Abstract
Mammalian palatogenesis is a complex process involving a temporally and spatially regulated myriad of factors. Together these factors control the 3 vital processes of proliferation, elevation and fusion of the developing palate. In this study, we show for the first time the unequivocally vital role of CCN2 in development of the mammalian palate. We utilized CCN2 knockout (KO) mice and cranial neural crest derived mesenchymal cells from these CCN2 KO mice to investigate the 3 processes crucial to normal palatogenesis. Similar to previously published reports, the absence of CCN2 inhibits proliferation of cells in the palate specifically at the G1/S transition. Absence of CCN2 also inhibited palatal shelf elevation from the vertical to horizontal position. CCN2 KO mesenchymal cells demonstrated deficiencies in adhesion and spreading owing to an inability to activate Rac1 and RhoA. On the contrary, CCN2 KO mesenchymal cells exhibited increased rates of migration compared to WT cells. The addition of exogenous CCN2 to KO mesenchymal cells restored their ability to spread normally on fibronectin. Finally, utilizing an organ culture model we show that the palatal shelves of the CCN2 KO mice demonstrate an inability to fuse when apposed. Together, these data signify that CCN2 plays an indispensible role in normal development of the mammalian palate and warrants additional studies to determine the precise mechanism(s) responsible for these effects.
Keywords: Palatogenesis, CCN2, Proliferation, Palate fusion, Palate elevation, Cranial neural crest
Introduction
Non-syndromic orofacial clefting is among the most common craniofacial birth defects with an overall incidence of roughly 1 in every 700 live births.(Arosarena 2007; Bush and Jiang 2012; Parada and Chai 2012) Cleft lip can occur with or without the presence of cleft palate (CL[P]), however cleft lip is associated with cleft palate in 68 % to 86 % of cases.(Arosarena 2007; Kirschner and LaRossa 2000) Cleft palate only (CP) is an etiologically distinct developmental deficit from CL[P] and is classified as non-syndromic in 50 % of cases.(Arosarena 2007; Kirschner and LaRossa 2000; Parada and Chai 2012) Children born with cleft palate typically have difficulty with feeding and talking, and therefore represent a population with a serious need for medical intervention.
Mammalian palatogenesis is a complex processes involving a tightly regulated sequence of cellular events. The sequence of processes involved in human and murine secondary palate development is identical.(Arosarena 2007; Bush and Jiang 2012) In mice the secondary palate forms as outgrowths of the oral side of the mammalian maxillary processes at embryonic day (E) 11.5.(Bush and Jiang 2012; Hill et al. 2015; Parada and Chai 2012; Smith et al. 2012) These newly formed palatal shelves grow vertically in the superior-inferior plane lateral to either side of the tongue in the oral cavity.(Bush and Jiang 2012; Hill et al. 2015; Parada and Chai 2012; Smith et al. 2012) As the mandible grows the tongue descends and flattens thereby allowing for continued elongation of the palatal shelves at E13.5 and subsequent elevation to occupy a horizontal position by E14.5.(Bush and Jiang 2012; Hill et al. 2015; Parada and Chai 2012; Smith et al. 2012) The palatal shelves continue to grow until contact is made in the midline at E15, followed by epithelium degeneration and extrusion and finally fusion of the underlying mesenchyme to complete formation of the secondary palate by E15.5.(Bush and Jiang 2012; Hill et al. 2015; Kim et al. 2015; Parada and Chai 2012; Smith et al. 2012) The mesenchymal cells in the anterior portion undergo intramembranous ossification to form the hard (bony) palate,(Bush and Jiang 2012; Levi et al. 2011) while those in the posterior portion differentiate into skeletal muscle to form the soft palate.(Bush and Jiang 2012)
The developing palate is predominantly composed of mesenchymal cells (neural crest origin), endothelial cells (mesodermal origin) and epithelium (ectodermal origin).(Yoshida et al. 2008) Palate formation involves the temporal and spatial coordination of cellular events including cell migration, proliferation, differentiation, and apoptosis.(Bush and Jiang 2012; Mishina and Snider 2014; Smith et al. 2012) Among the myriad of factors that have are expressed during development of the palate, transforming growth factor beta (TGF-β), fibroblast growth factor (FGF), and bone morphogenetic protein (BMP) signaling pathways have been shown to be essential for palate development.(Bush and Jiang 2012; Hill et al. 2015; Lane et al. 2015; Matsumura et al. 2011; Parada and Chai 2012; Smith et al. 2012; Yumoto et al. 2013)
Previous studies on the developmental deficits observed in the absence of CCN2 found craniofacial differences between CCN2 knockout (KO) compared to wild-type (WT) littermates. Allometric (size-based) and non-allometric shape differences were observed in CCN2 KO skulls, which were shorter and wider than their WT counterparts.(Lambi et al. 2012) Specific morphologic traits seen in CCN2 KO skulls included an increased curvature of the nasal bones, a serpentine shape of the mandibles, a lateral bend of the sphenoid pterygoid processes, a lateral kink in the vomer, and failure bony (hard) palate formation.(Lambi et al. 2012) These results regarding formation of the secondary palate were consistent with the initial description of the CCN2 KO mouse which showed that the palatal shelves failed to elevate in E15.5 embryos.(Ivkovic et al. 2003) What remains unclear from these studies is whether this aberrant palatogenesis represents a developmental delay or a failure of secondary palate formation. A temporal analysis of palatal development and cellular mechanisms contributing to aberrant palatogenesis remains to be studied.
In this paper, we expanded upon our previous studies of palatogenesis in CCN2 KO mice by analyzing underlying soft tissue structure through gross observation, micro-CT, and histological analysis. We show for the first time the vital role CCN2 plays in development of the mammalian secondary palate. We isolated neural crest derived primary mesenchymal cells from CCN2 KO and WT mice for in vitro studies to assess underlying cellular defects that would contribute to delayed and/or failed palate formation. We hypothesize that a lack of CCN2 in these cells will result in defects in underlying cell processes that contributes to failure of palatal shelf growth, elevation, and/or fusion. Understanding the role of CCN2 in palatogenesis is expected to provide novel information with the potential for development of new treatment strategies for the clinical management of patients with cleft palate.
Materials and methods
Source of animals
CCN2 heterozygous mice (CCN2+/LacZ) were used as breeders to obtain CCN2 KO (CCN2LacZ/LacZ) as previously described (Crawford et al. 2009). These mice are phenotypically identical to the original CCN2 KO mice(Ivkovic et al. 2003) but are generated by disruption of the CCN2 gene at a different location. Genotyping was determined by X-gal staining of tail clips (EMD Milipore, Billerica, MA). All animals were maintained and used according to the principles in the NIH Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services, Publ. No. 86–23, 1985) and guidelines established by the IACUC of the Lewis Katz School of Medicine at Temple University.
Cell isolation
Neural crest derived mesenchymal cells were isolated from newborn calvaria bone or embryonic day 14.5 (E14.5) secondary palatal shelves. Calvaria bone was digested in 1 mg/mL collagenase (C1764 Sigma, St. Louis, MO) and 0.25 % Trypsin (15090 Gibco, Grand Island, NY) at 37 °C to release mesenchymal cells. Palatal shelves were digested in 0.25 % trypsin with 2.21 mM EDTA (25-053-Cl Mediatech, Inc, Manassas, VA) at 37 °C to release mesenchymal cells. Calvaria bone derived mesenchymal cells were cultured in 1X MEM alpha modification (SH30265 Hyclone Laboratories, Logan Utah), 10 % FBS (S11150 Atlanta Biologicals, Flowery Branch, GA), 1X Penicillin/Streptomycin (Pen/Strep) (30-002-Cl Mediatech, Inc, Manassas, VA) and mouse embryonic palatal mesenchymal (MEPM) cells were cultured in 1X DMEM (10-013-CV Mediatech, Inc, Manassas, VA), 10 % FBS, 1X Pen/Strep at 37 °C 5 % CO2 in a humidified environment. Cells used for experiments were passage 2–4.
Phosphotungstic acid MicroCT analysis
Newborn pups (P0) were collected, euthanized by decapitation, and heads were fixed in 4 % paraformaldehyde (PFA) (P6148, Sigma, St. Louis, MO) in 1X PBS at 4 °C for 1 week with PFA changed at 48 and 96 h. Mandibles and tongues were excised to allow direct visualization of the secondary palate. Samples were washed in water for 24 h with water changed at hours 1, 4, and 8. Skin was removed from the head and samples were placed into phosphotungstic acid, hematoxylin solution (PTAH) (Electron Microscopy Sciences, Hatfield, PA, USA) for 2 weeks at 4 °C. Samples were rinsed in water and scanned in air with a Skyscan 1172, 11 megapixel camera model, high-resolution cone-beam micro-CT scanner (Bruker, Belgium). Heads were scanned at a pixel size of 6.95 μm with an X-ray tube potential of 59 kV and X-ray intensity of 149 μA with each slice equal to 7 μm. A 0.5-mm aluminum filter was used to remove image noise, with a ring artifact correction of 12 and a beam hardening correction of 20 %. After scanning, 3D image data was reconstructed using the SkyscanNRecon software. Images were visualized using the Skyscan CT Volume Rendering (CTvox) software.
Tissue preparation and histology
Animals used for this study were euthanized at embryonic 14.5 (E14.5) or birth (P0). Subsequently, tails were removed and used for X-gal staining (EMD Milipore, Billerica, MA). Heads were collected into 1X PBS and fixed in 4 % PFA in 1X PBS at 4 °C for 1 week with PFA changed at 48 and 96 h. Genomic DNA was isolated utilizing the REDExtract-N-Amp Tissue PCR kit according to manufacturer’s protocol (XNAT, Sigma, St. Louis, MO) from the E14.5 torsos and used for PCR genotyping. Forward Primer 5′-AAGACACATTTGGCCCAGAC-3′ and reverse primer 5′-TTTTCCTCCAGGTCAGCTTC-3′. P0 heads were decalcified in 14 % ethylenediaminetetraacetic acid (EDTA Acid) (BP118 Fisher Scientific, Fair Lawn, NJ) for 1 week at 4 °C with solution replaced every 24 h. Heads were then dehydrated in serial dilutions of 30–70 % ethanol for 48 h at 4 °C prior to paraffin embedding. Deparaffinized sections (5 μm) were stained with Mason’s trichrome stain. Images were acquired using a Nikon E1000 microscope.
Cell proliferation
Cell number was determined using the CyQUANT® NF Cell Proliferation Assay Kit (C35006, Molecular Probes, Eugene, OR) according to the manufacturer’s protocol. Briefly, CCN2 WT, Heterozygous, and KO MEPM cells were plated at 8 × 103 cells/well in 96 well plates (3603 Corning Incorporated, Corning, NY) in 1X DMEM, 10 % FBS, 1X Pen/Strep. At 72 h media was aspirated and replaced with DNA binding dye solution. Cells were incubated at 37 °C for 1 h and fluorescence was measured using a Wallac Victor 2 microplate reader (Perkin Elmer, Shelton, CT) with excitation at 485 nm and emission detection at 530 nm. Relative fluorescence units (RFUs) were converted to cell number using a standard curve of known numbers of cells.
Cell cycle analysis
Calvaria mesenchymal cells were cultured as previously described (Mundy et al. 2014). Cells were cultured at 37 °C, 5 % CO2 in humidified air. For experiments, cells were plated at 1X106 cells/plate in 100 mm dishes in α-MEM with 10 % FBS and allowed to adhere for 12 h. The cells were then serum starved for 24 h followed by a return to α-MEM with 10 % FBS for 24 h. Cells were lifted from the plates by treatment with 0.25 % Trypsin, 2.21 mM EDTA, 1X, washed once in 1X PBS, pelleted at 300 g at 4 °C, resuspended and fixed in 1 % PFA solution for 1 h at 4 °C. Cells were washed in 1X PBS, and resuspended in 70 % ethanol overnight at −20 °C. Cells were washed twice in 1X PBS. Cells were resuspended in 1X PBS containing 200ug/mL of Ribonuclease-A (R-5500 Sigma, St. Louis, MO), 0.1 % Triton X-100 (CS-282-4 Fisher Scientific, Fair Lawn, NJ), and 20ug/mL propridium iodide (P-4170 Sigma, St. Louis, MO) and incubated for 30 min at 37 °C in darkness. Flow Cytometry was performed on a BD FACSauto Flow Cytometer Ruo Special Order System (BD Biosciences, San Jose, CA). Data was analyzed using FlowJo software (FlowJo, Ashland, OR).
BrdU incorporation analysis
Pregnant timed E14.5 dams were given intraperitoneal injections (IP) with BrdU (228595000, Acros Organics, NJ) solution made in sterile 1X PBS at 50 mg/kg body weight. Embryos were harvested 4 h after injection and processed for paraffin embedding as described previously. Deparaffinized coronal sections (5 μm) were incubated in 1 N and 2 N HCl (UN1789 Macron, Center Valley, PA) to denature DNA and sections were blocked in 5 % donkey serum (D9663, Sigma, St. Louis, MO) diluted in 1X PBS and incubated overnight in anti-BrdU rat monoclonal antibody (ab6326, Abcam, Cambridge, MA) at 4 °C. Alexa Fluor 488-conjugated AffiniPure Donkey Anti-Rat IgG secondary antibody (712-545-153 Jackson Immunoresearch Laboratories, Inc, West Grove, PA) was incubated on sections for 2 h at R/T. Slides were coverslipped using Vectashield mounting medium containing DAPI (H-1200 Vector, Burlingame, CA). Fluorescence was observed using a Nikon E800 fluorescent microscope. BrdU positive and total nuclei in the mesenchymal tissue were counted using NIH ImageJ.
Western blotting
CCN2 WT and KO culture dishes were washed twice with cold 1X PBS and cells were lysed in 1X RIPA lysis buffer (N653, Amresco, Solon, OH) containing 1X protease inhibitor (P8340, Sigma, St. Louis, MO) and 1X HALT phosphatase inhibitors (78420, Thermo Scientific, Rockford, IL) then incubated with end-over-end tumbling for 1 h at 4 °C. Protein concentration of lysates was determined using Pierce BCA Protein Assay Kit (23225, Thermo Scientific, Rockford, IL). Membranes were blocked with 5 % bovine serum albumin (BSA) (BP1605, Fisher Scientific, Fair Lawn, NJ) solution in 1X TBS-T (0.1 % Tween 20 (X251-07, JT Baker, Center Valley, PA)) for 1 h at room temperature and then incubated with a goat polyclonal anti-CTGF antibody (sc-14939 Santa Cruz, Dallas, Tx) diluted 1:200, mouse monoclonal anti-Rac1 antibody (ARC03 Cytoskeleton, Denver, CO) diluted 1:500, mouse monoclonal anti-RhoA antibody (ARH04 Cytoskeleton, Denver, CO) diluted 1:500, or Rabbit anti-Actin antibody (A2066, Sigma, St. Louis, MO) in blocking buffer overnight at 4 °C. Membranes were washed with 1X TBS-T (0.1 % Tween 20) and incubated with Donkey anti-goat IRDye ® 800 CW (1:5000) (925–32214, Licor, Lincoln, NE), Donkey anti-Mouse HRP (1:5000) (715-035-50, Jackson ImmunoResearch Laboratories, Inc, West Grove, PA), or Donkey anti-Rabbit HRP (1:5000) (711-035-152, Jackson ImmunoResearch Laboratories, Inc, West Grove, PA) secondary antibodies diluted in blocking buffer. Membranes were scanned using the LI-COR Odyssey Infrared Imaging System or incubated with WesternBright Quantum HRP substrate (K-12042 Advansta, Menlo Park, CA) and imaged using a chemiluminescence detection system (Thermo Scientific). Densitometry was assessed utilizing ImageStudio 2.0 or NIH ImageJ.
Adhesion assay
Adhesion Assay was performed as previously described (Hendesi et al. 2015). Briefly, ninety six-well non-tissue culture treated plate (351172, Corning, Corning, NY) was coated with 1 % BSA, 0.2ug/cm2 fibronectin, or 0.2ug/cm2 rhCCN2 (9190-CC-050, R&D Systems, Minneapolis, MN) in 1X PBS and was left to dry in a tissue culture hood. To block non-specific binding sites, 1 % BSA was added to the wells and the plate was incubated at 4 °C for 1 h. Excess BSA was discarded and wells were washed with 1X PBS prior to plating 3 x104 mesenchymal cells to the wells and incubating at 37 °C for 45 min. Wells were washed with 1X PBS. CyQuant® NF dye was added to each well and the plate was incubated at 37 °C for 1 h. Fluorescence was measured using a microplate reader with excitation at ~485 nm and emission detection at ~530 nm. Relative fluorescence units (RFUs) were converted to cell number using standard curve made by performing adhesion assay for different cell numbers.
Immunofluorescent staining and cell spreading assay
Glass chamber slides (154534, Nalge Nunc International, Rochester, NY) were coated with 0.2ug/cm2 fibronectin (150025, MP Biomedicals, LLC, Solon, OH) in 1X PBS for 1 h at 37 °C and then washed twice with 1X PBS. 2 × 103 WT or CCN2 KO mesenchymal cells in serum free media, with or without addition of 100 ng/mL rhCCN2 (9190-CC-050, R&D Systems, Minneapolis, MN), were added to chambers and incubated at 37 °C for 24 h. Cells were fixed with 4 % PFA made in 1X PBS for 15 min, washed with washing buffer (1X PBS containing 0.05 % Tween20) and blocked with blocking solution (2.5 % BSA in 1X PBS) for 30 min at room temperature. TRITC-conjugated phalloidin (90228, Millipore, Temecula, CA) diluted 1:2000 in blocking solution WAS incubated for 1 h at room temperature. The slides were cover slipped using Vectashield ® Mounting Medium with DAPI (H-1200 Vector, Burlingame, CA). Fluorescence imaging was performed using a Nikon E1000. Cell spreading areas were measured using NIH ImageJ.
Migration assay
Glass 8X8mm cloning rings (CLS-1777-02 Chemglass Life Sciences, Vineland, NJ) were placed onto untreated cell culture plastic dishes. Cells were plated both inside and outside of the rings so that they were plated at a high density and allowed to adhere for a minimal amount of time in appropriate media containing 10 % FBS, and 1X Pen/Strep. Rings were removed vertically to yield a standardized cell free area 1 mm in width. Media was changed to 0.5 % heat inactivated FBS and 1X Pen/Strep for the remainder of the experiment. Images were taken of the cell free areas at time of ring removal (T0) and every 24 h thereafter using a Nikon TE300 inverted phase contrast microscope. Cell free area was measured using NIH ImageJ and calculated as a percent of the cell free area at T0.
Rac1 activity assay
Activation of Rac1 was performed using a Rac1 Activity Assay kit (BK035 Cytoskeleton, Inc, Denver, CO) according to the manufacturer protocol. Briefly, mesenchymal cells were grown to 80 % confluence on 100 mm tissue culture plastic dishes. Cells were serum deprived in medium containing 0.5 % heat inactivated FBS and 1X pen/strep 24 h prior to assay. The cells were lifted and 8X105 cells were plated in serum free conditions on 0.2ug/cm2 fibronectin coated 100 mm dishes. Cells were allowed to adhere for 15 min at 37 °C, 5 % CO2 in humidified air. Cells were then rapidly processed according to the kit protocol and lysates were snap frozen in liquid N2. 200ug of total lysate and 10ug of PAK-PBD beads were incubated together to pull down activated Rac1 for 1 h at 4 °C. After centrifugation, samples were prepared for western blot according to the kit protocol. 10ug of total protein lysate was used to assess for total Rac1 levels. A mouse monoclonal (1:500) anti-Rac1 primary antibody and 1:5000 horseradish peroxidase conjugated to a donkey anti-mouse secondary antibody were used to detect active and total Rac1. Bands were visualized with WesternBright Quantum HRP substrate and a chemiluminescence detection system.
RhoA activity assay
Activation of RhoA was performed using a RhoA Activity Assay kit (BK036 Cytoskeleton, Inc, Denver, CO) according to the manufacturer protocol. Briefly, mesenchymal cells were grown to 80 % confluence on 100 mm tissue culture plastic dishes. Cells were serum deprived in medium containing 0.5 % heat inactivated FBS and 1X pen/strep 24 h prior to assay. The cells were lifted and 8X105 cells were plated in serum free conditions on 0.2ug/cm2 fibronectin coated 100 mm dishes. Cells were allowed to adhere for 30 min at 37 °C, 5 % CO2 in humidified air. Cells were then rapidly processed according to the kit protocol and lysates were snap frozen in liquid N2. 200ug of total lysate and 50ug of Rhotekin-RBD beads were incubated together to pull down activated RhoA for 1 h at 4 °C. After centrifugation, samples were prepared for western blot according to the kit protocol. 10ug of total protein lysate was used to assess for total RhoA levels. A mouse monoclonal (1:500) anti-RhoA primary antibody and 1:5000 horseradish peroxidase conjugated to a donkey anti-mouse secondary antibody were used to detect active and total RhoA. Bands were visualized with WesternBright Quantum HRP substrate and a chemiluminescence detection system.
Organ culture
Animals used for this study were euthanized at embryonic E14.5. Embryos were dissected under sterile conditions to retain a portion of the maxillary tissue containing the secondary shelves and using an intact upper lip to stabilize the tissue in a U shape with the medial edge epithelium of each shelf in contact with the other. Palates were cultured using a modified grid method in serum free BGJb medium (12591–038 Life Technologies, Grand Island, NY) containing 1X Pen/Strep for 72 h in 5 % CO2 and 95 % room air at 37 °C in a humidified environment on sterile stainless steel grids. Following culture, palates were fixed and processed for paraffin embedding. 5 μm sections were cut in the coronal plane and stained with H&E. Images were visualized using a Nikon E1000 microscope.
Statistical analysis
Statistical analysis was performed by Student’s t test to compare two groups. Comparison of three or more groups was performed using a one-way ANOVA with a Bonferroni post-hoc test to compare means between two groups. Graphs were produced using Prism 6 (version 6.0 f). Densitometry was obtained using ImageStudio 2.0 or NIH ImageJ (version 1.48 v). Bar charts are displayed as mean ± SD.
Results
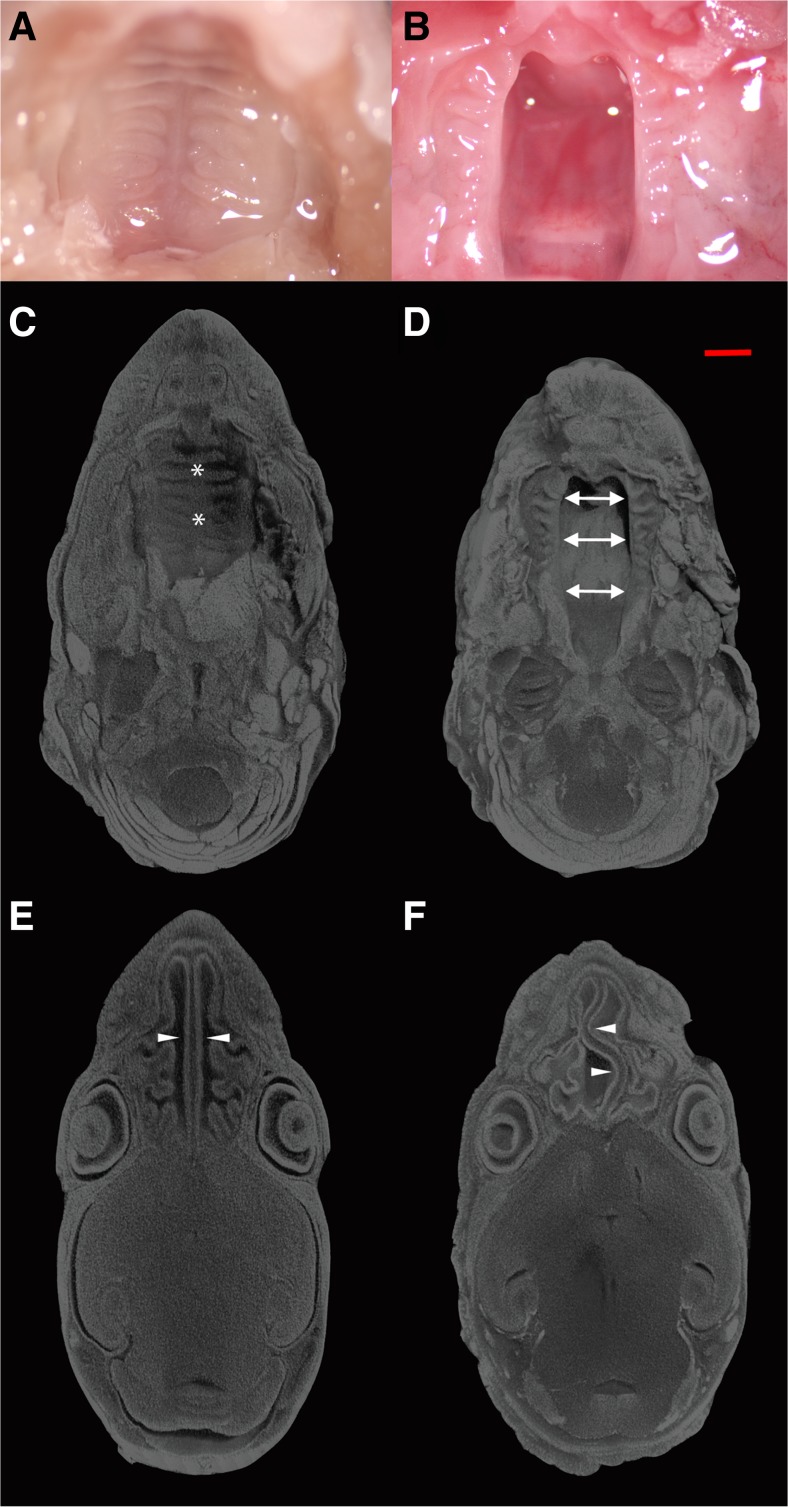
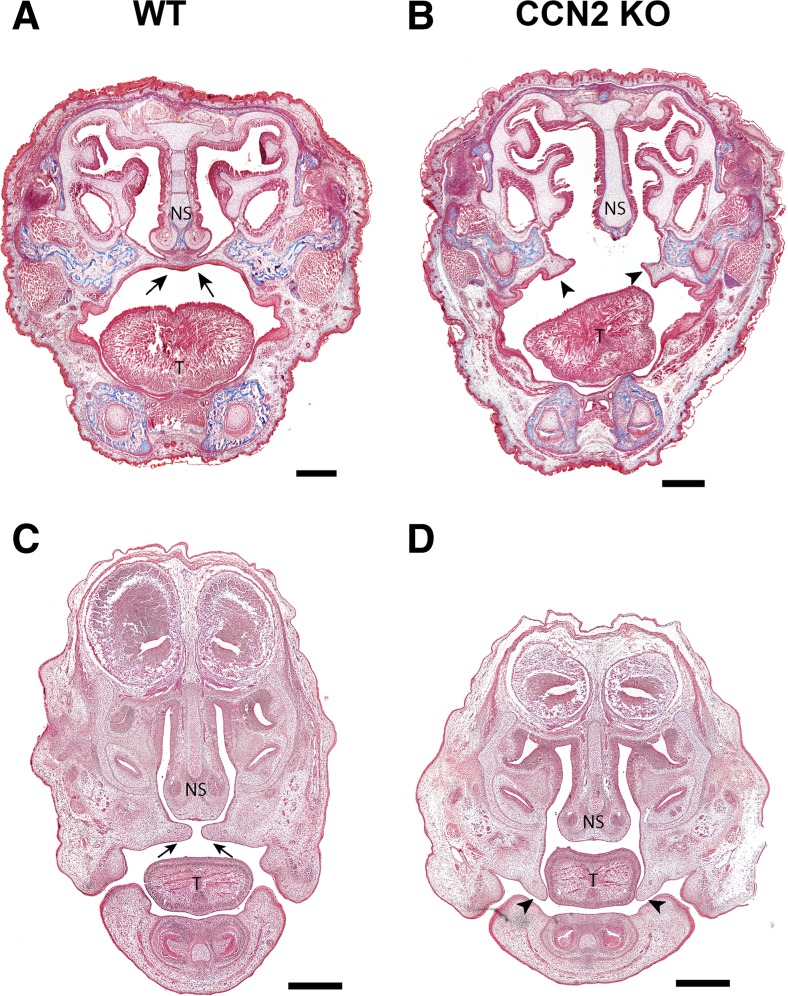
Based on an incidental finding in the original description of the skeletal phenotype in CCN2 knockout mice (Ivkovic et al. 2003; Lambi et al. 2012), we conducted a comprehensive assessment of palate development in CCN2 knockout (KO) mice and their wild-type (WT) littermates. Gross examination of the roof of the oral cavity in newborn (P0) KO mice from a total of 21 litters (n = 38) revealed a cleft palate (100 % penetrance) resulting from failure of secondary palate formation without lip involvement (Fig. 1a and b). MicroCT analysis of newborn heads stained with phophotungtistic acid (PTA), to allow for soft-tissue visualization and 3-D reconstruction of the craniofacial tissues, revealed a cleft palate extending throughout both the hard and soft portions of the secondary palate (Fig. 1c and d). Additionally, microCT analysis revealed a serpentine morphology of the nasal septum perhaps as a result of the shortened rostro-caudal length of the skull previously reported in the CCN2 knockout phenotype (Fig. 1e and f) (Lambi et al. 2012). Coronal sections of WT and KO P0 heads revealed absence of the secondary palate in KO mice (Fig. 2a and b). To examine elevation of the palatal shelves, we compared coronal sections of WT and KO heads harvested at E14.5 since others have shown that elevation has occurred at this stage in normal murine development (Bush and Jiang 2012; Bush et al. 2015). The KO palatal shelves failed to elevate compared to WT at E14.5 (Fig. 2c and d).
Fig. 1.
Gross specimen of wild type (WT; a) and CCN2 knockout (KO; b) palates in postnatal day 0 (P0) mice. MicroCT analysis of palate (top, c and d) and nasal septum/cavity (bottom, e and f) in (P0) heads from WT; (c and e) and KO; (d and f) mice. Specimens were stained with phosphotungstic acid to allow visualization and volumetric reconstruction of craniofacial tissues. The palate in WT mice (designated by * in c) is fully formed and fused, while remaining open in KO mice (designated by arrows in d). Nasal septum (arrowheads) and lateral wall of nasal cavity exhibit significant developmental deformities in KO (f) compared to WT (e) mice. Scale bar = 1 mm. (n = 38 WT and KO mice)
Fig. 2.
Coronal sections stained with Mason’s Trichrome stain of newborn (P0) (a and b) and E14.5 heads (c and d).WT sections show normal palate development (arrow, a and c) while CCN2 KO sections show failure of palate formation (arrow heads, b and d). T = Tongue, NS = Nasal Septum. Scale bar = 500 μm
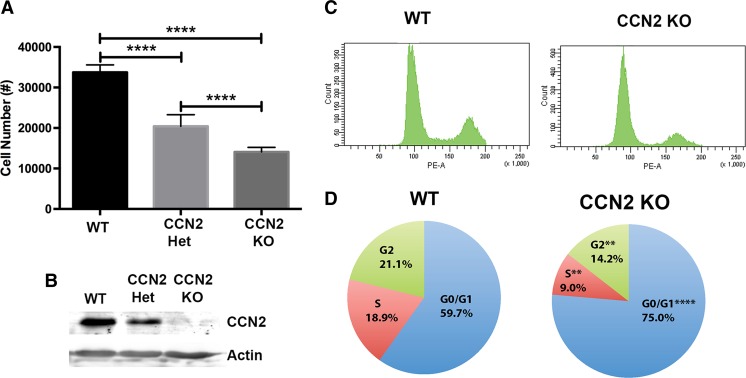
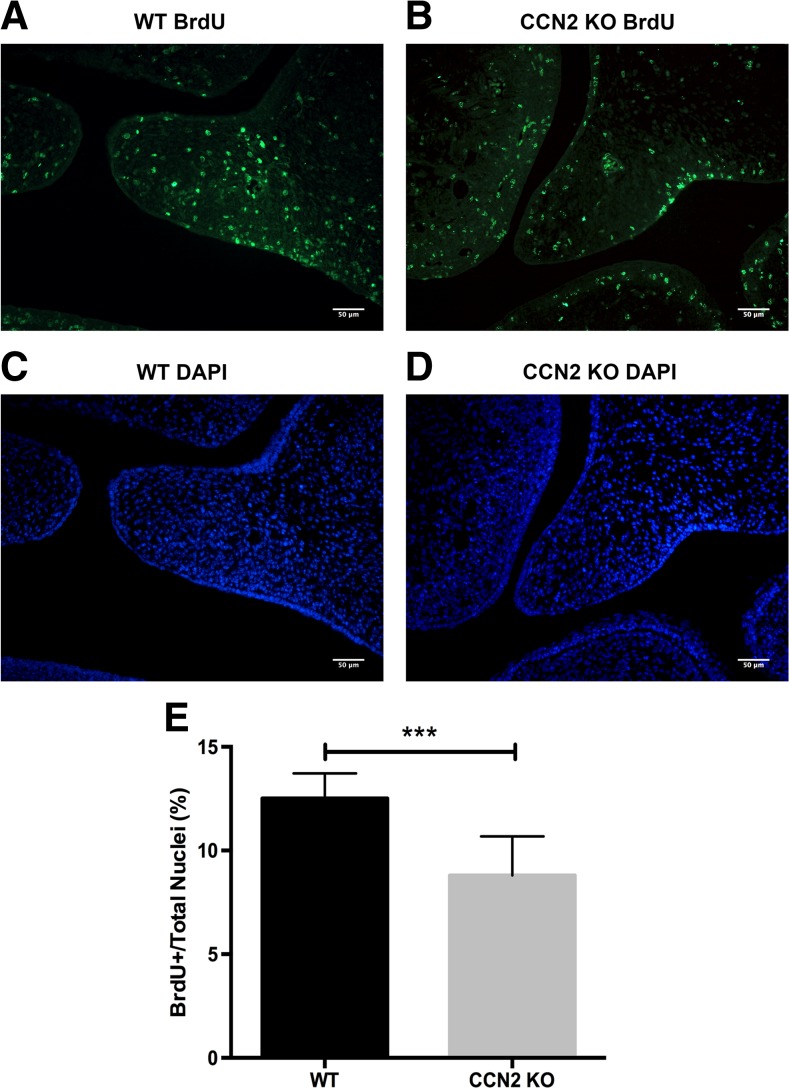
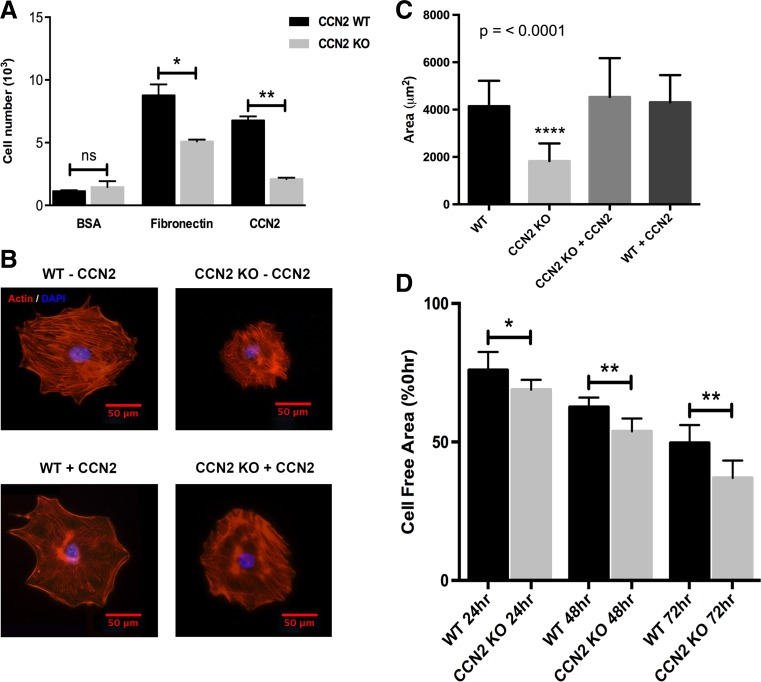
In the next series of experiments we utilized WT and KO embryonic mesenchymal cells derived from developing calvaria and palatal shelves both of which have a cranial neural crest origin. We first assessed cell proliferation comparing WT, CCN2 heterozygous, and KO cells (Fig. 3a). Cyquant NF analysis revealed a proliferation rate directly proportional to CCN2 protein expression (Fig. 3a and b). The WT cells proliferated at a rate nearly double of the KO cells and the CCN2 heterozygous cells proliferated at an intermediate rate. Next, cells were used to analyze cell cycle phase distribution via propridium iodide staining and flow cytometry (Fig. 3c and d). We found that a significantly higher percentage of the KO mesenchymal cells accumulated in the G0/G1 phase compared to WT cells (75.0 % ± 1.2 % versus 59.7 % ± 0.5 %). Significantly fewer KO cells entered the S phase compared to the WT (9.0 % ± 1.1 % versus 18.9 % ± 1.4 %) and as a result fewer KO cells existed in G2 phase compared to the WT (14.2 % ± 1.2 % versus 21.1 % ± 1.2 %). BrdU incorporation was utilized to assess in vivo proliferation of developing palatal shelves at E14.5. WT and KO sections showed an increase in BrdU incorporation in the WT versus KO shelves (Fig. 4a and b). Quantification of BrdU positive nuclei as a percentage of total nuclei (stained with DAPI) demonstrated a significant decrease in proliferating cells in the KO versus WT palatal shelves (Fig. 4c and d).
Fig. 3.
Cellular proliferation (a) demonstrating proliferation rates proportional to CCN2 expression (n = 9 wells per genotype). Representative Western blot (b) demonstrating differences in protein expression of CCN2 in cells used in assays. Cell cycle analysis of WT and KO cells (n = 4 plates of cells per genotype with assay repeated 3 times) (c and d). Representative raw flow cytometry data showing differences in cellular cycle in G0/G1 (left peak), S (middle plateau), and G2 (right peak) phases (c). Pie chart of mean proportion of cells residing in each cell phase (d). **P < 0.01, ****P < 0.0001
Fig. 4.
Proliferation in developing palatal shelves of E14.5 WT (a) and CCN2 KO (b) littermate embryos visualized by BrdU staining (n = 9 sections per genotype). Total nuclei are visualized with DAPI in WT (c) and KO (d) palatal shelves. Quantification of BrdU positive nuclei as a percentage of total nuclei demonstrates a significant decrease in proliferating cells in the KO compared to WT (e). ***P < 0.001. Scale bar = 50 μm
We examined cell adhesion, spreading and migration (Fig. 5) to assess the role of CCN2 in regulating cell/extracellular matrix (ECM) interactions in mesenchymal cells. First, we assessed the ability of WT and KO cells to adhere to BSA (negative control), fibronectin (FN), or recombinant CCN2 (Fig. 5a). A significantly greater number of WT cells adhered to either FN or CCN2 compared to KO cells with BSA serving as a negative control. Next, we assessed the ability of the cells to spread on FN with or without the addition of 100 ng/mL of recombinant CCN2 (Fig. 5b and c). The WT mesenchymal cells were more robust in their ability to spread on FN compared to KO cells (Fig. 5b) as confirmed by the significantly smaller average cell area in KO versus WT cells (Fig. 5c). Soluble recombinant CCN2 was able to restore the spreading capacity of the KO cells to the same degree as that of the WT cells while the addition of recombinant CCN2 to WT cells had no effect on their ability to spread (Fig. 5b and c). Finally, we assessed the ability of the cells to migrate at 24-h intervals for a total duration of 72 h. Interestingly, the KO cells migrated at a faster rate compared to WT cells (Fig. 5d), and this faster rate of migration was observed at all time points, although the relative difference between KO and WT cells progressively increased with each subsequent time point.
Fig. 5.
Adhesion, spreading and migration of WT and CCN2 KO mesenchymal cells on fibronectin (FN) or CCN2. Adhesion (a) and spreading (b and c) were significantly decreased in KO compared to WT cells. Soluble CCN2 added to the media was able to rescue spreading ability of the CCN2 KO cells. On the contrary, migration was increased in KO versus WT cells (d). *P < 0.05, **P < 0.01, ****P < 0.0001. Scale bar = 50 μm. For adhesion n = 5 wells per genotype per condition, for spreading n = 40 cells per genotype per condition, and for migration n = 10 wounds per genotype per time point
Rac1 and RhoA are two of the best-studied members of the Rho family of GTPases and are linked to the ability to cells to adhere and spread on a fibronectin matrix (Guo et al. 2006). Therefore, we investigated the activation of Rac1 and RhoA in WT and KO cells following plating on FN coated plates. Active (GTP bound) forms of Rac1 (Fig. 6a and b) and RhoA (Fig. 6c and d) were both dramatically reduced in the KO compared to WT cells. Although there were no differences in total levels of Rac1 between the WT and KO cells, there was a modest decrease in total levels of RhoA protein in the KO compared to WT cells. Quantification of activated forms of Rac1 and RhoA over normalized total Rac1 and RhoA, respectively, are shown in Fig. 6b and d.
Fig. 6.
Activation of Rac1 and RhoA in WT and CCN2 KO mesenchymal cells. Active (GTP bound) form of Rac1 is decreased in KO compared to WT mesenchymal cells plated on FN for 15 min, while total Rac1 is similar between KO and WT cells (a). Quantification of active Rac1 to normalized total Rac1 demonstrates a significant reduction in Rac1 activation in the KO versus the WT cells (b). Active (GTP bound) form of RhoA and total RhoA are both decreased in KO compared to WT mesenchymal cells plated on FN for 30 min (c). Quantification of active RhoA to normalized total RhoA demonstrates a dramatic reduction in RhoA activation in the KO versus the WT cells (d). Experiments were repeated three times with similar results
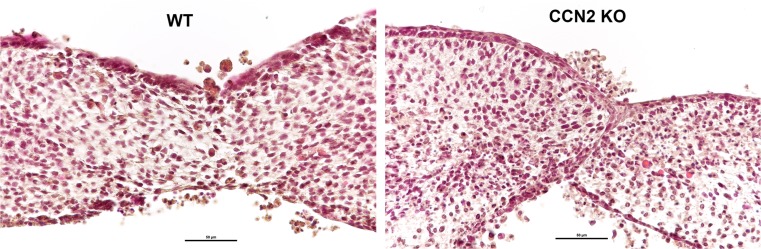
Finally, to examine if palate shelf fusion mechanisms are intact in the KO animals, we utilized palate organ culture (Fig. 7). The developing palates were isolated at E14.5 and cultured for 72 h. During this time, the WT palates fused normally in culture while KO palates failed to fuse (Fig. 7a and b). Failure of fusion is indicated by retention of the medial edge epithelium (MEE) between the two apposed palatal shelves in the KO cultures (Fig. 7b). This demonstrates that even when the shelves of the KO animals are apposed to one another, the mechanisms governing fusion are aberrant in the absence of CCN2. Collectively these results have demonstrated that the 3 essential processes in the development of the mammalian palate: growth (proliferation), elevation and fusion, are negatively affected in CCN2 KO mice.
Fig. 7.
Organ culture of E14.5 palatal shelves isolated from WT (a) and CCN2 KO (b) embryos (n = 5 organ cultures per genotype). The medial edge of palatal shelves were placed in juxtaposition to one another and grown in organ culture for 72 h. H&E stained sections demonstrate fusion of palatal shelves in WT cultures (a), while they failed to fuse in KO cultures (b). Scale bar = 50 μm
Discussion
Development of the mammalian palate is dependent on multiple signaling factors that must be organized both temporally and spatially (Bush and Jiang 2012; Parada and Chai 2012; Parada et al. 2013; Smith et al. 2012). Examples include transcription factors such as Msx1, Shox2, Dlx5 and Tbx22, cell surface receptors such as BMPR1a, TGF-βRs, FGFRs, and EGFR, and secreted molecules such as BMPs, TGF-βs, FGFs, hedgehogs, collagens, and Wnts (Bush and Jiang 2012; Smith et al. 2012). Together, these factors control the 3 processes vital to development of the palate: cellular proliferation, shelf elevation and fusion (reviewed in (Bush and Jiang 2012; Smith et al. 2012)). Deficits or abnormalities in many of these factors lead to some form of orofacial clefting.
CCN2 is known as a matricellular protein; it is secreted from cells and becomes incorporated into the surrounding extracellular matrix (ECM), where it modulates the binding of several regulatory proteins to their receptors, including TGF-β1, BMP2, EGF, FGF2/4 and Fibronectin (Abreu et al. 2002; Aoyama et al. 2012; Hoshijima et al. 2006; Mundy et al. 2014; Rayego-Mateos et al. 2013). Our findings demonstrate that CCN2 possesses an important biological role in regulating the development of the mammalian palate in that absence of CCN2 leads to a cleft secondary palate without cleft lip with 100 % phenotypic penetrance. This observation is noteworthy since few global knockout models that reach birth (P0) demonstrate 100 % penetrance of orofacial clefting (Gritli-Linde 2008). However, these CCN2 KO mice die shortly after birth from hypoxia similar to those generated by Dr. Karen Lyons (Ivkovic et al. 2003). Our findings show that CCN2 KO mice suffer clefting of both the skeletal and muscular portions of the palate. Thus, CCN2 transcends the anterior and posterior division of secondary palatal development (Smith et al. 2012). Previously, it has been shown that Tgfbr2 or Smad4 deletion in the palate mesenchymal cells utilizing condition KO via Wnt1-cre;Tgfbr2fl/fl and osr2-cre;Smad4fl/fl resulted in clefting of the secondary palate that was capable of being rescued by addition of exogenous CCN2 (Parada et al. 2013). Although the knockouts used in the Parada study had decreased levels of CCN2, our model has a complete absence of CCN2 and thus allows for the direct examination of the functional consequences of CCN2 during palate development.
The presence of partially developed palatal shelves indicates that palatal agenesis is not the mechanism of clefting in this model as compared with the Col2a1 transgenic mouse model (Li et al. 1995). Examination of the palatal shelves in the CCN2 KO newborns reveals that rugae are present on each shelf indicating that sonic hedgehog (SHH) signaling is, at least in part, intact as SHH expression is limited to the thickened palatal oral epithelium that will develop into the rugae (Rice et al. 2006). Furthermore, the CCN2 KO animals exhibit a serpentine nasal septum. Previous characterization of craniofacial development of this mouse model by our lab revealed that midline structures were more dramatically affected (e.g. vomer and pterygoid processes) than peripheral structures (Lambi et al. 2012). Since the nasal septum is normally anchored to the palate at the midline, the absence of a midline structure for the septum to anchor to in KO mice is one possible cause for its serpentine appearance.
Examination of CCN2 KO newborn and embryonic coronal sections also reveals palatal shelves that fail to elevate. The mouse palatal shelves normally elevate by embryonic day 14.5 (E14.5) but the shelves in the KO mice are not elevated at this developmental time point, and they fail to elevate to a horizontal position even at P0 indicating that shelf elevation is inhibited rather than delayed in the absence of CCN2. The mechanism(s) regulating shelf elevation is/are poorly understood. A previous study elucidated a potential mechanism of palatal shelf elevation by relating the presence of ECM proteins, in this case hyaluronic acid, to the ability to produce turgor pressure within the shelves (Ferguson 1978). CCN2 is well known to stimulate ECM protein synthesis as well as facilitate interactions between ECM proteins and cells (Gao and Brigstock 2004; Hendesi et al. 2015; Hoshijima et al. 2006; Nishida et al. 2007). Therefore, the lack of shelf elevation in the KO mice may be related to a decrease in synthesis of ECM proteins and/or absence of the function of CCN2 as a molecular bridge between cells and their ECM, questions that warrant future study into the causative mechanism for failure of shelf elevation in KO mice.
Cell proliferation is one of the 3 crucial processes that govern development of the mammalian palate (Bush and Jiang 2012; Smith et al. 2012). Analysis of cell proliferation rates reveal that CCN2 levels correspond directly to the rate of cellular proliferation, particularly with regards to progression through the G1 phase and G1/S checkpoint. Our findings are in agreement with previous reports that CCN2 stimulates proliferation (Battula et al. 2013; Gao et al. 2004; Shimo et al. 1999). The decreased number of KO cells found in the G2 phase is likely an incidental finding resulting from fewer cells progressing into S phase rather than a direct result of the absence of CCN2. Our results clearly demonstrate that in neural crest derived mesenchymal cells of the murine palate, CCN2 plays a critical role in regulating cell proliferation. To ensure that the proliferative deficit observed in the KO cells ex vivo translates to what is occurring in vivo, we demonstrated decreased cell proliferation (assessed via BrdU incorporation) in the KO compared to WT palatal shelves at E14.5. Future studies on proliferation will focus on examining differences between KO and WT cells in proteins vital to the G1 phase and G1/S checkpoint such as Cyclins, CDKs, and proto-oncogenes (e.g. p21, p53, and RB).
The cranial neural crest cells that compose the mesenchyme of the developing palatal shelves must migrate into the first pharyngeal arch which subsequently develops into the maxillary region (Arosarena 2007). We examined how the mesenchymal cells from the WT and KO animals interact with the ECM. KO cells demonstrated a reduced ability to adhere and spread on a matrix consisting of FN or CCN2. Both adhesion and spreading are integrin-dependent processes and our findings support the previous studies indicating that CCN2 promotes integrin-dependent adhesion to FN (Gao and Brigstock 2004, 2006; Hendesi et al. 2015). CCN2 has also been documented to interact with and facilitate many cell-ECM protein interactions both directly, but more commonly, indirectly through modulation of β1 containing integrin function (Gao and Brigstock 2004; Hendesi et al. 2015; Hoshijima et al. 2006). Briefly, CCN2 is believed to act as a molecular bridging protein that increases the affinity of integrins to ECM components such as fibronectin (Chen et al. 2004). The property of CCN2 to increase binding is believed to be primarily accomplished through the ability of CCN2 to bind to heparan sulfate proteoglycans through its fourth domain (CT domain) (Gao and Brigstock 2004) which act as co-receptors for integrins to bind to ECM proteins (Carey 1997). CCN2 thus allows cells to adhere and spread more efficiently to ECM proteins. We did not observe a restoration in the ability of KO cells to adhere to immobilized CCN2, thus further highlighting the bridging role CCN2 plays in promoting integrin to ECM protein interactions. The addition of recombinant CCN2 was able to restore the ability of KO cells to spread to a similar extent as that observed in WT cells, suggesting that exogenous CCN2 is sufficient to rescue at least some of the abnormal cellular functions in KO cells. However, we have unpublished preliminary data showing that a number of key regulatory genes/proteins involved in palatogenesis are adversely affected in the absence of CCN2, and therefore, we cannot conclude that the addition of exogenous CCN2 alone will restore normal palate development in the KO mice. Additional studies are required to elucidate the intrinsic versus extrinsic cellular effects of CCN2 in palatogenesis. Our data also showed that KO cells migrated faster than the WT cells. This was at first an unexpected result given the wealth of information suggesting that CCN2 promotes cell migration (Gao and Brigstock 2006; Mao et al. 2011; Shimo et al. 1999; Tsai et al. 2014; Xiu et al. 2012). However, some recent studies have demonstrated that CCN2 decreases migration leading to a reduction in metastasis in different cancer types (Lin et al. 2005; Zhen et al. 2014). Our results suggest that the role of CCN2 in regulating cell migration is cell-type specific.
To begin exploring the mechanism governing the decreased adhesion and spreading in KO cells, we explored the activity levels of Rac1 and RhoA. Rac1 and RhoA are two of the best-studied members of the Rho family of GTPases and are (Guo et al. 2006) both known to contribute to lamellipodia-mediated cellular spreading and adhesion to ECM proteins such as FN (Guo et al. 2006; Machacek et al. 2009). Rac1 and RhoA both demonstrated dramatic decreases in activation in the KO cells, a finding that is consistent with the observation that the KO cells adhere and spread less when compared to WT cells. These results support the role of Rac1 in cell adhesion and spreading and highlight the understudied aspect of RhoA in these processes. Rac1 promotes actin polymerization and microtubule elongation locally at the level of the cell membrane (Daub et al. 2001). RhoA has been implicated in having a role in formation of focal adhesions, and the ability of cells to spread has been linked to activation of RhoA (Machacek et al. 2009; McBeath et al. 2004). Our finding that active RhoA is reduced in KO cells is consistent with the literature because at the time of assessment the KO cells had not yet begun spreading while the WT cells had. Collectively, both Rac1 and RhoA are important in regulating cell adhesion and spreading of mesenchymal cells of neural crest origin. Interestingly, Rac1, but not RhoA, is associated with TGF-β1 induced CCN2 expression via activation of JNK, a component of the MAPK pathway (Black and Trackman 2008). Thus, while reduced adhesion and spreading can be associated reduced cytoskeletal activity as a result of reduced Rac1 and RhoA activity, investigations of other mechanisms involving these proteins is warranted.
In embryonic development neural crest cells are known to migrate via collective migration (Scarpa and Mayor 2016). Collective migration varies from typical cellular migration in that only the cells on the leading edge of the migrating cell mass form lamellipodia and filopodia whereas formation of these processes is actively suppressed in lagging cells (Scarpa and Mayor 2016). Thus, examination of Rac1 or RhoA in migration cannot be accomplished by the pull-down methods used here as this would assess the activated Rac1 and RhoA of all cells. Rac1 is typically found at high activity levels in the leading edge of a migrating cell whereas RhoA is typically highest on the lagging aspect. Thus, whole cell lysate does not allow for accurate examination of activated states of these Rho family GTPases on a single cell basis. Thus, live cell imaging will be utilized in future studies examining Rac1 and RhoA in cellular migration.
To establish if the mechanisms governing fusion were intact, E14.5 palatal shelves obtained from KO and WT mice were cultured with the medial edge epithelium (MEE) of adjacent shelves placed in close proximity at the midline and grown in culture. The WT shelves fused normally while the KO shelves retained the MEE and failed to fuse. TGF-β3 signaling is known to be important for fusion of the palatal shelves and TGF-β signaling is negatively impacted by the absence of CCN2 (Abreu et al. 2002; Cui and Shuler 2000; Yang and Kaartinen 2007). Our results indicate that the signaling pathways surrounding the process of palatal fusion are aberrant in the absence of CCN2 and should be assessed in future studies, particularly the role of CCN2 in TGF-β3 signaling in palatal fusion. The ability of CCN2 to augment signaling of a TGF-β family member (Abreu et al. 2002) represents potential for similar interactions to occur between CCN2 and TGF-β3.
In conclusion, our findings clearly demonstrate the pivotal role of CCN2 in the development of the mammalian secondary palate. In the absence of CCN2, each of the 3 processes vital to palatal development is negatively impacted resulting in 100 % penetrance of clefting. CCN2 interacts with and impacts the function of a number of proteins that have been shown to play a role in palate development. Future studies will screen for RNA and protein expression differences between KO and WT palatal mesenchyme to assess for abnormal expression of factors known to be both important for palate development and impacted by CCN2. These include TGF-β, BMP, FGF and EGF, as well as integrin-mediated signaling pathways. We propose that the identification of aberrant expression of factors regulated by CCN2 in the KO may help elucidate common abnormalities underlying non-syndromic cases of cleft palate.
Acknowledgments
Authors would like to thank Dr. Alex G. Lambi from the University of California, Los Angeles for his guidance and intellectual feedback. Authors would also like to thank Mamta Amin for her technical contributions and assistance.
Abbreviations
- CCN2
Connective tissue growth factor
- WT
Wild type
- KO
Knockout
- CL[P]
Cleft lip with or without palate involvement
- CP
Cleft palate only
- E
Embryonic day of development
- P
Days after birth
- TGF-β
Transforming growth factor beta
- FGF
Fibroblast growth factor
- BMP
Bone morphogenetic protein
- MEPM
Mouse embryonic palate mesenchymal
- DMEM
Dulbecco’s modified eagle medium
- FBS
Fetal bovine serum
- PFA
Paraformaldehyde
- PTA
Phosphotungstic acid
- ECM
Extracellular matrix
- MEE
Medial edge epithelium
- FN
Fibronectin
Compliance with ethical standards
Conflict of interest
All authors declare that there are no conflicts of interest.
References
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama E, Kubota S, Takigawa M. CCN2/CTGF binds to fibroblast growth factor receptor 2 and modulates its signaling. FEBS Lett. 2012;586:4270–4275. doi: 10.1016/j.febslet.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Arosarena OA. Cleft lip and palate. Otolaryngol Clin N Am. 2007;40:27–60. doi: 10.1016/j.otc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Battula VL, Chen Y, Cabreira Mda G, Ruvolo V, Wang Z, Ma W, Konoplev S, Shpall E, Lyons K, Strunk D, Bueso-Ramos C, Davis RE, Konopleva M, Andreeff M. Connective tissue growth factor regulates adipocyte differentiation of mesenchymal stromal cells and facilitates leukemia bone marrow engraftment. Blood. 2013;122:357–366. doi: 10.1182/blood-2012-06-437988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black SA, Jr, Trackman PC. Transforming growth factor-beta1 (TGFbeta1) stimulates connective tissue growth factor (CCN2/CTGF) expression in human gingival fibroblasts through a RhoA-independent, Rac1/Cdc42-dependent mechanism: statins with forskolin block TGFbeta1-induced CCN2/CTGF expression. J Biol Chem. 2008;283:10835–10847. doi: 10.1074/jbc.M710363200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JO, Jiang R. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development. 2012;139:231–243. doi: 10.1242/dev.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush RA, Wei LL, Sieving PA. Convergence of human genetics and animal studies: gene therapy for x-linked retinoschisis. Cold Spring Harb Perspect Med. 2015;5:a017368. doi: 10.1101/cshperspect.a017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DJ. Syndecans: multifunctional cell-surface co-receptors. Biochem J. 1997;327(Pt 1):1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Abraham DJ, Shi-Wen X, Pearson JD, Black CM, Lyons KM, Leask A. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol Biol Cell. 2004;15:5635–5646. doi: 10.1091/mbc.E04-06-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LA, Guney MA, Oh YA, Deyoung RA, Valenzuela DM, Murphy AJ, Yancopoulos GD, Lyons KM, Brigstock DR, Economides A, Gannon M. Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Mol Endocrinol. 2009;23:324–336. doi: 10.1210/me.2008-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XM, Shuler CF. The TGF-beta type III receptor is localized to the medial edge epithelium during palatal fusion. Int J Dev Biol. 2000;44:397–402. [PubMed] [Google Scholar]
- Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem. 2001;276:1677–1680. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- Ferguson MW. Palatal shelf elevation in the Wistar rat fetus. J Anat. 1978;125:555–577. [PMC free article] [PubMed] [Google Scholar]
- Gao R, Ball DK, Perbal B, Brigstock DR. Connective tissue growth factor induces c-fos gene activation and cell proliferation through p44/42 MAP kinase in primary rat hepatic stellate cells. J Hepatol. 2004;40:431–438. doi: 10.1016/j.jhep.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J Biol Chem. 2004;279:8848–8855. doi: 10.1074/jbc.M313204200. [DOI] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. A novel integrin alpha5beta1 binding domain in module 4 of connective tissue growth factor (CCN2/CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut. 2006;55:856–862. doi: 10.1136/gut.2005.079178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A. The etiopathogenesis of cleft lip and cleft palate: usefulness and caveats of mouse models. Curr Top Dev Biol. 2008;84:37–138. doi: 10.1016/S0070-2153(08)00602-9. [DOI] [PubMed] [Google Scholar]
- Guo F, Debidda M, Yang L, Williams DA, Zheng Y. Genetic deletion of Rac1 GTPase reveals its critical role in actin stress fiber formation and focal adhesion complex assembly. J Biol Chem. 2006;281:18652–18659. doi: 10.1074/jbc.M603508200. [DOI] [PubMed] [Google Scholar]
- Hendesi H, Barbe MF, Safadi FF, Monroy MA, Popoff SN. Integrin mediated adhesion of osteoblasts to connective tissue growth factor (CTGF/CCN2) induces cytoskeleton reorganization and cell differentiation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CR, Jacobs BH, Brown CB, Barnett JV, Goudy SL. Type III transforming growth factor beta receptor regulates vascular and osteoblast development during palatogenesis. Dev Dyn. 2015;244:122–133. doi: 10.1002/dvdy.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima M, Hattori T, Inoue M, Araki D, Hanagata H, Miyauchi A, Takigawa M. CT domain of CCN2/CTGF directly interacts with fibronectin and enhances cell adhesion of chondrocytes through integrin alpha5beta1. FEBS Lett. 2006;580:1376–1382. doi: 10.1016/j.febslet.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lewis AE, Singh V, Ma X, Adelstein R, Bush JO. Convergence and extrusion are required for normal fusion of the mammalian secondary palate. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner RE, LaRossa D. Cleft lip and palate. Otolaryngol Clin N Am. 2000;33:1191–1215. doi: 10.1016/S0030-6665(05)70277-2. [DOI] [PubMed] [Google Scholar]
- Lambi AG, Pankratz TL, Mundy C, Gannon M, Barbe MF, Richtsmeier JT, Popoff SN. The skeletal site-specific role of connective tissue growth factor in prenatal osteogenesis. Dev Dyn. 2012;241:1944–1959. doi: 10.1002/dvdy.23888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J, Yumoto K, Azhar M, Ninomiya-Tsuji J, Inagaki M, Hu Y, Deng CX, Kim J, Mishina Y, Kaartinen V. Tak1, Smad4 and Trim33 redundantly mediate TGF-beta3 signaling during palate development. Dev Biol. 2015;398:231–241. doi: 10.1016/j.ydbio.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi B, James AW, Nelson ER, Brugmann SA, Sorkin M, Manu A, Longaker MT. Role of Indian hedgehog signaling in palatal osteogenesis. Plast Reconstr Surg. 2011;127:1182–1190. doi: 10.1097/PRS.0b013e3182043a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Prockop DJ, Helminen H, Fassler R, Lapvetelainen T, Kiraly K, Peltarri A, Arokoski J, Lui H, Arita M, et al. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995;9:2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- Lin BR, Chang CC, Che TF, Chen ST, Chen RJ, Yang CY, Jeng YM, Liang JT, Lee PH, Chang KJ, Chau YP, Kuo ML. Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterol. 2005;128:9–23. doi: 10.1053/j.gastro.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Ma X, Rong Y, Cui L, Wang X, Wu W, Zhang J, Jin D. Connective tissue growth factor enhances the migration of gastric cancer through downregulation of E-cadherin via the NF-kappaB pathway. Cancer Sci. 2011;102:104–110. doi: 10.1111/j.1349-7006.2010.01746.x. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Taketomi T, Yoshizaki K, Arai S, Sanui T, Yoshiga D, Yoshimura A, Nakamura S. Sprouty2 controls proliferation of palate mesenchymal cells via fibroblast growth factor signaling. Biochem Biophys Res Commun. 2011;404:1076–1082. doi: 10.1016/j.bbrc.2010.12.116. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Snider TN. Neural crest cell signaling pathways critical to cranial bone development and pathology. Exp Cell Res. 2014;325:138–147. doi: 10.1016/j.yexcr.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy C, Gannon M, Popoff SN. Connective tissue growth factor (CTGF/CCN2) negatively regulates BMP-2 induced osteoblast differentiation and signaling. J Cell Physiol. 2014;229:672–681. doi: 10.1002/jcp.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Kawaki H, Baxter RM, Deyoung RA, Takigawa M, Lyons KM. CCN2 (Connective Tissue Growth Factor) is essential for extracellular matrix production and integrin signaling in chondrocytes. J Cell Commun Signal. 2007;1:45–58. doi: 10.1007/s12079-007-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada C, Chai Y. Roles of BMP signaling pathway in lip and palate development. Front Oral Biol. 2012;16:60–70. doi: 10.1159/000337617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada C, Li J, Iwata J, Suzuki A, Chai Y. CTGF mediates Smad-dependent transforming growth factor beta signaling to regulate mesenchymal cell proliferation during palate development. Mol Cell Biol. 2013;33:3482–3493. doi: 10.1128/MCB.00615-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayego-Mateos S, Rodrigues-Diez R, Morgado-Pascual JL, Rodrigues Diez RR, Mas S, Lavoz C, Alique M, Pato J, Keri G, Ortiz A, Egido J, Ruiz-Ortega M. Connective tissue growth factor is a new ligand of epidermal growth factor receptor. J Mol Biol. 2013;5:323–335. doi: 10.1093/jmcb/mjt030. [DOI] [PubMed] [Google Scholar]
- Rice R, Connor E, Rice DP. Expression patterns of Hedgehog signalling pathway members during mouse palate development. Gene Expr Patterns. 2006;6:206–212. doi: 10.1016/j.modgep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Scarpa E, Mayor R. Collective cell migration in development. J Cell Biol. 2016;212:143–155. doi: 10.1083/jcb.201508047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- Smith TM, Lozanoff S, Iyyanar PP, Nazarali AJ. Molecular signaling along the anterior-posterior axis of early palate development. Front Physiol. 2012;3:488. doi: 10.3389/fphys.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Su HL, Huang CY, Fong YC, Hsu CJ, Tang CH. CTGF increases matrix metalloproteinases expression and subsequently promotes tumor metastasis in human osteosarcoma through down-regulating miR-519d. Oncotarget. 2014;5:3800–3812. doi: 10.18632/oncotarget.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu M, Liu YH, Brigstock DR, He FH, Zhang RJ, Gao RP. Connective tissue growth factor is overexpressed in human hepatocellular carcinoma and promotes cell invasion and growth. World J Gastroenterol. 2012;18:7070–7078. doi: 10.3748/wjg.v18.i47.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LT, Kaartinen V. Tgfb1 expressed in the Tgfb3 locus partially rescues the cleft palate phenotype of Tgfb3 null mutants. Dev Biol. 2007;312:384–395. doi: 10.1016/j.ydbio.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev. 2008;125:797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Yumoto K, Thomas PS, Lane J, Matsuzaki K, Inagaki M, Ninomiya-Tsuji J, Scott GJ, Ray MK, Ishii M, Maxson R, Mishina Y, Kaartinen V. TGF-beta-activated kinase 1 (Tak1) mediates agonist-induced Smad activation and linker region phosphorylation in embryonic craniofacial neural crest-derived cells. J Biol Chem. 2013;288:13467–13480. doi: 10.1074/jbc.M112.431775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Ye Y, Yu X, Mai C, Zhou Y, Chen Y, Yang H, Lyu X, Song Y, Wu Q, Fu Q, Zhao M, Hua S, Wang H, Liu Z, Zhang Y, Fang W. Reduced CTGF expression promotes cell growth, migration, and invasion in nasopharyngeal carcinoma. PLoS One. 2014;8 doi: 10.1371/journal.pone.0064976. [DOI] [PMC free article] [PubMed] [Google Scholar]