Abstract
The expression of Ccn2 (CTGF) has been linked to fibrosis in many tissues and pathologies, although its activities in fibroblastic cells and precise mechanism of action in fibrogenesis are still controversial. Here, we showed that CCN2 can induce cellular senescence in fibroblasts both in vitro and in vivo, whereupon senescent cells express an anti-fibrotic “senescence-associated secretory phenotype” (SASP) that includes upregulation of matrix metalloproteinases and downregulation of collagen. Mechanistically, CCN2 induces fibroblast senescence through integrin α6β1-mediated accumulation of reactive oxygen species, leading to activation of p53 and induction of p16INK4a. In cutaneous wound healing, Ccn2 expression is highly elevated only during the initial inflammatory phase and quickly declines thereafter to a low level during the proliferation and maturation phases of healing when myofibroblasts play a major role. Consistent with this expression kinetics, knockdown of Ccn2 has little effect on the rate of wound closure, formation of senescent cells, or collagen content of the wounds. However, application of purified CCN2 protein on cutaneous wounds leads to induction of senescent cells, expression of SASP, and reduction of collagen content. These results show that CCN2 can induce cellular senescence in fibroblasts and is capable of exerting an anti-fibrotic effect in a context-dependent manner.
Keywords: CCN1/CYR61, CCN2/CTGF, Integrins, Wound healing, Cellular senescence
Introduction
Cellular senescence is a stress response to a variety of cellular insults that include DNA damage, mitochondrial dysfunction, and oncogenic activation (Campisi 2013; Wiley et al. 2016). Senescent cells lose the ability to proliferate even in the presence of growth factors and nutrients, although they remain viable and metabolically active. A hallmark of senescent cells is expression of the senescence-associated secretory phenotype (SASP) that includes upregulation of a number of cytokines, growth factors, and matrix metalloproteinases (MMPs), as well as downregulation of extracellular matrix (ECM) proteins and their regulators such as collagen and TGF-β (Coppe et al. 2008). Substantial evidence indicates that senescent cells play a role in physiological changes associated with degenerative phenotypes of aging, and in the suppression or promotion of cancer (Campisi 2013; Chang et al. 2016). Recent studies have also shown that senescent cells contribute to tissue injury repair (Kim et al. 2013; Krizhanovsky et al. 2008) and wound healing (Demaria et al. 2014; Jun and Lau 2010b), where senescent cells can play an anti-fibrotic role.
Wound healing is a complex process that includes three temporally overlapping but functionally distinct phases: it begins with an inflammatory response with infiltration of neutrophils and macrophages, followed by granulation tissue formation with deposition of ECM by myofibroblasts, and concludes with a maturation phase in which the provisional ECM is degraded and remodeled to rebuild the architecture of the original uninjured tissue (Gurtner et al. 2008). In cutaneous wound healing, cellular senescence occurs initially in the proliferation phase in fibroblasts and endothelial cells to promote the formation of granulation tissue by the secretion of mitogenic growth factors (Demaria et al. 2014), whereas in the maturation phase myofibroblasts undergo senescence to initiate matrix remodeling and limit fibrosis through the secretion of MMPs and downregulation of collagen (Jun and Lau 2010b). The matricellular protein CCN1 (CYR61) plays a critical role in wound healing by inducing myofibroblast senescence in the maturation phase through interaction with integrin α6β1, thus activating the anti-fibrotic SASP to limit fibrosis (Jun and Lau 2010a; Jun and Lau 2010b).
CCN1 and CCN2 (CTGF) are two highly homologous members of the CCN family of matricellular proteins that share common structural features including conservation of four structural domains, alignment of all 38 cysteine residues, and binding sites for a number of integrin receptors (Chen and Lau 2009; Jun and Lau 2011). Not surprisingly, CCN1 and CCN2 exhibit many similar biochemical activities and biological functions in various cell types. For example, both CCN1 and CCN2 induce integrin α6β1-mediated adhesive signaling in fibroblasts (Chen et al. 2001) and αvβ3-dependent angiogenic functions in endothelial cells (Babic et al. 1999; Leu et al. 2002). However, Ccn1 and Ccn2 knockout mice show different phenotypes (Ivkovic et al. 2003; Mo et al. 2002), and various studies have suggested that Ccn1 and Ccn2 may serve distinct functions in diverse physiological and pathological contexts (Hall-Glenn and Lyons 2011; Kubota and Takigawa 2015; Lau 2011). Thus, it is plausible to hypothesize that some of the distinct biological functions of CCN1 and CCN2 may be due to differences in their expression patterns, including the cell type and/or time course of their expression in a given context. However, there has not been specific data to support this hypothesis to date.
Here we show that CCN2, similar to CCN1, is capable of inducing cellular senescence in human skin fibroblasts through integrin α6β1-mediated accumulation of reaction oxygen species (ROS) and activation of p53 and p16INK4a (Jun and Lau 2010b). However, Ccn2 does not play a significant role in senescence induction in cutaneous wound healing because its expression is induced only in the inflammatory phase and not in the proliferation and maturation phases when myofibroblasts form the granulation tissue and subsequently stimulate matrix remodeling. By contrast, Ccn1 is highly expressed in the maturation phase when matrix remodeling occurs, during which CCN1 induces myofibroblast senescence (Jun et al. 2015; Jun and Lau 2010b). Remarkably, when purified CCN2 protein is applied on cutaneous wounds during the maturation phase, it is able to induce cellular senescence and elicit the senescence-associated anti-fibrotic response. These findings illustrate that CCN2 can play a context-dependent role in restricting fibrosis, and provide new insights into the function and biology of CCN2.
Materials and Methods
CCN proteins and reagents
Recombinant CCN1 and CCN2 proteins were produced in Sf9 insect cells using a baculovirus expression system and purified by ion-exchange chromatography (Chen et al. 2001; Kireeva et al. 1997). Immunoblots of purified proteins were probed with specific anti-CCN1 and anti-CCN2 antibodies (Fig. 1a). Rabbit polyclonal antibodies against CCN1 and CCN2 were raised against the CCN1 vWC domain (Leu et al. 2003) and a glutathione-S-transferase (GST)-CCN2 fusion protein (Kireeva et al. 1997), respectively. Function blocking monoclonal antibody against α6 integrin (GoH3) were from Chemicon, and rabbit and mouse IgGs were from Sigma. HRP-conjugated anti-rabbit secondary antibodies were from Amersham Biosciences. Adenoviral vectors Ad-LacZ and Ad-CCN2 (Yoon et al. 2010) were kind gifts from Dr. Woo Jin Park (Gwangju Institute of Science and Technology, South Korea) and amplified in 293 T cells.
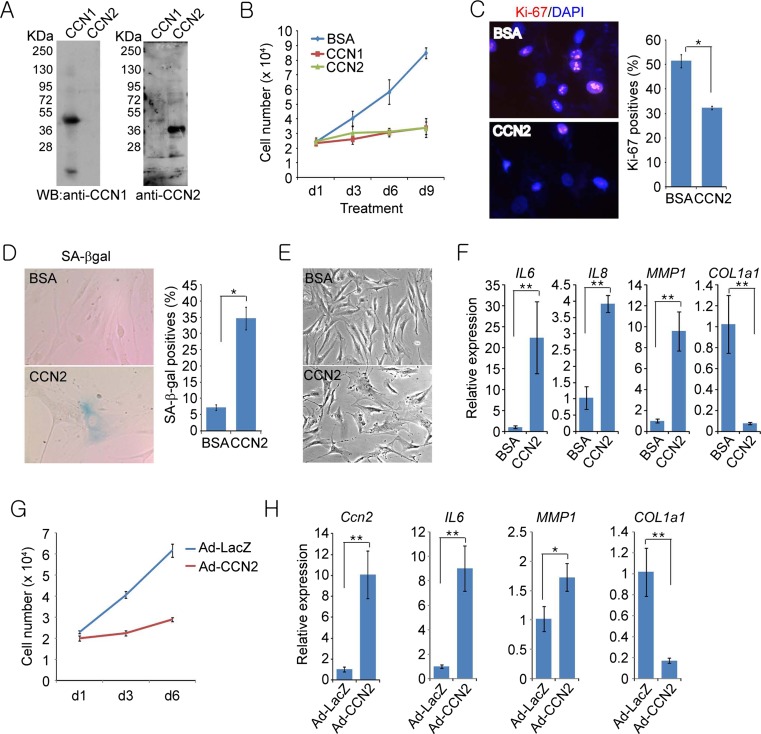
Fig. 1.
CCN2 induces cellular senescence in normal human BJ fibroblasts. a Immunoblots of purified CCN1 and CCN2 protein probed with either anti-CCN1 or anti-CCN2 antibodies. b Human BJ fibroblasts treated with BSA, purified recombinant CCN1, or CCN2 (2.5 μg/ml each) were grown for indicated days and cell numbers counted using a haemocytometer. c After indicated treatment for 3 days, cells were subjected to immunostaining for Ki-67 and counterstained with DAPI. Cells positive for Ki-67 were counted in ten random fields and expressed as percentages of total cell number. d Cells were stained for SA-β-gal activity; positive cells were counted in ten random fields and expressed as percentages of total cell number. e Phase-contrast microscopy showing enlarged and flattened senescence morphology in cells treated with CCN2. f Total RNA was isolated from BJ cells treated with either BSA or CCN2 for 4 days and mRNAs for IL6, IL8, MMP1, and COL1a1 were quantified by qPCR (n = 4 per time point). g BJ cells were infected with recombinant adenoviral vectors (Ad-LacZ and Ad-CCN2) at a multiplicity of infection of 10 for 2 h. Subsequently, cell numbers were counted at indicated days. h Gene expression at day 4 after adenoviral infection was quantified using qPCR (n = 3 per time point). Experiments were done in triplicates and data presented as means ± s.d. *p < 0.05, **p < 0.01
Cell culture
Human BJ foreskin fibroblasts (ATCC# CRL-2522) were maintained at 37 °C in 5 % CO2 in Eagle’s minimum essential medium (EMEM) containing 10 % fetal bovine serum (FBS; Hyclone), 1 % penicillin/streptomycin, non-essential amino acids and sodium pyruvate. BJ cells stably infected with control lentivirus (LV) or lentivirus expressing shp53 or shp16 cells were as described (Jun and Lau 2010b). For adenoviral expression of CCN2, BJ cells were infected with recombinant adenoviral vectors for 2 h at a multiplicity of infection of 10 and cultured for 6 days. The cumulative population doubling (CPD) was calculated using the formula n = (log10 F – log10 I)/log102, where F is the number of cells at the end of one passage, I is the number of cells that were seeded at the beginning of one passage, and n is the number of population doublings occurring in that passage. BJ cells were used between population doublings 30–45.
Analysis of cell proliferation and senescence
Senescence associated-β galactosidase (SA-βgal) assay was performed as described (Debacq-Chainiaux et al. 2009). Frozen wound tissues (6 μm) were fixed in 1 % formalin before staining, and then counterstained with Eosin. For growth curves, cells (1 × 104 cells per well) were grown in 12-well plates and treated as indicated. Cells were stained with 1 % Trypan blue (Invitrogen) and the total cell numbers counted. For Ki-67 immunostaining, cells in 8-well chamber slides (2 × 103 cells per well) were treated with CCN2 for 3 days and fixed using Carnoy’s fixative (ethanol:acetic acid =3:1), washed and cells were stained with anti-Ki67 antibody (Abcam), followed by Alexafluor594-conjugated anti-rabbit IgGs (Invitrogen). Fluorescence microscopy images were taken from five random fields in each well using a Leica DM4000B microscope with QImaging QIClick camera (Image pro Insight software) and Ki-67-positive cells were scored and normalized against 4,6-diamidino-2-phenylindole (DAPI; 1 mg/ml)-positive cells. All assays were done in triplicate, and approximately 300 cells were counted in each sample from randomly selected fields.
ROS measurement
H2DCF-DA (Invitrogen) was used for ROS measurement as described (Chen et al. 2007). Briefly, cells plated on 8-well chamber slides were treated with CCN2 for indicated times. Cells were then incubated with either H2DCF-DA (10 μM) for 10 min at 37 °C, washed with 0.5 % BSA-containing HANKS’ balanced salt solution (HBSS). The fluorescence images were acquired using Leica DM IRB microscope and fluorescence intensity was calculated using ImageJ software (NIH).
Animals, cutaneous wound healing, and wound analysis
All experimental procedures with animals were approved by the Institutional Animal Care Committee of the University of Illinois at Chicago, where mice were housed in an AAALAC-accredited barrier facility. Ccn1 dm/dm mice were generated as described (Jun and Lau 2010b). C57BL/6 mice and Ccn1 dm/dm mice (10 to 12-week-old male mice with 25–28 g body weight) were used for cutaneous wound healing. Full thickness excisional wounds (6 mm) through the panniculus carnosus were created as described (Jun and Lau 2010b). Wound diameters were measured from digital images of wounds using Photoshop CS2 (Adobe). For histological analyses, wound tissues were snap-frozen in OCT compound (Tissue-Tek) and sectioned serially (6 μm) using a Leica CM1950 UV cryostat. Sections were stained for SA-β-gal activity for senescence or with Sirius-red (Sigma) for collagen deposition, and counterstained with Hematoxylin/Eosin (H&E; Sigma). The number of SA-β-gal positives in the wound tissues were counted in 5 randomly-selected high powered fields.
Measurement of collagen deposition in wounds
For hydroxyproline assay, skin wounds were collected at indicated days and dried in 110 °C oven overnight, placed in a screw-capped glass vessel and hydrolyzed in 6 N hydrochloric acid at 110 °C for 18 h. The lysates were processed and hydroxyproline content determined as described (Edwards and O’Brien 1980), using purified hydroxyproline (Sigma) to generate a standard curve. Alternatively, frozen section of wound tissues were processed for Sirius red staining (Sigma) and viewed under polarized light. The Sirius red positive areas were calculated using Image J software (NIH).
Antisense oligonucleotide treatment
Antisense oligonucleotide against Ccn2 was prepared in PBS and injected intravenously (I.V.) (200 μg per injection) through the retro-orbital sinus starting from one day before wound creation to day 2 post-wounding. Sense oligonucleotides were used as control. The sequences of oligonucleotides are: antisense, 5′-CGACGGAGGCGAGCAT-3′ and sense, 5′-ATGCTCGCCTCCGTCGC-3′.
Total RNA isolation and qRT-PCR
BJ cells or skin wound tissues were homogenized using TRIzol reagent (Invitrogen) and total RNA from each sample was isolated using RNeasy® Mini Kit (Quiagen). After reverse transcription using MMLV-Reverse Transcriptase (Promega), qRT-PCR was performed with the iCycler Thermal Cycler (Bio-Rad) using iQ SYBR Green Supermix (Bio-Rad). The specificity of qRT-PCR was confirmed by agarose gel electrophoresis and melting-curve analysis. A housekeeping gene (CyclophilinE) was used as an internal standard. Primers for qRT-PCR are as follows: IL6: forward 5′-AAATTCGGTACATCCTCGACGGCA-3′, reverse 5′- AGTGCCTCTTTGCTGCTTTCACAC-3′; IL8: forward 5′- AGCCTTCCTGATTTCTGCAGCTCT-3′, reverse 5′-AATTTCTGTGTTGGCGCAGTGTGG-3′; MMP1: forward 5′- AGTGACTGGGAAACCAGATGCTGA-3′, reverse 5′-TCAGTGAGGACAAACTGAGCCACA-3′; COL1A1: forward 5′-ACGAAGACATCCCACCAATCACCT-3′, reverse 5′- AGATCACGTCATCGCACAACACCT-3′; Cyclophillin E (human): forward 5′-TTCACAAACCACAATGGCACAGGG-3′, reverse 5′-CACCCTGACACATAAACCCTG-3′; Ccn2: forward 5′-TGAGGCTGAGTCCAGCTGTTCTTT-3′, reverse 5′-ACTTGCCACAAGCTGTCCAGTCTA-3′; Ccn1: forward 5′-GGAGGTGGAGTTAACGAGAAAC-3′, reverse 5′-GTGGTCTGAACGATGCATTTC-3′; Mmp2: forward 5′- TCTGGTGCTCCACCACATACAACT-3′, reverse 5′- CTGCATTGCCACCCATGGTAAACA-3′; for Mmp3: forward 5′-TGGAACAGTCTTGGCTCATGCCTA-3′, reverse 5′- TGGGTACATCAGAGCTTCAGCCTT-3′; Mmp9: forward 5′- TGAGCTGGACAGCCAGACACTAAA-3′, reverse 5′- TCGCGGCAAGTCTTCAGAGTAGTT-3′, for Tgfb1; forward 5′-GTGCGGCAGCTGTACATTGACTTT-3′, reverse 5′- TGTACTGTGTGTCCAGGCTCCAAA-3′; Cyclophilin E (mouse): forward 5′- TTCACAAACCACAATGGCACAGGG-3′, reverse 5′- TGCCGTCCAGCCAATCTGTCTTAT-3′.
Topical CCN2 treatment during wound healing
Recombinant CCN2 protein was diluted in PBS to 0.1 μg/μl, and 50 μl of protein or saline control was directly applied daily to each full-thickness excisional wound in Ccn1 dm/dm mice. Wound tissues were harvested 9 days post-wounding and processed for analysis.
Statistics
Results are expressed as mean ± standard deviation (S.D.), and statistical analysis was performed by one-way ANOVA analysis of variance and Student’s t-test. A p < 0.05 was considered significant. All experiments were performed in triplicate unless otherwise indicated.
Results
CCN2 induces cellular senescence in human skin fibroblasts
To determine whether CCN2 can induce cellular senescence in fibroblasts, we assessed the effect of purified CCN2 protein on the proliferation of human BJ skin fibroblasts cultured in 10 % serum with CCN1 as a positive control. CCN2 inhibited the proliferation of BJ fibroblasts with similar efficiency as CCN1 based on cell numbers counted over several days, whereas cells treated with BSA continued to proliferate as expected (Fig. 1b). Consistently, CCN2-treated fibroblasts exhibited >40 % reduction in Ki-67-positive cells (Fig. 1c), indicating that CCN2 inhibited BJ cell proliferation. Nearly 35 % of cells treated with CCN2 expressed the lysosomal enzyme senescence-associated β-galactosidase (SA-β-gal)(Fig. 1d), a marker for cellular senescence (Rodier and Campisi 2011), compared to ~6 % in BSA-treated control. BJ cells treated with CCN2 also exhibited enlarged and flattened cell morphologies, characteristics of senescent cells (Fig. 1e). Furthermore, CCN2 treatment of BJ fibroblasts induced the expression of SASP, with up-regulation of cytokines (IL6 and IL8) and matrix metalloproteinase 1 (MMP1) and down-regulation of collagen (COL1a1; Fig. 1f). To confirm these results further, we also expressed Ccn2 in BJ cells via an adenoviral vector, which increased Ccn2 mRNA by ~10 fold in BJ cells (Fig. 1g,h). Adenoviral expression of Ccn2 in BJ cells also inhibited cell proliferation and induced expression of SASP, showing upregulation of IL6 and MMP1 but down-regulation of COL1a1. Taken together, these results indicate that CCN2 can induce cellular senescence in human BJ skin fibroblasts.
CCN2 induces reactive oxygen species (ROS) through integrin α6β1, leading to cellular senescence
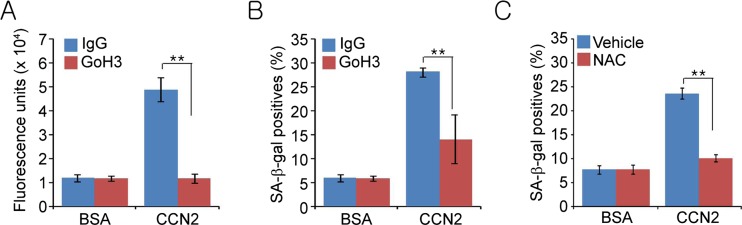
CCN1 was previously shown to induce cellular senescence through its binding to integrin α6β1, leading to the accumulation of ROS (Jun and Lau 2010b). Thus, we assessed the potential role of α6 integrin and ROS in CCN2-induced senescence. We found that fibroblasts treated with CCN2 for 1 h showed high levels in ROS as measured using H2-DCFDA, and ROS accumulation was efficiently blocked when cells were pre-treated with a function-blocking mAb against integrin α6 (GoH3) but not by treatment with control IgG (Fig. 2a). Importantly, pretreatment with either GoH3 or N-acetyl cysteine (NAC), a ROS scavenger, abrogated CCN2-induced cellular senescence (Fig. 2b,c). These results are consistent with previous observation that CCN2 can induce ROS accumulation in human skin fibroblasts as measured by flow cytometry (Juric et al. 2009). ROS can initiate a DNA damage response that may include senescence (Catalano et al. 2005).
Fig. 2.
CCN2 induces cellular senescence through α6 integrin-mediated ROS accumulation. a BJ cells were pre-treated with either IgG or function-blocking monoclonal antibodies against integrin α6 (GoH3, 50 μg/ml) for 1 h, followed by the addition of BSA or CCN2 for an additional 1 h and then stained with H2DCF-DA (10 μM). ROS was quantified by fluorescence measurements using ImageJ (NIH). b Three days after addition of BSA or CCN2, cells positive for SA-β-gal were counted in ten random fields and expressed as percentages of total cell number. c Cells were treated with either NAC (2.5 mM) or vehicle 1 h prior to BSA or CCN2 addition and SA-β-gal activity was assayed 3 days thereafter. Experiments were done in triplicates and data presented as means ± s.d. ** indicates p < 0.01
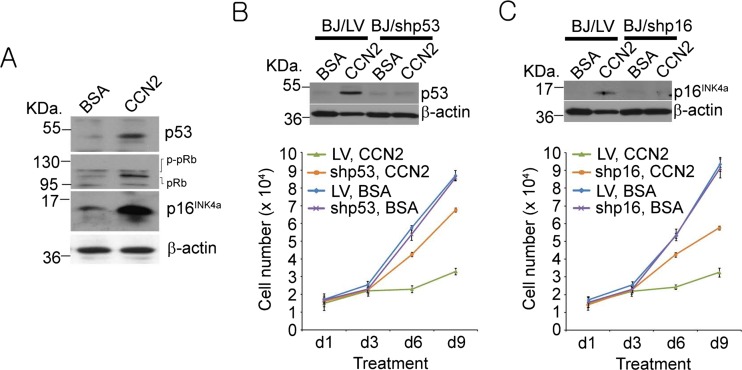
CCN2-induced senescence requires p53 and p16INK4a
Cellular senescence is known to be established and maintained by pathways involving either p53 or p16INK4a, or both (Campisi and d’Adda di Fagagna 2007; Collado et al. 2007). Immunoblot analysis showed that CCN2 treatment in BJ fibroblasts led to the activation of p53 and induction of p16INK4a (Fig. 3a). p16INK4a can in turn activate pRb by inhibiting Cdk4 and Cdk6, leading to accumulation of hypophosphorylated pRb (Fig. 3a). To further confirm the functional role of p53 and p16INK4a in CCN2-induced senescence, we expressed short hairpin RNAs (shRNAs) against p53 and p16INK4a using the lentiviral expression system (Fig. 3b,c). Whereas CCN2 efficiently blocked cell proliferation in cells infected with control lentivirus (LV), knockdown of p53 restored cell proliferation by more than 80 % (Fig. 3b). Likewise, p16INK4a knockdown also mitigated CCN2-induced growth arrest (Fig. 3c). These results indicate that CCN2-induced senescence is dependent on activation of p53 and p16INK4a.
Fig. 3.
CCN2-induced senescence requires p53 and p16INK4a. a Cell lysates from BJ fibroblasts treated with either BSA or CCN2 were electrophoresed and immunoblotted to show increases in p53, p16INK4a, and hypophosphorylated pRb. b Cells were infected with either empty lentivirus (LV) or lentivirus driving expression of shRNAs against p53; cells were then treated with BSA or CCN2 for indicated times before cell numbers counted. c Cells were infected with either LV or lentivirus driving expression of shRNAs against p16INK4a; cells were then treated with BSA or CCN2 for indicated times and cell numbers counted. Knockdown effects of shp53 and shp16 were confirmed using Western blotting. Experiments were done in triplicates and data presented as means ± s.d
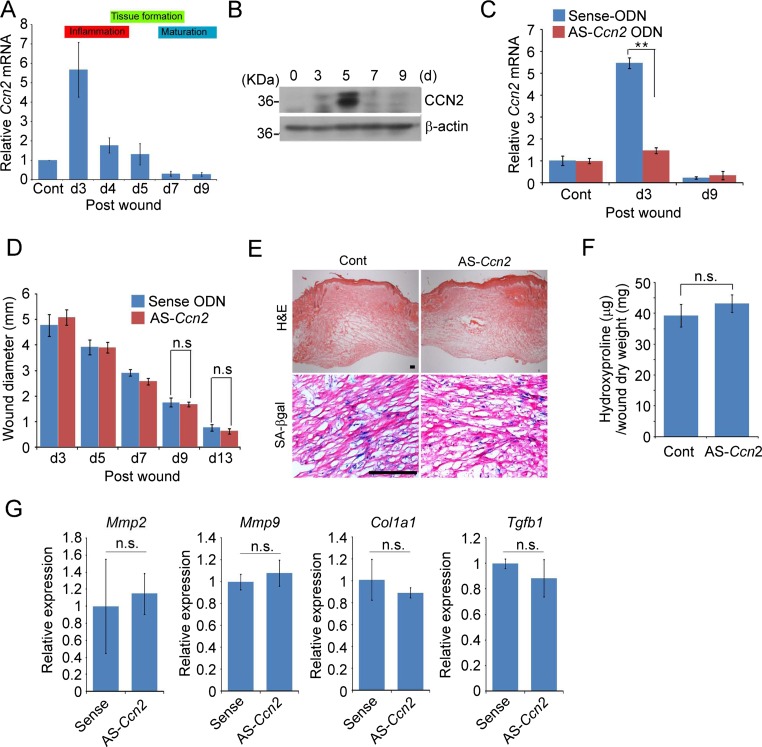
CCN2 is dispensable for cellular senescence during cutaneous wound healing
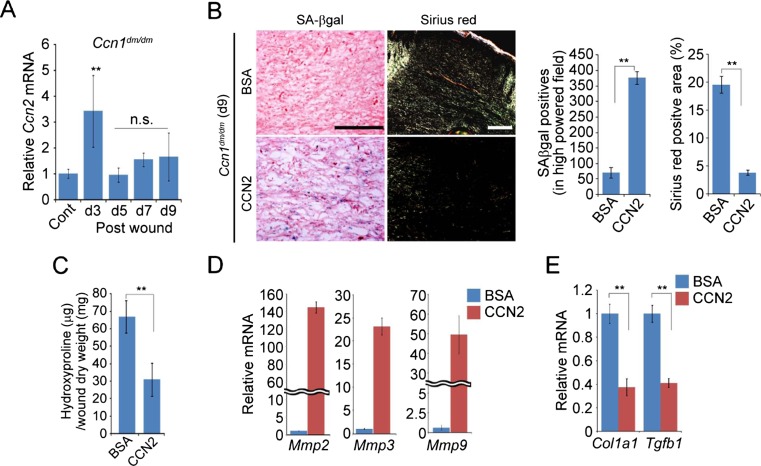
Since myofibroblast senescence occurs as part of the healing process in skin wounds (Demaria et al. 2014; Jun and Lau 2010b), we assessed whether Ccn2 contributes to senescence in wounds. Interestingly, Ccn2 expression in cutaneous wound healing was greatly induced only in the early inflammatory phase (day 3) and is substantially decreased thereafter to a low level in the proliferation phase (day 4–7) and below the uninjured control in the maturation phase (day 6–9; Fig. 4a). Consistently, immunoblot analysis showed that CCN2 protein accumulation peaked at day 5 post-wounding and declined thereafter (Fig. 4b). The low level of Ccn2 expression during the maturation phase suggests that Ccn2 may not play an important role in cellular senescence induction during matrix remodeling. Indeed, although antisense oligonucleotides (AS-ODN) effectively knocked down Ccn2 expression over the 9 day experimental period (Fig. 4c), they did not affect the kinetics of wound closure nor reduce cellular senescence as judged by SA-β-gal activity staining (Fig. 4d,e). Furthermore, Ccn2 knockdown had no effect on collagen accumulation during the maturation phase, as shown by measurements of hydroxyproline content (Fig. 4f). Expression of SASP genes, including Mmp2, Mmp9, Tgfb1, and Col1a1 was not affected by Ccn2 knockdown as analyzed by qPCR (Fig. 4g). Thus, consistent with the low level of Ccn2 expression in the proliferation and maturation phases of wound healing, knockdown of Ccn2 had no appreciable effect on the kinetics of wound closure, accumulation of senescent cells, or collagen content of the wounds.
Fig. 4.
CCN2 is dispensable for cellular senescence during skin wound healing. Cutaneous wounds were created in mice using a 6 mm biopsy punch and healing process observed. a Ccn2 mRNA was quantified by qPCR in total RNA isolated from wounds of WT C57BL/6 mice at indicated days post wounding (n = 4 per time point). b Immunoblots of protein lysates from wounds of C57BL/6 mice probed with anti-CCN2 or anti-β actin antibodies. c Ccn2 mRNA levels were quantified in wounds treated daily with sense or antisense ODN against Ccn2 (AS-Ccn2). d Wound diameters were measured in C57BL/6 mice treated with sense or antisense Ccn2 ODN; n = 6. e Wounds treated as above were harvested at day 9, sectioned, and stained for SA-β-gal activity and counterstained with H&E. f Hydroxyproline content of wounds treated with sense or antisense Ccn2 oligonucleotides was measured 9 days post-wounding. n = 4. g Relative expression of Mmp2, Mmp9, Col1a1, and Tgfb1 in wounds at day 9 was quantified using qPCR and normalized to Cyclophilin E (n = 4). *p < 0.05, **p < 0.01; n.s., not statistically significant. Scale bar = 100 μm
Administration of CCN2 induces cellular senescence and associated anti-fibrotic phenotype in vivo
To test whether CCN2 is capable of inducing cellular senescence in vivo, we applied purified recombinant CCN2 protein directly on cutaneous wounds. For this purpose, we have used Ccn1 dm/dm knockin mice, which encode a mutant CCN1 unable to bind integrin α6β1 and therefore defective for senescence induction, to eliminate CCN1-mediated senescence in skin wounds (Jun and Lau 2010b). The expression of Ccn2 during wound healing in Ccn1 dm/dm mice, as measured by qPCR analysis, was similar to that in WT mice (Fig. 5a). As expected, Ccn1 dm/dm mice showed a dearth of cellular senescence during the maturation phase at day 9, concomitant with a substantial accumulation of collagen as shown by Sirius red staining (Fig. 5b). Strikingly, application of CCN2 on the wounds resulted in a large increase (~8-fold) in the number of senescent cells as well as decreased collagen accumulation (Fig. 5b). Direct measurement of hydroxyproline content confirmed and quantified the decreased of collagen accumulation as a results of CCN2 treatment (Fig. 5c). CCN2-treated wounds also exhibited greatly increased expression of Mmp2, Mmp3, and Mmp9, and decreased expression of Col1a1 and Tgfb1, characteristics of SASP (Fig. 5d,e). Together, these results indicate that CCN2 is capable of inducing cellular senescence in cutaneous wounds and thereby exerting an anti-fibrotic effect.
Fig. 5.
Administration of CCN2 in skin wounds induces senescence and reduces fibrosis. a Expression of Ccn2 in Ccn1 dm/dm mice during wound healing was quantified using qPCR. b Ccn1 dm/dm knockin mice were subjected to cutaneous excisional wounds, and purified recombinant CCN2 protein (0.1 mg/ml; 50 μl per dose) in PBS was applied topically daily. Wounds were sectioned and stained for SA-β-gal activity, or stained with Sirius red and visualized under polarized light. Scale bar = 100 μm. c Wounds treated with CCN2 as above were harvested at day 9 and hydroxyproline content was quantified and normalized over the total dry weight (n = 4). d Relative expression of Mmp2, Mmp3 and Mmp9 in day 9 wounds, as judged by qPCR analysis, was greatly increased in mice treated with CCN2 protein vs. BSA control. e Expression of Col1a1 and Tgfb1 from wounds at day 9 from mice treated with CCN2 or BSA was quantified using qPCR and normalized to Cyclophilin E (n = 4). Data are expressed as mean ± s.d in triplicate determinations.*p < 0.05, **p < 0.01. n.s., not statistically significant
Discussion
Many matricellular proteins genes are highly expressed at sites of tissue injury and wound repair (Kubota and Takigawa 2015; Roberts and Lau 2011). Although the expression of both Ccn1 and Ccn2 are associated with wound repair and fibrosis, these homologous genes appear to have distinct biological effects. Here we show that CCN2, like CCN1, is capable of inducing fibroblast senescence and the anti-fibrotic SASP. However, their divergent roles in cutaneous wound healing appear to be dictated in part by their distinct expression kinetics.
Given the high degree of homology between CCN1 and CCN2, it is not surprising that they share similar biochemical activities, including α6β1-mediated functions in fibroblasts (Chen et al. 2001; Chen et al. 2007). Early studies have shown that both CCN1 and CCN2 support fibroblast adhesion through direct binding to integrin α6β1 and heparin sulfate proteoglycans, thus inducing the formation of filopodia and lamellipodia, α6β1-containing focal adhesion complexes, activation of focal adhesion kinases (FAK), paxillin, Rac, and p42/p44 MAPK (Chen et al. 2001). It was also shown that fibroblast adhesion to CCN2 induces expression of MMP1 and MMP3 (Chen et al. 2001), which are now appreciated to be part of the SASP signature (Coppe et al. 2008). The mechanism by which CCN2 induces senescence involves α6β1-dependent accumulation of ROS, leading to p53 activation and p16INK4a induction (Figs. 2 and 3), similar to the signaling pathway for CCN1-induced senescence (Jun and Lau 2010b).
Previous studies showed that deletion of Ccn2 in fibroblasts had no effect on the kinetics of cutaneous wound healing, the collagen content, or accumulation of α-smooth muscle actin-positive myofibroblasts in the wounds (Liu et al. 2014b). Our results with Ccn2 knockdown in cutaneous wounds are in agreement with these observations, showing that depletion of Ccn2 in the wound tissue had no effect on the wound closure rate, collagen content, induction of cellular senescence, or expression of the SASP (Fig. 4). These findings may reflect high expression of Ccn2 only in the initial inflammatory phase of wound healing (day 3); Ccn2 expression rapidly declines thereafter in the proliferation and maturation phases when myofibroblasts are most active (Fig. 4a). Thus, it is not surprising that antisense knockdown (Fig. 4) or deletion of Ccn2 (Liu et al. 2014b) had little effect on myofibroblast functions during granulation tissue formation or matrix remodeling.
Despite the strong association of Ccn2 with fibrosis, the precise mechanism by which CCN2 promotes fibrogenesis is not well understood (Jun and Lau 2011; Lipson et al. 2012). Given the ability of CCN2 to directly induce cellular senescence in fibroblasts, the pro-fibrotic effects of CCN2 may be mediated through cell types different from, or in addition to, myofibroblasts. For example, Ccn2 has been found to promote fibroblast differentiation from mesenchymal stem cells, although it does not by itself induce myofibroblast differentiation in these cells (Lee et al. 2010). Ccn2 plays an important role in the recruitment of Sox2-expressing cells to sites of cutaneous wound healing or bleomycin-induced skin injury, and these cells serve as progenitors for myofibroblasts (Liu et al. 2014a; Tsang and Leask 2015). Thus, it is possible that recruitment of Sox2-expressing progenitor cells by Ccn2 in cutaneous wounds occurs only when Ccn2 is highly expressed; however, Ccn2 expression declines to a low level when differentiated myofibroblasts proliferate in granulation tissue and progress to matrix remodeling in the maturation phase (Fig. 4a). In this model, Ccn2 is pro-fibrotic by recruiting progenitors for myofibroblasts (Liu et al. 2014a; Tsang and Leask 2015), but retains the capability to trigger cellular senescence in myofibroblasts and induce the associated anti-fibrotic genetic program (Fig. 5). This dichotomy of pro-fibrotic and anti-fibrotic functions may be found in CCN1 as well, which has been shown to promote matrix remodeling and limit fibrosis by inducing myofibroblast senescence in cutaneous wound healing, liver injuries, and mouse models of cardiac diseases (Borkham-Kamphorst et al. 2014; Jun and Lau 2010a; Kim et al. 2013; Meyer et al. 2016). However, Ccn1 has also been reported to exhibit pro-fibrotic properties in lung fibrosis (Kurundkar et al. 2016), suggesting a context-dependent effect on fibrogenesis.
It is of interest that direct application of purified CCN2 protein on skin wounds is able to induce cellular senescence and the associated SASP, including upregulation of MMPs and downregulation of collagen and TGF-β, resulting in reduced accumulation of collagen (Fig. 5). These findings suggest a therapeutic potential for CCN2 for the treatment of fibrotic conditions when delivered during specific stages in fibrotic progression.
Acknowledgments
We are grateful to Dr. Woo Jin Park for gifts of adenoviral vectors. This work was supported by a grant from the NIH (R01 AR061791) to L.F.L.
References
- Babic AM, Chen C-C, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin αvβ3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/MCB.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkham-Kamphorst E, Schaffrath C, Van de Leur E, Haas U, Tihaa L, Meurer SK, Nevzorova YA, Liedtke C, Weiskirchen R. The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-beta signaling. Biochim Biophys Acta. 2014;1843:902–914. doi: 10.1016/j.bbamcr.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Catalano A, Rodilossi S, Caprari P, Coppola V, Procopio A. 5-lipoxygenase regulates senescence-like growth arrest by promoting ROS-dependent p53 activation. EMBO J. 2005;24:170–179. doi: 10.1038/sj.emboj.7600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Chen N, Lau LF. The angiogenic factors Cyr61 and CTGF induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001;276:10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. EMBO J. 2007;26:1257–1267. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CA, O’Brien J. Modified assay for determination of hydroxyproline in tissue hydrolyzate. Clin Chim Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Hall-Glenn F, Lyons KM. Roles for CCN2 in normal physiological processes. Cell Mol Life Sci. 2011;68:3209–3217. doi: 10.1007/s00018-011-0782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2010;2:627–631. doi: 10.18632/aging.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Kim KH, Lau LF. The matricellular protein ccn1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat Commun. 2015;6:7386. doi: 10.1038/ncomms8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juric V, Chen CC, Lau LF. Fas-mediated apoptosis is regulated by the extracellular matrix protein CCN1 (CYR61) in vitro and in vivo. Mol Cell Biol. 2009;29:3266–3279. doi: 10.1128/MCB.00064-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Chen CC, Monzon RI, Lau LF. The matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol. 2013;33:2078–2090. doi: 10.1128/MCB.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva ML, Latinkic BV, Kolesnikova TV, Chen C-C, Yang GP, Abler AS, Lau LF. Cyr61 and Fisp12 are both signaling cell adhesion molecules: comparison of activities, metablism, and localization during development. Exp Cell Res. 1997;233:63–77. doi: 10.1006/excr.1997.3548. [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Cellular and molecular actions of CCN2/CTGF and its role under physiological and pathological conditions. Clin Sci (Lond) 2015;128:181–196. doi: 10.1042/CS20140264. [DOI] [PubMed] [Google Scholar]
- Kurundkar AR, Kurundkar D, Rangarajan S, Locy ML, Zhou Y, Liu RM, Zmijewski J, Thannickal VJ. The matricellular protein CCN1 enhances TGF-beta1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury. FASEB J. 2016;30:2135–2150. doi: 10.1096/fj.201500173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci. 2011;68:3149–3163. doi: 10.1007/s00018-011-0778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120:3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu S-J, Lam SC-T, Lau LF. Proangiogenic activities of CYR61 (CCN1) mediated through integrins αvβ3 and α6β1 in human umbilical vein endothelial cells. J Biol Chem. 2002;277:46248–46255. doi: 10.1074/jbc.M209288200. [DOI] [PubMed] [Google Scholar]
- Leu S-J, Liu Y, Chen N, Chen CC, Lam SC, Lau LF. Identification of a novel integrin α6β1 binding site in the angiogenic inducer CCN1 (CYR61) J Biol Chem. 2003;278:33801–33808. doi: 10.1074/jbc.M305862200. [DOI] [PubMed] [Google Scholar]
- Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Herault Y, Pavlovic G, Leask A. Skin progenitor cells contribute to bleomycin-induced skin fibrosis. Arthritis Rheum. 2014;66:707–713. doi: 10.1002/art.38276. [DOI] [PubMed] [Google Scholar]
- Liu S, Thompson K, Leask A. CCN2 expression by fibroblasts is not required for cutaneous tissue repair. Wound Repair Regen. 2014;22:119–124. doi: 10.1111/wrr.12131. [DOI] [PubMed] [Google Scholar]
- Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J Am Coll Cardiol. 2016;67:2018–2028. doi: 10.1016/j.jacc.2016.02.047. [DOI] [PubMed] [Google Scholar]
- Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DD, Lau LF. Matricellualr Proteins. In: Mecham RP, editor. The extracellular matrix: an overview. Berlin: Springer-Verlag; 2011. pp. 369–413. [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M, Leask A. CCN2 is required for recruitment of Sox2-expressing cells during cutaneous tissue repair. J Cell Commun Signal. 2015;9:341–346. doi: 10.1007/s12079-014-0245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016;23:303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon PO, Lee MA, Cha H, Jeong MH, Kim J, Jang SP, Choi BY, Jeong D, Yang DK, Hajjar RJ, Park WJ. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J Mol Cell Cardiol. 2010;49:294–303. doi: 10.1016/j.yjmcc.2010.04.010. [DOI] [PubMed] [Google Scholar]