Abstract
Purpose
This study aimed to analyze G3BP1 and VEZT expression profiles in patients with gastric cancer, and examine the possible relationship between the expressions of each gene and clinicopathological factors.
Materials and Methods
Expression of these genes in formalin-fixed paraffin embedded (FFPE) tissues, collected from 40 patients with gastric cancer and 40 healthy controls, was analyzed. Differences in gene expression among patient and normal samples were identified using the GraphPad Prism 5 software. For the analysis of real-time polymerase chain reaction products, GelQuantNET software was used.
Results
Our findings demonstrated that both VEZT and G3BP1 mRNA expression levels were downregulated in gastric cancer samples compared with those in the normal controls. No significant relationship was found between the expression of these genes and gender (P-value, 0.4835 vs. 0.6350), but there were significant changes associated with age (P-value, 0.0004 vs. 0.0001) and stage of disease (P-value, 0.0019 vs. 0.0001). In addition, there was a direct relationship between VEZT gene expression and metastasis (P-value, 0.0462), in contrast to G3BP1 that did not demonstrate any significant correlation (P-value, 0.1833).
Conclusions
The results suggest that expression profiling of VEZT and G3BP1 can be used for diagnosis of gastric cancer, and specifically, VEZT gene could be considered as a biomarker for the detection of gastric cancer progression.
Keywords: Stomach neoplasms, G3BP1, VEZT, Gene expression
INTRODUCTION
Although the incidence of gastric cancer has been dramatically declined due to lifestyle and environmental changes, like Helicobacter pylori eradication and smoking cessation, this cancer still remains the 5th most common malignancy in the world. Moreover, it is the third leading cause of cancer-related death in both sexes worldwide [1]; in 2011, it accounted for 4% of cancer death [2]. The distribution of gastric cancer varies across geographical regions, which illustrates the multitude of factors that are associated with the incidence, survival, and mortality rates of the disease [3]; even marital status, low educational attainment, and low income increase the risk of gastric cancer [4]. In 2012, 70% of gastric cancer cases occurred in developing countries, and about half of them occurred in eastern Asia [3,5]. Similar to other cancers, epigenetic changes, including DNA methylation, epigenetic gene silencing, and histone modifications play a key role in the development of gastric cancer [6].
As classified by Laurén, the 2 main histologic subtypes of gastric cancer are intestinal and diffuse type, which are different in molecular profiles, epidemiology, etiology, pathogenesis, and behavior of the disease [1,7]. For example, the intestinal gastric cancer commonly arises from a premalignant gastric change, such as atrophic gastritis followed by intestinal metaplasia and dysplasia, which in turn, develops into a chronic inflammatory condition that is usually induced by H. pylori infection [1,7]. Because of gastric cancer heterogeneous properties [1,7], the identified biomarkers for diagnosis of this disease are rare. There are several molecular markers associated with the early diagnosis of gastric cancer, including the carcinoembryonic antigen, cancer antigen 19-9, and recently, some of microRNAs (miRNAs) and DNA hypomethylation. Other molecular markers, such as growth factors, cytokines, cell cycle regulators, apoptosis‑associated factors, and epigenetic alterations are associated with the prognosis of gastric cancer [8].
Modification of downstream or upstream effectors of Ras signaling, or activating mutations in Ras genes, lead to aberrant activation of Ras signaling [9]; this phenomenon has been regularly reported in several types of tumors. G3BP1 is a downstream effector of Ras signaling [10]. Overexpression of G3BP1 protein has been reported in several types of human tumors, such as gastric cancer, colon cancer, head and neck cancers, pancreatic cancer, breast cancer, and esophageal squamous carcinoma [10,11,12,13,14,15]. G3BP1 protein can bind to various proliferation-related proteins, like RasGAP through its conserved N-terminal nuclear transport factor 2-like domain [10,16,17,18]. In addition, G3BP1 protein can mediate metabolism of mRNA through its phosphorylation-dependent RNase activity, which originates from its RNA recognition motif [19]. Inactivation of tumor suppressor genes is an essential step in the development of gastric cancer [20,21]. miRNAs and promoter methylation plays a fundamental role in gene inactivation. Particularly, miRNAs regulate the expression of a protein-coding gene, via degrading its mRNA or inhibiting its translation [21]. VEZT gene encodes an adherens junctions transmembrane protein, called VEZATIN. This protein has 3 domains, extracellular, transmembrane, and intracellular. The intracellular domain of VEZATIN is long and binds directly to myosin VIIA [22], while it is indirectly connected with actin cytoskeleton and E-cadherin-catenin complex [23]. Recently, miRNAs and promoter methylation were reported as 2 significant mechanisms of the transcriptional inactivation of genes in human cancer [24]. The VEZT gene has been identified as a tumor suppressor gene; therefore, the role of promoter hypermethylation of this gene and miRNAs was analyzed [20,22,24].
In this study, we examined the expression of G3BP1 gene, which has been suggested to modulate the proliferation, migration, and invasion of gastric cancer tumor cells [10], as well as the expression of VEZT gene that has been identified as a tumor suppressor gene [20]. We also analyzed the correlation between the expression of these genes and disease progression, cell differentiation, age, and gender in Iranian patients with gastric cancer.
MATERIALS AND METHODS
In this study, formalin-fixed paraffin embedded (FFPE) samples derived from 2 different patient groups, were used to examine the expression signature of the genes. With the collaboration of government and public sector hospitals, a total of 40 clinical files of patients with gastric cancer from 2014 to 2016, were investigated. In the cascade of genomic and phenotypic changes, which have been described as “multistep oncogenesis,” invasive gastric cancer is the last step. This process includes a variety of gradually dedifferentiated phenotypes, which may result in a new cell, characterized by independent and potentially metastatic growth (termed as plasia). The World Health Organization has redefined dysplasia as non-invasive neoplasia. In the natural history of gastric cancer, dysplasia precedes invasive adenocarcinoma. A total of 40 paraffin-embedded tissue samples from patients with gastric cancer and 40 samples from healthy individuals for alternative reasons were selected. The histologic grade is a qualitative assessment of the differentiation of the tumor expressed, as the extent to which a tumor resembles the normal tissue at that site. Tumors with well-differentiated cancer cells are less aggressive than undifferentiated or poorly differentiated cancer cells. In most types of cancer, tumors have moderately differentiated cancer cells; these cells are somewhere between well-differentiated and un-differentiated cells (Table 1). Patients who developed recurrent gastric cancer or metastasis, underwent ultrasound, radiography, bone scan, and pathology investigations, and their clinical information were utilized for further analysis. RNA was extracted from FFPE samples and analyzed via real-time reverse transcription polymerase chain reaction (RT-PCR). The study was approved by ethical committee of Deputy of Research Affairs of Shahid Beheshti University of Medical Sciences.
Table 1. Summary statistics for clinical variables among the patient population.
| Variables | No. of patients | |
|---|---|---|
| Age (yr) | ||
| ≥50 | 20 | |
| <50 | 20 | |
| Chemotherapy | ||
| Yes | 40 | |
| No | 0 | |
| Radiation therapy | ||
| Yes | 40 | |
| No | 0 | |
| H. pylori | ||
| Patient (+/−) | 40/0 | |
| Normal (+/−) | 0/40 | |
| Disease metastasis (M) | ||
| M1 | 25 | |
| M0 | 15 | |
| TNM classification | ||
| I | 15 | |
| II | 8 | |
| III | 7 | |
| IV | 10 | |
| FFPE | ||
| P | 40 | |
| N | 40 | |
TNM = tumor, node, metastasis; FFPE = formalin-fixed paraffin embedded.
Real-time PCR assay, gene selection, and designing of primers
G3BP1 and VEZATIN genes, which were reported to have a significant association with gastric cancer, were selected from gene expression databases [2,5,16,21,25]. The expression level of G3BP1 and VEZATIN genes in tumor and marginal non-tumor samples was investigated via specific G3BP1 primers, F: 5ʹ-AAACGTTTGTCCTTGCTCCT-3ʹ and R: 5ʹ-TTCAGACTCCTCCTGAGGCT-3ʹ); and VEZATIN primers, F: 5ʹ-ACCGAAGTGATTTCCAGAGG-3ʹ and R: 5ʹ-AGATGCTGACTTGGATGCTG-3ʹ. Furthermore, GAPDH gene was used as an internal control, and its primer sequences were as follow; GAPDH F: 5ʹ-ATGGAGAAGGCTGGGGCT-3ʹ and GAPDH R: 5ʹ-ATCTTGAGGCTGTTGTCATACTTCTC-3ʹ. Quantitative real-time PCR was performed using SYBR® Green Master mix (Bio-Rad Laboratories, Inc., Hercules, CA, USA), according the manufacturer's instructions. The PCR program was as follow: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 10 seconds, and annealing/extension for 30 seconds at 54.5°C, 58°C, and 59°C for G3BP1, VEZT, and GAPDH, respectively. To minimize experimental variability of the cycle threshold (Ct) values, defined as the cycle number where the fluorescent signal is higher than the background level in the exponential phase of the PCR amplification, those values were determined via the second derivative maximum method. The PCR product size was verified by electrophoresis on agarose gel, and the authenticity of amplified fragment was confirmed via direct sequencing of the PCR product.
RNA extraction
After paraffin removal stage using xylene, tissue digestion was performed by proteinase K (Fermentas, Waltham, MA, USA). Then, the total RNA was isolated using RNX-Plus (SinaClon, Karaj, Iran), according to the protocol supplied by the manufacturer (CinnaGen Co., Tehran, Iran) [26]. Quality assessment of the total RNA was established using NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA). After RNA isolation using RNeasy® Kit (Applied Biosystems, Foster City, CA, USA), the RNA quality was assessed using Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA, USA). Total RNA extracted from the tissue section was reverse transcribed in a reaction mixture containing 250 mM Tris-HCl buffer (pH 8.3; 375 mM KCl and 15 mM MgCl2) (Applied Biosystems), 0.1 MDTT (Applied Biosystems), 10 mM dNTPs (Fermentas), 20 U/reaction of RNasin™ ribonuclease inhibitor (Applied Biosystems), and 200 U/reaction of Superscript™ III RT (Applied Biosystems). The cDNA obtained, was diluted by 10-fold in 2 ng/μL polyinosinic acid, and used in quantitative PCR (qPCR) reactions [26].
Real-time qPCR
The qPCR reactions were performed in 96-well plates on a real-time PCR 7500 (Applied Biosystems) instrument. Typically, a total 20 µL of the reaction mixture contained 100 ng of cDNA, 12.5 µL of assays-on-demand SYBR Green PCR Master Mix (Applied Biosystems), of these genes were used, 1 µL each of 10 mmol/µL forward and reverse primers, and 4.5 µL nuclease-free water. All PCRs were performed using the ABI Prism 7500 System (Applied Biosystems) under the conditions recommended by the manufacturers. A standard curve was constructed with at least 4 different concentrations in triplicate, using a control cDNA for both the control gene (GAPDH) and the genes of interest. These 2 genes were analyzed in 40 paraffin-embedded tissue. Differences in gene expression among metastatic and normal samples were estimated using Student GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) with the t-test analysis. The relative gene expression levels were determined using the comparative Ct (ΔΔCt) method. Another analysis was performed using the GelQuantNET software (BiochemLabSolutions, San Francisco, CA, USA) to examine the real-time RT-PCR products.
RESULTS
To examine whether G3BP1 and VEZT expression levels are correlated with clinicopathological factors of patients with gastric cancer, G3BP1 and VEZT expression levels were evaluated in gastric cancer and normal specimens; GAPDH gene expression was used as internal control.
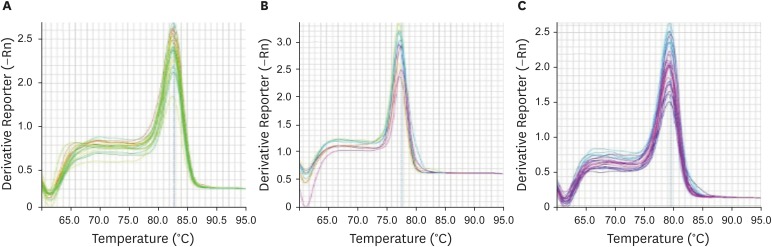
To analyze the specificity of qPCR products and absence of unspecific PCR products and dimer primers, the melt curves of each gene were drawn, independently (Fig. 1).
Fig. 1.
Melting curves of (A) GAPDH, (B) VEZT, and (C) G3BP1 genes in normal and cancerous samples show specific qPCR products for each gene.
qPCR = quantitative polymerase chain reaction.
After converting the calculated Ct for each sample to relative quantitation (RQ), gene expression was assessed via the ΔΔCt method. Expression of each gene was compared between normal and cancerous samples. A graph for each gene was generated, independently.
Analysis of both G3BP1 and VEZT expression levels and clinicopathological variables was performed via the t-test. We analyzed the correlation between expression of each of G3BP1 and VEZT genes, individually, with clinicopathological factors in patients with gastric cancer. A summary of the results is presented in Table 2.
Table 2. Correlation of G3BP1 and VEZT expression with clinicopathological factors in patients with gastric cancer.
| Variables | No. | G3BP1 mean RQ | VEZT mean RQ | G3BP1 P-value | VEZT P-value | |
|---|---|---|---|---|---|---|
| Samples | 0.6344 | 0.0004 | ||||
| Patients | 40 | 0.9308 | 0.4322 | |||
| Normal | 40 | 1.0116 | 1.0292 | |||
| Gender | 0.4835 | 0.6350 | ||||
| Male | 21 | 0.8861 | 0.5247 | |||
| Female | 19 | 0.9783 | 0.4737 | |||
| Age (yr) | 0.0004 | 0.0001 | ||||
| <50 | 20 | 1.1909 | 0.4402 | |||
| ≥50 | 20 | 0.7961 | 0.7885 | |||
| TNM Classification | 0.0001 | 0.0019 | ||||
| I | 15 | 0.8883 | 0.4204 | |||
| II | 8 | 0.6687 | 0.4534 | |||
| III | 7 | 0.3655 | 0.2667 | |||
| IV | 10 | 0.1603 | 0.1334 | |||
| Disease metastasis (M) G3BP1 and VEZT | 0.1833 | 0.0462 | ||||
| M1 | 25 | 0.6606 | 0.4308 | |||
| M0 | 15 | 0.8735 | 0.6540 | |||
RQ = relative quantitation; TNM = tumor, node, metastasis.
All cancerous samples showed a decrease in mRNA expression level of VEZT compared with the normal samples. Correlation between mean RQ of VEZT in patients lower than 50 years-old and above 50 years-old was significant (P-value, 0.0001). Similarly, there was a significant relationship between the stage of the disease, metastasis, and gene expression (P-value, 0.0019 and 0.0462). However, no significant relationship was observed between gender and VEZT gene expression (P-value, 0.6350).
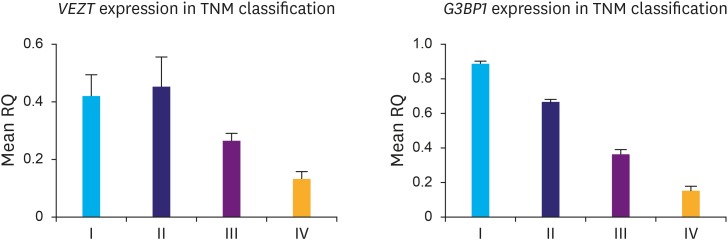
Although the expression of G3BP1 mRNA level in most of gastric cancer samples were downregulated, our analysis showed no significant correlation between G3BP1 expression and gender and disease metastasis (Table 2). As it is shown in Fig. 2, there is a significant relation between G3BP1 gene expression and the disease stage.
Fig. 2.
VEZT and G3BP1 gene expression mRNA level in TNM calssification.
The achieved results were analyzed with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Each experiment was repeated 3 times. The P-value for VEZT and G3BPI are 0.0019 and 0.0001, respectively.
TNM = tumor, node, metastasis; RQ = relative quantitation.
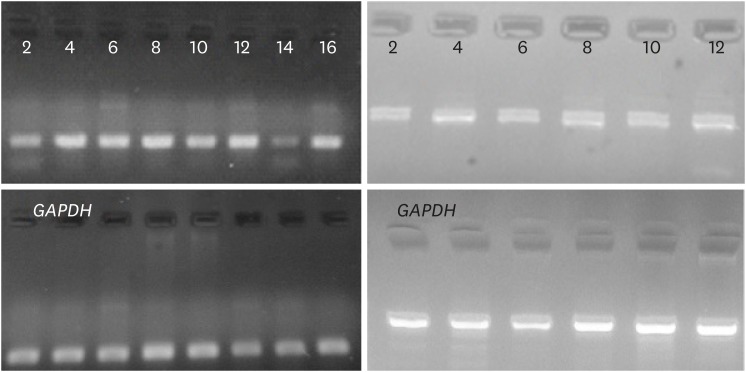
After electrophoresis and imaging of the qPCR products, the findings were analyzed using GelQuantNET software (BiochemLabSolutions; Fig. 3). Again, the only significant relationship was related to the stage of disease and down-regulation of VEZT gene, and all the results were consistent with the qPCR results.
Fig. 3.
G3BP1 and GAPDH real-time PCR products were loaded in upper and lower lane, respectively. The results obtained after GelQuantNET software (BiochemLabSolutions, San Francisco, CA, USA) and GraphPad Prism 5 analysis (GraphPad Software, Inc., La Jolla, CA, USA), suggest no significant correlation between the G3BP1 expression and disease progression (P-value, 0.0886), while VEZT expression was found to be significantly correlated (P-value, 0.0175).
PCR = polymerase chain reaction.
DISCUSSION
Gastric cancer is the second most common cancer in the world. It is now obvious that various genetic alterations, including H. pylori infection, activation of oncogenes, and inactivation of tumor suppressor genes, are necessary stages in gastric cancer development [20]. Studies have shown that people who carry high-risk genetic variants and have specific dietary habits can have an increased risk of gastric cancer, compared with those who do not carry high-risk genetic variants, which may justify the higher incidence rates of gastric cancer in specific countries [27]. VEZT and G3BP1 genes are 2 biomarkers, which their expression in gastric cancer tumors was evaluated, separately.
Modification of G3BP1, as a downstream effector of Ras, can cause aberrant activation of Ras signaling, which has been reported in various types of tumors [9]. The significant role of G3BP1 in promotion of proliferation, migration, invasion, and survival of tumor cells has been also reported in several studies [10]. A previous study demonstrated up-regulation of G3BP1 at the post-transcriptional level in gastric cancer tumors, although in most gastric cancer cases the mRNA levels were decreased [10]. Another study demonstrated that activation of the heregulin-human epidermal growth factor receptor 2 (HRG-HER2) signaling pathway in breast cancer cells might contribute to the up-regulation of G3BP1 at the mRNA and protein levels [13]. Molecular and functional studies indicate that the interaction of G3BP1 with β-F1 mRNA inhibits its translation, supporting a role for G3BP1 in the glycolytic switch that occurs in cancer [25]. Two previous studies demonstrated that Y-box binding protein 1 regulates a ribonucleoprotein complex, known as stress granules, as well as formation [28,29] and tumor progression by translationally activating G3BP1. Moreover, down-regulation of G3BP1 in sarcoma xenografts, which prevents tumor invasion and blocks lung metastasis in mouse models, has been reported [28]. Finally, inactivation of the p53 tumor suppressor pathway is a critical step in human tumorigenesis and isoforms of G3BP, including G3BP1 and G3BP2, were suggested as negative regulators of p53 [30]. In line with a previous study [10], our results showed a decrease in G3BP1 mRNA expression level of most patients' samples in comparison with the normal samples, but no significant correlation between G3BP1 mRNA expression level and age, gender, cell differentiation, and cancer stage, was observed.
As previously mentioned, the inactivation of tumor suppressor genes is an essential step in the development of gastric cancer. Promoter hypermethylation of gastric cancer cell lines was demonstrated using methylation specific PCR [24] and bisulfite sequence-PCR methods [20,24]. Moreover, a luciferase reporter assay demonstrated that miR-43c suppresses VEZT protein expression [24]. In another study, epigenetic regulation and biological functions of VEZT in gastric cancer tumors were examined, showing that VEZT was hypermethylated in tissues and blood of patients with gastric cancer compared with those of healthy controls. Additionally, H. pylori was reported as an inducing factor of methylation and silencing of VEZT gene in GES-1 cells, since promoter methylation of VEZT in H. pylori positive patients was 2.4-fold higher than in the healthy controls [20]. Although the correlation between expression of VEZT and age, gender, cell differentiation, tumor size, and tumor site were not reported, a significant association was observed with lymphatic metastasis, tumor, node, metastasis (TNM) stage, depth of cancer, and longer overall survival [20,22]. Inhibition of cell proliferation, migration, invasion, and tumorigenesis in vivo and in vitro via VEZT expression restoration in gastric cancer cell lines, suggests that VEZT can be considered as a therapeutic agent in the treatment of gastric and/or other cancers [20,22,24].
Similar to the previous studies [20,22] the genetic variation in different populations, as well as the role of epigenetic factors and the single nucleotide polymorphisms, demonstrate the need of similar studies of VEZT and G3BP1 genes in patients with gastric cancer in the Iranian population; for this reason, this study investigated the expression levels of these genes in samples from Iranian individuals. Our results showed that VEZT mRNA levels were decreased in all gastric cancer samples and expression of this gene had a significant correlation with the status of gastric cancer progression. Therefore, evaluation of VEZT and G3BP1 gene expression could be used as a molecular technique for diagnosis of gastric cancer. Finally, this study suggests that VEZT gene could be considered as a biomarker for detection of gastric cancer progression.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11:664–674. doi: 10.1038/nrgastro.2014.143. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Lagergren J, Andersson G, Talbäck M, Drefahl S, Bihagen E, Härkönen J, et al. Marital status, education, and income in relation to the risk of esophageal and gastric cancer by histological type and site. Cancer. 2016;122:207–212. doi: 10.1002/cncr.29731. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer. 2015;51:1164–1187. doi: 10.1016/j.ejca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Fu DG. Epigenetic alterations in gastric cancer. Mol Med Rep. 2015;12:3223–3230. doi: 10.3892/mmr.2015.3816. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carcas LP. Gastric cancer review. J Carcinog. 2014;13:14. doi: 10.4103/1477-3163.146506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Z, Jiang W, Wang L. Biomarkers for gastric cancer: progression in early diagnosis and prognosis. Oncol Lett. 2015;9:1502–1508. doi: 10.3892/ol.2015.2959. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 10.Min L, Ruan Y, Shen Z, Jia D, Wang X, Zhao J, et al. Overexpression of Ras-GTPase-activating protein SH3 domain-binding protein 1 correlates with poor prognosis in gastric cancer patients. Histopathology. 2015;67:677–688. doi: 10.1111/his.12695. [DOI] [PubMed] [Google Scholar]
- 11.Guitard E, Parker F, Millon R, Abecassis J, Tocqué B. G3BP is overexpressed in human tumors and promotes S phase entry. Cancer Lett. 2001;162:213–221. doi: 10.1016/s0304-3835(00)00638-8. [DOI] [PubMed] [Google Scholar]
- 12.Taniuchi K, Nishimori I, Hollingsworth MA. The N-terminal domain of G3BP enhances cell motility and invasion by posttranscriptional regulation of BART. Mol Cancer Res. 2011;9:856–866. doi: 10.1158/1541-7786.MCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 13.Barnes CJ, Li F, Mandal M, Yang Z, Sahin AA, Kumar R. Heregulin induces expression, ATPase activity, and nuclear localization of G3BP, a Ras signaling component, in human breast tumors. Cancer Res. 2002;62:1251–1255. [PubMed] [Google Scholar]
- 14.Winslow S, Leandersson K, Larsson C. Regulation of PMP22 mRNA by G3BP1 affects cell proliferation in breast cancer cells. Mol Cancer. 2013;12:156. doi: 10.1186/1476-4598-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang HZ, Liu JG, Wei YP, Wu C, Cao YK, Wang M. Expression of G3BP and RhoC in esophageal squamous carcinoma and their effect on prognosis. World J Gastroenterol. 2007;13:4126–4130. doi: 10.3748/wjg.v13.i30.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker F, Maurier F, Delumeau I, Duchesne M, Faucher D, Debussche L, et al. A Ras-GTPase-activating protein SH3-domain-binding protein. Mol Cell Biol. 1996;16:2561–2569. doi: 10.1128/mcb.16.6.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy D, French J, Guitard E, Ru K, Tocque B, Mattick J. Characterization of G3BPs: tissue specific expression, chromosomal localisation and rasGAP(120) binding studies. J Cell Biochem. 2001;84:173–187. doi: 10.1002/jcb.1277. [DOI] [PubMed] [Google Scholar]
- 18.Tourrière H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, et al. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003;160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Gallouzi IE, Parker F, Chebli K, Maurier F, Labourier E, Barlat I, et al. A novel phosphorylation-dependent RNase activity of GAP-SH3 binding protein: a potential link between signal transduction and RNA stability. Mol Cell Biol. 1998;18:3956–3965. doi: 10.1128/mcb.18.7.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao R, Guo X, Zhi Q, Shi Y, Li L, Mao X, et al. VEZT, a novel putative tumor suppressor, suppresses the growth and tumorigenicity of gastric cancer. PLoS One. 2013;8:e74409. doi: 10.1371/journal.pone.0074409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 22.Li YS, Chen YZ, Guo XB, Liu X, Li LP. VEZT as a novel independent prognostic factor in gastric cancer. Cancer Biomark. 2015;15:375–380. doi: 10.3233/CBM-150476. [DOI] [PubMed] [Google Scholar]
- 23.Bahloul A, Simmler MC, Michel V, Leibovici M, Perfettini I, Roux I, et al. Vezatin, an integral membrane protein of adherens junctions, is required for the sound resilience of cochlear hair cells. EMBO Mol Med. 2009;1:125–138. doi: 10.1002/emmm.200900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo X, Jing C, Li L, Zhang L, Shi Y, Wang J, et al. Down-regulation of VEZT gene expression in human gastric cancer involves promoter methylation and miR-43c. Biochem Biophys Res Commun. 2011;404:622–627. doi: 10.1016/j.bbrc.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Ortega ÁD, Willers IM, Sala S, Cuezva JM. Human G3BP1 interacts with β-F1-ATPase mRNA and inhibits its translation. J Cell Sci. 2010;123:2685–2696. doi: 10.1242/jcs.065920. [DOI] [PubMed] [Google Scholar]
- 26.Izadi A, Moslemi E, Poorhosseini SM, Yassaee VR, Kheiri HR, Elikai HR. UBD identify in paraffin tissues in patients with colorectal cancer. J Isfahan Med Sch. 2014;32:1–10. [Google Scholar]
- 27.Kim J, Cho YA, Choi WJ, Jeong SH. Gene-diet interactions in gastric cancer risk: a systematic review. World J Gastroenterol. 2014;20:9600–9610. doi: 10.3748/wjg.v20.i28.9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somasekharan SP, El-Naggar A, Leprivier G, Cheng H, Hajee S, Grunewald TG, et al. YB-1 regulates stress granule formation and tumor progression by translationally activating G3BP1 . J Cell Biol. 2015;208:913–929. doi: 10.1083/jcb.201411047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annibaldi A, Dousse A, Martin S, Tazi J, Widmann C. Revisiting G3BP1 as a RasGAP binding protein: sensitization of tumor cells to chemotherapy by the RasGAP 317-326 sequence does not involve G3BP1 . PLoS One. 2011;6:e29024. doi: 10.1371/journal.pone.0029024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MM, Wiederschain D, Kennedy D, Hansen E, Yuan ZM. Modulation of p53 and MDM2 activity by novel interaction with Ras-GAP binding proteins (G3BP) Oncogene. 2007;26:4209–4215. doi: 10.1038/sj.onc.1210212. [DOI] [PubMed] [Google Scholar]