Abstract
Purpose
Perioperative chemotherapy improves survival outcomes in locally advanced (LA) gastric cancer.
Materials and Methods
We retrospectively analyzed patients with LA gastric cancer who were offered perioperative chemotherapy consisting of epirubicin, oxaliplatin, and capecitabine (EOX) from May 2013 to December 2015 at Tata Memorial Hospital in Mumbai.
Results
Among the 268 consecutive patients in our study, 260 patients (97.0%) completed neoadjuvant chemotherapy, 200 patients (74.6%) underwent D2 lymphadenectomy, and 178 patients (66.4%) completed adjuvant chemotherapy. The median follow-up period was 17 months. For the entire cohort, the median overall survival (OS), 3-year OS rate, median progression-free survival (PFS), and 3-year PFS rate were 37 months, 64.4%, 31 months, and 40%, respectively. PFS and OS were significantly inferior in patients who presented with features of obstruction than in those who did not (P=0.0001). There was no difference in survival with respect to tumor histology (well to moderately differentiated vs. poorly differentiated, signet ring vs. non-signet ring histology) or location (proximal vs. distal). Survival was prolonged in patients with an early pathological T stage and a pathological node-negative status. In a multivariate analysis, postoperative pathological nodal status and gastric outlet obstruction on presentation significantly correlated with survival.
Conclusions
EOX chemotherapy with curative resection and D2 lymphadenectomy is a suggested alternative to the existing perioperative regimens. The acceptable postoperative complication rate and relatively high resection, chemotherapy completion, and survival rates obtained in this study require further evaluation and validation in a clinical trial.
Keywords: Epirubicin, Oxaliplatin, Capecitabine, Stomach neoplasms, Resection, Gastrectomy, Lymph node, Involved
INTRODUCTION
Two-thirds of gastric cancers are locally advanced (LA) at presentation, with initially guarded outcomes following unimodality surgical management [1,2,3,4,5,6]. To improve outcomes and evaluate the usefulness of perioperative chemotherapy, several randomized control trials were conducted in the last 15 years, thereby changing the management paradigm for LA gastric cancer. The phase III Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) and French Fédération Nationale des Centres de Lutte Contre le Cancer and the Fédération Francophone de Cancérologie Digestive trial (FNCLCC/FFCD) European trials conclusively showed benefits with perioperative chemotherapy, as did the US Intergroup (INT)-116 study, the Adjuvant Capecitabine and Oxaliplatin for Gastric Cancer After D2 Gastrectomy (CLASSIC) and the Adjuvant Chemoradiotherapy in Stomach Tumors (ARTIST) trials in Southeast Asia, which examined chemotherapy and chemoradiotherapy in the adjuvant setting [7,8,9,10,11]. Although perioperative therapy is now the standard treatment for LA gastric cancer, its advantages in patients undergoing D2 gastrectomy are unclear, as are those of radiotherapy. Most high-volume centers in Southeast Asia routinely perform D2 gastrectomy for LA gastric cancer, whereas only 10% to 40% of patients with this disease undergo this procedure in Western countries [7,9,12].
The infusional epirubicin, cisplatin, and 5-fluorouracil (ECF) protocol used in the MAGIC trial has requirements that limit its use in non-trial situations (e.g., the need for a central line and constant specialized handling) and poor completion rates (42%) [7]. An alternative regimen that may overcome these issues is epirubicin, oxaliplatin, and capecitabine (EOX), in which oxaliplatin and capecitabine replace cisplatin and 5-fluorouracil, respectively. The REAL 2 analysis showed that the ECF and EOX regimens were equally effective, albeit for advanced tumors, whereas a meta-analysis of the data from the REAL 2 and ML17032 trials suggested better response rates and overall survival (OS) rates with capecitabine combinations [13,14,15,16]. These encouraging data for EOX, coupled with the ease of EOX administration as performed at our center [17], support the use of EOX for treatment of LA gastric cancer in the perioperative setting, thereby maximize its benefits. The analysis presented herein describes the treatment patterns and outcomes of patients with LA gastric cancer who received EOX at the Tata Memorial Hospital (TMH), a tertiary cancer center.
MATERIALS AND METHODS
The study is a retrospective analysis of patients with LA gastric cancer who were offered perioperative chemotherapy (neoadjuvant plus adjuvant) with EOX from May 2013 to December 2015 in the Department of Medical Oncology at TMH in Mumbai. These patients were extracted from a prospectively maintained stomach cancer database at TMH. Those included in the study satisfied the following criteria:
T3/T4 and/or node-positive, as determined by radiological assessment and the multidisciplinary joint clinic (MDJC)
No evidence of metastatic disease, as determined by staging laparoscopy (to rule out peritoneal metastases and palpable liver nodules)
Eastern Cooperative Oncology Group performance status 0–2
All patients were assessed and optimized by the nutrition clinic department. Standard doses of epirubicin (50 mg/m2, day 1), oxaliplatin (130 mg/m2, day 1), and capecitabine (625 mg/m2, orally, twice daily, days 1–21) at 3-week intervals were administered.
Toxicity was assessed at all patient visits and recorded in accordance with the Common Terminology Criteria for Adverse Events, version 4.03, of the National Cancer Institute [18]. Response to treatment was evaluated via mapping endoscopy and contrast-enhanced computed tomography after 3 cycles of neoadjuvant chemotherapy (NACT). All visits included documentation of the patient's medical history and a physical examination.
Additional management strategies after NACT including the extent of surgery were discussed by members of the MDJC. Resection was performed by 4 surgeons trained in D2 lymphadenectomy and consisted of subtotal or total gastrectomy with extension depending on the location of the primary tumor along with D2 lymphadenectomy. To completely remove the primary tumor and any suspicious lymph nodes, nearby organs or tissues were also removed. The gastrointestinal passage was reconstructed in accordance with our institutional guidelines. Patients undergoing surgery received adjuvant chemotherapy as previously planned. Patients whose tumors were considered unresectable continued receiving chemotherapy or received radiotherapy with or without concurrent chemotherapy with a palliative intent.
As this is a retrospective audit of charts and records, no ethics committee clearance was required.
Clinical data collection and statistics
Demographic data, baseline clinical and tumor characteristics, chemotherapy regimens, surgical procedures, and outcomes were collected retrospectively from charts maintained prospectively using the Gastrointestinal Medical Oncology information system and the electronic medical record system. Clinical and radiological data were obtained from the patient's hospital files and electronic medical records. All data were analyzed by using IBM SPSS Statistics ver. 21 (IBM Co., Armonk, NY, USA). Descriptive statistics were used to compare categorical variables such as age, sex, treatment, and response to treatment, which were expressed as median values or percentages. Survival outcomes in terms of progression-free survival (PFS) and OS were determined. PFS extended from the date of diagnosis to the date of clinical or radiological evidence of disease progression or the last follow-up. OS extended from the date of diagnosis to the date of the last follow up or death. The survival analysis was performed by using Kaplan-Meier estimates and the log-rank test for bivariate comparisons.
RESULTS
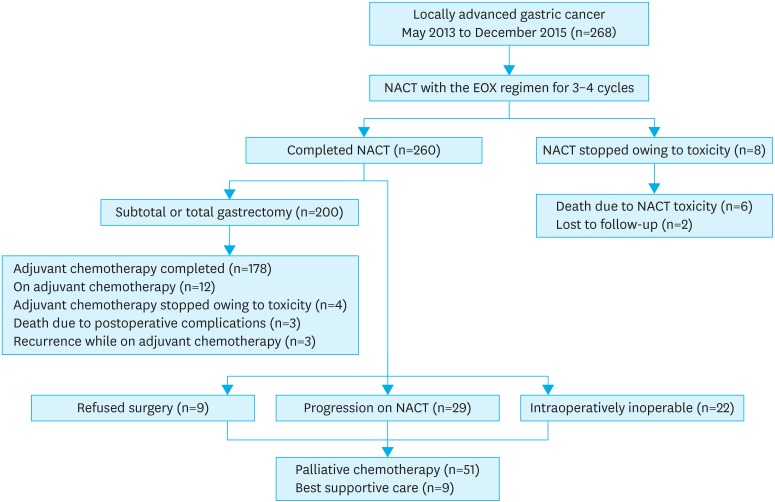
Two hundred sixty-eight consecutive patients (Fig. 1) with LA gastric cancer received NACT at TMH between May 2013 and December 2015. The median age at diagnosis was 54 years (21–80 years). Table 1 summarizes the baseline patient characteristics.
Fig. 1.
Outline of study.
NACT = neoadjuvant chemotherapy; EOX = epirubicin, oxaliplatin, and capecitabine.
Table 1. Baseline patient characteristics.
| Characteristic | Total (n=268) | |
|---|---|---|
| Age (yr) | Median | 54 |
| Range | 21–80 | |
| Sex | Female | 71 (26.5) |
| Male | 197 (73.5) | |
| ECOG PS | 0, 1 | 260 (97.0) |
| 2 | 8 (3.0) | |
| Disease site | Proximal (GEJ, cardia, fundus) | 79 (29.5) |
| Body | 65 (24.3) | |
| Distal (antral and antropyloric) | 124 (46.3) | |
| Gastric outlet obstruction | Present | 73 (27.2) |
| Absent | 195 (72.8) | |
| Histology | WD/MD adenocarcinoma | 82 (30.6) |
| PD adenocarcinoma | 100 (37.3) | |
| Signet ring adenocarcinoma | 86 (32.1) | |
Values are presented as median, range, or number (%).
ECOG PS = Eastern Cooperative Oncology Group Performance Status; GEJ = gastro-esophageal junction; WD/MD = well differentiated/moderately differentiated; PD = poorly differentiated.
NACT and responses
Among the 268 patients who received NACT with EOX, 260 patients (97.0%) completed the treatment. Owing to NACT-related toxicity, treatment was discontinued in 8 patients (3.0%), 6 patients of whom (6/268, 2.2%) died with adverse events during NACT. The median number of NACT cycles received was 3 (Table 2).
Table 2. NACT, adjuvant chemotherapy, and toxicity.
| Variable | No. (%) of patients | ||
|---|---|---|---|
| NACT | Adjuvant | ||
| Chemotherapy | |||
| Median number of cycles | 3 | 3 | |
| Dose reductions required | 32/268 (11.9) | 20/268 (7.4) | |
| Toxicity (grade 3 or 4*) | |||
| Diarrhea | 30/268 (11.2) | 6/200 (3.0) | |
| Vomiting | 16/268 (6.0) | 5/200 (2.5) | |
| Hand-foot syndrome | 11/268 (4.1) | 3/200 (1.5) | |
| Mucositis | 5/268 (1.9) | 0/200 (0.0) | |
| Neutropenia | 8/268 (3.0) | 2/200 (1.0) | |
| Thrombocytopenia | 7/268 (2.6) | 1/200 (0.5) | |
| Anemia | 2/268 (0.7) | 0/200 (0.0) | |
| Febrile neutropenia | 2/268 (0.7) | 0/200 (0.0) | |
| Neuropathy | 2/268 (0.7) | 0/200 (0.0) | |
| Hyponatremia | 3/268 (1.1) | 1/200 (0.5) | |
| Non-neutropenic sepsis | 3/268 (1.1) | 0/200 (0.0) | |
Values are presented as median or number (%).
NACT = neoadjuvant chemotherapy.
*Classification according to the Common Terminology Criteria for Adverse Events (CTCAE) 4.03_2010-06-14.
Surgery and complications
Among the 260 patients who completed NACT, 222 patients underwent exploratory surgery. Two hundred patients underwent resection (resection rate, 74.6%, 200/268); R0 resection was achieved in 186 patients (69.4%) and R+ resection in 14 patients (5.2%). The tumors in 22 of the patients (8.2%) were deemed unresectable intraoperatively, and all attempts at resection were abandoned for the mutiple reasons. These reasons were intra-operative peritoneal disease, poor response to chemotherapy with status quo, with involvement of the pancreatic head or D2 (second part of the duodenum) requiring Whipple's surgery, nodal involvement requiring more than D2 resection which was missed on the post-NACT imaging, or intra-operative liver lesions those were positive on frozen sections. Exploratory surgery was not performed in 29 patients (10.8%) owing to disease progression during NACT. In addition, 9 patients (3.4%) refused surgery. Hence, 38 patients (14.2%) did not undergo exploratory surgery. In patients undergoing surgery, postoperative complications occurred in 20 patients (7.5%), whereas, mortality was noted in 3 patients (1.1%). The postoperative complications are listed in Table 3.
Table 3. Postoperative complications.
| Complications | Value |
|---|---|
| Postoperative complications | 20 (7.5) |
| Mortalities due to postoperative complications | 3 (1.1) |
| Duodenal leak | 1 |
| Bile leak | 1 |
| Stump bleed | 1 |
| Chyle leak | 1 |
| Wound infection | 5 |
| Post-operative hypotension | 3 |
| Splenic abscess | 1 |
| Hematemesis | 1 |
| Intra-abdominal bleeding | 1 |
| Peritonitis | 2 |
| Intra-abdominal collection | 1 |
| Postoperative pneumonia | 2 |
| Wound dehiscence | 1 |
| Liver failure | 1 |
| Thyrotoxic crisis | 1 |
| Intussusceptions | 1 |
Values are presented as number (%) or number only.
Pathological complete responses were seen in 13 of the patients (4.9%). The median tumor regression grade (TRG) was 3 (Supplementary Table 1). One hundred seventy-eight patients (66.4%) completed NACT, surgery, and adjuvant chemotherapy, while 12 patients (4.5%) were still receiving adjuvant chemotherapy on the cut-off date for the analysis. In the 200 patients who underwent curative surgery after NACT, the median number of dissected nodes was 20 (range, 5–67) and the median number of positive nodes was 1 (range, 0–29). Among the 60 patients who did not undergo resection, 51 patients (19.0%) continued to receive palliative chemotherapy, while 9 patients (3.4%) received best supportive care.
Survival analysis
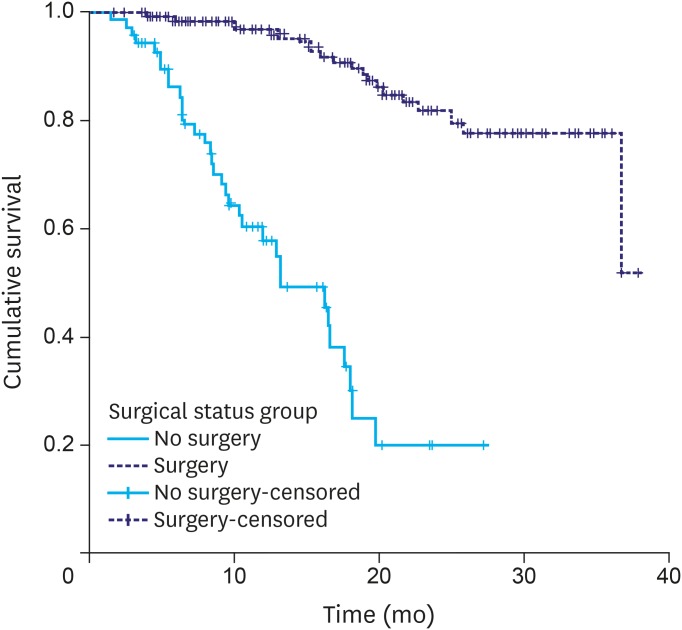
The median follow-up time was 17 months. The median OS time was 37 months for the entire cohort, and the 3-year OS rate was 64.4%. OS was significantly better in patients who underwent surgical resection vs. those deemed inoperable after NACT or who refused surgery (median OS time, not reached vs. 13 months; 3-year OS rate, 77.4% vs. 20.0%; P<0.001) (Fig. 2). OS was significantly worse in patients presenting with features of obstruction vs. those without such features (median OS time, 27.8 months vs. not reached; 3-year OS rate, 72.9% vs. 43.9%; P=0.0001). There was no difference in OS with respect to tumor histology (well and moderately differentiated vs. poorly differentiated, signet ring histology vs. non-signet ring histology) or location (proximal vs. distal). For the purpose of analysis, tumors in the body of stomach were considered to be proximal tumors.
Fig. 2.
Overall survival: surgery vs. no surgery.
OS was significantly better in patients with early-T stage tumors (post-chemotherapy pathological stage [yp]T1 or ypT2) and a pathological node-negative status (Table 4). Three-year OS rates were better in patients with a TRG of 1–3 than in those with a TRG of 4–5, but this difference was not significant. In a multivariate analysis, pathological nodal status and obstruction correlated significantly with survival.
Table 4. Subgroup comparisons of OS and PFS.
| Variable | OS | P-value | PFS | P-value | |||
|---|---|---|---|---|---|---|---|
| Median OS | 3-yr OS | Median PFS | 3-yr PFS | ||||
| Pathological T stage* | 0.04 | 0.02 | |||||
| ypT0–2 | Not reached | 97.4 | Not reached | 82.6 | |||
| ypT3–4 | 36 mo | 72.5 | 35 mo | 45.4 | |||
| Pathological node stage | 0.01 | 0.01 | |||||
| ypNode + | 36 mo | 67.0 | 35 mo | 54.0 | |||
| ypNode − | Not reached | 90.7 | Not reached | 81.0 | |||
| TRG | 0.23 | 0.44 | |||||
| 1–3 | Not reached | 86.8 | Not reached | 62.8 | |||
| 4–5 | Not reached | 68.8 | Not reached | 60.8 | |||
Values are presented as percentage.
OS = overall survival; PFS = progression-free survival; yp = post chemotherapy pathological stage; TRG = tumor regression grade.
*Classification according to the Union for International Cancer Control/American Joint Committee on Cancer 7th edition.
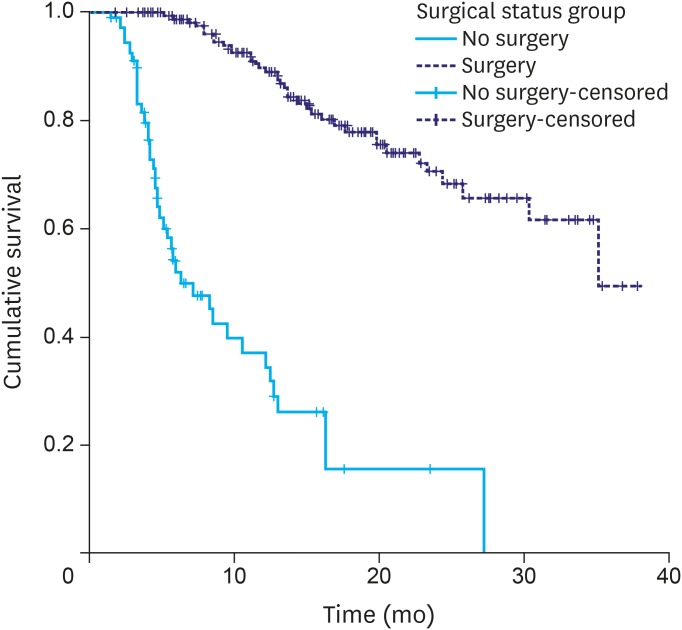
The median PFS time for the entire cohort was 31 months, and the 3-year PFS rate was 40%. Patients who underwent surgery had a significantly longer median PFS time than did those who did not (not reached vs. 6.4 months; P<0.001)(Fig. 3). PFS was unaffected by the location or histology of the tumor. Patients without features of gastric outlet obstruction at presentation had a significantly longer median PFS than did those with such features (not reached vs. 16.3 months; P<0.001).
Fig. 3.
Progression-free survival: surgery vs. no surgery.
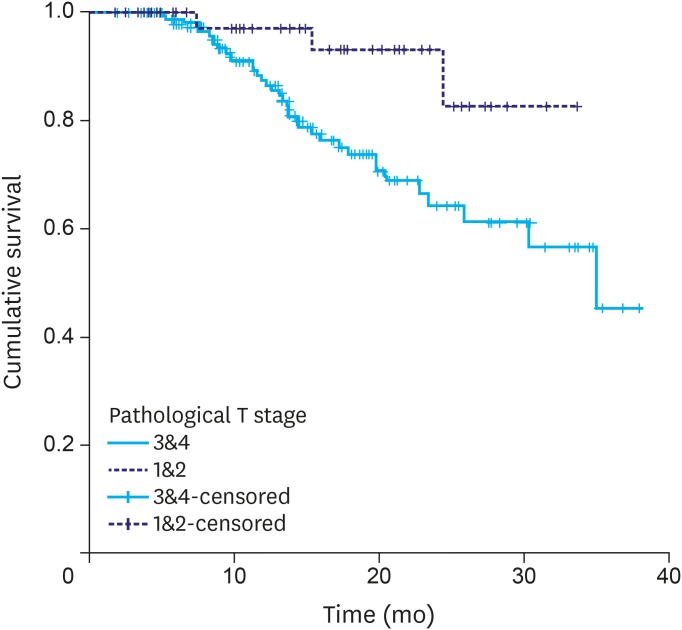
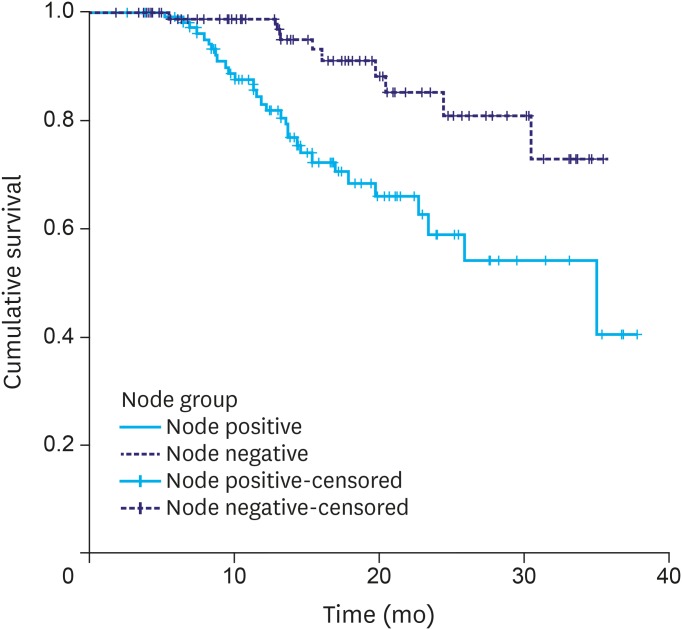
PFS differed significantly according to pathological T stage (pT0–2 vs. pT3–4) and pathological node status (Figs. 4, 5, and Table 3). It was unaffected by the TRG.
Fig. 4.
Progression-free survival: pathological T staging.
Fig. 5.
Progression-free survival: pathological nodal status.
Toxicity analysis
Grade 3 or 4 toxicity was observed in only 58 of the patients (21.6%) during NACT. Grade 3–4 diarrhea was the most common post-NACT adverse event, occurring in 30 of the patients (11.1%). The toxicity profiles are shown in Table 2. NACT was discontinued in 8 patients (3.0%) owing to NACT-related toxicity. NACT-related toxicity resulted in 6 deaths; 3 patients died while receiving NACT, and 3 patients died after completing 3 cycles of NACT. Doses were modified during NACT in 32 of the patients (11.9%) owing to poor tolerance or toxicity (Table 2). Doses were modified during adjuvant chemotherapy in 20 of the patients (7.4%), and adjuvant chemotherapy was terminated in 4 of the patients (1.5%) owing to life-threatening toxicity.
Delay in adjuvant therapy
The median time between the day of surgery and the initiation of adjuvant chemotherapy was 20 days. The 3-year PFS rate was significantly lower when the delay was >3 weeks after surgery vs. ≤3 weeks after surgery (55% vs. 65%; P=0.049). The 3-year OS rate was also inferior, although not significantly so, when the delay was >3 weeks vs. ≤3 weeks (62% vs. 83%; P=0.099).
Relapse patterns
Tumors recurred in 39 of the patients (19.5%). Recurrence was localized in 4 of the patients (10.3%) and systemic in 35 of the patients (89.7%). Peritoneal involvement was the most common site of recurrence, occurring in 21 of the patients (53.8%).
DISCUSSION
Perioperative therapy is now the standard of care for gastric cancer, despite ongoing discussion about the usefulness of external beam radiation therapy and the sequencing of therapy. Treatment strategies vary according to region owing to region-specific differences in disease incidence, tumor histology (diffuse vs. intestinal), tumor location (proximal vs. distal vs. the gastroesophageal junction), surgical approach (D1 vs. D2 gastrectomy), and trial data (the INT 116 trial in the USA, the MAGIC trial in the UK, and ARTIST and CLASSIC trials in Southeast Asia) [7,9,10,11]. Hence, whether a uniform management protocol for LA gastric cancer will be attained is questionable. Regardless, the performance of systemic perioperative therapy should be optimized, as it has a biological basis and has been repeatedly shown to improve outcomes. For example, in a Cochrane meta-analysis, perioperative chemotherapy increased the 5-year survival rate in patients with LA gastric cancer by 9% compared with surgery alone [16].
Early data from an epidemiological study of gastric cancer and its treatment in India suggest that distal site to proximal site migration is observed in many parts of the world except India. The data also suggests that performance of D2 gastrectomy in high-volume centers is feasible, with manageable perioperative morbidity and mortality rates [17,19]. Currently, D2 gastrectomy is the standard surgical treatment for gastric cancer at our institution. Our institution is the largest volume referral center for gastric cancer resection in India, and we and the National Cancer Institute in Japan perform D2 lymphadenectomies in a similar manner. Our prospectively audited database and previous publications have consistently highlighted the importance of a standardized technique meticulously performed by specialized surgeons and thereby showing excellent perioperative outcomes [19]. Our resection rate in this study was 74.6%, as compared with only 69.3% (for curative surgery in a perioperative cohort) in the MAGIC study. Our postsurgical complication rate of 7.5% is manageable and supports the feasibility of performing an extensive surgical procedure. Supplementary Table 2 briefly compares the results of our studies with those of the French FNCLCC/FFCD and MAGIC studies. This comparison shows that our study had better completion rates for NACT and adjuvant chemotherapy and fewer postoperative complications.
Major concerns regarding perioperative therapy include the low completion rates of chemotherapy and chemoradiotherapy and the inability to reap maximal benefits from systemic therapy. A low completion rate was observed in multiple trials: 41.0% in the MAGIC trial, 64.0% in the INT 116 trial, and 67.0% in the CLASSIC trial [9,10,13]. In addition to toxicity and refusal by patients to undergo chemotherapy even in trials, logistic constraints also dictated treatment strategies in the Indian study. Constant handling of a central line or peripherally inserted central line requires specialized skill and increases complication and infection rates, which may hamper delivery of the chemotherapeutic agents. The data from the REAL 2 [13] analysis show that the EOX and ECF regimens are almost equally effective, and thus suggest that the former is a feasible and efficacious perioperative regimen.
To the best of our knowledge, the present study is the largest published study of perioperative EOX administration in patients receiving D2 resections. Our results are heartening and may encourage further use of this well-tolerated regimen. Almost all (97.0%) of the patients in this study completed all 3 cycles of NACT, which is higher than the completion rate (86.0%) in the MAGIC trial. This difference likely reflects the use of oral capecitabine in our study rather than the continuous infusion of 5-fluorouracil in the MAGIC trial. The post-surgery completion rate of adjuvant chemotherapy in our study was 178 of the 268 patients (66.4%). More importantly, 178 of the 200 patients (89.0%) who underwent curative resection completed the planned 3 cycles of adjuvant chemotherapy. This completion rate is unprecedented and allows 2 conclusions: EOX chemotherapy is well tolerated and, more strategically, it is feasible when combined with extensive D2 resection, as previously reported by members of our center [17]. Our adverse event profile highlights the tolerability of EOX: only 21.6% of the patients had grade 3 or 4 toxicities. This rate is markedly lower than that obtained for the ECF regimen in MAGIC trial and the EOX regimen in the REAL 2 analysis.
The survival values obtained during our limited follow-up period of 17 months are in between those reported in studies [7,8,9,10,11] of Western populations and Southeast Asian populations. While a longer follow-up period and prospective data are required to validate our data, identification of the factors that contribute to the outcomes of LA gastric cancer (e.g., those related to etiology, histology, advanced presentation, and differential responses to chemotherapy) may provide insight into this disease.
Our study attempted to address the potential prognostic factors for LA gastric cancer and their impact on outcome. In agreement with the results of a previous study [20], post-therapy pathological staging correlated with outcome in our study. Both T stage (ypT1/ypT2 vs. ypT3/ypT4) and nodal status (ypN0 vs. ypN+) significantly correlated with both PFS and OS. This finding is important as it provides a means of quantifying responses to chemotherapy; at present, assessment of radiological responses to chemotherapy in gastric cancer is not well standardized. It also adds fuel to the growing debate on whether intensification of adjuvant chemotherapy in poor responders is a viable option. The post-NACT TRG did not significantly correlate with PFS or OS in our study, which contradicts its value in predicting survival after chemoradiotherapy as shown by Mandard et al. [21].
In addition to pathological T and N downstaging, the absence of obstruction features at presentation also correlated with improved OS (P=0.0001). Whether obstruction is associated with a more advanced disease stage or impedes food intake, which may contribute to a poor outcome, remains open to question. In a retrospective study of patients who underwent radical surgery for distal cancers, gastric outlet obstructions significantly reduced the survival time from 50.8 to 30.6 months (P=0.001) [22].
Surprisingly, our study showed that survival outcomes were significantly worse in patients starting chemotherapy >3 weeks (compared with ≤3 weeks) after surgery. Whether this time limit will apply to gastric cancers in larger studies remains to be determined.
Although our study is the largest cohort study of the EOX regimen in LA gastric cancer to date and assesses safety, efficacy, and D2 gastrectomy, its retrospective design limits its general applicability. Another limitation is the lack of accurate pretreatment TNM staging via endoscopic ultrasonography. The prognostic factors identified in our analysis (ypT and ypN stage, delay in adjuvant chemotherapy, symptoms of obstruction) are hypothesis-generating, requiring prospective data for validation. We also acknowledge the lack of grade 1 and 2 toxicity data, as such data are important when considering wider usage of EOX, especially in the curative setting with quality of life assessment.
EOX chemotherapy with curative resection and D2 lymphadenectomy is a suggested alternative to existing perioperative regimens. The acceptable postoperative complication rate and relatively high resection, chemotherapy completion, and survival rates obtained in this study require further evaluation and validation in a clinical trial.
Acknowledgments
Dr. Shripad Banavali, Head of Department of Medical Oncology and Dr. Sudeep Gupta have constantly supported us throughout the study inception to the end.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Parts of this study were presented at the Asia Pacific Gastroenterology Cancer Summit in 2016 as an oral presentation.
Supplementary Materials
Postoperative pathology
Comparison of our results with those of 2 prominent studies
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Nashimoto A, Nakajima T, Furukawa H, Kitamura M, Kinoshita T, Yamamura Y, et al. Gastric Cancer Surgical Study Group, Japan Clinical Oncology Group Randomized trial of adjuvant chemotherapy with mitomycin, Fluorouracil, and Cytosine arabinoside followed by oral Fluorouracil in serosa-negative gastric cancer: Japan Clinical Oncology Group 9206-1. J Clin Oncol. 2003;21:2282–2287. doi: 10.1200/JCO.2003.06.103. [DOI] [PubMed] [Google Scholar]
- 4.Rivera F, Vega-Villegas ME, López-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev. 2007;33:315–324. doi: 10.1016/j.ctrv.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald JS. Treatment of localized gastric cancer. Semin Oncol. 2004;31:566–573. doi: 10.1053/j.seminoncol.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, EUROCARE Working Group EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. MAGIC Trial Participants Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 8.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 9.Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. CLASSIC trial investigators Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 12.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362:858–859. doi: 10.1056/NEJMc0911925. [DOI] [PubMed] [Google Scholar]
- 14.Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 15.Xiong BH, Cheng Y, Ma L, Zhang CQ. An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer. Cancer Invest. 2014;32:272–284. doi: 10.3109/07357907.2014.911877. [DOI] [PubMed] [Google Scholar]
- 16.Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, et al. GE Adenocarcinoma Meta‐analysis Group Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev. 2013:CD008107. doi: 10.1002/14651858.CD008107.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Sirohi B, Barreto SG, Singh A, Batra S, Mittra A, Rastogia S, et al. Epirubicin, oxaliplatin, and capectabine is just as “MAGIC”al as epirubicin, cisplatin, and fluorouracil perioperative chemotherapy for resectable locally advanced gastro-oesophageal cancer. J Cancer Res Ther. 2014;10:866–870. doi: 10.4103/0973-1482.146122. [DOI] [PubMed] [Google Scholar]
- 18.Common Terminology Criteria for Adverse Events (CTCAE) [Internet] Bethesda (MD): US Department of Health and Human Services; NIH Cancer Institute; 2010. [cited 2010 Jun 14]. Available from: https://stacks.stanford.edu/file/druid:nw036fx4646/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Google Scholar]
- 19.Shrikhande SV, Shukla PJ, Qureshi S, Siddachari R, Upasani V, Ramadwar M, et al. D2 lymphadenectomy for gastric cancer in Tata Memorial Hospital: Indian data can now be incorporated in future international trials. Dig Surg. 2006;23:192–197. doi: 10.1159/000094537. [DOI] [PubMed] [Google Scholar]
- 20.Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 21.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 22.Park SH, Mok YJ, Kim JH, Park SS, Kim SJ, Kim CS. Clinical significance of gastric outlet obstruction on the oncologic and surgical outcomes of radical surgery for carcinoma of the distal stomach. J Surg Oncol. 2009;100:215–221. doi: 10.1002/jso.21256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Postoperative pathology
Comparison of our results with those of 2 prominent studies