1199. [PubMed] [Google Scholar]
- 115.Chaturvedi N. Modulation of renin-angiotensin system and retinopathy. Heart. 2000;84:i29–i31. doi: 10.1136/heart.84.suppl_1.i29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clermont A., Bursell S.E., Feener E.P. Role of the angiotensin II type 1 receptor in the pathogenesis of diabetic retinopathy: effects of blood pressure control and beyond. J Hypertens. 2006;24:S73–S80. doi: 10.1097/01.hjh.0000220410.69116.f8. [DOI] [PubMed] [Google Scholar]
- 117.Mauer M., Zinman B., Gardiner R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilkinson-Berka J.L., Tan G., Jaworski K., Ninkovic S. Valsartan but not atenolol improves vascular pathology in diabetic ren-2 rat retina. Am J Hypertens. 2007;20:423–430. doi: 10.1016/j.amjhyper.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 119.Fernandez L.A., Twickler J., Mead A. Neovascularization produced by angiotensin II. J Lab Clin Med. 1985;105:141–145. [PubMed] [Google Scholar]
- 120.Aiello L.P., Bursell S.E., Clermont A. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 121.Funatsu H., Yamashita H., Ikeda T., Nakanishi Y., Kitano S., Hori S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. Am J Ophthalmol. 2002;133:537–543. doi: 10.1016/s0002-9394(02)01323-5. [DOI] [PubMed] [Google Scholar]
- 122.Funatsu H., Yamashita H., Nakanishi Y., Hori S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Bri J Ophthalmol. 2002;86:311–315. doi: 10.1136/bjo.86.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schiffman R.M., Fisher L., Nussbaum J., Edwards P., Scicli G. Prorenin and renin levels in the vitreous of human eyes with and without proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1992;33:1362. [Google Scholar]
- 124.Pradhan R., Fong D., March C. Angiotensin-converting enzyme inhibition for the treatment of moderate to severe diabetic retinopathy in normotensive type 2 diabetic patients a pilot study. J Diabetes Complications. 2002;16:377–381. doi: 10.1016/s1056-8727(02)00188-5. [DOI] [PubMed] [Google Scholar]
- 125.Chaturvedi N., Porta M., Klein R. Direct programme study group. Effect of candesartan on prevention (Direct Prevent 1) and progression (Direct Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394–1402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 126.Gilbert R.E., Vranes D., Berka J.L. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab Invest. 1998;78:1017–1027. [PubMed] [Google Scholar]
- 127.Vaajanen A., Vapaatalo H. Local ocular renin–angiotensin system – a target for glaucoma therapy? Basic Clin Pharmacol Toxicol. 2011;9:217–224. doi: 10.1111/j.1742-7843.2011.00729.x. [DOI] [PubMed] [Google Scholar]
- 128.Bathija R., Gupta N., Zangwill L., Weinreb R.N. Changing definition of glaucoma. J Glaucoma. 1998;7:165–169. [PubMed] [Google Scholar]
- 129.Bonomi L., Marchini G., Marraffa M., Bernardy P., Morbio R., Varotto A. Vascular risk factors for primary open angle glaucoma: the Enga-Neumarkt study. Ophthalmology. 2000;107:1287–1293. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 130.Drance S., Anderson D.R., Schulzer M. Collaborative normal-tension glaucoma study group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 131.Flammer J., Haefl Iger I.Q., Orgul S., Resink T. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999;8:212–219. [PubMed] [Google Scholar]
- 132.Quigley H.A., Pitha I.F., Welsbie D.S. Losartan treatment protects retinal ganglion cells and alters scleral remodeling in experimental glaucoma. PLoS One. 2015;10:e0141137. doi: 10.1371/journal.pone.0141137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang H., Hirooka K., Fukuda K., Shiraga F. Neuroprotective effects of angiotensin II type 1 receptor blocker in a rat model of chronic glaucoma. Invest Ophthalmol Vis Sci. 2009;50:5800–5804. doi: 10.1167/iovs.09-3678. [DOI] [PubMed] [Google Scholar]
- 134.Espinosa-Heidmann D.G., Suner I.J., Hernandez E.P., Monroy D., Csaky K.G., Cousins S.W. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 135.Sakurai E., Anand A., Ambati B.K., Rooijen V.N., Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 136.Tsutsumi C., Sonoda K.H., Egashira K. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32. doi: 10.1189/jlb.0902436. [DOI] [PubMed] [Google Scholar]
- 137.Gragoudas E.S., Adamis A.P., Cunningham E.T., Feinsod M., Guyer D.R. Pegaptanib for neovascular age-related macular degeneration. New Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 138.Rosenfeld P.J., Brown D.M., Heier J.S. Ranibizumab for neovascular age-related macular degeneration. New Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 139.Praddaude F., Cousins S.W., Pecher C., Marin-Castano M.E. Angiotensin II induced hypertension regulates AT1 receptor subtypes and extracellular matrix turnover in mouse retinal pigment epithelium. Exp Eye Res. 2009;89:109–118. doi: 10.1016/j.exer.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Striker G.E., Praddaude F., Alcazar O., Cousins S.W., Marin-Castano M.E. Regulation of angiotensin II receptors and extracellular matrix turnover in human retinal pigment epithelium: role of angiotensin II. Am J Physiol Cell Physiol. 2008;295:C1633–C1646. doi: 10.1152/ajpcell.00092.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nabah Y.N., Mateo T., Estelles R. Angiotensin II induces neutrophil accumulation in vivo through generation and release of CXC chemokines. Circulation. 2004;110:3581–3586. doi: 10.1161/01.CIR.0000148824.93600.F3. [DOI] [PubMed] [Google Scholar]
- 142.Alcazar O., Cousins S.W., Striker G.E., Marin-Castano M.E. (Pro)renin receptor is expressed in human retinal pigment epithelium and participates in extracellular matrix remodeling. Exp Eye Res. 2009;89:638–647. doi: 10.1016/j.exer.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 143.Nahmod K.A., Vermeulen M.E., Raiden S. Control of dendritic cell differentiation by angiotensin II. FASEB J. 2003;17:491–493. doi: 10.1096/fj.02-0755fje. [DOI] [PubMed] [Google Scholar]
- 144.Pastore L., Tessitore A., Martinotti S. Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation. 1999;100:1646–1652. doi: 10.1161/01.cir.100.15.1646. [DOI] [PubMed] [Google Scholar]
- 145.Gupta S.K., Selvan V.K., Agrawal S.S., Saxena R. Advances in pharmacological strategies for the prevention of cataract development. Indian J Ophthalmol. 2009;57:175–183. doi: 10.4103/0301-4738.49390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sabanayagam C., Wang J.J., Mitchell P. Metabolic syndrome components and age-related cataract: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2011;52:2397–2404. doi: 10.1167/iovs.10-6373. [DOI] [PubMed] [Google Scholar]
- 147.Phipps J.A., Wilkinson-Berka J.L., Fletcher E.L. Retinal dysfunction in diabetic ren-2 rats is ameliorated by treatment with valsartan but not atenolol. Invest Ophthalmol Vis Sci. 2007;48:927–934. doi: 10.1167/iovs.06-0892. [DOI] [PubMed] [Google Scholar]
- 148.Strain W.D., Chaturvedi N. The renin-angiotensin-aldosterone system and the eye in diabetes. J Renin Angiotensin Aldosterone Syst. 2002;3:243–246. doi: 10.3317/jraas.2002.045. [DOI] [PubMed] [Google Scholar]