Figure 4.
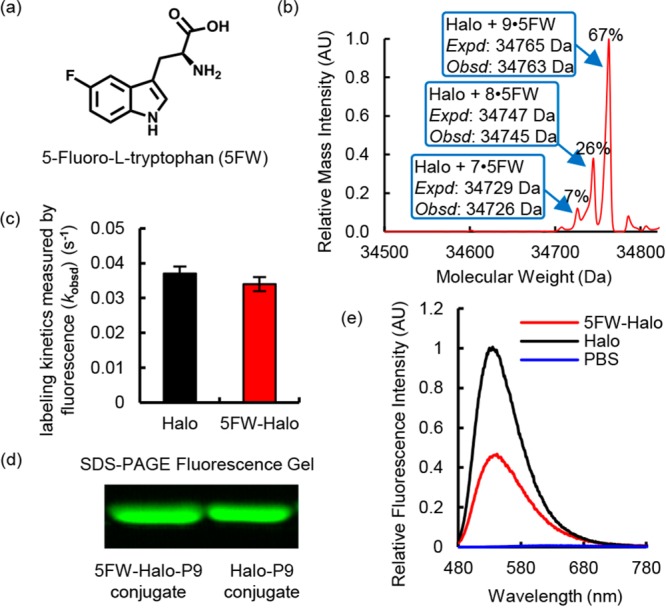
Incorporation of 5-fluorotryptophan (5FW) reduced the fluorescence intensity of the P9–Halo conjugate. (a) Molecular structure of 5FW. (b) Mass spectroscopic evidence of incorporation of 5FW into the Halo protein (5FW-Halo). (c) 5FW-Halo reacts with P9 at an observed rate similar to that observed with wild-type Halo. The reaction mixture contained 10 μM P9 and 30 μM Halo or 5FW-Halo in DPBS buffer. The labeling reaction was monitored at 450 nm excitation and 530 nm emission at 25 °C. (d) 5FW-Halo can be covalently labeled with P9, like wild-type Halo. The labeling reaction was performed using 10 μM P9 and 30 μM Halo or 5FW-Halo for 10 min in DPBS buffer at 25 °C. The Halo–P9 conjugates were visualized on an SDS–PAGE gel using the Bio-Rad Gel Doc EZ imager. (e) The 5FW-Halo–P9 conjugate (red) exhibits an ∼50% decrease in fluorescence intensity, compared to that of the wild-type Halo–P9 conjugate (black). The samples contain 30 μM protein incubated with 10 μM P9 in DPBS buffer at 25 °C for 1 h. Fluorescence emission spectra were recorded at 450 nm excitation.
