Abstract
Background: Lesch–Nyhan disease (LND) is an X-chromosomal disorder of purine metabolism characterized by hyperuricemia, dystonia, and self-mutilation, leading to an extremely high burden of disease in affected patients and families. Although allopurinol therapy can control hyperuricemia, it has no effect on self-mutilation and neurological symptoms. Single reports describe a beneficial effect of S-adenosylmethionine (SAM) on the neurological symptoms, which motivated us to evaluate this alternative treatment.
Methods: We performed a double-blind placebo-controlled trial to analyze the effects of SAM on self-mutilation attempts in a male patient affected by LND. The trial lasted for 282 days and comprised three alternating verum and placebo periods of 50 days each. The mother of the patient recorded attempts of self-mutilation during the entire trial.
Results: While verum and placebo were both well tolerated, a total of 1,762 events of self-mutilation were recorded, of which 1,281 events were in the placebo period and 481 in the verum period. The daily mean of events was 8.6 with placebo and 4.5 with SAM corresponding to a 50 % decrease in self-mutilation events under SAM treatment (p < 0.05).
Conclusion: The results of this double-blind placebo-controlled single-case trial suggest that SAM can have a beneficial effect on self-mutilation in patients with LND, possibly by replenishing the purine pool in affected brain cells.
Keywords: Lesch–Nyhan disease, Placebo-controlled double-blind trial, S-Adenosylmethionine, Self-mutilation
Introduction
Lesch–Nyhan disease (LND, OMIM #308000) is a severe metabolic disorder due to a lack of hypoxanthine-guanine phosphoribosyltransferase (HPRT E.C. 2.4.2.8). The disease is characterized by hyperuricemia and neurological symptoms including dystonia, psychomotor retardation, and self-mutilation. In humans, the main role of HPRT is to salvage hypoxanthine and guanine and, hereby, to replenish the intracellular purine pool (Fig. 1). In LND, hypoxanthine is degraded to uric acid by xanthine oxidase, thus resulting in hyperuricemia. The disease is X-linked with an incidence of 1 in 380,000 (Bertelli et al. 2004). The defective enzyme is encoded by the HPRT1 gene, and more than 615 mutations are known (Fu et al. 2014; Jinnah et al. 2004). There are several variants of the disease with different clinical severity (Jinnah et al. 2000, 2010; Puig et al. 2001; Schretlen et al. 2005). HPRT is expressed in all tissues with high levels found in many regions of the brain.
Fig. 1.
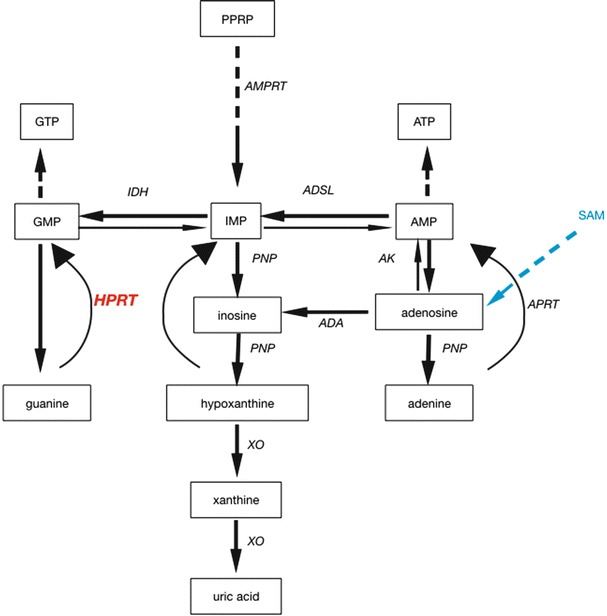
Scheme of the metabolic pathway of purines and their relation to hypoxanthine-guanine-phosphoribosyltransferase (HPRT). In Lesch–Nyhan disease (LND), the lack of HPRT activity leads to a failure in recycling of both guanine and hypoxanthine, thus creating a deficiency of both inosine monophosphate (IMP) and guanosine monophosphate (GMP). Hypoxanthine (and guanine) concentrations are elevated in LND and will be metabolized to uric acid by xanthine oxidase (XO). With S-adenosylmethionine (SAM, depicted in blue), an adenosine donor, the lack of purines in LND can be recovered by turning adenosine to adenosine monophosphate (AMP) by adenine phosphoribosyltransferase (APRT) and then to IMP. ADA adenosine deaminase, ADSL adenylosuccinate lyase, AK adenosine kinase, AMPRT amido phosphoribosyltransferase, ATP adenosine triphosphate, GTP guanosine triphosphate, IDH isocitrate dehydrogenase, PNP purine nucleoside phosphorylase, PPRP phosphoribosylpyrophosphate
The clinical phenotype manifests mainly in males, but there are also female patients with LND (Chen et al. 2014; De Gregorio et al. 2000). Manifestation of LND is often by developmental delay in infancy, followed by dystonia and spasticity, which may provoke misdiagnosis as cerebral palsy. The presence of orange crystals in the diapers may raise suspicion of hyperuricemia.
LND usually leads to several severe complications along each of the clinical presentations, and all have a huge impact on the (social) life of patients and their families. The movement disorder is often severe and may necessitate the use of a wheelchair from early childhood. Self-mutilation and autoaggressive behavior are almost pathognomonic and may require wearing of gloves and/or dental removal to prevent bite wounds to fingers/hands and lips/cheeks. Overproduction of uric acid causes nephrolithiasis leading to renal insufficiency, which is a main cause of death. Furthermore, there are several sudden death cases related to LND (Neychev and Jinnah 2006).
The pathophysiology of LND is not fully understood, but the general hypothesis includes depletion of adenosine (Criswell et al. 1988; Green et al. 1982; Kopin 1981; Stone 1981). Lack of purines due to HPRT deficiency in LND may be rescued by salvaging purines via adenosine → inosine monophosphate (IMP) → guanosine monophosphate (GMP). S-adenosylmethionine (SAM) is thought to replenish the purine pool by serving as an adenosine donor (Fig. 1).
SAM, previously used in another purine deficiency disease, Arts syndrome (de Brouwer et al. 2007; Duley et al. 2011), has been beneficial for treatment of self-mutilation in a single LND patient who initially received SAM for liver detoxification and elevated transaminases (Bottiglieri 2002; Glick 2006) and was tested in 14 patients from the Italian LND cohort with unequivocally opposite effects on patients (Dolcetta et al. 2013). A recent study in four additional patients (Chen et al. 2014) demonstrated a positive effect of SAM on the neurological symptoms, mainly self-mutilation.
Here, we prospectively investigated the effect of SAM in a double-blind placebo-controlled trial in a single patient with LND. From the findings of this trial, we suggest that SAM therapy could be beneficial for control of self-mutilation in LND.
Methods
Patient Presentation and Clinical Course
We describe a firstborn male child from unrelated healthy Swiss parents with normal postnatal adaptation. After normal development during his first 6 months of life, parents noticed first signs of developmental delay and muscular hypotonia. Evolution of dystonia and spasticity as well as orange crystals in the diapers and elevations of uric acid in blood and urine led to the suspicion of LND. This was confirmed by enzymatic testing in leukocytes (Laboratory of Genetic Metabolic Disease, Erasmus Medical Center, Rotterdam) at an age of 18 months. Mutation analysis of the HPRT1 gene showed a c.319-1G>A de novo mutation in the patient, but not in the mother, probably leading to a splice defect of the gene. Allopurinol therapy was started at a low dose (as the only additional pharmacological treatment at the time of the SAM trial) and adapted in relation to the uric acid levels in serum and urine (Fig. 2). The patient showed first self-mutilation attempts at age 2.5 years by biting his fingers, and this symptom was progressive leading to severe destruction of lips and repeated deep oral wounds. Physiotherapy led to some improvement of hypotonia and spasticity. The patient became wheelchair bound at age four years. To prevent malnutrition, supportive gastric tube feeding was initiated at age 3.7 years. For treatment of sleep disorders and anxiety, risperidone seemed beneficial.
Fig. 2.
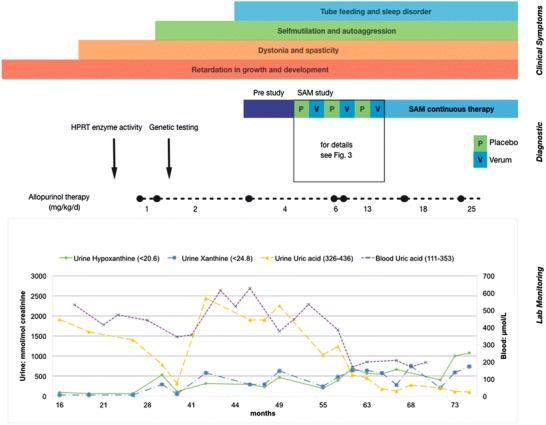
Details of the patient divided into three main groups: clinical symptoms, diagnostic interventions, and lab monitoring. In the top in differently colored bars, the clinical manifestations of the disease in the patient are depicted. Below another bar shows the pre-study period followed by the SAM trial. The dotted black line indicates the adjustments of allopurinol therapy. A small drop in uric acid concentrations can be seen after start of allopurinol therapy, but the dose had to be adapted several times until urine and blood uric acid decreased to therapeutic ranges, while urinary xanthine and hypoxanthine increased
Planning of Therapeutic Trial and Study Design
Self-mutilation became the most stressful symptom for patient and parents and led to restraining of hands and legs for most of the time during days and nights. In an attempt to quantify self-mutilation attempts, recording by the parents was started as a pre-study period (Fig. 2). Self-mutilation attempts were defined as abrupt arm movements of the patient aimed at biting his fingers or hands. This pre-study period comprised 165 days, during which 212 self-mutilation attempts were documented confirming that recording of these events was feasible and tolerated by the parents. Later analysis of these recordings showed a possible association of self-mutilation attempts with pain and/or infections. In order to evaluate a possible effect of SAM on the number of self-mutilation events, a double-blind placebo-controlled trial was performed over eight months in which the parents continued to record self-mutilation events. Informed consent of parents was obtained. The trial consisted of alternating periods of 50 days on either placebo or SAM (verum) (Figs. 2 and 3). The patient was given capsules with 100 mg SAM twice daily (corresponding to 17 mg/kg/day) or placebo via his nasogastric tube. SAM (Abbot AG, Baar, Switzerland) and placebo were prepared as capsules indistinguishable by appearance and smell/taste. As this was a therapeutic trial with a licensed drug in a single patient, there was no need to obtain formal approval by the local ethical board.
Fig. 3.
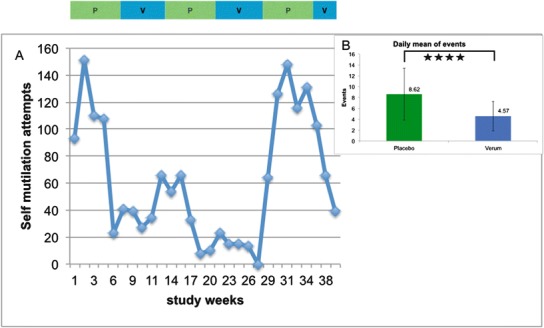
Graph illustrating the number of self-mutilation events during the SAM trial. Panel A: Events during trial periods (a bar above the graph shows placebo periods in green and SAM periods in blue). For clarity of presentation, each 50-day period is calculated as 5 parts of 10 days each. Panel B: Daily mean ± SD of self-mutilation events; green column represents placebo periods (total days, 154), and blue column represents verum period (total days, 128). The difference between the two periods was significant (p < 0.001)
Statistical Analysis
To analyze results, an unpaired t-student test was used with average of recorded events ± standard deviation. Results were considered significant in case of p < 0.05.
Results
The study was tolerated without adverse events, and total study duration was 9 months. All periods of the study (placebo and verum) could be completed as planned with 50 days each. As the only exception, capsules taken in the last study period changed appearance and smell probably because of the high temperature and/or humidity in the air during summer months. Therefore, the study was finished slightly ahead of schedule. Before unblinding of the study, the parents mentioned their suspicion of having lastly received verum based on the recent smell of the compound and their impression of a clinical improvement during every second period.
During the entire study period, a total of 1,762 self-mutilation events were recorded. Of these, 1,281 events occurred during the placebo periods and 481 during the verum periods of the study. This corresponds to a significant reduction of self-mutilation events from 8.6 ± 4.75 mean daily events in the placebo periods to 4.5 ± 2.57 mean daily events when SAM was taken (p < 0.001). Based on this almost 50 % reduction of self-mutilation events (Fig. 3) and based on the parental impression of a clear therapeutic benefit of SAM when study periods were unblinded, SAM was continued at the same dose (100 mg twice daily) as long-term therapy.
Along increasing allopurinol dosages, levels of uric acid in blood and urine decreased, while concentrations of xanthine and hypoxanthine, the latter the best soluble of the three, increased (Fig. 2).
Discussion
Treatment of LND is symptomatic and often of only limited efficacy. The result from our placebo-controlled trial in a single patient showed a clear reduction in self-mutilation attempts. Self-mutilation is a key symptom in LND and most often includes biting lips or fingers (Robey et al. 2003). Most patients experience this as involuntary and request help or try to protect themselves by wearing gloves (Nyhan 1976). Our patient started self-mutilation with about 2.5 years, which is in line with other reports (Anderson and Ernst 1994).
Although we only studied one patient, we tried to compensate this with a careful pre-study period and a strict study protocol. In the pre-study period, we ensured quantification of self-mutilation events as good as this can be achieved with this partly subjective symptom that seemed to be associated with tiredness and pain, which is typical for LND patients. The study itself was double blind and placebo controlled, lasted 282 days, and was thus the optimum we could achieve in this n = 1 situation. Another limitation of our study is the fact that self-injury varies over time and some of the changes may not be related to SAM. Nevertheless, while we are aware of these limitations, we still consider the improvement of the clinical situation under SAM therapy, as demonstrated by the significant decrease in self-mutilating events, as very encouraging because it relieved the physical and psychological distress for the patient and his family. The result may be further valuable for the understanding of the pathophysiology of LND and may open the way for further improvement of treatment.
SAM treatment has previously been reported in only few patients (Chen et al. 2014; Dolcetta et al. 2013; Glick 2006); in some of these, supplementation with SAM led to a drastic reduction in self-mutilation events and very good clinical outcome. This was investigated with (Dolcetta et al. 2013) or without (Chen et al. 2014; Glick 2006) formal assessment tools, and we decided here to base effect on parental judgment and documentation.
Action of LND is not fully understood, but SAM may replenish adenosine in cells, which can then be formed into adenosine monophosphate by adenosine kinase, and adenosine triphosphate. Furthermore, IMP can be formed by adenylosuccinate lyase, which can then be formed to GMP by isocitrate dehydrogenase, thus replenishing guanosine triphosphate purines. Purine depletion in tissue culture models and selective vulnerability of basal ganglia in HPRT-deficient mice was shown in several studies (Dunnett et al. 1989; Finger et al. 1988; Jinnah et al. 1992, 1994, 1999), and SAM could act on this by replenishing adenosyl stores in LND. Other authors discuss a role of SAM in neurotransmitter synthesis and metabolism (Dolcetta et al. 2013; Miller 2008), but this clearly needs more studies.
Although self-mutilation events cause most stress in LND, many patients die from renal failure due to nephrolithiasis. Prevention or treatment of nephrolithiasis should therefore be a priority, and a lesson can be learned from management in our patient. Lowering urinary uric acid concentration, the main therapeutic goal in LND, is coupled to an increase of renal xanthine and hypoxanthine excretion, which are a risk of urolithiasis. However, the solubility of these three metabolites should guide dosing of allopurinol: uric acid has by far the lowest solubility in water (10.5 mmol/L, HMBD, http://www.hmdb.ca/), while hypoxanthine is much better soluble (95.5 mmol/L, HMBD; this concentration will even with high-dose allopurinol hardly ever be reached). We thus suggest that a high dose of allopurinol should be administered from the beginning of treatment in LND.
In conclusion, our n = 1 trial could show that SAM significantly reduces self-mutilating behavior in LND and may offer an additional therapeutic means adding to current symptomatic therapy. Furthermore, the course of purine metabolites in blood and urine underline the recommendation for an early start of high-dose allopurinol to prevent development of kidney stones. Finally, the findings of our study point toward adenosine depletion as a factor in the still dubious pathophysiology of LND.
Acknowledgments
We appreciate the help of the family of our patient in conducting this trial and specifically the excellent recording of self-mutilation events. We also thank Mrs. Ursina Spörri, Zurich, for her help in data analysis.
Compliance with Ethics Guidelines
Conflict of Interest
Matthias Lauber, Barbara Plecko, Miriam Pfiffner, Jean-Marc Nuoffer, and Johannes Häberle declare that they have no conflict of interest.
This article does contain studies with a human subject. The patient received a medication as compassionate use after approval was obtained from the SwissMedic/Schweizerisches Heilmittelinstitut. As this was a therapeutic trial with a licensed drug in a single patient, there was no need to obtain formal approval by the local ethical board.
Jean-Marc Nuoffer and Johannes Häberle have together planned the study that was performed under the supervision of Johannes Häberle. Analysis and interpretation of data, drafting of the article, and revision were performed by Matthias Lauber, Barbara Plecko, Miriam Pfiffner, Jean-Marc Nuoffer, and Johannes Häberle.
Contributor Information
Johannes Häberle, Email: Johannes.Haeberle@kispi.uzh.ch.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Anderson LT, Ernst M. Self-injury in Lesch–Nyhan disease. J Autism Dev Disord. 1994;24:67–81. doi: 10.1007/BF02172213. [DOI] [PubMed] [Google Scholar]
- Bertelli M, Randi D, Micheli V, Gallo S, Andrighetto G, Parmigiani P, Jacomelli G, Carella M, Lievore C, Pandolfo M. Molecular basis of hypoxanthine-guanine phosphoribosyltransferase deficiency in Italian Lesch–Nyhan patients: identification of nine novel mutations. J Inherit Metab Dis. 2004;27:767–773. doi: 10.1023/B:BOLI.0000045799.78633.23. [DOI] [PubMed] [Google Scholar]
- Bottiglieri T. S-Adenosyl-L-methionine (SAMe): from the bench to the bedside—molecular basis of a pleiotrophic molecule. Am J Clin Nutr. 2002;76:1151S–1157S. doi: 10.1093/ajcn/76/5.1151S. [DOI] [PubMed] [Google Scholar]
- Chen BC, Balasubramaniam S, McGown IN, O'Neill JP, Chng GS, Keng WT, Ngu LH, Duley JA. Treatment of Lesch–Nyhan disease with S-adenosylmethionine: experience with five young Malaysians, including a girl. Brain Dev. 2014;36:593–600. doi: 10.1016/j.braindev.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Criswell H, Mueller RA, Breese GR. Assessment of purine-dopamine interactions in 6-hydroxydopamine-lesioned rats: evidence for pre- and postsynaptic influences by adenosine. J Pharmacol Exp Ther. 1988;244:493–500. [PubMed] [Google Scholar]
- de Brouwer AP, Williams KL, Duley JA, van Kuilenburg AB, Nabuurs SB, Egmont-Petersen M, Lugtenberg D, Zoetekouw L, Banning MJ, Roeffen M, Hamel BC, Weaving L, Ouvrier RA, Donald JA, Wevers RA, Christodoulou J, van Bokhoven H. Arts syndrome is caused by loss-of-function mutations in PRPS1. Am J Hum Genet. 2007;81:507–518. doi: 10.1086/520706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio L, Nyhan WL, Serafin E, Chamoles NA. An unexpected affected female patient in a classical Lesch–Nyhan family. Mol Genet Metab. 2000;69:263–268. doi: 10.1006/mgme.2000.2967. [DOI] [PubMed] [Google Scholar]
- Dolcetta D, Parmigiani P, Salmaso L, Bernardelle R, Cesari U, Andrighetto G, Baschirotto G, Nyhan WL, Hladnik U. Quantitative evaluation of the clinical effects of S-adenosylmethionine on mood and behavior in Lesch–Nyhan patients. Nucleosides Nucleotides Nucleic Acids. 2013;32:174–188. doi: 10.1080/15257770.2013.774012. [DOI] [PubMed] [Google Scholar]
- Duley JA, Christodoulou J, de Brouwer AP. The PRPP synthetase spectrum: what does it demonstrate about nucleotide syndromes? Nucleosides Nucleotides Nucleic Acids. 2011;30:1129–1139. doi: 10.1080/15257770.2011.591747. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Sirinathsinghji DJ, Heavens R, Rogers DC, Kuehn MR. Monoamine deficiency in a transgenic (Hprt-) mouse model of Lesch–Nyhan syndrome. Brain Res. 1989;501:401–406. doi: 10.1016/0006-8993(89)90659-8. [DOI] [PubMed] [Google Scholar]
- Finger S, Heavens RP, Sirinathsinghji DJ, Kuehn MR, Dunnett SB. Behavioral and neurochemical evaluation of a transgenic mouse model of Lesch–Nyhan syndrome. J Neurol Sci. 1988;86:203–213. doi: 10.1016/0022-510X(88)90099-8. [DOI] [PubMed] [Google Scholar]
- Fu R, Chen CJ, Jinnah HA. Genotypic and phenotypic spectrum in attenuated variants of Lesch–Nyhan disease. Mol Genet Metab. 2014;112:280–285. doi: 10.1016/j.ymgme.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick N. Dramatic reduction in self-injury in Lesch–Nyhan disease following S-adenosylmethionine administration. J Inherit Metab Dis. 2006;29:687. doi: 10.1007/s10545-006-0229-8. [DOI] [PubMed] [Google Scholar]
- Green RD, Proudfit HK, Yeung SM. Modulation of striatal dopaminergic function by local injection of 5'-N-ethylcarboxamide adenosine. Science. 1982;218:58–61. doi: 10.1126/science.7123218. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Langlais PJ, Friedmann T. Functional analysis of brain dopamine systems in a genetic mouse model of Lesch–Nyhan syndrome. J Pharmacol Exp Ther. 1992;263:596–607. [PubMed] [Google Scholar]
- Jinnah HA, Wojcik BE, Hunt M, Narang N, Lee KY, Goldstein M, Wamsley JK, Langlais PJ, Friedmann T. Dopamine deficiency in a genetic mouse model of Lesch–Nyhan disease. J Neurosci. 1994;14:1164–1175. doi: 10.1523/JNEUROSCI.14-03-01164.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Jones MD, Wojcik BE, Rothstein JD, Hess EJ, Friedmann T, Breese GR. Influence of age and strain on striatal dopamine loss in a genetic mouse model of Lesch–Nyhan disease. J Neurochem. 1999;72:225–229. doi: 10.1046/j.1471-4159.1999.0720225.x. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, De Gregorio L, Harris JC, Nyhan WL, O'Neill JP. The spectrum of inherited mutations causing HPRT deficiency: 75 new cases and a review of 196 previously reported cases. Mutat Res. 2000;463:309–326. doi: 10.1016/S1383-5742(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Harris JC, Nyhan WL, O'Neill JP. The spectrum of mutations causing HPRT deficiency: an update. Nucleosides Nucleotides Nucleic Acids. 2004;23:1153–1160. doi: 10.1081/NCN-200027400. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Ceballos-Picot I, Torres RJ, Visser JE, Schretlen DJ, Verdu A, Larovere LE, Chen CJ, Cossu A, Wu CH, Sampat R, Chang SJ, de Kremer RD, Nyhan W, Harris JC, Reich SG, Puig JG, Lesch–Nyhan Disease International Study Group (2010) Attenuated variants of Lesch–Nyhan disease. Brain 133:671–689. doi:10.1093/brain/awq013 [DOI] [PMC free article] [PubMed]
- Kopin IJ. Neurotransmitters and the Lesch–Nyhan syndrome. N Engl J Med. 1981;305:1148–1150. doi: 10.1056/NEJM198111053051910. [DOI] [PubMed] [Google Scholar]
- Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008;13:216–226. [PubMed] [Google Scholar]
- Neychev VK, Jinnah HA. Sudden death in Lesch–Nyhan disease. Dev Med Child Neurol. 2006;48:923–926. doi: 10.1017/S0012162206002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhan WL. Behavior in the Lesch–Nyhan syndrome. J Autism Child Schizophr. 1976;6:235–252. doi: 10.1007/BF01543464. [DOI] [PubMed] [Google Scholar]
- Puig JG, Torres RJ, Mateos FA, Ramos TH, Arcas JM, Buno AS, O'Neill P. The spectrum of hypoxanthine-guanine phosphoribosyltransferase (HPRT) deficiency. Clinical experience based on 22 patients from 18 Spanish families. Medicine (Baltimore) 2001;80:102–112. doi: 10.1097/00005792-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Robey KL, Reck JF, Giacomini KD, Barabas G, Eddey GE. Modes and patterns of self-mutilation in persons with Lesch–Nyhan disease. Dev Med Child Neurol. 2003;45:167–171. doi: 10.1111/j.1469-8749.2003.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Ward J, Meyer SM, Yun J, Puig JG, Nyhan WL, Jinnah HA, Harris JC. Behavioral aspects of Lesch–Nyhan disease and its variants. Dev Med Child Neurol. 2005;47:673–677. doi: 10.1017/S0012162205001374. [DOI] [PubMed] [Google Scholar]
- Stone TW. Physiological roles for adenosine and adenosine 5'-triphosphate in the nervous system. Neuroscience. 1981;6:523–555. doi: 10.1016/0306-4522(81)90145-7. [DOI] [PubMed] [Google Scholar]


