Abstract
To simultaneously quantify and profile the complex mixture of short-, median-, and long-chain CPs (SCCPs, MCCPs, and LCCPs) in Australian sewage sludge, we applied and further validated a recently developed novel instrumental technique, using quadrupole time-of-flight high resolution mass spectrometry running in the negative atmospheric pressure chemical ionization mode (APCI-qTOF-HRMS). Without using an analytical column the cleaned extracts were directly injected into the qTOF-HRMS followed by quantification of the CPs by a mathematical algorithm. The recoveries of the four SCCP, MCCP and LCCP-spiked sewage sludge samples ranged from 86 to 123%. This APCI-qTOF-HRMS method is a fast and promising technique for routinely measuring SCCPs, MCCPs, and LCCPs in sewage sludge. Australian sewage sludge was dominated by MCCPs with concentrations ranging from 542 to 3645 ng/g dry weight (dw). Lower SCCPs concentrations (<57–1421 ng/g dw) were detected in the Australian sewage sludge, which were comparable with the LCCPs concentrations (116–960 ng/g dw). This is the first time that CPs were reported in Australian sewage sludge. The results of this study gives a first impression on the distribution of the SCCPs, MCCPs, and LCCPs in Australia wastewater treatment plants (WWTPs).
Introduction
Chlorinated paraffins (CPs), also known as polychlorinated n-alkanes (PCAs), are complex mixtures of carbon chains of variable length and a variable number of chlorine atoms. They are currently produced in high volumes (>1 million tons year–1 in China alone1). Concerns are rising regarding their ubiquitous occurrence and high persistence in the environment,2 including detection in remote areas.3 So-called short-chain CPs (SCCPs), CPs with carbon chain lengths between 10 and 13, are under scrutiny as these compounds have a high bioaccumulation potential4−6 and are chronically toxic to aquatic organisms.7,8 They are, therefore, classified as substances of very high concern and are listed as key compounds for monitoring in several legislations and guidelines. They are currently candidates to be designated as persistent organic pollutants (POPs) under the Stockholm Convention. In the case of the medium-chain CPs (MCCPs) (C14–C17) and long-chain CPs (LCCPs) (≥C18), relevant information on their levels, fates, and potential hazards to the environment is still insufficient to facilitate international regulations. However, the current restrictions on the use of SCCPs are expected to result mainly in a replacement by MCCPs in many applications.9
The insufficient data about CPs is partly due to limitations regarding the reliability of analytical methods to identify and quantify these compounds. To date, the existing analytical methods for the determination of CPs suffer all from serious drawbacks that makes a reliable quantification difficult. Recently, Bogdal et al.10 developed a promising instrumental technique to determine SCCPs, MCCPs, and LCCPs in environmental matrices in a single measurement run with a particularly fast method using high resolution mass spectrometry. Reliable CP exposure data is needed, particularly in regions where the CP levels and fate are still unknown. In Australia, for example, limited data on CPs exists, and the capability of analyzing CPs is lacking. Until today, only two nonpeer reviewed studies describe the presence of CPs in the Australian environment. Gillet et al.11 reported SCCPs in indoor and outdoor air in Melbourne with median concentrations of 52 ng/m3 and 81 ng/m3, respectively. Kemmlein et al.12 reported SCCPs, MCCPs, and LCCPs in mussels, crabs, and sediments collected near a CP manufacturer in Australia. The levels were, respectively, 2300 μg/kg lipid weight (lw), 23 200 μg/kg lw, and 9300 μg/kg lw in mussels, and 64 900 μg/kg lw, 30 500 μg/kg lw and 14 300 μg/kg lw in crabs. In the sediment, the concentrations ranged from 61 to 440 μg/kg dry weight (dw) for the SCCPs; from 1100 to 16 400 μg/kg dw for the MCCPs; and from 900 to 3190 μg/kg dw for the LCCPs. Despite the lack of information, the presence of at least local CP contamination is highly likely. Australia manufactures MCCPs and LCCPs (e.g., in Orica, Melbourne) with ongoing investments for expansion of CP manufacturing facilities.13 According to a government desktop study,14 SCCPs were imported into Australia in large quantities (e.g., 360 tonnes) between 1998 and 2000 and were still imported at approximately 25 tonnes/year in 2002. MCCPs and LCCPs were imported into Australia in 2002 in even higher quantities at approximately 475 tonnes/year. NICNAS14 reported that it is most likely that the use of MCCPs and LCCPs in Australia have increased due to their replacement of the SCCPs. While current information is lacking, to our knowledge, SCCP import or use is still permitted in Australia. In 2002 SCCPs were mainly used as lubricants in the Australian metal working industry (70%),14 and therefore, major releases of CPs in Australia are thought to be from production, both from spills or facility wash-down, and from industrial usage, either from improper disposal of used metalworking lubricants or carry-off from work pieces.14 Such releases of CPs occur either directly or through wastewater treatments plants (WWTPs).15 As CPs are emerging POPs, determining the levels of CPs near suspected sources (i.e., WWTPs) would facilitate a first understanding on their release, presence, and potential risks of these compounds. Therefore, this study aims to determine CPs in sewage sludge samples (also known as biosolids) from various WWTPs in order to allow a first evaluation of SCCP, MCCP, and LCCP levels in Australia.
MaterialS and Methods
Information about the standards, chemicals and suppliers is provided in the Supporting Information (SI) of this manuscript.
Sample Collection
Pooled (eight subsamples from each WWTP) sewage sludge samples were collected in 2014 at 15 different WWTPs in Australia (Figure 1). The WWTPs were located in five of eight states and territories of Australia, servicing populations of between 25 000 and 600 000 people, representing a combined population of approximately 2.5 million people (over 10% of the Australian population). Further details regarding sampling and sample composition are provided in the SI.
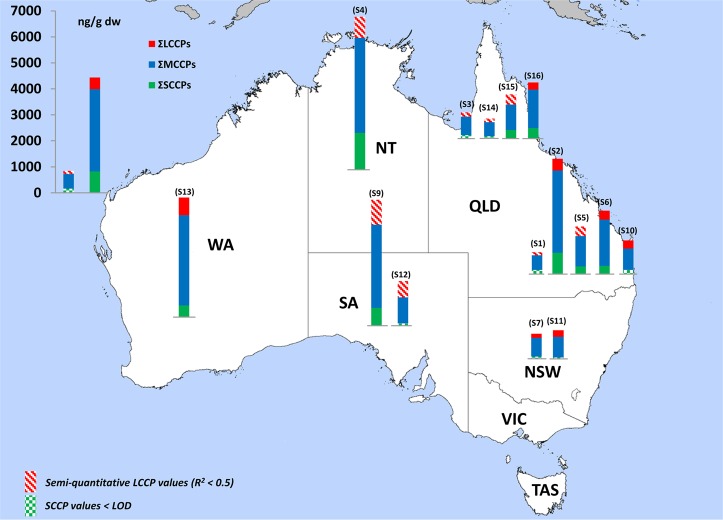
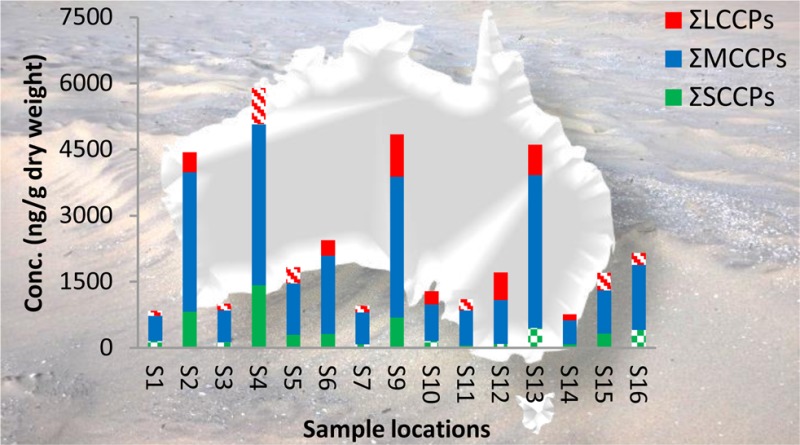
Figure 1.
Total CP concentrations in ng/g dry weight measured in the sewage sludge samples from Australia. The LCCP values with an R2 lower than 0.5 are highlighted with red stripes and are semiquantitative values. The SCCP values lower that the LOD are highlighted with green blocks. (WA = Western Australia; NT = Northern Australia; SA = South Australia; QLD = Queensland; NSW = New South Wales; VIC = Victoria; TAS = Tasmania.
Sample Pretreatment
After freeze-drying, approximately 0.2–0.3 g of the sewage sludge sample was extracted by pressurized liquid extraction (PLE) on an ASE350 (Dionex, Sunyvale, CA). Extraction was performed with n-hexane/acetone (3:1, v/v) at 100 °C and 1500 psi with a heat-, and static-time of 5 min using three extraction cycles. Cleanup was performed in two steps first with aluminum oxide (8% H20) and second with silicagel (1.5% H20) columns. Briefly, 15 g of aluminum oxide (8% H20) was weighed into a glass column. After washing the aluminum oxide column with 25 mL of n-hexane, the extracts were brought on top of the column followed by elution of the CPs with 170 mL of n-hexane. During the second cleanup, 1.8 g of silicagel (1.5% H20) was weighed into a glass column. The silicagel column was washed with 6 mL of n-hexane before the sample extract was added. Fractionation was performed in two fractions, the first one consisted of 14 mL of n-hexane (waste fraction) and the second one of 15 mL 15% diethyl ether in n-hexane (the CPs fraction). The fraction was evaporated under nitrogen and solvent changed to 0.5 mL acetonitrile (ACN) and transferred to an LC-vial followed by addition of 100 μL of injection standard 13C6–PCP (700 pg/μL in ACN).
Measurement of CPs
Measurement of the CPs in the Australian sewage sludge samples was performed with a slightly adopted analytical method recently developed by Bogdal et al.10 Briefly, without using an analytical column, 10 μL of the cleaned sewage sludge extract was directly injected into the qTOF-HRMS (Triple TOF 5600+ Sciex, Concord, Ontario, Canada) running in the negative atmospheric pressure chemical ionization (APCI) mode. The injection was performed with an Shimadzu Nexera HPLC system (Shimadzu Corp., Kyoto, Japan) using ACN as eluent with an isocratic flow of 250 μL/min. To increase the sensitivity of the CP detection in negative APCI mode, dichloromethane (DCM) at a flow rate of 40 μL/min was used as a dopant and mixed with the eluent just before it entered the ion source (Bogdal et al., (10). Addition of DCM results in an excess Cl- ions in the ion source and significantly enhances the formation of [M+Cl]-.
CPs were analyzed with the APCI-qTOF-HRMS using the following settings. The nebulizer temperature was optimized and set to 200 °C. The declustering potential (DP) was set at −90 V, and collision energy (CE) of −10 V was used. The mass spectrometer was operated in TOF-MS mode measuring the full scan range of m/z 200–1500. The minimal resolution was 10 000 (minimum of 7000 is required for the separation of the m/z values of the most important [M+Cl]- ions).10 External mass calibration was performed with the Sciex APCI Negative Calibration solution 5600, which consist of a mix of known molecular weight polypropylene glycols (PPGs). In total, 558 m/z ratios were extracted from the full scan mass spectra using MultiQuant 3.0 software (Sciex). The 558 m/z ratios are related to the two most abundant m/z signals of the CP isotope cluster corresponding to the CP congener groups with chain lengths of C10Cl4 to C27Cl27 (SI Table S10).
Quantification and Deconvolution
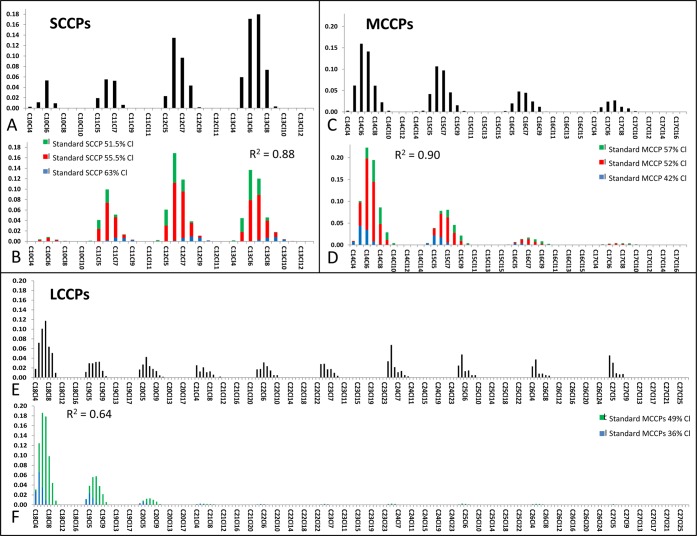
Quantification of the SCCPs, MCCPs, and LCCPs was based on a mathematical algorithm recently applied by Bogdal et al.10 for the quantification of CPs in various environmental matrices. A brief description of the deconvolution and quantification is given in the SI. An example of the deconvolution whereby the CP pattern measured in the sludge sample S2 was reconstructed into a linear combination of patterns of CPs of the technical mixtures is shown in Figure 2. As example for the reconstruction, the total MCCP concentration in sample S2 is 3150 ng/g dw, which is attributed to 1082 ng/g dw MCCP 42% Cl, 1582 ng/g dw MCCP 52% Cl, and 486 ng/g dw MCCP 57% Cl (Table 1).
Figure 2.
An example of chlorinated paraffins (SCCPs, MCCPs, and LCCPs) patterns in sewage sludge sample S2. For each chlorinated paraffin group (SCCPs, MCCPs, and LCCPs) the measured pattern is given (A, C, E) and the reconstructed pattern based on deconvolution of the technical chlorinated paraffin mixtures (B, D, F). Deconvolution of the SCCP pattern in the sewage sample was performed with the 51.5% Cl, 55.5% Cl, and 63% SCCP standards, for the deconvolution of the MCCP pattern the 42% Cl, 52% Cl, and 57% Cl MCCP standards and for the deconvolution of the LCCP pattern the 36% Cl and 49% CL LCCP standards were used. The goodness of fit (R2) for the deconvolution is also given.
Table 1. A, B, C. Composition Profile of the SCCPs, MCCPs, and LCCPs, Sample Concentration Attributed (ng/g dw), Total SCCP, MCCP, and LCCP Concentration in ng/g dw, Goodness of fit (R2) and the Chlorination Degree in the Sewage Sludge Samplesa.
| SCCPs
percent contribution |
sample
conc. (ng/g dw) attributed |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | C10 | C11 | C12 | C13 | SCCP 63% Cl | SCCP 55.5% Cl | SCCP 51.5% Cl | total SCCP conc. (ng/g dw) | R2 | Cl-degree (%) |
| S1 | 6 | 22 | 21 | 50 | 80 | 64 | 12 | <156 | 0.65 | 60.9% |
| S2 | 8 | 13 | 30 | 49 | 34 | 479 | 306 | 820 | 0.84 | 57.9% |
| S3 | 28 | 45 | 8 | 19 | 57 | 73 | 0 | <130 | 0.16 | 61.6% |
| S4 | 10 | 24 | 21 | 45 | 229 | 738 | 454 | 1421 | 0.76 | 59.5% |
| S5 | 8 | 21 | 24 | 46 | 180 | 116 | 0 | 296 | 0.72 | 62.2% |
| S6 | 3 | 20 | 26 | 51 | 137 | 178 | 0 | 315 | 0.70 | 61.2% |
| S7 | 4 | 16 | 25 | 55 | 55 | 30 | 0 | <85 | 0.75 | 62.4% |
| S9 | 10 | 13 | 23 | 54 | 60 | 422 | 202 | 685 | 0.64 | 58.6% |
| S10 | 17 | 32 | 15 | 37 | 25 | 122 | 0 | <147 | 0.60 | 58.9% |
| S11 | 18 | 0 | 15 | 67 | 23 | 34 | 0 | <57 | 0.24 | 60.8% |
| S12 1 | 1 | 4 | 55 | 39 | 32 | 54 | 0 | <86 | 0.59 | 60.7% |
| S12 2 | 0 | 0 | 59 | 41 | 22 | 38 | 0 | <60 | 0.58 | 61.0% |
| S13 | 2 | 4 | 22 | 72 | 6 | 411 | 17 | 435 | 0.55 | 57.8% |
| S14 | 5 | 21 | 26 | 48 | 56 | 12 | 16 | <83 | 0.69 | 62.4% |
| S15 | 5 | 20 | 22 | 52 | 177 | 148 | 0 | 325 | 0.68 | 61.6% |
| S16 | 13 | 28 | 22 | 37 | 218 | 186 | 1 | 406 | 0.65 | 63.0% |
| Bl1 | 25 | 59 | 12 | 4 | 6** | 0** | 3** | 9** | 0.002 | 66.2% |
| Bl2 | 29 | 53 | 12 | 7 | 7** | 0** | 9** | 16** | 0.001 | 64.0% |
| MCCPs
percent contribution |
sample
conc. (ng/g dw) attributed |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | C14 | C15 | C16 | C17 | MCCP 42% Cl | MCCP 52% Cl | MCCP 57% Cl | total MCCP conc. (ng/g dw) | R2 | Cl-degree(%) |
| S1 | 47 | 29 | 14 | 9 | 159 | 0 | 402 | 561 | 0.89 | 56.7% |
| S2 | 45 | 31 | 15 | 8 | 1082 | 1582 | 486 | 3150 | 0.86 | 53.5% |
| S3 | 32 | 38 | 19 | 11 | 114 | 250 | 345 | 710 | 0.61 | 55.6% |
| S4 | 51 | 32 | 11 | 6 | 1303 | 1656 | 686 | 3645 | 0.95 | 54.0% |
| S5 | 42 | 32 | 15 | 10 | 264 | 0 | 898 | 1162 | 0.85 | 56.5% |
| S6 | 39 | 32 | 18 | 11 | 662 | 0 | 1110 | 1772 | 0.82 | 55.3% |
| S7 | 39 | 35 | 16 | 9 | 216 | 16 | 484 | 716 | 0.78 | 56.5% |
| S9 | 45 | 35 | 13 | 7 | 107 | 2555 | 529 | 3192 | 0.89 | 54.3% |
| S10 | 40 | 34 | 16 | 11 | 0 | 386 | 427 | 813 | 0.81 | 54.9% |
| S11 | 29 | 38 | 20 | 14 | 69 | 21 | 700 | 790 | 0.56 | 56.0% |
| S12 1 | 33 | 39 | 17 | 11 | 0 | 181 | 814 | 995 | 0.71 | 55.9% |
| S12 2 | 29 | 40 | 19 | 11 | 74 | 266 | 676 | 1016 | 0.60 | 55.2% |
| S13 | 40 | 36 | 14 | 9 | 0 | 2242 | 1208 | 3449 | 0.81 | 54.6% |
| S14 | 35 | 33 | 18 | 15 | 204 | 0 | 338 | 542 | 0.68 | 56.3% |
| S15 | 42 | 33 | 15 | 9 | 0 | 0 | 974 | 974 | 0.84 | 57.5% |
| S16 | 44 | 33 | 14 | 9 | 600 | 0 | 875 | 1474 | 0.86 | 56.0% |
| Bl1 | 78 | 21 | 1 | 0 | 24** | 0** | 0** | 24** | 0.93 | 49.5% |
| Bl2 | 78 | 20 | 2 | 0 | 22** | 0** | 0** | 22** | 0.90 | 49.0% |
| LCCPs
percent contribution |
sample
conc. (ng/g dw) attributed |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | C18 | C19 | C20 | C21 | C22 | C23 | C24 | C25 | C26 | C27 | LCCP 36% Cl | LCCP 49% Cl | total LCCP conc. (ng/g dw) | R2 | Cl-degree (%) |
| S1 | 26 | 13 | 13 | 7 | 6 | 5 | 9 | 6 | 7 | 7 | 0 | 116 | 116 | 0.42 | 44.1% |
| S2 | 29 | 10 | 10 | 6 | 8 | 7 | 10 | 7 | 6 | 7 | 129 | 317 | 446 | 0.64 | 42.3% |
| S3 | 29 | 12 | 9 | 5 | 7 | 4 | 10 | 9 | 7 | 7 | 0 | 156 | 156 | 0.47 | 44.9% |
| S4 | 16 | 6 | 6 | 4 | 5 | 4 | 5 | 5 | 4 | 45 | 260 | 554 | 814 | 0.05 | 39.9% |
| S5 | 22 | 11 | 10 | 5 | 7 | 11 | 11 | 10 | 6 | 7 | 0 | 364 | 364 | 0.29 | 45.0% |
| S6 | 34 | 12 | 11 | 5 | 8 | 6 | 7 | 6 | 6 | 6 | 0 | 349 | 349 | 0.71 | 45.1% |
| S7 | 32 | 18 | 12 | 6 | 8 | 7 | 6 | 5 | 4 | 3 | 0 | 154 | 154 | 0.50 | 48.3% |
| S9 | 18 | 8 | 8 | 6 | 9 | 12 | 13 | 11 | 9 | 6 | 172 | 788 | 960 | 0.32 | 42.7% |
| S10 | 27 | 12 | 11 | 6 | 9 | 10 | 10 | 7 | 4 | 4 | 0 | 305 | 305 | 0.57 | 43.9% |
| S11 | 30 | 11 | 8 | 6 | 7 | 8 | 10 | 7 | 7 | 5 | 0 | 250 | 250 | 0.53 | 47.1% |
| S12 1 | 11 | 6 | 4 | 5 | 8 | 13 | 17 | 15 | 12 | 9 | 0 | 625 | 625 | 0.11 | 42.5% |
| S12 2 | 12 | 5 | 4 | 4 | 7 | 14 | 17 | 17 | 11 | 9 | 0 | 527 | 527 | 0.09 | 43.2% |
| S13 | 31 | 12 | 8 | 6 | 7 | 10 | 10 | 7 | 6 | 4 | 0 | 683 | 683 | 0.67 | 46.0% |
| S14 | 24 | 13 | 12 | 6 | 8 | 8 | 10 | 7 | 6 | 6 | 0 | 137 | 137 | 0.33 | 47.2% |
| S15 | 24 | 13 | 11 | 7 | 9 | 9 | 8 | 7 | 6 | 6 | 0 | 398 | 398 | 0.40 | 46.5% |
| S16 | 29 | 14 | 13 | 7 | 8 | 6 | 7 | 7 | 5 | 5 | 0 | 270 | 270 | 0.51 | 46.4% |
| Bl1 | 0 | 0 | 0 | 0 | 0 | 0 | 42 | 29 | 29 | 0 | 0.2** | 0.2** | 0.4 | 0.00 | 33.2% |
| Bl2 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 41 | 38 | 0 | 0.3** | 0.2** | 0.5 | 0.00 | 33.4% |
** = levels for the blanks are reported in ng abs. Italic = R2 lower than 0.5 therefore reported as tentative values.
In our study, the variation in response factors observed for SCCPs, MCCPs, and LCCPs were considerable less depending on their chain length and chlorination degree than in the study of Bogdal et al.,.10 Therefore, the quantification results are also less impacted by the goodness of fit (R2) between the CP patterns in the technical mixtures and the sample. With their analytical setup, Bogdal et al.,10 tested different technical CP mixtures with known concentration and reported that an R2 of 0.50 for SCCPs results in a quantification differences by a factor of 4 from the expected concentration. For MCCPs with an R2 > 0.60 the differences between calculated and expected concentration is a factor of 2 and for the LCCPs with an R2 > 0.50 results in an differences of an factor less than 1.2. Because in our study the response differences for the SCCPs, MCCPs, and LCCPS were comparable with those reported for MCCPs and LCCPs by Bogdal et al.,10 the differences between calculated and expected concentration here are also estimated to be below a factor of 2 for a R2 > 0.50. Therefore, values with an R2 < 0.50 are reported as tentative values. An example of the measured and reconstructed SCCP, MCCP, and LCCP patterns in three sewage sludge samples are provided in the Figures 2 and SI S5–S7.
Performance Characteristics of the Analytical Method
Three recovery standards, containing all eight commercial CP standards with known concentrations (120–160 ng absolute, were included in this study and underwent the same treatment as the 15 sewage sludge samples. Acceptable recoveries were observed for all three CP categories with mean SCCPs, MCCPs, and LCCPs recoveries of 79%, 81%, and 92%, respectively (SI Tables S3 and S8). The goodness of fit (R2) was higher than 0.93 for all mixtures. Although acceptable recoveries were observed for the CP categories (SCCPs, MCCPs, LCCPs), the recoveries of the lowest chlorinated CP formulations within a CP category were somewhat low, with recoveries down to 56% for the SCCP 51.5% Cl, and down to 42% for the MCCP 42% Cl. These lower recoveries are related to the somewhat reduced sensitivity of the APCI-qTOF-HRMS for CPs with a lower chlorination degree and shorter chain length.
Matrix interferences were investigated by spiking four of the analyzed sewage sludge samples with environmentally realistic concentrations of three SCCP, three MCCP, and two LCCP mixtures in the concentration range of 120−160 ng absolute. The mean recoveries of the four SCCP, MCCP, and LCCP-spiked sewage sludge samples ranged from 86 to 123%, and the R2 was higher than 0.93, with the exception of the LCCP-spiked S2 sample, which had an R2 of 0.54 (SI Tables S3 and S8). Overall, the results of the spiked sewage sludge samples were comparable with the results of the recovery standards which indicate that the results were not influenced by matrix interferences.
The stability of the APCI-qTOF-HRMS was investigated by performing an 8-fold measurement of a cleaned S9 sewage sludge sample (SI Table S9). Whereas, Bogdal et al.,10 reported that multiple injections are required to obtain a good estimate of the average response factor (RF), in this study an injection standard (13C6 -PCP) was applied to correct for the variability. The relative standard deviation (RSD) of the injection standard (13C6-PCP) of the 8-fold sewage sludge sample was 5%. The RSD of the sum of the CP congeners corrected for the internal standard was 5%, 2%, and 4%, for respectively for the SCCPs (685 ng/g dw), MCCPs (3192 ng/g dw), and LCCPs (960 ng/g dw). The calculated RSDs indicate that the variability of the APCI-qTOF-HRMS was low, and by using an injection standard, multiple injections were not needed. As expected at levels close to the LOQ (peak area around 200 counts), the RSD went up to a maximum of 46%, and decreased to 20% if the peak area increased 10 times and decreased to 5% if the peak area increased 100 times (SI Table S10). One sewage sludge sample (S12) was also extracted, cleaned, and analyzed in duplicate. Comparable levels were observed for this duplicate sample with SCCPs of <86 and <60 ng/g dw, MCCPs of 995 and 1016 ng/g dw, and LCCPs of 625 and 527 ng/g dw (Table 1).
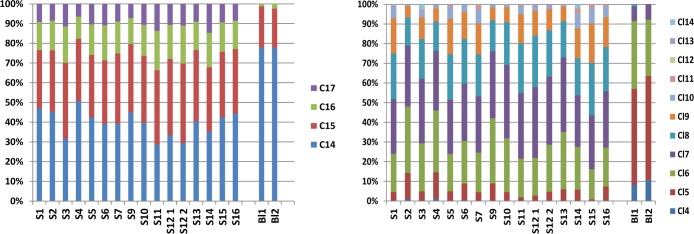
Regardless of the effort to reduce background contamination, aluminum oxide was heated to 250 °C; silica was prerinsed with dichloromethane and methanol before use; and all glassware was rinsed with both n-hexane and acetone prior to use, low CP levels were observed in the two blank samples (Table 1). The total CP levels observed in the two blanks were up to 16 ng absolute for the SCCPs, up to 24 ng absolute for the MCCPs, and up to 0.5 ng absolute for the LCCPs. Therefore, all CP values observed in the sewage sludge samples were blank corrected, and only the corrected levels higher than 3 times the blank were reported in this study. All MCCP and LCCP values observed in the 15 sewage sludge samples were at least 10 times higher than the CP values in the blank. For some SCCP values, the levels were lower than 3 times the blank and therefore reported as < LOQ. The CP patterns observed in the blanks were different from the patterns observed in the sewage sludge samples, which indicates that the contamination is not related to cross-contamination during sample treatment but more likely to another source(s) (Figures 3 and S1). The patterns in the blank were mainly dominated by the short chain CPs, C10, and C11 for the SCCPs and C14 for the MCCPs.
Figure 3.
Composition of MCCPs in the 15 sewage sludge samples and the two blanks (the composition of the SCCPs and LCCPs is given in SI Figure S1). Composition based on carbon number (left) or chlorine number (right).
The chlorination degree of the eight standard technical CP formulations we measured in this study were compared with the stated chlorination degree of the manufacturer (SI Table S2). For the higher chlorinated CP formulations, the calculated chlorination degree was close to the stated chlorination degree. However, for the lower chlorinated CP formulations, the calculated values were somewhat higher (SCCP; 51.5% Cl, calculated Cl 55.9%, MCCP; 42% Cl, calculated 49.4% Cl, LCCP; 36% Cl, calculated 41.4% Cl). Congener patterns of the eight technical CP mixtures are shown in SI Figures S2–S4.
Bogdal et al.,10 reported that the sensitivity of the APCI-qTOF-HRMS method increased with chain length and chlorination degree. The authors observed a differences in response by a factor of 50 between the SCCP 49% Cl and the SCCP 70% formulations; whereas the response of MCCP 45% Cl and MCCP 56% Cl formulations differed only by a factor 4. In our study we used the same analytical technique, but with different instrumental equipment and adapted instrumental setup conditions. The differences in sensitivity in our study was less depending on the chain length and chlorine degree. For example the differences in response between SCCP 51.5% Cl and SCCP 63% Cl was only a factor of 1.9, between MCCP 42% and MCCP 57% a factor 3.2, and between LCCP 36% Cl and LCCP 49% a factor of 1.2. This indicates that the sensitivity of the APCI-qTOF-HRMS method is influenced by the analytical set up and the instrumental equipment used.
Calibration standards were prepared in ACN from the eight CP stock solutions and ranged from 0.1 ng/μL to 10 ng/μL for the SCCPs and MCCPs and from 0.05 ng/μL to 10 ng/μL for the LCCPs. The linear range of the calibration curve were determined using the Excel linear regression tool. The linear range of the calibration curve for the SCCPs and MCCPs ranged from 0.1 to 10 ng/μL; whereas for the LCCP, it ranged from 0.05–5.0 ng/μL with an R2 > 0.99. For the SCCPs, the lowest concentration of the calibration curve, 0.1 ng/μL, corresponds to the LOQ (10 times the signal/noise), whereas for both the MCCPs and the LCCPs, the LOQ could easily be lowered by a factor of 4. However, all SCCPs and MCCPs concentrations detected in the sewage sludge samples was above 0.1 ng/μL and above 0.05 ng/μL for the LCCPs.
Results and Discussion
CP Concentrations in Australian Sewage Sludge
MCCPs were the dominant CPs and were detected in all sludge samples with concentrations ranging from 542 to 3645 ng/g dw (Table 1). In eight of the 15 samples SCCPs were detected with concentrations ranging from <57 to 1421 ng/g dw. The LCCPs concentrations (116–960 ng/g dw) were comparable to the SCCP concentrations (Table 1). Nine of the 15 LCCPs levels were reported as tentative values due to a deconvolution result with an R2 < 0.5. The results of the total CPs (sum of SCCPs, MCCPs, and LCCPs) detected in sewage sludge collected at the various WWTPs around Australia are illustrated in Figure 1. The highest total CP concentration (5880 ng/g dw) were detected in sewage sludge from location S4 in the Northern Territory of Australia. However, elevated levels were also detected in South-East Queensland (location S2; 4416 ng/g dw), South Australia (location S9; 4737 ng/g dw) and Western Australia (location S13; 4567 ng/g dw). The total CP concentrations were higher than the mean α-, β-, γ-HBCDD concentrations (0.11–129 ng/g) and mean BDE209 concentrations (67–1340 ng/g dw) detected in the same sewage sludge samples. BDE209 was the predominant PBDE and contributed to 61–88% of the ∑PBDE.16
To our knowledge, this is the first time that CPs were detected in Australian sewage sludge. The SCCP and MCCP concentrations observed in the Australian sewage sludge were comparable with the SCCP and MCCP concentrations observed in Swedish sewage sludge collected between 2004 and 2010 (median SCCPs; 1100 ng/g dw, median MCCPs; 3800 ng/g dw),17 Czech sludge samples collected in 2004 near a chemical company in Usti (SCCPs ranged from 205 to 396 ng/g dw, MCCPs ranged from 736 to 2301 ng/g dw),18 and Swiss sewage sludge collected in 2007 around Zürich (SCCP; 135–581 ng/g dw, MCCPs; 1070–8960 ng/g dw).10 Maulshagen et al.19 measured only SCCPs (75–859 ng/g dw) in sewage sludge from Germany collected in the early 2000s, which were also comparable with the SCCP concentrations observed in our study. Higher SCCP concentrations were found in municipal and industrial sewage sludge in China with concentrations from 800 to 52 700 ng/g dw.15 The highest CP concentrations were found in municipal and industrial sewage sludge from the UK reported by Stevens el al.,5 with SCCP concentrations ranging from 6900 to 200 000 ng/g dw and MCCPs concentrations from 30 to 9700 ng/g and by Nicholls et al.,20 with sum SCCP and MCCPs concentrations ranged from 1800 to 93 100 ng/g dw. These concentrations were up to 200-fold higher than those reported in our study.
Data about the longer chain CPs in sewage sludge is limited. Only Olofsson et al.17 has reported LCCPs in sewage sludge samples from Sweden with median concentrations of 31 000 ng/g dw. The LCCP concentrations were 8-fold higher than the median MCCPs reported in that study and 28-fold higher than the median SCCPs in that study. These concentrations are 300-fold higher than the LCCP concentrations observed in Australian sewage sludge in this study. This may indicate a higher usage and demand of CPs in Sweden compared to Australia. Other reasons for the difference could be a different composition of the technical CP mixtures, or a different treatment of WWTP system.
Despite the huge challenges and uncertainties in quantification of CPs, the order of magnitude differences observed indicate that point sources of CPs can be very important. In two studies,5,15 no correlation was observed between the CP concentration and the WWTP location, treatment capacity and serving population. In our study the correlation between the CP concentrations in the sewage sludge samples and the WWTP size (population), geographical location (Southern Queensland versus Northern Queensland) and sewage sludge treatment (anaerobic versus aerobic/activated) was investigated by comparing the CP concentrations (multiple t test). Taken into account the relative small data set, only a significant higher mean CP concentration was observed in the anaerobic treated sewage sludge compared to the aerobic/activated sludge treatment (p < 0.05). However, other factors may have influenced this result; as for example, all anaerobic WWTPs were of large size. A more extensive study is needed to draw more solid conclusions regarding CP concentrations and WWTP characteristics.
Carbon and Chlorine Homologue Groups of CPs in Sewage Sludge
The carbon and chlorine homologue groups for the SCCPs, MCCPs and LCCPs in the Australian sewage sludge are given in Figure 3 and SI Figure S1. Among the sewage sludge samples collected at different locations in Australia, similar carbon and chlorine homologue patterns were observed for the SCCPs, MCCPs, and LCCPs. The calculated chlorination degree for the sewage sludge ranged from 57.8 to 63% for the SCCPs, from 53.5 to 57.5% for the MCCPs, and from 39.9 to 48.3% for the LCCPs. This finding was consistent with the study of Zeng et al.,15 who studied the SCCP composition in various sewage sludge samples from China and observed similar carbon and chlorine homologue profiles among different WWTPs. However, the SCCP homologue pattern observed in the sewage sludge from China was different than the pattern observed in the Australian sewage sludge. In the Chinese sludge, the SCCP homologue groups were dominated by C11 (37%) followed by C10 (27%) and C12 (23%) with Cl7 (37%) and Cl8 (29%), whereas in our study the SCCP homologue groups were dominated by C13 (51%) followed by C12 (24%) and C11 (18%) with Cl7 (37%) and Cl6 (26%). Taking into account the different instrumental techniques used, these findings may indicate that the CP composition of the commercial SCCP mixtures used in China are different than used in Australia. CPs in China are not divided in SCCPs, MCCPs, and LCCPs, but into different technical products. The most common technical products are CP-42 (42% Cl), CP-52 (52% Cl), and CP-70 (70% Cl). CP-42 and CP-52 are the most widely produced in China (over 80%) in 2005 and contain apparently 3–4% and 25–40% SCCPs, respectively.21,22 Zeng et al.15 only detected some MCCPs in sewage sludge from China, and these were at low concentrations, which is in contrast to our study where MCCPs were dominant. This may indicate that MCCPs are probably used in lesser amounts in China compared to Australia. However, in soil and sediment of highly industrial areas in China (Pearl River Delta), higher MCCP levels were observed compared to SCCPs, and the opposite was observed across more rural areas.23,24 This, together with an increased MCCPs/SCCPs ratio in a sediment core in time, suggests an increased usage to MCCPs in recent years in the Pearl River Delta.24 Overall, the carbon homologue group patterns for the SCCPs and MCCPs in the Australian sewage sludge were more comparable with the patterns found in sewage sludge from the UK and Switzerland5,10 which were also dominated by C11–C13 for the SCCPs and C14–C15 for the MCCPs.
Until today, LCCPs were only reported by Olofsson et al.17 in Swedish sewage sludge as total LCCP concentration (median of 31 000 ng/g dw) and by Bogdal et al.10 in sewage sludge form Zürich in Switzerland with tentative levels varying from 17.4–98.7 ng/g dw. The total LCCP levels reported by Bogdal et al.10 were only based on the C19–C27 congener groups. The C18 was excluded by Bogdal et al.10 after studying the complete C10-C27 pattern whereby the technical MCCP (52% Cl) formulation contained up to 10% of C18 of the LCCP congener groups. They observed that after subtracting the C18 congener group, the remaining pattern in the sewage sludge was more comparable with the pattern in the technical LCCP formulations (40, 49, and 70% Cl), which would improve the deconvolution process. In both standard technical LCCP mixtures (36% Cl and 49% Cl) used in this study, C18 was the most dominant congener group (see SI Figure S4). Also the technical standard MCCP mixtures (42%, 52%, and 57% Cl) used in our study contain only up to 1.3% LCCPs as an impurity. Therefore, LCCPs could be calculated through deconvolution using only two standard LCCP technical mixtures (36% Cl and 49% Cl) instead of three for the SCCPs and MCCPs (Figure 2). LCCP homologue groups in the Australian sewage sludge were dominated by C18 (30%) followed by C19 (13%) and C20 (10%), with Cl7 (22%), Cl6 (18%), Cl8 (17%), and Cl4, Cl5, Cl9 (each 11%). The only two exceptions were sewage sludge sample S4 from the Northern Territory of Australia, which had a pattern dominated by C27 (45%), and sewage sludge sample S12 from South Australia, which was dominated by C23–C26. No clear explanation could be given, but it may be related to local sources. The LCCPs technical mixtures contain mainly C18–C20 and almost no long chain LCCPs (C21–C27). Therefore, samples which are dominated by the longer chain LCCPs (C21–C27), cannot be entirely reconstructed by the LCCP technical mixtures. This explains the low R2 calculated between the measured and reconstructed pattern for the sewage sludge samples S4 and S12, which were dominated with the longer chain LCCPs. For both samples the LCCP levels are reported as tentative levels.
This is the first study that applies the promising and particularly fast instrumental technique recently develop by Bogdal et al.,10 routinely for simultaneously quantifying and profiling SCCPs, MCCPs, and LCCPs in Australian sewage sludge. Due the toxicity, bioaccumulation potency and persistency SCCPs are already regulated and phased-out in Europe and targeted by the Stockholm Convention.25,26 However, LCCPs and MCCPs are hardly regulated and the knowledge about them is poor. First studies show that the MCCP/SCCP ratios in sediment, soil, and biota increased in recent years, which may be influenced by the replacement of SCCPs by MCCPs.23,24,27 However, data on MCCPs and LCCPs in the environment is rare, and more research is needed to further understand the risk and fate of CPs in the environment. Especially, because more than 59% of all the sewage sludge in Australia is reused in agriculture and through landfilling, the CPs re-enter into the environment.16
Acknowledgments
We gratefully acknowledge the European Commission as the work was part of the EU-funded PEOPLE-2011 IRSES INTERFLAME Project (PIRSES-GA-2011-295138).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.6b05318.
Additional information on materials, sample collection, the participating WWTPs (Table S1), calculated chlorination degree for the eight technical CP mixtures (Table S2), recovery and spike experiments (Tables S3 to S8), results of the 8-fold measurement of a cleaned sewage sludge sample (Table S9), list of m/z ratios considered for CPs (Table S10), composition profile of the CPs in the sewage sludge samples (Figure S1), congener pattern of the CPs in the technical mixtures (Figures S2–S4), example of the measured and deconvoluted congener pattern (Figures S5–S7) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- WCC, 2014. WCC World Chlorine Council International Chlorinated Alkanes Industry Association (ICAIA) newsletter. World Chlorine Council (2014), http://www.eurochlor.org/media/88258/20140908_icaia_newsletter_03_final.pdf.
- Wei G. L.; Liang X. L.; Li D. O.; Zhuo M. N.; Zhang S. Y.; Huang O.X.; Liao Y. S.; Xie Z. Y.; Guo T. L.; Yuan Z. J. Occurrence, fate and ecological risk of chlorinated paraffins in Asia: A review. Environ. Int. 2016, 92–93, 373–387. 10.1016/j.envint.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Ma X.; Wang Z.; Yao Z.; Chen J.; Chen J. Bioaccumulation and trophic transfer of short chain chlorinated paraffins in a marine food web from liaodong bay, north china. Environ. Sci. Technol. 2014, 48, 5964–5971. 10.1021/es500940p. [DOI] [PubMed] [Google Scholar]
- Zeng L.; Wang T.; Wang P.; Liu Q.; Han S.; Yuan B.; Zhu N.; Wang Y.; Jiang G. Distribution and trophic transfer of short-chain chlorinated paraffins in an aquatic ecosystem receiving effluents from a sewage treatment plant. Environ. Sci. Technol. 2011, 45, 5529–5535. 10.1021/es200895b. [DOI] [PubMed] [Google Scholar]
- Stevens J. L.; Northcott G. L.; Stern G. A.; Tomy G. T.; Jones K. C. PAHs, PCBs, PCNs, organochlorine pesticides, synthetic musks, and polychlorinated n-alkanes in UK sewage sludge: survey results and implications. Environ. Sci. Technol. 2003, 37, 462–467. 10.1021/es020161y. [DOI] [PubMed] [Google Scholar]
- Van Mourik L. M.; Gaus C.; Leonards P. E. G.; de Boer J. Chlorinated paraffins in the environment: A review on their production, fate, levels and trends between 2010 and 2015. Chemosphere 2016, 155 (2016), 415–428. 10.1016/j.chemosphere.2016.04.037. [DOI] [PubMed] [Google Scholar]
- Shaw S. D.; Blum A.; Weber R.; Kannan K.; Rich D.; Lucas D.; Koshland C. P.; Dobraca D.; Hanson S.; Birnbaum L. S. Halogenated flame retardants: Do the fire safety benefits justify the risks? Rev. Rev. Environ. Health 2010, 25, 261–305. 10.1515/REVEH.2010.25.4.261. [DOI] [PubMed] [Google Scholar]
- El-Sayed T.; Legler. Overview of the Mammalian and Environmental Toxicity of Chlorinated Paraffins. In Chlorinated Paraffins de Boer J., Ed.; 2010; pp 135–154. [Google Scholar]
- ESWI. ESWI Study on Waste Related Issues of Newly Listed POPs and Candidate POPs (2011) Consortium ESWI (Bipro, Umweltbundesamt and Enviroplan) for the European Commission, 2011.
- Bogdal C.; Alsberg T.; Diefenbacher P. S.; MacLeod M.; Berger U. Fast quantification of chlorinated paraffins in environmental samples by direct injection high-resolution mass spectrometry with pattern deconvolution. Anal. Chem. 2015, 87, 2852–2860. 10.1021/ac504444d. [DOI] [PubMed] [Google Scholar]
- Gillett R. W.; Keywood M. D.; Galbally I. E.. Indoor air project: persistent organic pollutants and metals. A Report to the Air Quality Section, Environment Standards Branch, Department of the Environment, Water, Heritage and the Arts Commonwealth of Australia. Australian Bureau of Meteorology and CSIRO Marine and Atmospheric Research, 2010. [Google Scholar]
- Kemmlein S.; Hermeneit A.; Rotard W. Carbon skeleton analysis of chloroparaffins in sediment, mussels and crabs. Organohalogen Compd. 2002, 59, 279–282. [Google Scholar]
- Orica, 2015. Melbourne. http://www.orica-watercare.com/?page=10http://www.orica-watercare.com/?page=10.
- NICNAS. Environmental exposure assessment of short chain chlorinated paraffins (SCCPs) in Australia. National Industrial Chemicals Notification and Assesment Scheme Report; Department of Health and Ageing: Canberra, 2004. [Google Scholar]
- Zeng L.; Wang T.; Ruan T.; Liu Q.; Wang Y.; Jiang G. Levels and distribution patterns of short chain chlorinated paraffins in sewage sludge of wastewater treatment plants in China. Environ. Pollut. 2012, 160, 88–94. 10.1016/j.envpol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Gallen C.; Drage D.; Kaserzon S.; Baduel C.; Gallen M.; Banks A.; Broomhall S.; Mueller J. F. Occurrence and distribution of brominated flame retardants and perfluoroalkyl substances in Australian landfill leachate and biosolids. J. Hazard. Mater. 2016, 313, 55–64. 10.1016/j.jhazmat.2016.03.031. [DOI] [PubMed] [Google Scholar]
- Olofsson U.; Bignert A.; Haglund P. Time-trends of metals and organic contaminants in sewage sludge. Water Res. 2012, 46, 4841–4851. 10.1016/j.watres.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Přibylová P.; Klánová J.; Holoubek I. Screening of short- and medium-chain chlorinated paraffins in selected riverine sediments and sludge from the Czech Republic. Environ. Pollut. 2006, 144, 248–254. 10.1016/j.envpol.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Maulshagen A.; Hamm S.; Petersen M.; Elsholz O.; Fengler S.; Seel P. Analysis of Short-chain Chlorinated Paraffins (C10-C13) in German River Suspended Particulate Matter, Sewage Sludge and Industrial Sludge Samples. Organohalogen Compd. 2003, 62, 371–374. [Google Scholar]
- Nicholls C. R.; Allchin C. R.; Law R. J. Levels of short and median chain length polychlorinated n-alkanes in environmental samples from selected industrial areas in England and Wales. Environ. Pollut. 2001, 114, 415–430. 10.1016/S0269-7491(00)00230-X. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Zhang H.; Su F.; Tian Y.; Chen J. Environmental occurrence and distribution of short chain chlorinated paraffins in sediments and soils from the Liaohe river basin, PR China. Environ. Sci. Technol. 2012, 46, 3771–3778. 10.1021/es2041256. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Zhang H.; Zou L.; Wu P.; Yu Z.; Lu X.; Chen J. Quantification of short-chain chlorinated paraffins by deuterodechlorination combined with gas chromatography-mass spectrometry. Environ. Sci. Technol. 2016, 50, 3746–3753. 10.1021/acs.est.5b05115. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li J.; Cheng Z.; Li Q.; Pan X.; Zhang R.; Liu D.; Luo C.; Liu X.; Katsoyiannis A.; Zhang G. Short- and medium-chain chlorinated paraffins in air and soil of subtropical terrestrial environment in the Pearl River Delta, South China: distribution, composition, atmospheric deposition fluxes, and environmental fate. Environ. Sci. Technol. 2013, 47, 2679–2687. 10.1021/es304425r. [DOI] [PubMed] [Google Scholar]
- Chen M. Y.; Luo X. J.; Zhang X. L.; He M. J.; Chen S. J.; Mai B.X. Chlorinated paraffins in sediments from the Pearl River Delta, South China: spatial and temporal distributions and implication for processes. Environ. Sci. Technol. 2011, 45, 9936–9943. 10.1021/es202891a. [DOI] [PubMed] [Google Scholar]
- EC, 2011. European Commission, European Union Risk Assessment Report: Alkanes, C14–17, chloro - Addendum to the final report (2007) of the risk assessment - Part I - Environment part. http://publications.jrc.ec.europa.eu/repository/bitstream/JRC63780/lbnb21640enn.pdf.
- UNEP, 2007. United Nations Environment Programme, Stockholm Convention on Persistent Organic Pollutants, Draft risk profile for short-chained chlorinated paraffins. United Nations Environment Programme; http://www.pops.int/documents/meetings/poprc/drprofile/drp/DraftRiskProfile_SCCP.pdf. [Google Scholar]
- Zeng L.; Lam J. C. W.; Wang Y.; Jiang G.; Lam P. K. S. Temporal trends and pattern changes of short and medium chain chlorinated paraffins in marine mammals from the South China Sea over the past decade. Environ. Sci. Technol. 2015, 49, 11348–11355. 10.1021/acs.est.5b02473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.