Abstract
Background: The state of newborn screening (NBS) programmes for organic acidurias in Europe was assessed by a web-based questionnaire in the EU programme of Community Action in Public Health 2010/2011 among the – at that time – 27 EU member states, candidate countries, potential candidates and three EFTA countries.
Results: Thirty-seven data sets from 39 target countries were analysed. Newborn screening for glutaric aciduria type I (GA-I) was performed in ten, for isovaleric aciduria (IVA) in nine and for methylmalonic aciduria including cblA, cblB, cblC and cblD (MMACBL) as well as for propionic aciduria (PA) in seven countries. Samples were obtained at a median age of 2.5 days and laboratory analysis began at median age of 4.5 days. Positive screening results were mostly confirmed in specialised centres by analysis of organic acids in urine. Confirmation of a positive screening result usually did not start before the second week of life (median ages: 9.5 days [IVA], 9 days [GA-I], 8.5 days [PA, MMACBL]) and was completed early in the third week of life (median ages: 15 days [IVA, PA, MMA], 14.5 days [GA-I]). Treatment was initiated in GA-I and IVA at a median age of 14 days and in MMACBL and PA at a median age of 15 days.
Conclusion: NBS for organic acidurias in Europe is variable and less often established than for amino acid disorders. While for GA-I its benefit has already been demonstrated, there is room for debate of NBS for IVA and especially PA and MMACBL.
Keywords: cblA, cblB, cblC and cblD; Europe; Glutaric aciduria type I; Isovaleric aciduria; Methylmalonic acidurias; Newborn screening; Propionic aciduria
Introduction
In 2010/2011 regulation and practice of population newborn screening (NBS) for rare disorders were surveyed among – at that time – 27 EU member states (NBS in Belgium is different in the Flemish and in the French part, therefore contributed two data sets), five candidate countries (Croatia, Former Yugoslav Republic of Macedonia, Iceland, Montenegro, Turkey), four potential candidates (Albania, Bosnia Herzegovina, Kosovo, Serbia) and three EFTA countries (Norway, Switzerland and Liechtenstein) through a tender of the European Commission within the EU programme of Community Action in Public Health (Burgard et al. 2012; Loeber et al. 2012).
Data collection covered five domains of an NBS programme, and results have been reported previously (Burgard et al. 2012; Loeber et al. 2012; Cornel et al. 2011).
This article specifies the state of NBS programmes for organic acidurias in Europe in 2010, regarding four organic acidurias: glutaric aciduria type I (GA-I), isovaleric aciduria (IVA), propionic aciduria (PA) and methylmalonic acidurias including inherited deficiencies of methylmalonyl-CoA mutase as well as of cblA, cblB, cblC and cblD deficiency (MMACBL).
NBS for these disorders aims to detect elevations of characteristic acylcarnitines by tandem mass spectrometry and subsequent calculation of acylcarnitine ratios (Lindner et al. 2006, 2008; Ensenauer et al. 2011; Chace et al. 2001).
In IVA, MMACBL and PA patients may already develop life-threatening metabolic decompensation during the first days of life (Deodato et al. 2006; Vockley and Ensenauer 2006; Kölker et al. 2015a, b). Therefore, NBS process times are key performance indicators.
Methods
A web-based questionnaire asked for current practice of NBS and its possible regulation by directives and/or by guidelines (Burgard et al. 2012; Loeber et al. 2012).
In each country respondents nominated by European professional societies (ISNS; SSIEM; ESPE) reported data for all disorders screened for according to the national NBS panel (Loeber et al. 2012). The survey started in August 2010 and was closed on January 14, 2011; reference date for all data is September 1, 2010. Values given for time points of obtaining NBS samples, conforming diagnosis and start of treatment are mostly experts’ estimates for single disorders or groups of disorders, but not calculated measures based on individual measurements. Therefore standard deviations cannot be communicated. Process times were not differentiated by different methods to confirm a diagnosis.
Results
Description of the Data Set
From 39 target countries, 37 data sets were available for analysis (response rate 92.5%). Data sets were not available for Albania (NBS was not established), Liechtenstein (NBS is performed in Switzerland and data are included in the Swiss data set) and Kosovo (the questionnaire was not returned), but it was known by personal communication that newborn screening, if functional, does not include organic acidurias. Newborn screening for GA-I was performed in ten countries and for IVA in nine countries; MMACBL and PA were screened for in seven countries (Table 1).
Table 1.
Confirmation of newborn screening and feedback of screening results
| Countries | Point of confirmation | Method of confirmation | Feedback of screening results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Specialised centre | Local hospital | GP/Paed. | Metabolites | Enzyme activity | Mutation analysis | To screening lab | To registry | Only diagnosis | Detailed results | |
| Disorder: GA-I | ||||||||||
| Austria | 50% | 50% | X | X | X | X | X | |||
| Belgium (Flemish part) | X | X | X | X | ||||||
| Czech Republic | X | X | X | X | X | X | ||||
| Denmark | X | X | X | X | X | X | ||||
| Germany | X | X | X | X | X | X | ||||
| Hungary | 50% | 50% | X | X | X | X | ||||
| Netherlands | na | X | X | X | na | na | na | na | ||
| Portugal | X | X | X | X | X | X | ||||
| Spain | X | X | X | X | X | |||||
| Iceland | X | na | na | na | X | X | ||||
| Disorder: IVA | ||||||||||
| Austria | 50% | 50% | X | X | X | X | X | |||
| Belgium (Flemish part) | X | X | X | X | ||||||
| Czech Republic | X | X | X | X | X | X | ||||
| Germany | X | X | X | X | X | |||||
| Hungary | 50% | 50% | X | X | X | X | ||||
| Netherlands | 50% | 50% | X | X | X | na | na | na | na | |
| Portugal | X | X | X | X | X | X | ||||
| Spain | X | X | X | X | X | |||||
| Iceland | X | na | na | na | X | X | ||||
| Disorder: MMACBLC | ||||||||||
| Austria | 50% | 50% | X | X | X | X | X | |||
| Belgium (Flemish part) | X | X | X | X | ||||||
| Denmark | X | X | X | X | X | X | ||||
| Hungary | 50% | 50% | X | X | X | X | ||||
| Portugal | X | X | X | X | X | X | ||||
| Spain | X | X | X | X | X | |||||
| Iceland | X | na | na | na | X | X | ||||
| Disorder: PA | ||||||||||
| Austria | 50% | 50% | X | X | X | |||||
| Belgium (Flemish part) | X | X | X | X | ||||||
| Denmark | X | X | X | X | X | X | ||||
| Hungary | 50% | 50% | X | X | X | X | ||||
| Portugal | X | X | X | X | X | X | ||||
| Spain | X | X | X | X | X | |||||
| Iceland | X | na | na | na | X | X | ||||
na data not available, GP general practitioner, Paed. paediatrician
Confirmation of Screening Results
Nine of ten countries answered that positive screening results for GA-I were confirmed by specialised centres but rarely also by local hospitals or by general practitioners/paediatricians in practice (2/10) (Table 1). Similar results were obtained for IVA: in 9/9 countries positive screening results are confirmed by specialised centres (Table 1). MMACBL and PA screening results are confirmed by specialised centres (7/7 countries), but rarely also in local hospitals (1/7 countries) or by general practitioners or paediatricians in private practice (1/7 countries) (Table 1). Screening results are confirmed most frequently by analyses of organic acids in urine. However, in the majority of countries, additionally mutation analysis and/or enzyme analysis is performed for the confirmation of diagnosis (Table 1).
Process Times
In most countries there is (written) information for prospective parents and informed consent is asked at the time of blood sampling (Burgard et al. 2012). Blood spots are taken at a median age of 2.5 days and analysis in the screening laboratory starts at median age of 4.5 days (Table 2).
Table 2.
Process times of newborn screening
| Countries | DBS sampling | NBS lab analysis | Confirmation | Treatment | |
|---|---|---|---|---|---|
| Mean age (days) | Age at start (days) | Mean start (days) | Mean end (days) | Mean start (days) | |
| Disorder: GA-I | |||||
| Austria | 2.3 | 3.8 | 11 | 14 | 14 |
| Belgium (Flemish part) | 2.2 | 4.2 | 10 | 12 | 14 |
| Czech Republic | 2.5 | 4.5 | 6 | 8 | 8 |
| Denmark | 2.5 | 4.5 | 5 | 12 | na |
| Germany | 2.2 | 3.2 | 11 | 16 | 14 |
| Hungary | 2.5 | 5.5 | na | na | na |
| Netherlands | 3.8 | 4.8 | 10 | 21 | 10 |
| Portugal | 2.2 | 4.2 | 8 | na | na |
| Spain | 3.8 | 7.8 | 8 | 15 | 15 |
| Iceland | 3 | 6 | 9 | 23 | 23 |
| Median | 2.5 | 4.5 | 9 | 14.5 | 14 |
| Disorder: IVA | |||||
| Austria | 2.3 | 3.8 | 11 | 14 | 14 |
| Belgium (Flemish part) | 2.2 | 4.2 | 10 | 12 | 14 |
| Czech Republic | 2.5 | 4.5 | 6 | 8 | 8 |
| Germany | 2.2 | 3.2 | 11 | 16 | 14 |
| Hungary | 2.5 | 5.5 | na | na | na |
| Netherlands | 3.8 | 4.8 | 10 | 21 | 10 |
| Portugal | 2.2 | 4.2 | 8 | na | na |
| Spain | 3.8 | 7.8 | 8 | 15 | 15 |
| Iceland | 3 | 6 | 9 | 23 | 23 |
| Median | 2.5 | 4.5 | 9.5 | 15 | 14 |
| Disorder: MMACBL | |||||
| Austria | 2.3 | 3.8 | 11 | 14 | 14 |
| Belgium (Flemish part) | 2.2 | 4.2 | 10 | 14 | 14 |
| Denmark | 2.5 | 4.5 | 5 | 15 | 15 |
| Hungary | 2.5 | 5.5 | na | na | na |
| Portugal | 2.2 | 4.2 | 8 | na | na |
| Spain | 3.8 | 7.8 | 8 | 15 | 15 |
| Iceland | 3 | 6 | 9 | 23 | 23 |
| Median | 2.5 | 4.5 | 8.5 | 15 | 15 |
| Disorder: PA | |||||
| Austria | 2.3 | 3.8 | 11 | 14 | 14 |
| Belgium (Flemish part) | 2.2 | 4.2 | 10 | 12 | 14 |
| Denmark | 2.5 | 4.5 | 5 | 15 | 15 |
| Hungary | 2.5 | 5.5 | na | na | na |
| Portugal | 2.2 | 4.2 | 8 | na | na |
| Spain | 3.8 | 7.8 | 8 | 15 | 15 |
| Iceland | 3 | 6 | 9 | 23 | 23 |
| Median | 2.5 | 4.5 | 8.5 | 15 | 15 |
na data not available, DBS dried blood spot
Confirmation of a positive screening result usually does not start before the second week of life and is completed early in the third week of life (Table 2). Treatment starts in GA-I and IVA at a median age of 14 days (range 8–23 days) and in MMACBL and PA at a median age of 15 days (range 14–23 days) (Table 2).
Information Flow Between NBS Domains
In all countries with NBS for organic acidurias, results of the diagnostic confirmatory process are reported back to NBS laboratories (Table 1), following local guidelines (Table 3 Part II). Type and extent of transmitted data are varied, ranging from communication of only the confirmed diagnosis to reporting detailed results (Table 1).
Table 3.
Regulation of the confirmation process
| Countries | How to confirm NBS | Authors | Where to confirm diagnosis? | Authors | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Guideline | Application | Directive | Application | Guideline | Application | Directive | Application | |||
| Disorder: GA-I PART I | ||||||||||
| Austria | X | National | PS | X | National | PS | ||||
| Belgium (Flemish part) | na | X | National | PS | ||||||
| Czech Republic | X | Regional | LH | X | National | X | National | HA | ||
| Denmark | X | National | X | National | HA, LH | X | National | X | National | HA, LH |
| Germany | X | National | PS | X | National | X | National | HA, PS | ||
| Hungary | X | National | LH | X | Regional | LH | ||||
| Netherlands | na | na | na | na | ||||||
| Portugal | X | National | X | Regional | ||||||
| Spain | na | na | na | na | ||||||
| Iceland | X | Regional | X | Regional | LH | X | X | National | LH | |
| Disorder: IVA PART I | ||||||||||
| Austria | X | PS | X | National | PS | |||||
| Belgium (Flemish part) | na | na | X | National | PS | |||||
| Czech Republic | X | Regional | LH | X | National | X | National | HA | ||
| Germany | X | National | PS | X | National | X | National | HA, PS | ||
| Hungary | X | National | LH | X | Regional | LH | ||||
| Netherlands | X | X | X | HA,PS | ||||||
| Portugal | X | National | X | Regional | ||||||
| Spain | na | na | na | na | ||||||
| Iceland | X | Regional | X | Regional | LH | X | X | National | LH | |
| Disorder: MMACBL PART I | ||||||||||
| Austria | X | National | PS | X | National | PS | ||||
| Belgium (Flemish part) | na | na | X | National | PS | |||||
| Denmark | X | National | X | National | HA, LH | X | National | X | National | HA, LH |
| Hungary | X | National | LH | X | Regional | LH | ||||
| Portugal | X | National | X | Regional | ||||||
| Spain | na | na | na | na | ||||||
| Iceland | X | Regional | X | Regional | LH | X | X | National | LH | |
| Disorder: PA PART I | ||||||||||
| Austria | X | National | PS | X | National | PS | ||||
| Belgium (Flemish part) | na | na | X | National | PS | |||||
| Denmark | X | National | X | National | HA, LH | X | National | X | National | HA, LH |
| Hungary | X | National | LH | X | Regional | LH | ||||
| Portugal | X | National | X | Regional | ||||||
| Spain | na | na | ||||||||
| Iceland | X | Regional | X | Regional | LH | X | X | National | LH | |
| Countries | Recommended age to confirm a suspicious NBS result | Authors | Feedback of final diagnosis | Authors | ||||||
| Guideline | Minimum age (days) | Maximum age (days) | Application | Guideline | Application | Directive | Application | |||
| Disorder: GA-I PART II | ||||||||||
| Austria | na | X | National | PS | ||||||
| Belgium (Flemish part) | X | 1 | National | LH | X | Regional | X | Regional | HA, PS | |
| Czech Republic | na | X | Regional | LH | ||||||
| Denmark | na | X | National | X | National | HA, LH | ||||
| Germany | X | National | HA,PS | X | National | HA | ||||
| Hungary | na | X | National | LH | ||||||
| Netherlands | na | na | na | |||||||
| Portugal | na | X | National | |||||||
| Spain | X | 3 | 20 | National | PS | X | Regional | X | Regional | LH |
| Iceland | X | National | LH | X | National | LH | ||||
| Disorder: IVA PART II | ||||||||||
| Austria | na | X | National | PS | ||||||
| Belgium (Flemish part) | X | 1 | National | LH | X | Regional | X | Regional | HA, PS, LH | |
| Czech Republic | na | X | Regional | LH | ||||||
| Germany | X | National | HA, PS | X | National | HA | ||||
| Hungary | na | X | National | LH | ||||||
| Netherlands | X | 14 | National | PS | X | PS | ||||
| Portugal | na | X | National | |||||||
| Spain | X | 3 | 20 | National | PS | X | Regional | X | Regional | LH |
| Iceland | X | National | LH | X | National | LH | ||||
| Disorder: MMACBL PART II | ||||||||||
| Austria | na | X | National | PS | ||||||
| Belgium (Flemish Part) | X | 1 | National | LH | X | Regional | X | Regional | HA, PS, LH | |
| Denmark | na | X | National | X | National | HA, LH | ||||
| Hungary | na | X | National | LH | ||||||
| Portugal | na | X | National | |||||||
| Spain | X | 3 | 20 | National | PS | X | Regional | X | Regional | LH |
| Iceland | X | National | LH | X | National | LH | ||||
| Disorder: PA PART II | ||||||||||
| Austria | na | X | National | PS | ||||||
| Belgium (Flemish part) | X | 1 | National | LH | X | Regional | X | regional | HA, PS, LH | |
| Denmark | na | X | National | X | National | HA, LH | ||||
| Hungary | na | X | National | LH | ||||||
| Portugal | na | X | National | |||||||
| Spain | X | 3 | 20 | National | PS | X | Regional | X | Regional | LH |
| Iceland | X | National | LH | X | National | LH | ||||
na data not available, LH local head of institution responsible for confirmation of NBS, PS professional society, HA health authority
Regulations
A guideline on how to confirm the diagnosis is usually available mostly on a national level, but usually written by local heads/directors of the institution executing the confirmation test – in contrast a national directive concerning the same topic is rarely available (Table 3 Part I). Where to confirm a diagnosis is also predominantly regulated by guidelines, mostly on a national level and less frequently by a directive (Table 3 Part I). The same holds true for the time needed to confirm a diagnosis (Table 3 Part II). Reporting positive results is also more often regulated by guidelines than by directives; few countries have guidelines as well as directives (Table 3 Part II). In Germany, for example, there is a national directive concerning NBS for GA-I, but a guideline endorsed by a national professional society is also in place (Kölker et al. 2011).
Discussion
The major aim of this study was to describe the concepts and state of established NBS programmes for organic acidurias in Europe. In contrast to amino acid disorders, organic acidurias are less often included in European NBS disease panels (Burgard et al. 2012). Therefore experience about the impact of different logistics on outcome of these disorders is scarce.
Our study reveals that performance and, confirmation of positive NBS results, and start of treatment for organic acidurias in Europe is variable. This may result in inappropriate interventions, delayed treatment, increased disease burden and finally health inequities and increased costs. Further studies shall provide systematic evaluation of existing NBS programmes for their impact on patients’ outcomes and also costs and health equity. Particular attention should be paid to the time schedule of the NBS process for these conditions (Kelm and Tanksley 2015).
Since patients with a classical organic aciduria such as IVA, MMACBL and PA are at risk to already present early in the neonatal period with a putatively life-threatening metabolic decompensation, it is important to accomplish NBS as early as possible in order to start treatment and care ideally in the still asymptomatic patient. In our survey, treatment in these disorders was reported to start after 15 days of life. This is clearly too late for most patients with a neonatal metabolic decompensation. Furthermore the confirmatory diagnostic process needs to be tailored to avoid therapy in patients with mild phenotypes which is especially recognised in IVA (Ensenauer et al. 2004).
The European Network of Experts on Newborn Screening (EUNENBS) delivered a consensus document with recommendations how to develop NBS in the European Union based on the results of the survey (Cornel et al. 2014). Within these recommendations GA-I is considered as a disorder with lower prevalence, not too difficult to test and prove health gain, IVA is a candidate disorder where NBS is more challenging according to the criteria by Wilson and Jungner (1968), and NBS for MMACBL or PA is not recommended at all.
NBS and subsequent treatment of GA-I according to an international guideline have significantly improved the neurological outcome of affected individuals (Heringer et al. 2010; Kölker et al. 2006, 2007, 2011), and NBS is considered a cost-effective diagnostic strategy for countries with healthcare systems comparable to Germany (Pfeil et al. 2013). According to this international guideline, it is crucial to start treatment early before the manifestation of irreversible neurologic damage (Kölker et al. 2011). Treatment has to be started early in the diagnostic process, i.e. immediately after demonstration of elevated concentrations of 3-hydroxyglutaric acid and already in advance of the final confirmation of the diagnosis by demonstration of two disease-causing mutations or impaired enzyme activity (Kölker et al. 2011). In our survey only in the Netherlands and in Germany, pre-emptive treatment of GA-I patients starts before the end of the confirmation process as recommended (Kölker et al. 2011). In Fig. 1a we illustrate the current practice mostly employed and show our proposed model (Fig. 1b). In concordance with an international guideline (Kölker et al. 2011), we strongly advocate starting pre-emptive treatment in the setting of a metabolic centre as soon as the suspicion of an organic aciduria arises from newborn screening and before the diagnosis is finally confirmed. This is important in GA-I, but may even be more crucial in MMACBL, PA and IVA, where neonatal decompensation is more frequent.
Fig. 1.
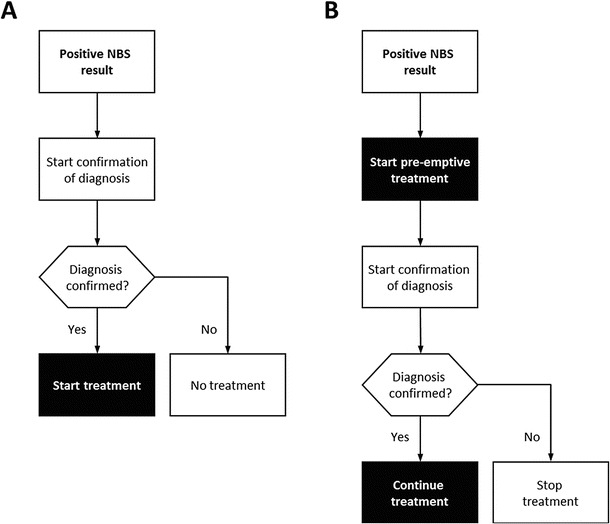
Current practice after positive NBS result (a) versus a proposed model recommending pre-emptive treatment after a positive NBS result is received (b). This should be initiated and supervised by a metabolic centre with the major aim to minimise the risk of a neonatal metabolic decompensation in patients with organic acidurias during the confirmatory diagnostic process
For IVA, successful experience with NBS programmes has been published (Ensenauer et al. 2011; Grünert et al. 2012a, b; Lindner et al. 2011), and the natural history is favourable as long as early neonatal brain damage can be prevented by adequate therapy (Grünert et al. 2012a, b). However, about half of the individuals with a positive screening result for IVA are homozygous for mutation c.932C>T in the IVA gene, presumably associated with a benign phenotype (Ensenauer et al. 2004), for whom the benefit of screening is questionable.
The impact of NBS on history and outcome of PA and MMACBL was thought to be low due to the fact that many patients will have already presented clinically before the results of NBS are available (Leonard et al. 2003). In line with this, a study demonstrated that NBS for PA did not affect the outcome of these patients (Grünert et al. 2012a, b). However, there still is controversy whether PA and MMACBL patients with a late disease onset, i.e. presenting with first symptoms after the newborn period, might benefit from NBS (Dionisi-Vici et al. 2006; Couce et al. 2011). Follow-up studies on a larger number of patients are required to address this question.
In 2011, E-IMD (EAHC no. 2010 12 01; www.eimd-registry.org), an observational patient registry including organic acidurias, has been initiated. It currently includes detailed follow-up data of more than 1100 patients (Kölker et al. 2015a, b).
Taking the start of confirmation as a surrogate parameter for the time when an organic aciduria can be suspected from NBS, percentages of patients identified before onset of symptoms can be estimated. For example, in Denmark confirmation of NBS for MMACBL starts on day 5 after birth (Table 2), which is the earliest among all European countries performing NBS screening for MMACBL. At that time at least 25% of all MMACBL patients have already been diagnosed by clinical symptoms [total number of patients: 101; median age at diagnosis (interquartile range): 21 (3–210) days] in the E-IMD sample. Similar results were obtained for PA with at least 25% of all patients being diagnosed until day 5 [total number of patients: 78; median age at diagnosis (interquartile range): 14 (4–90) days].
For IVA, confirmation of NBS is at first initiated at day 6 in the Czech Republic (Table 2); at that time at least 25% of patients have been diagnosed [total number of patients, 29; median age at diagnosis (interquartile range), 8 (5–465) days]. In contrast, since GA-I patients rarely present with symptoms before age 3 months (Kölker et al. 2006), neonatal onset in GA-I has been rarely observed until day 5 in the E-IMD survey. On day 5 confirmation of positive NBS results for GA-I is first started in Denmark [total number of patients: 52; median age at diagnosis (interquartile range): 300 (145–428) days].
Although being preliminary, these data support the call for NBS to be fast in intoxication-type organic acidurias such as MMACBL, PA and IVA and to initiate treatment early before completing follow-up investigations. Patients with late-onset forms of these disorders will most likely benefit the most from NBS programmes. This notion is supported by a recently published E-IMD study demonstrating that (1) NBS significantly lowers the age of diagnosis in classic organic acidurias compared to patients with late onset of symptoms identified by selective metabolic testing, (2) improves the neurological outcome in patients with GA-I and cobalamin-nonresponsive MMA and (3) reduces the probability of cardiac manifestation with increasing age in PA patients (Heringer et al. 2015).
However, the decision whether or not to screen for these diseases must be based on many more criteria, including long-term follow-up studies and health economic evaluations.
Conclusion
NBS for organic acidurias in Europe is variable and less often established than for amino acid disorders. While for GA-I its benefit has already been demonstrated, there is room for debate of NBS for IVA and especially PA and MMACBL patients. NBS for intoxication-type organic acidurias has to be fast and treatment has to be started pre-emptively, i.e. even before the diagnosis is finally confirmed.
Acknowledgements
We thank all respondents for contributing their data to the survey. Collection of data underlying this publication was funded by the European Union contract number 2009 6206 of the Executive Agency for Health and Consumers.
Abbreviations
- EFTA
European Free Trade Association
- ESPE
European Society for Paediatric Endocrinology
- FYROM
Former Yugoslav Republic of Macedonia
- GA-I
Glutaric aciduria type I, OMIM 231670 deficiency of glutaryl-CoA dehydrogenase
- GP
General practitioner
- IVA
Isovaleric aciduria, OMIM 243500 deficiency of isovaleryl-CoA dehydrogenase
- ISNS
International Society for Neonatal Screening
- MMACBL
-
Methylmalonic aciduria including cblA, cblB, cblC and cblD defects
OMIM 251000 methylmalonic aciduria, methylmalonyl-CoA mutase deficiency
OMIM 251100 methylmalonic aciduria, cblA type
OMIM 251110 methylmalonic aciduria, cblB type
OMIM 277400 methylmalonic aciduria and homocystinuria, cblC type
OMIM 277410 methylmalonic aciduria and homocystinuria, cblD type
- NBS
Newborn screening
- PA
-
Propionic aciduria
OMIM 232050 deficiency of propionyl-CoA carboxylase subunit β
OMIM 232000 deficiency of propionyl-CoA carboxylase subunit α
- SSIEM
Society for the Study of Inborn Errors of Metabolism
Synopsis
NBS for organic acidurias in Europe is variable and less often established than for amino acid disorders. While for GA-I its benefit has already been demonstrated, there is room for debate of NBS for IVA and especially PA and MMACBL defects. For optimal benefit NBS for intoxication-type organic acidurias has to be fast and treatment has to be started pre-emptively, i.e. even before the diagnosis is confirmed.
Authors’ Contributions
Designing, planning and conducting the study: All authors
Collection of data and statistical analysis: Peter Burgard
Manuscript writing: All authors
Guarantor
Peter Burgard
Conflict of Interest
Friederike Hörster, Stefan Kölker, J. Gerard Loeber, Martina C. Cornel, Georg F. Hoffmann and Peter Burgard declare that they have no conflict of interests.
Details of Funding
Collection of data underlying this publication was funded by the European Union contract number 2009 6206 of the Executive Agency for Health and Consumers. All authors declare that the content of the article has not been influenced by the sponsors.
Ethics Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. No data on individual patients are included in this study; therefore no informed consent had to be obtained.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Competing interests: None declared
Contributor Information
Friederike Hörster, Email: friederike.hoerster@med.uni-heidelberg.de.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Burgard P, Rupp K, Lindner M, et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 2. From screening laboratory results to treatment, follow-up and quality assurance. J Inherit Metab Dis. 2012;35(4):613–625. doi: 10.1007/s10545-012-9484-z. [DOI] [PubMed] [Google Scholar]
- Chace DH, DiPerna JC, Kalas TA, et al. Rapid diagnosis of methylmalonic and propionic acidemias: quantitative tandem mass spectrometric analysis of propionylcarnitine in filter-paper blood specimens obtained from newborns. Clin Chem. 2001;47:2040–2044. [PubMed] [Google Scholar]
- Cornel MC, Rigter T, Weinreich SS, et al. A framework to start the debate on neonatal screening policies in the EU: an Expert Opinion Document. Eur J Hum Genet. 2014;22:12–17. doi: 10.1038/ejhg.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couce ML, Castiñeiras DE, Bóveda MD, et al. Evaluation and long-term follow-up of infants with inborn errors of metabolism identified in an expanded screening programme. Mol Genet Metab. 2011;104(4):470–475. doi: 10.1016/j.ymgme.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Deodato F, Boenzi S, Santorelli FM, et al. Methylmalonic and propionic aciduria. Am J Med Genet C Semin Med Genet. 2006;142C:104–112. doi: 10.1002/ajmg.c.30090. [DOI] [PubMed] [Google Scholar]
- Dionisi-Vici C, Deodato F, Roschinger W, et al. ‘Classical’ organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J Inherit Metab Dis. 2006;29:383–389. doi: 10.1007/s10545-006-0278-z. [DOI] [PubMed] [Google Scholar]
- Ensenauer R, Vockley J, Willard JM, et al. A common mutation is associated with a mild, potentially asymptomatic phenotype in patients with isovaleric acidemia diagnosed by newborn screening. Am J Hum Genet. 2004;75:1136–1142. doi: 10.1086/426318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensenauer R, Fingerhut R, Maier EM, et al. Newborn screening for isovaleric acidemia using tandem mass spectrometry: data from 1.6 million newborns. Clin Chem. 2011;57(4):623–626. doi: 10.1373/clinchem.2010.151134. [DOI] [PubMed] [Google Scholar]
- Grünert SC, Wendel U, Lindner M, et al. Clinical and neurocognitive outcome in symptomatic isovaleric acidemia. Orphanet J Rare Dis. 2012;7:9. doi: 10.1186/1750-1172-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünert SC, Müllerleile S, de Silva L, et al. Propionic acidemia: neonatal versus selective metabolic screening. J Inherit Metab Dis. 2012;35:41–49. doi: 10.1007/s10545-011-9419-0. [DOI] [PubMed] [Google Scholar]
- Heringer J, Boy SP, Ensenauer R, et al. Use of guidelines improves the neurological outcome in glutaric aciduria type I. Ann Neurol. 2010;68(5):743–752. doi: 10.1002/ana.22095. [DOI] [PubMed] [Google Scholar]
- Heringer J, Valayannopoulos V, Lund AM, et al. Impact of age at onset and newborn screening on outcome in organic acidurias. J Inherit Metab Dis. 2015 doi: 10.1007/s10545-015-9907-8. [DOI] [PubMed] [Google Scholar]
- Kölker S, Garbade SF, Greenberg CR, et al. Natural history, outcome, and treatment efficacy in children and adults with glutaryl-CoA dehydrogenase deficiency. Pediatr Res. 2006;59:840–847. doi: 10.1203/01.pdr.0000219387.79887.86. [DOI] [PubMed] [Google Scholar]
- Kölker S, Garbade SF, Boy N, et al. Decline of acute encephalopathic crises in children with glutaryl-CoA dehydrogenase deficiency identified by newborn screening in Germany. Pediatr Res. 2007;62:357–363. doi: 10.1203/PDR.0b013e318137a124. [DOI] [PubMed] [Google Scholar]
- Kölker S, Christensen E, Leonard JV, et al. Diagnosis and management of glutaric aciduria type I – revised recommendations. J Inherit Metab Dis. 2011;34:677–694. doi: 10.1007/s10545-011-9289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölker S, Garcia Cazorla A, Valayannopoulos V et al (2015a) The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J Inherit Metab Dis 38:1041–1057 [DOI] [PubMed]
- Kölker S, Valayannopoulos V, Burlina AB, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving phenotype. J Inherit Metab Dis. 2015;38:1159–1174. doi: 10.1007/s10545-015-9892-y. [DOI] [PubMed] [Google Scholar]
- Leonard JV, Vijayaraghavan S, Walter JH. The impact of screening for propionic and methylmalonic acidaemia. Eur J Pediatr. 2003;162(Suppl 1):S21–S24. doi: 10.1007/s00431-003-1345-1. [DOI] [PubMed] [Google Scholar]
- Lindner M, Ho S, Fang-Hoffmann J, et al. Neonatal screening for glutaric aciduria type I: strategies to proceed. J Inherit Metab Dis. 2006;29:378–382. doi: 10.1007/s10545-006-0284-1. [DOI] [PubMed] [Google Scholar]
- Lindner M, Ho S, Kolker S, et al. Newborn screening for methylmalonic acidurias-optimization by statistical parameter combination. J Inherit Metab Dis. 2008;31:379–385. doi: 10.1007/s10545-008-0892-z. [DOI] [PubMed] [Google Scholar]
- Lindner M, Gramer G, Haege G, et al. Efficacy and outcome of expanded newborn screening for metabolic diseases--report of 10 years from South-West Germany. Orphanet J Rare Dis. 2011;6:44. doi: 10.1186/1750-1172-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber JG, Burgard P, Cornel MC, et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 1. From blood spot to screening result. J Inherit Metab Dis. 2012;35(4):603–611. doi: 10.1007/s10545-012-9483-0. [DOI] [PubMed] [Google Scholar]
- Pfeil J, Listl S, Hoffmann GF, et al. Newborn screening by tandem mass spectrometry for glutaric aciduria type 1: a cost-effectiveness analysis. Orphanet J Rare Dis. 2013;8:167. doi: 10.1186/1750-1172-8-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vockley J, Ensenauer R. Isovaleric acidemia: new aspects of genetic and phenotypic heterogeneity. Am J Med Genet C Semin Med Genet. 2006;142C(2):95–103. doi: 10.1002/ajmg.c.30089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JMG, Jungner G (1968) The principles and practice of screening for disease. Public health papers, vol 34. World Health Organization, Geneva. http://whqlibdoc.who.int/php/WHO_PHP_34.pdf. Accessed 9 Oct 2015
Internet Documents
- Cornel M, Rigter T, Weinreich S et al (2011) Newborn screening in Europe: expert opinion document. http://www.iss.it/cnmr/index.php?lang=1&id=1621&tipo=72. Accessed 5 Oct 2015
- E-IMD Consortium. www.eimd-registry.org. Accessed 5 Oct 2015
- Kelm K, Tanksley S (2015) Timeliness of newborn screening: suggested recommendations from DACHDNC laboratory standards and procedures subcommittee. Accessed 12 Feb 2015


