Abstract
CCN3 is a matricellular protein that belongs to the CCN family. CCN3 consists of 4 domains: insulin-like growth factor-binding protein-like domain (IGFBP), von Willebrand type C-like domain (VWC), thrombospondin type 1-like domain (TSP1), and the C-terminal domain (CT) having a cysteine knot motif. Periostin is a secretory protein that binds to extracellular matrix proteins such as fibronectin and collagen. In this study, we found that CCN3 interacted with periostin. Immunoprecipitation analysis revealed that the TSP1-CT interacted with the 4 repeats of the Fas 1 domain of periostin. Immunofluorescence analysis showed co-localization of CCN3 and periostin in the periodontal ligament of mice. In addition, targeted disruption of the periostin gene in mice decreased the matricellular localization of CCN3 in the periodontal ligament. Thus, these results indicate that periostin was required for the matricellular localization of CCN3 in the periodontal ligament, suggesting that periostin mediated an interaction between CCN3 and the extracellular matrix.
Keywords: Periostin, CCN3, Periodontal ligament
Introduction
Matricellular proteins that are secreted into the extracellular milieu are localized on the surface of cells and/or extracellular matrix to regulate cell states by interacting with cell-surface receptors, cytokines, and hormones (Bornstein 2009). Matricellular proteins are ubiquitously expressed during embryogenesis, and are also expressed in injured tissues in adulthood (Bornstein 2009; Chatterjee et al. 2014; Krupska et al. 2015). These proteins include SPARC, BM-40, myocilin, thrombospondin-1 (TSP-1), TSP-2, osteopontin, tenascins, βig-h3 (TGFBI), CCN family, and periostin (Norris et al. 2009; Kudo 2011; Conway et al. 2014; Chatterjee et al. 2014; Krupska et al. 2015).
The CCN family is composed of 6 proteins, CCN1 to 6 (Perbal 2013; Krupska et al. 2015), each of which is expressed in different tissues (Li et al. 2015). CCN3, also known as NOV (Nephroblastoma Overexpressed), is expressed in osteoblasts, smooth muscle cells, nerve cells, and tumor cells (Su et al. 2001; Ouellet and Siegel 2012; Krupska et al. 2015; Li et al. 2015). CCN3 is N-glycosylated and secreted into the extracellular milieu as a dimer, becoming localized on the cell surface and extracellular matrix (Joliot et al. 1992; Chevalier et al. 1998; Kyurkchiev et al. 2004; Perbal 2006, 2013). CCN3 interacts with extracellular matrix proteins such as Fibulin-1C (Perbal et al. 1999) and cell-surface heparan sulfate proteoglycans (Ouellet and Siegel 2012).
The pathophysiological roles of CCN3 have been demonstrated in Ccn3 −/− mice. Ccn3 −/− mice exhibit a mild skeletal phenotype (Canalis et al. 2010) and a hyper-proliferative response to vascular injury (Leask 2010; Shimoyama et al. 2010). In the murine model of abdominal aortic aneurysm, Ccn3 −/− mice exhibit severe phenotypes characterized by elastin fragmentation, vessel dilation, vascular inflammation, dissection, heightened ROS generation, and smooth muscle cell loss, suggesting that CCN3 is a regulator of abdominal aortic aneurysm (Zhang et al. 2016). Ccn3 −/− mice also show metabolic phenotypes such as improved glucose tolerance and insulin sensitivity on a high-fat diet, suggesting that CCN3 can act as an adipocytokine (Martinerie et al. 2016). Thus, CCN3 is an attractive target for therapies of several diseases (Boehm 1989; McCallum et al. 2012; Van Roeyen et al. 2012; Riser et al. 2014, 2015; Li et al. 2015). CCN3 also acts as an anti-fibrotic factor (Abd El Kader et al. 2013; Riser et al. 2014; Leask 2015; Riser et al. 2015). On the other hand, in Ccn3 −/− mice, interstitial renal fibrosis is reduced in a chronic kidney disease model, suggesting CCN3 as a fibrotic mediator (Marchal et al. 2015). Therefore, the molecular function of CCN3 regarding the progression of fibrosis is controversial (Abd El Kader et al. 2013).
CCN3 has been demonstrated to activate the Notch signaling pathway. CCN3 regulates osteoblastic differentiation via Notch signal activation (Ouellet and Siegel 2012). In osteosarcoma, CCN3 is highly expressed and correlated with metastasis and poor prognosis (Manara et al. 2002; Perbal et al. 2008). In addition, Notch is linked to metastasis and a worse prognosis in osteosarcoma patients (Hughes 2009). In Notch signaling, extracellular CCN3 is a key regulator: CCN3 interacts with the extracellular domain of Notch 1 and induces its cleavage (Sakamoto et al. 2002; Minamizato et al. 2007). The released Notch intracellular domain localizes into nuclei and acts there as a transcription factor (Schroeter et al. 1998). Thus, the matricellular localization of CCN3 is important for cellular regulation via Notch signaling.
Periostin is expressed in adult tissues such as periodontal ligament, periosteum, and cardiac valves (Kudo 2011). Targeted disruption of the periostin gene in mice leads to non-lethal phenotypes such as eruption disturbance of their incisors and disrupted collagen fibrillogenesis (Kii et al. 2006, 2010). Nevertheless, expression of periostin is markedly induced during wound healing, myocardial infarction, and also in cancer stroma (Kikuchi et al. 2008; Shimazaki et al. 2008; Nishiyama et al. 2011; Kikuchi et al. 2014). Periostin −/− mice exhibit severe phenotypes in these situations (Shimazaki et al. 2008; Nishiyama et al. 2011; Kikuchi et al. 2014). Periostin binds to the cell surface (Horiuchi et al. 1999) and also to components of the extracellular matrix, such as fibronectin, collagen, and tenascin-C (Takayama et al. 2006; Kii et al. 2010). Immuno-electron microscopic analysis also showed that periostin is localized on the collagen fibrils in the periodontal ligament and heart valve, as well as on the cell surface of fibroblasts in the colon (Kii et al. 2006; Norris et al. 2007; Kikuchi et al. 2008).
Earlier, we revealed the functions of periostin in the promotion of localization of extracellular matrix proteins. Periostin interacts with type I collagen, fibronectin, tenascin-C, and laminin γ2 chain (Kii et al. 2010; Nishiyama et al. 2011; Kii et al. 2016). These interactions allow the extracellular matrix proteins to be located in the appropriate locus (Kii et al. 2010; Nishiyama et al. 2011; Kii et al. 2016). This appropriate localization of fibronectin, tenascin-C and laminin γ2 chain proteins plays important roles in mechanical adaption and cell proliferation (Kii et al. 2010; Nishiyama et al. 2011).
Previously, in the purification of periostin from the culture supernatant of MC3T3-E1 cells, we had found that CCN3 co-purified with periostin (unpublished results). In this present study, we revealed the interaction between CCN3 and periostin, and identified the binding domains of these proteins. Periostin deficiency in mice resulted in decreased extracellular localization of CCN3 in the periodontal ligament. Thus, this study suggests that periostin was required for the matricellular localization of CCN3.
Materials and methods
Mice
Care and experiments with animals were in accordance with the guidelines of the Animal Care and Use Committee at Tokyo Institute of Technology. The wild-type C57BL6 mice were purchased from CLEA Japan, Inc. (Tokyo, Japan). Generation of C57BL6 background periostin −/− mice was described previously (Kii et al. 2006; Shimazaki et al. 2008).
Plasmid
The HA tag in the pCAGIPuro-periostin-HA (Kii et al. 2010) was replaced with the FLAG tag by the standard PCR method. The ΔEMIΔCT-FLAG, CCN3-FLAG, and CCN3-d5-HA expression vectors were newly constructed with the following primers: for ΔEMIΔCT-FLAG, 5′-ACCGAATTCAAGCTGCAGATTTGAAAGATCTCC-3′ and 5′-ACCGGATCCGCGGCCGCTTACTACTTGTCATCGTCGTCCTTGTAGTCTGCTGGATAGAGGAGTTTGTCC-3′; for CCN3-FLAG, 5′-ACCGGATCCCTCGAGGCCACCATGAGCCTCTTCCTGCGAAAGC-3′ and 5′-ACCGGATCCGAATTCGCGGCCGCTTACTACTTGTCATCGTCGTCCTTGTAGTCAATTTCTCCTCTGCTTGTCTTCAGC-3′; for CCN3-d5-HA, 5′-ACCGGATCCCTCGAGGCCACCATGAGCCTCTTCCTGCGAAAGC-3′ and 5′-ACCGGATCCGAATTCGCGGCCGCTTACTAAGCGTAGTCTGGGACGTCGTATGGGTAAGAGACTTCAACTCCTACGG-3′. The other constructs used were described previously (Sakamoto et al. 2002; Tanabe et al. 2010; Kii et al. 2010). The protein tags, FLAG, HA, and human Fc (hFc), were placed in-frame at the C-terminus. All expression vectors were based on the pCXN2, pCAGIPuro, which was provided by Dr. Niwa (Niwa et al. 1998) or on the pCAGIHygro backbone, in which the puromycin resistance cassette of pCAGIPuro was replaced with the hygromycin resistance one. Full details will be provided upon request.
Cell culture and transfection
HEK293T cells were obtained from the Cell Bank of RIKEN BioResource Center and cultured in low-glucose Dulbecco’s modified Eagle’s medium (Nacalai Tesque, Inc., Kyoto, Japan) supplemented with 10% FBS (MultiSer™; Thermo Fisher Scientific Inc., Beverly, MA or JRH Biosciences, Inc.,Lenexa, KS), 100 U/ml penicillin G, and 100 μg/ml streptomycin. One day before transfection, 2.4 × 105 cells or 1.0 × 106 cells were plated in 12- well plates or 3.5 cm dish, respectively. After having been grown to 90% confluence, the cells were transfected with expression vectors by using polyethylenimine (Sigma-Aldrich, St. Louis, MO), as previously described (Boussif et al. 1995) or with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Immunoprecipitation
Cells in 12-well plates or 3.5-cm dishes were scraped on ice into PBS and lysed in 200 μL or 600 μL of lysis buffer (100 mM Tris, pH 7.4, containing 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% TritonX-100, 0.5% NP-40, 1 μg/mL leupeptin and 0.5 mM PMSF), respectively. For immunoprecipitation of HA- or FLAG-tagged protein, the clarified extracts were incubated at 4 °C for 30 min with anti-HA antibody-conjugated agarose (Sigma-Aldrich) or anti-FLAG antibody-conjugated one (Sigma-Aldrich). For immunoprecipitation of hFc-tagged protein, the extracts were incubated with Protein A Sepharose (GE Healthcare Life Sciences, Piscataway, NJ) at 4 °C for 1 h. The bound proteins were eluted in SDS sample buffer (50 mM Tris (pH 6.8), 2% SDS, 0.1% Bromophenol Blue, and 10% glycerol with 50 or 100 mM DTT) with boiling for 5 min.
Western blotting
Samples were separated by SDS-PAGE and blotted onto nitrocellulose membranes. Non-specific binding was blocked by immersion of the membranes for 30 min in 5% skim milk in Tris-buffered saline (TBS) containing 0.1% Tween (TBS-T) at room temperature. The membranes were washed with TBS-T and then incubated at 4 °C for overnight with primary antibodies (Table 1). Signals were detected by using appropriate HRP-conjugated secondary antibodies.
Table 1.
Antibodies used in this study
| Antibody | Cat no. | Supplier | Host/clonality | Dilution |
|---|---|---|---|---|
| FLAG | A4596 | Sigma-Aldrich | mouse monoclonal | 1:2000 (agarose beads, IP) |
| FLAG | F7425 | Sigma-Aldrich | rabbit polyclonal | 1:2000 (W) |
| GFP | 598 | MBL | rabbit polyclonal | 1:3000 (W) |
| HA | A2095 | Sigma-Aldrich | mouse monoclonal | 1:2000 (agarose beads, IP) |
| HA | H6908 | Sigma-Aldrich | rabbit polyclonal | 1:2000 (W) |
| mPeriostin (RD1) | N/A | N/A (raised in our laboratory) | rabbit polyclonal | 1:100 (IHC) |
| mPeriostin (CT) | N/A | N/A (raised in our laboratory) | rabbit polyclonal | 1:2000 (W) |
| hCCN3 | AF1640 | R&D | goat polyclonal | 1:200 (IHC) |
| hCCN3 (K19 M) | N/A | N/A (gift from Dr. B. Perbal) | rabbit polyclonal | 1:150 (IHC) |
W Western blotting, IHC Immunohistochemistry, IP Immunoprecipitation. N/A indicates not applicable
Immunohistochemistry
Wild-type and periostin −/− mice were sacrificed under anesthesia, fixed with 4% paraformaldehyde (PFA) in 0.1 M cacodylate buffer (pH 7.4) by perfusion through the cardiac left ventricle. The mandibles from the mice were post-fixed in 4% PFA in PBS at 4 °C for overnight, and then decalcified in 10% EDTA in PBS for 1 month. The mandibles were next embedded in paraffin and sectioned into 4-μm-thick sections. After dewaxing, the sections were pretreated with 0.3% hydrogen peroxide for 20 min, and then with 1% bovine serum albumin (BSA; Serological Proteins Inc.) in PBS for 30 min. They were then incubated with primary antibodies (Table 1) in 1% BSA in PBS at room temperature (RT) for 2 h: anti-RD1 (Shimazaki et al. 2008) or anti-K19 M (Chevalier et al. 1998) (for single-immunostaining) or anti-RD1 and goat anti-CCN3 (for double-immunostaining). After having been rinsed with PBS, the sections were incubated with appropriate secondary antibodies at RT for 1 h. They were then washed with PBS and visualized with diaminobenzidine. Counterstaining was done with methyl green (for single-immunostaining) or DAPI (double-immunostaining).
Results
CCN3 interacted with periostin
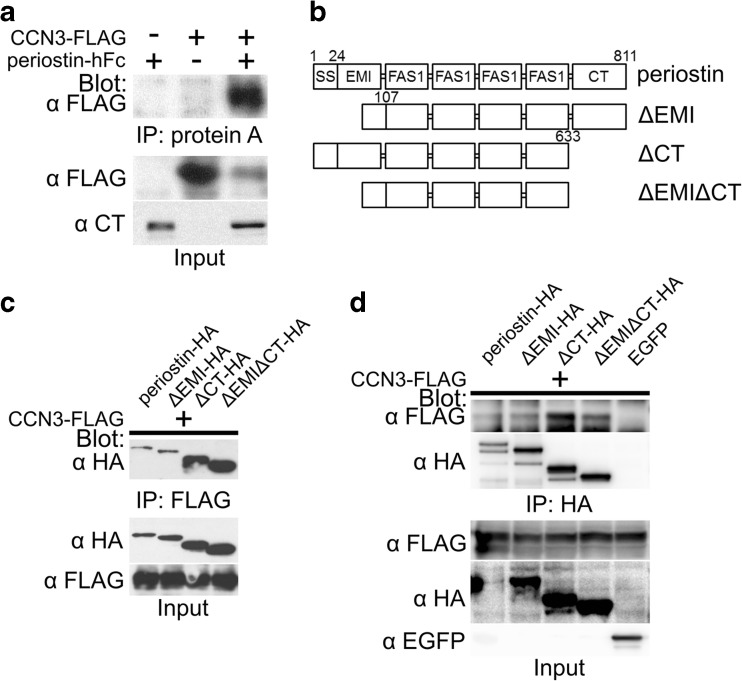
To confirm the co-purification of CCN3 and periostin, we performed a co-immunoprecipitation assay. We co-transfected 293 T cells with the vectors for periostin fused with human Fc (periostin-hFc) and CCN3 tagged with FLAG (CCN3-FLAG). The cells were lysed and pulled-down with protein A Sepharose. CCN3-FLAG was detected in the co-immunoprecipitated fraction from the lysate of the cells co-expressing CCN3-FLAG and periostin-hFC (Fig. 1a). This result indicated the interaction between CCN3 and periostin.
Fig. 1.
CCN3 interacts with the 4 repeats of the Fas 1 domain of periostin. a Co-immunoprecipitation assay for CCN3-FLAG and periostin-hFc. 293 T cells were co-transfected with the combinations of CCN3-FLAG, periostin-hFc, and their control vectors. The cell lysates were co-immunoprecipitated with protein A Sepharose. Blot, antibody used for Western blot analysis; IP, antibody used for immunoprecipitation; Input, crude transfected 293 T cell lysates. b Domain structures of intact and domain-deleted forms of periostin. All the forms were expressed as HA or FLAG fusion proteins with the tag at their carboxyl-terminal. The numbers denote the positions of the amino acids of the periostin protein. SS, signal sequence; EMI, EMILIN domain; FAS1, Fasciclin I domain; CT, C-terminal region. (c, d) Co-immunoprecipitation assays for CCN3-FLAG and HA-tagged intact or deleted forms of periostin, tagged with HA. 293 T cells were co-transfected with CCN3-FLAG and intact or mutant forms of periostin-HA (c, d) or EGFP as a control (d). The total cell lysates were co-immunoprecipitated with anti-FLAG (c) or anti-HA (d) Agarose
CCN3 interacted with the 4 repeats of the Fas 1 domain of periostin
Periostin is composed of an N-terminal signal sequence (SS) and 6 domains: the EMI domain, 4 repeats of the Fas 1 domain, and C-terminal region (CT; Fig. 1b; Kudo 2011). To clarify the domain of periostin that bound to CCN3, first we constructed various periostin deletion mutants tagged with HA (Fig. 1b). Then we performed a co-immunoprecipitation assay using anti-FLAG Agarose and lysates of 293 T cells co-transfected with the expression vectors for CCN3-FLAG and the intact periostin-HA or its deletion mutants. CCN3-FLAG interacted with all of the periostin-HA mutants (Fig. 1c). To confirm these bindings, we next performed co-immunoprecipitation assay using anti-HA Agarose under the same conditions. Just the same as in Fig. 1c, all of the periostin-HA mutants interacted with CCN3-FLAG (Fig. 1d). These data demonstrate that CCN3 interacted with the 4 repeats of the Fas 1 domain of periostin.
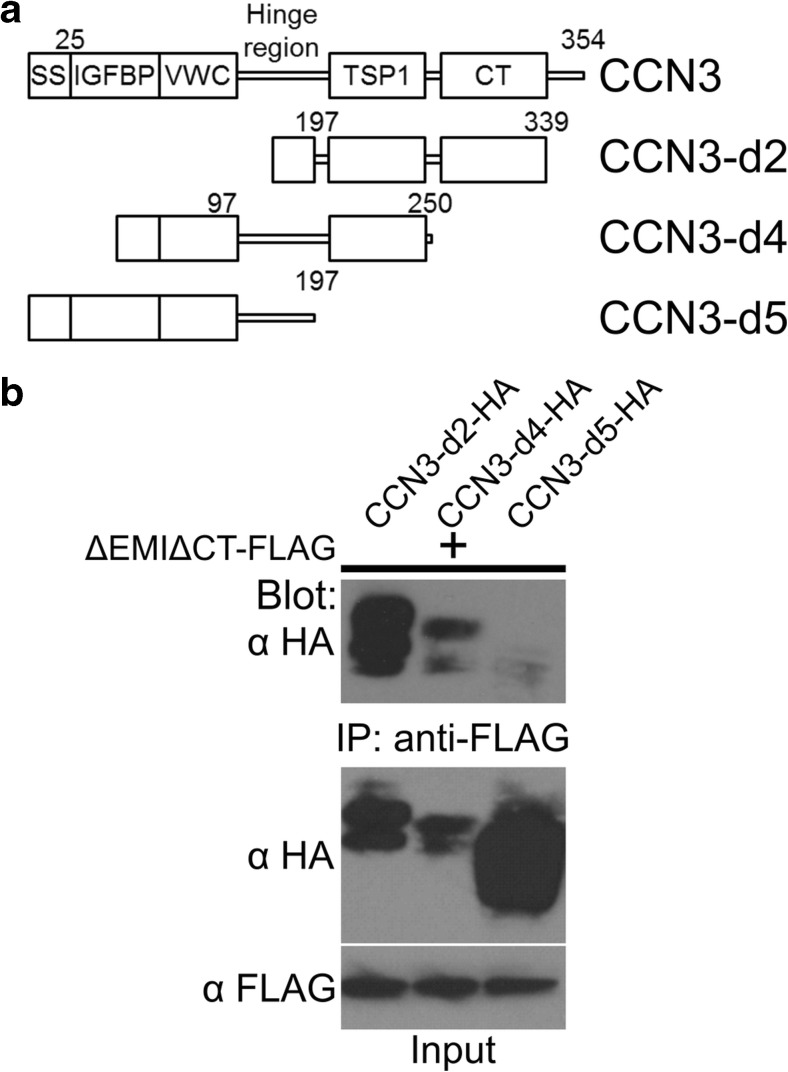
TSP1-CT domain of CCN3 interacted with the 4 repeats of the Fas 1 domain of periostin
CCN3 possesses an N-terminal signal sequence (SS), IGFBP, VWC, TSP1, and CT domains (Fig. 2a; Krupska et al. 2015). To investigate the binding domain of CCN3 to periostin, we designed the CCN3 deletion constructs shown in Fig. 2a. We performed co-immunoprecipitation assay using anti-FLAG Agarose and 293 T cells co-transfected with the expression vectors for the FLAG-tagged 4 repeats of Fas 1 domain (ΔEMIΔCT) and HA-tagged CCN3-d2, CCN3-d4, or CCN3-d5. ΔEMIΔCT-FLAG interacted with CCN3-d2-HA and CCN3-d3-HA, but not with CCN3-d5-HA (Fig. 2b). These data indicate that the TSP1-CT domain of CCN3 interacted with the 4 repeats of the Fas 1 domain of periostin (Fig. 3).
Fig. 2.
TSP1 domain of CCN3 interacts with periostin. a Domain structures of intact and domain deletion forms of CCN3 protein. All the forms were expressed as HA or FLAG tagged proteins with the tag at their C-terminal. The signal sequence from IgG was fused to the N-terminal deletion constructs (CCN3-d2 and CCN3-d4). The numbers denote the positions of the amino acids of the CCN3 protein. SS, signal sequence; IGFBP, insulin-like growth factor binding protein-like domain; VWC, von Willebrand factor type C-like domain; TSP1, thrombospondin type 1-like domain; CT, C-terminal domain. b Co-immunoprecipitation assay for ΔEMIΔCT-FLAG and HA-tagged CCN3-d2, CCN3-d4, or CCN3-d5. 293 T cells were co-transfected with the expression vectors. The total cell lysates were co-immunoprecipitated with anti-FLAG Agarose
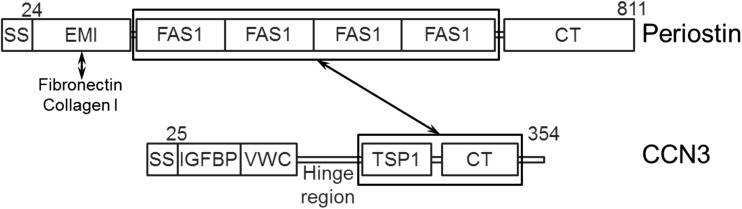
Fig. 3.
Interaction domains between CCN3 and periostin. Summary of the co-immunoprecipitation assay in 293 T cells described in Figs. 1 and 2. The TSP1-CT domain of CCN3 interacts with the 4 repeats of the Fas 1 domain of periostin. The EMI domain of periostin interacts with extracellular matrix proteins, fibronectin, and type I collagen (Collagen I)
CCN3 co-localized with periostin in the periodontal ligament of mice
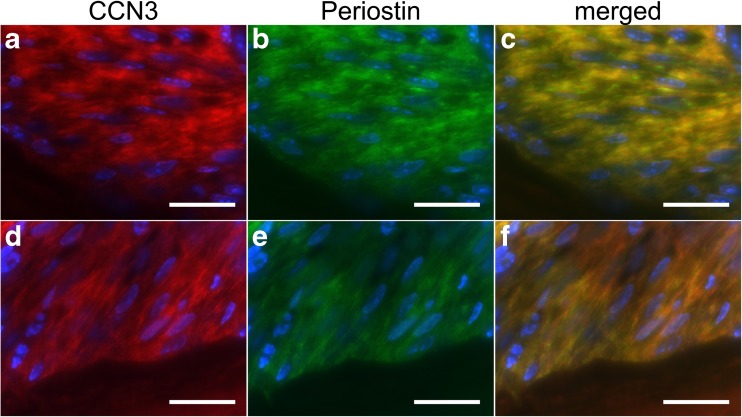
We next examined whether CCN3 co-localized with periostin. We performed double-immunofluorescence analysis with thin sections of molars of mice. Immunofluorescent signals for CCN3 were detected as a fibrillar structure in the periodontal ligament (Fig. 4a, d). Signals for periostin were also detected as a fibrillar structure, indicating its matricellular localization (Fig. 4b, e). Almost all of the signals for CCN3 merged with those for periostin (Fig. 4c, f), suggesting that CCN3 interacted with periostin in the periodontal ligament.
Fig. 4.
CCN3 co-localizes with periostin in the mouse periodontal ligament. Fluorescence immunohistochemical analysis of periodontal ligaments of the molars from wild-type C57BL/6 mice. Paraffin sections of mandibles from 8-week-old mice were stained with anti-CCN3 antibody (a, d), anti-periostin antibody (b, e), and DAPI (nuclei, blue signal). Merged images are shown in “c” and “f.” Scale, 50 μm
Decreased matricellular localization of CCN3 in the periodontal ligament of periostin−/− mice
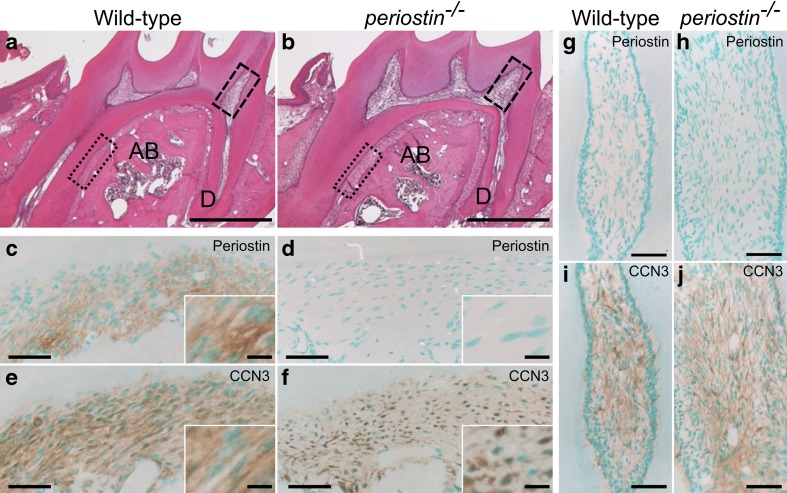
The possible co-localization of CCN3 and periostin on the extracellular matrix encouraged us to examine whether CCN3 would become localized on the extracellular matrix in the absence of periostin. We compared the localization of CCN3 in the molar periodontal ligament (dotted-line boxes in Fig. 5a, b) and dental pulp (dashed-line boxes in Fig. 5a, b) from wild-type and periostin −/− mice. Periostin was expressed in the periodontal ligament (Fig. 5c, Kii et al. 2006), but not in the dental pulp (Fig. 5g, Zhou et al. 2015). In the molar periodontal ligament, periostin was detected in the wild-type tissue (Fig. 5c), but not in the periostin −/− tissue (Fig. 5d). Like periostin, CCN3 was also detected as a fibrillar structure as in the molar periodontal ligament from the wild-type mice (Fig. 5e). On the other hand, CCN3 was not detected as a fibrillar structure in the periostin −/− tissue (Fig. 5f). The localization of CCN3 seemed to be inside the cells, not on extracellular matrix. In contrast, in the dental pulp, in which periostin was not expressed (Fig. 5g, h), the localization of CCN3 was not different between the wild-type and the periostin −/− tissues (Fig. 5i, j), indicating a possibility that another secretory factor compensated the function of periostin in the dental pulp. Thus, these data indicate that periostin deficiency in mice decreased the matricellular localization of CCN3.
Fig. 5.
Decreased matricellular localization of CCN3 in the periostin -deficient periodontal ligament. Hematoxylin and eosin staining (a, b) and immunohistochemical analysis (c–j) of the mandibles of the mice. Paraffin sections of mandibles from 8-week-old wild-type (a, c, e, g, i) and periostin −/− (b, d, f, h, j) mice were stained with hematoxylin and eosin (a, b), anti-periostin (c, d, g, h), or anti-CCN3 (e, f, i, j) antibodies. High magnifications of dotted-line boxes and dashed-line boxes in “a” and “b” indicate the periodontal ligament ( c–f) and the dental pulp (g–j), respectively. Typical immunoreactivities in “c”-“f” are represented as high-magnification images in the insets (white-lined boxes). AB, alveolar bone; D, dentin. Scale, 500 μm (a, b); 50 μm (c–f); 20 μm (insets of c–d); 100 μm (g–j)
Discussion
This study demonstrated the interaction between the TSP1-CT domain of CCN3 and the 4 repeats of the Fas 1 domain of periostin. Periostin directly binds to the extracellular matrix proteins (collagen I and fibronectin) via its EMI domain (Kii et al. 2010; Fig. 3). This EMI domain is a conserved domain that functions as a protein-protein interaction module, and it is frequently found in secretory and extracellular matrix proteins (Doliana et al. 2000; Callebaut et al. 2003). Thus, this study indicated the possible existence of a CCN3-periostin-extracellular matrix complex. CCN3 was detected on the extracellular matrix in the periodontal ligament, which CCN co-localized with periostin (Fig. 4). Deletion of the periostin gene in mice reduced the matricellular localization of CCN3 (Fig. 5e, f), indicating that periostin was required for the matricellular localization of CCN3. The interaction between CCN3 and periostin may have facilitated this matricellular localization.
Our previous study also demonstrated that periostin facilitates the incorporation of tenascin-C into the extracellular matrix and that deletion of the periostin gene in mice reduces the tenascin-C content in the extracellular matrix and disrupts the extracellular meshwork structure (Kii et al. 2010). As found presently for CCN3, tenascin-C also interacts with the 4 repeats of the Fas 1 domain (Kii et al. 2010). Thus, periostin may promote the appropriate localization of the matricellular proteins by mediating the interaction between these proteins and the extracellular matrix.
Periostin −/− mice exhibit severe eruption disturbance in their incisors (Kii et al. 2006), which is due to defects in remodeling of the extracellular matrix in the periodontal ligament (Lv et al. 2014). Previously we reported that the expression of cathepsin K, matrix metalloproteinase 1 (MMP1), and MMP2 was significantly decreased in the periodontal ligament of periostin −/− mice compared with that in their wild-type counterparts (Lv et al. 2014), suggesting that periostin was required for the expression of these enzymes. CCN3 has been demonstrated to play roles in the expression of MMPs (Laurent et al. 2003; Benini et al. 2005; Lin et al. 2005; Fukunaga-Kalabis et al. 2008; Tzeng et al. 2011; Kular et al. 2012). As examples, the interaction between CCN3 and αv integrin promotes MMP13 expression (Tzeng et al. 2011); and CCN3 mediates the expression of MMP3 via the PDGF signaling pathway (Laurent et al. 2003). These results suggest that CCN3 was partly responsible for the eruption disturbance seen in periostin −/− mice.
Periostin is occasionally co-expressed with CCN3 in tissues and diseases, such as periodontal ligament (Fig. 4), aorta (Fu et al. 2009; Zhang et al. 2016), abdominal aortic aneurysm (Yamashita et al. 2013; Zhang et al. 2016), wound healing (Lin et al. 2005; Nishiyama et al. 2011), osteosarcoma (Perbal et al. 2008; Hu et al. 2014, 2016), and vascular injury (Lindner et al. 2005). In these situations, CCN3 plays important roles in the mitigation of abdominal aortic aneurysm (Zhang et al. 2016), the angiogenesis in wound healing (Lin et al. 2005), and the cell migration in osteosarcoma (Huang et al. 2011). Our present data indicated that periostin facilitated the matricellular localization of CCN3. Thus, some parts of the pathophysiological functions of periostin could be explained by CCN3 involvement.
Acknowledgements
The authors thank Dr. H. Niwa (RIKEN, Japan) for the pCAGIPuro expression vector and Dr. B. Perbal (University of Paris) for K19 M antibody. This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science, Culture, and Sports of Japan (KAKENHI Grant No. 19791362 (IK) and 19370093 (AK)) and partly by the Project for Cancer Research And Therapeutic Evolution (P-CREATE) (IK) from the Japan Agency for Medical Research and Development (AMED).
Author’s contributions
IT, HT, KK, and AK designed the research. IT, HT, HI, NA, and ML performed experiments. IT, HT, TN, and IK analyzed and interpreted the data. TN and IK wrote the manuscript.
Abbreviations
- BMP
Bone morphogenetic protein
- CT
Carboxyl-terminal (C-terminal) domain
- EMI
EMILIN
- Fas 1
Fasciclin I
- IGFBP
Insulin-like growth factor-binding protein
- TSP1
Thrombospondin type 1
- VWC
von Willebrand factor type C
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Footnotes
Issei Takayama, Hideyuki Tanabe and Takashi Nishiyama contributed equally.
Contributor Information
Isao Kii, Phone: +81-78-304-7162, Email: isao.kii@riken.jp.
Akira Kudo, Phone: +81-45-924-5718, Email: akudo@bio.titech.ac.jp.
References
- Abd El Kader T, Kubota S, Janune D, et al. Anti-fibrotic effect of CCN3 accompanied by altered gene expression profile of the CCN family. J Cell Commun Signal. 2013;7:11–18. doi: 10.1007/s12079-012-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benini S, Perbal B, Zambelli D, et al. In Ewing’s sarcoma CCN3(NOV) inhibits proliferation while promoting migration and invasion of the same cell type. Oncogene. 2005;24:4349–4361. doi: 10.1038/sj.onc.1208620. [DOI] [PubMed] [Google Scholar]
- Boehm S. Patient contracting. Annu Rev Nurs Res. 1989;7:143–153. [PubMed] [Google Scholar]
- Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal. 2009;3:163–165. doi: 10.1007/s12079-009-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, MAntoniet Z, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut I, Mignotte V, Souchet M, Mornon JP. EMI domains are widespread and reveal the probable orthologs of the Caenorhabditis elegans CED-1 protein. Biochem Biophys Res Commun. 2003;300:619–623. doi: 10.1016/S0006-291X(02)02904-2. [DOI] [PubMed] [Google Scholar]
- Canalis E, Smerdel-Ramoya A, Durant D, et al. Nephroblastoma overexpressed (Nov) inactivation sensitizes osteoblasts to bone morphogenetic protein-2, but nov is dispensable for skeletal homeostasis. Endocrinology. 2010;151:221–233. doi: 10.1210/en.2009-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Villarreal G, Rhee DJ. Matricellular proteins in the trabecular meshwork: review and update. J Ocul Pharmacol Ther. 2014;30:447–463. doi: 10.1089/jop.2014.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier G, Yeger H, Martinerie C, et al. novH: differential expression in developing kidney and Wilm’s tumors. Am J Pathol. 1998;152:1563–1575. [PMC free article] [PubMed] [Google Scholar]
- Conway SJ, Izuhara K, Kudo Y, et al. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014;71:1279–1288. doi: 10.1007/s00018-013-1494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doliana R, Bot S, Bonaldo P, Colombatti A. EMI, a novel cysteine-rich domain of EMILINs and other extracellular proteins, interacts with the gC1q domains and participates in multimerization. FEBS Lett. 2000;484:164–168. doi: 10.1016/S0014-5793(00)02140-2. [DOI] [PubMed] [Google Scholar]
- Fu Z, Wang M, Gucek M, et al. Milk fat globule protein epidermal growth factor-8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circ Res. 2009;104:1337–1346. doi: 10.1161/CIRCRESAHA.108.187088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga-Kalabis M, Martinez G, Telson SM, et al. Downregulation of CCN3 expression as a potential mechanism for melanoma progression. Oncogene. 2008;27:2552–2560. doi: 10.1038/sj.onc.1210896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Hu F, ang W W, Zhou H-C, Shang X-F. High expression of periostin is dramatically associated with metastatic potential and poor prognosis of patients with osteosarcoma. World J Surg Oncol. 2014;12:287. doi: 10.1186/1477-7819-12-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Shang X-F, ang W W, et al. High-level expression of periostin is significantly correlated with tumour angiogenesis and poor prognosis in osteosarcoma. Int J Exp Pathol. 2016;97:86–92. doi: 10.1111/iep.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Lee CY, Chen MY, et al. Nephroblastoma overexpressed gene (NOV) enhances cell motility and COX-2 upregulation of human osteosarcoma involves αvβ5 integrin, ILK and AP-1-dependent pathways. Biochem Pharmacol. 2011;81:577–585. doi: 10.1016/j.bcp.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Hughes DPM. How the NOTCH pathway contributes to the ability of osteosarcoma cells to metastasize. Cancer Treat Res. 2009;152:479–496. doi: 10.1007/978-1-4419-0284-9_28. [DOI] [PubMed] [Google Scholar]
- Joliot V, Martinerie C, Dambrine G, et al. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992;12:10–21. doi: 10.1128/MCB.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kii I, Amizuka N, Minqi L, et al. Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun. 2006;342:766–772. doi: 10.1016/j.bbrc.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kii I, Nishiyama T, Li M, et al. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem. 2010;285:2028–2039. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kii I, Nishiyama T, Kudo A. Periostin promotes secretion of fibronectin from the endoplasmic reticulum. Biochem Biophys Res Commun. 2016;470:888–893. doi: 10.1016/j.bbrc.2016.01.139. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Kashima TG, Nishiyama T, et al. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem. 2008;56:753–764. doi: 10.1369/jhc.2008.951061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Kunita A, Iwata C, et al. The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am J Pathol. 2014;184:859–870. doi: 10.1016/j.ajpath.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Krupska I, Bruford EA, Chaqour B. Eyeing the Cyr61/CTGF/NOV (CCN) group of genes in development and diseases: highlights of their structural likenesses and functional dissimilarities. Hum Genomics. 2015;9:1–13. doi: 10.1186/s40246-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 2011;68:3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kular L, Rivat C, Lelongt B, et al. NOV/CCN3 attenuates inflammatory pain through regulation of matrix metalloproteinases-2 and -9. J Neuroinflammation. 2012;9:36. doi: 10.1186/1742-2094-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyurkchiev S, Yeger H, Bleau A-M, Perbal B. Potential cellular conformations of the CCN3(NOV) protein. Cell Commun Signal. 2004;2:9. doi: 10.1186/1478-811X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent M, Martinerie C, Thibout H, et al. NOVH increases MMP3 expression and cell migration in glioblastoma cells via a PDGFR-alpha-dependent mechanism. FASEB J. 2003;17:1919–1921. doi: 10.1096/fj.02-1023fje. [DOI] [PubMed] [Google Scholar]
- Leask A. It,s a knockout: CCN3 suppresses neointimal thickening. J Cell Commun Signal. 2010;4:109–110. doi: 10.1007/s12079-010-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. Yin and Yang revisited: CCN3 as an anti-fibrotic therapeutic? J Cell Commun Signal. 2015;9:97–98. doi: 10.1007/s12079-015-0281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ye L, Owen S, et al. Emerging role of CCN family proteins in tumorigenesis and cancer metastasis (review) Int J Mol Med. 2015;36:1451–1463. doi: 10.3892/ijmm.2015.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CG, Chen CC, Leu SJ, et al. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem. 2005;280:8229–8237. doi: 10.1074/jbc.M404903200. [DOI] [PubMed] [Google Scholar]
- Lindner V, Wang Q, Conley BA, et al. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol. 2005;25:77–83. doi: 10.1161/01.ATV.0000149141.81230.c6. [DOI] [PubMed] [Google Scholar]
- Lv S, Liu H, Cui J, et al. Histochemical examination of cathepsin K, MMP1 and MMP2 in compressed periodontal ligament during orthodontic tooth movement in periostin deficient mice. J Mol Histol. 2014;45:303–309. doi: 10.1007/s10735-013-9548-x. [DOI] [PubMed] [Google Scholar]
- Manara MC, Perbal B, Benini S, et al. The expression of ccn3(nov) gene in musculoskeletal tumors. Am J Pathol. 2002;160:849–859. doi: 10.1016/S0002-9440(10)64908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal PO, Kavvadas P, Abed A, et al. Reduced NOV/CCN3 expression limits inflammation and interstitial renal fibrosis after obstructive nephropathy in mice. PLoS One. 2015;10:1–12. doi: 10.1371/journal.pone.0137876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinerie C, Garcia M, Do TTH, et al. NOV/CCN3: a new adipocytokine involved in obesity-associated insulin resistance. Diabetes. 2016;65:2502–2515. doi: 10.2337/db15-0617. [DOI] [PubMed] [Google Scholar]
- McCallum L, Lu W, Price S, et al. CCN3 suppresses mitogenic signalling and reinstates growth control mechanisms in chronic myeloid leukaemia. J Cell Commun Signal. 2012;6:27–35. doi: 10.1007/s12079-011-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamizato T, Sakamoto K, Liu T, et al. CCN3/NOV inhibits BMP-2-induced osteoblast differentiation by interacting with BMP and notch signaling pathways. Biochem Biophys Res Commun. 2007;354:567–573. doi: 10.1016/j.bbrc.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Kii I, Kashima TG, et al. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One. 2011;6:e18410. doi: 10.1371/journal.pone.0018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris R, Damon B, Mironov V, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR. The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal. 2009;3:275–286. doi: 10.1007/s12079-009-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet V, Siegel PM. CCN3 modulates bone turnover and is a novel regulator of skeletal metastasis. J Cell Commun Signal. 2012;6:73–85. doi: 10.1007/s12079-012-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. NOV story: the way to CCN3. Cell Commun Signal. 2006;4:3. doi: 10.1186/1478-811X-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: a centralized communication network. J Cell Commun Signal. 2013;7:169–177. doi: 10.1007/s12079-013-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B, Martinerie C, Sainson R, et al. The C-terminal domain of the regulatory protein NOVH is sufficient to promote interaction with fibulin 1C: a clue for a role of NOVH in cell-adhesion signaling. Proc Natl Acad Sci U S A. 1999;96:869–874. doi: 10.1073/pnas.96.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B, Zuntini M, Zambelli D, et al. Prognostic value of CCN3 in osteosarcoma. Clin Cancer Res. 2008;14:701–709. doi: 10.1158/1078-0432.CCR-07-0806. [DOI] [PubMed] [Google Scholar]
- Riser BL, Najmabadi F, Garchow K, et al. Treatment with the matricellular protein CCN3 blocks and/or reverses fibrosis development in obesity with diabetic nephropathy. Am J Pathol. 2014;184:2908–2921. doi: 10.1016/j.ajpath.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Riser BL, Barnes JL, Varani J. Balanced regulation of the CCN family of matricellular proteins: a novel approach to the prevention and treatment of fibrosis and cancer. J Cell Commun Signal. 2015;9:327–339. doi: 10.1007/s12079-015-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Yamaguchi S, Ando R, et al. The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via notch signaling pathway. J Biol Chem. 2002;277:29399–29405. doi: 10.1074/jbc.M203727200. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- Shimazaki M, Nakamura K, Kii I, et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama T, Hiraoka S, Takemoto M, et al. CCN3 inhibits neointimal hyperplasia through modulation of smooth muscle cell growth and migration. Arterioscler Thromb Vasc Biol. 2010;30:675–682. doi: 10.1161/ATVBAHA.110.203356. [DOI] [PubMed] [Google Scholar]
- Su BY, Cai WQ, Zhang CG, et al. The expression of ccn3 (nov) RNA and protein in the rat central nervous system is developmentally regulated. Mol Pathol. 2001;54:184–191. doi: 10.1136/mp.54.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Tanabe H, Takayama I, Nishiyama T, et al. Periostin associates with Notch1 precursor to maintain Notch1 expression under a stress condition in mouse cells. PLoS One. 2010;5:e12234. doi: 10.1371/journal.pone.0012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng H-E, Chen J-C, Tsai C-H, et al. CCN3 increases cell motility and MMP-13 expression in human chondrosarcoma through integrin-dependent pathway. J Cell Physiol. 2011;226:3181–3189. doi: 10.1002/jcp.22672. [DOI] [PubMed] [Google Scholar]
- Roeyen CRC, Boor P, Borkham-Kamphorst E, et al. A novel, dual role of CCN3 in experimental glomerulonephritis: pro-angiogenic and antimesangioproliferative effects. Am J Pathol. 2012;180:1979–1990. doi: 10.1016/j.ajpath.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Yamashita O, Yoshimura K, Nagasawa A, et al. Periostin links mechanical strain to inflammation in abdominal aortic aneurysm. PLoS One. 2013;8:e79753. doi: 10.1371/journal.pone.0079753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Voort D, Shi H, et al. Matricellular protein CCN3 mitigates abdominal aortic aneurysm. J Clin Invest. 2016;126:1282–1299. doi: 10.1172/JCI82337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Kawashima N, Suzuk N, et al. Periostin is a negative regulator of mineralization in the dental pulp tissue. Odontology. 2015;103:152–159. doi: 10.1007/s10266-014-0152-7. [DOI] [PubMed] [Google Scholar]